Abstract
Morning blood pressure (BP) surge is considered to be an independent risk factor for cardiovascular diseases. We tested the hypothesis that increased large-artery stiffness and impaired sympathetic baroreflex sensitivity (BRS) contribute to augmented morning surge in elderly hypertensive subjects. Morning surge was assessed as morning systolic BP averaged for 2 h just after waking up minus minimal sleeping systolic BP by using ambulatory BP monitoring (ABPM) in 40 untreated hypertensive [68 ± 1 (SE) yr] and 30 normotensive (68 ± 1 yr) subjects. Beat-by-beat finger BP and muscle sympathetic nerve activity (MSNA) were recorded in the supine position and at 60° upright tilt. We assessed arterial stiffness with carotid-to-femoral pulse wave velocity (cfPWV) and sympathetic BRS during spontaneous breathing. Awake and asleep ABPM-BPs and morning surge were higher in hypertensive than normotensive subjects (all P < 0.001). cfPWV was higher (P = 0.002) and sympathetic BRS was lower (P = 0.096) in hypertensive than normotensive subjects. Hypertensive subjects with morning surge ≥35 mmHg (median value) had higher cfPWV (11.9 ± 0.5 vs. 9.9 ± 0.4 m/s, P = 0.002) and lower sympathetic BRS (supine: −2.71 ± 0.25 vs. −3.73 ± 0.29, P = 0.011; upright: −2.62 ± 0.22 vs. −3.51 ± 0.35 bursts·100 beats−1·mmHg−1, P = 0.052) than those with morning surge <35 mmHg. MSNA indices were similar between groups (all P > 0.05), while upright total peripheral resistance was higher in hypertensive subjects with greater morning surge than those with lesser morning surge (P = 0.050). Morning surge was correlated positively with cfPWV (r = 0.59, P < 0.001) and negatively with sympathetic BRS (r = 0.51, P < 0.001) in hypertensive subjects only. Thus, morning BP surge is associated with arterial stiffness and sympathetic BRS, as well as vasoreactivity during orthostasis in hypertensive seniors.
Keywords: circadian rhythm, sympathetic nerve activity, hypertension, aging
in humans, blood pressure (BP) shows circadian variations; it decreases during sleep and increases in the morning (19, 36). Cardiovascular events also have a distinct circadian pattern with a peak incidence in the morning (5, 19). The degree of morning BP surge from the nadir during the night has been found to be an independent predictor for cardiovascular events (12, 15) and, therefore, elderly hypertensive patients who have poor morning BP control (26) may be at higher risk of these events. Thus, it is important to evaluate the pathophysiological mechanisms underlying the morning surge in elderly hypertensive subjects.
Some studies have demonstrated that the morning surge is associated with arterial stiffness and autonomic function. For example, hypertensive patients with an exaggerated morning surge ≥50 mmHg in systolic BP (SBP) or ≥22 mmHg in diastolic BP (DBP) had higher levels of carotid intima-media thickness and urinary catecholamine excretion than those with lesser morning surge (17). Cardiovagal baroreflex sensitivity (BRS), which regulates BP at the heart through the autonomic nervous system, was negatively correlated with morning SBP (4). Moreover, it was found that a decreased cardiovagal BRS was caused by a reduction in distensibility of the carotid artery, especially during the BP rise in the morning (33). Therefore, it seems that hypertensive patients with greater arterial stiffness have a lesser ability to buffer against BP increases in the morning. Based on the finding that the contribution of total peripheral resistance (TPR) to BP change by orthostasis became greater, while the contribution of heart rate (HR) decreased with age in hypertensive subjects (16), the sympathetic baroreflex, which regulates BP via vasoconstriction, may play a more important role in control of morning BP in elderly hypertensive subjects. The morning surge is actually assessed with night time BP and morning BP for 2 h after waking up (12), suggesting that the morning surge includes BP rises upon standing. Therefore, sympathetic control of BP during upright posture may also affect the magnitude of the morning surge. However, there is no information available regarding the effect of supine or upright sympathetic BRS on the morning surge in humans.
We recently reported that sympathetic BRS was correlated with the stiffness of the barosensory arteries, the carotid artery and aorta in elderly individuals (21). Because it seems that elderly hypertensive subjects with higher arterial stiffness have lower sympathetic BRS, we hypothesized that impaired sympathetic BRS and higher arterial stiffness would be observed in elderly hypertensive subjects with greater morning surge and that the level of morning surge would be correlated with sympathetic BRS. Additionally, elderly hypertensive subjects with enhanced α1-adrenergic responsiveness were found to have a greater increase in BP by norepinephrine infusion even with a similar increase of sympathetic nerve activity compared with elderly normotensive subjects (32). Therefore, we also evaluated muscle sympathetic nerve activity (MSNA) during head-up tilt to test whether the efficacy of change in MSNA on TPR by head-up tilt is higher and/or upright sympathetic BRS is lower in elderly hypertensive subjects with greater morning surge than those with lesser morning surge or normotensive subjects.
METHODS
Subjects
Two hundred and ninety-four elderly individuals in the Dallas-Fort Worth area were contacted between August 2008 and May 2011, and 138 of them were interested in participating in research and were screened for our study. Ultimately, 70 elderly volunteers [40 hypertensive subjects (awake SBP: 135–159 and/or awake DBP: 85–99 mmHg according to 24-h ambulatory BP monitoring, ABPM) (2) and 30 normotensive subjects] participated in and completed the study. All participants were screened with a careful medical history, physical examination, 12-lead electrocardiogram, fasting blood samples, 24-h urine collection, and cardiovascular- and abdominal-echogram to confirm that they had no overt history of chronic diseases (e.g., cardiopulmonary, neurological, renal diseases, renal artery stenosis, primary aldosteronism, diabetes mellitus, and sleep apnea) that can cause secondary hypertension. They were excluded if they were smokers, regularly exercised at moderate- to high-intensity levels for >30 min/day for >3 times/wk, or their body mass index was >35 kg/m2. Women taking hormone replacement treatment were also excluded. Subjects gave their written informed consent to a protocol approved by the Institutional Review Boards of University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. Subjects' characteristics are presented in Table 1.
Table 1.
Physical characteristics
| Variables | Hypertensive Subjects | Normotensive Subjects |
|---|---|---|
| n | 40 | 30 |
| Men:women | 20:20 | 15:15 |
| Age, yr | 68 ± 1 | 68 ± 1 |
| Height, cm | 169 ± 1 | 168 ± 2 |
| Weight, kg | 77 ± 2 | 76 ± 3 |
| Body surface area, m2 | 1.90 ± 0.03 | 1.90 ± 0.04 |
| Body mass index, kg/m2 | 26.9 ± 0.4 | 26.8 ± 0.6 |
Values are means ± SE; n, no. of subjects.
Measurements
Muscle sympathetic nerve activity.
MSNA signals were obtained with microneurography (30, 31). Briefly, a recording electrode was placed in the peroneal nerve at the popliteal fossa, and a reference electrode was placed subcutaneously 2–3 cm apart from the recording electrode. The nerve signals were amplified (70,000 to 160,000-fold), band-pass filtered (700 to 2,000 Hz), full-wave rectified, and integrated with a resistance-capacitance circuit (time constant 0.1 s). Criteria for adequate MSNA recordings include: pulse synchrony; facilitation during the hypotension phase of the Valsalva maneuver, and suppression during the hypertensive overshoot phase after release; and insensitivity to emotional stimuli (37).
Hemodynamics.
HR was determined from the electrocardiogram (lead II), and beat-by-beat BP was derived by finger photoplethysmography (Nexfin; BMEYE, Amsterdam, The Netherlands). Arm cuff BP was measured by electrosphygmomanometry (model 4240; SunTech Medical Instruments, Raleigh, NC) with a microphone placed over the brachial artery to detect Korotkoff sounds. Carotid and femoral arterial pressure waveforms were obtained with a pencil-sized tonometer (SphygmoCor; AtCor Medical, Sydney, Australia). Cardiac output was measured via the modified acetylene rebreathing technique (10). Stroke volume was calculated from cardiac output divided by HR, and TPR was calculated as the quotient of mean BP and cardiac output, multiplied by 80, where variables were measured during rebreathing. Respiratory excursions were detected by a nasal cannula.
Twenty four-hour ambulatory blood pressure monitoring.
The ABPM device (Oscar 2; SunTech Medical Instruments) automatically measured BP and HR using the oscilometric method every 30 min during the awake period and every 30–60 min during the sleep period throughout the 24-h cycle (12, 15).
Protocol
Patients who had been taking antihypertensive medications were progressively weaned from these drugs for ∼2 wk. All subjects underwent a 3-wk run-in period before testing, and they were instructed to maintain a healthy lifestyle according to the Seventh Report of the Joint National Committee standard guidelines (2). Twenty four-hour ABPM was repeated after this period. We used 24-h ABPM with the subjects' report and diary documenting the waking and sleeping time to evaluate morning surge. If they had poor sleep quality, they repeated 24-h ABPM. Before testing, all subjects consumed a 3-day isocaloric constant diet consisting of: 100 meq sodium, 100 meq potassium, and 1,000 mg calcium daily to minimize the effects of salt intake on MSNA. Fluid intake was ad libitum.
The experiment was performed in a quiet, environmentally controlled laboratory with an ambient temperature of ∼25°C. Subjects came to the laboratory early in the morning, ≥72 h after the last caffeinated or alcoholic beverage and ≥24 h after strenuous physical activity, and were placed in the supine position. At least 10 min after a satisfactory nerve recording site had been found, MSNA signals were recorded for 6 min during spontaneous breathing. All participants then performed two Valsalva maneuvers at 40 mmHg for 20 s with ∼5 min apart. Thereafter, 60° upright tilt was conducted for 10 min, and MSNA data were collected during the last 3 min of tilting. Because the day-to-day variability of MSNA is small and the reproducibility of the measurement is high (31), arterial stiffness was assessed the next morning to avoid the effect of fatigue on the autonomic nervous system and then arterial stiffness because of the lengthy study. After >30 min of supine rest, baseline hemodynamics were measured. Carotid and femoral arterial pressure waveforms were obtained simultaneously with the electrocardiogram.
Data Analysis
Data were sampled at 625 Hz and stored on a personal computer with a commercial data acquisition system (AcqKnowledge; Biopac System, Santa Barbara, CA). Off-line data analyses were performed using signal-processing software (LabView; National Instruments, Austin, TX). Sympathetic bursts were identified by a computer program (21) and then confirmed by an experienced microneurographer. The integrated neurogram was normalized by assigning a value of 100 to the largest amplitude of a sympathetic burst during the 6-min data collection (13). Burst area was measured as the area under the curve of each sympathetic burst on a beat-by-beat basis. The number of bursts per minute (burst frequency), the number of bursts per 100 heart beats (burst incidence), and total burst area per minute (total activity) were used as quantitative indexes. The efficacy of MSNA for vasoconstriction was assessed with a percent change in TPR divided by percent change in MSNA burst frequency by upright tilt.
Morning surge.
We defined morning surge as the morning SBP (averaged SBP for 2 h just after wake-up) minus the lowest nocturnal SBP by using ABPM after the run-in period (sleep-through morning surge) (12). We found similar results by using sleep-through morning surge and preawakening morning surge calculated with averaged SBP for 2 h before waking up instead of the lowest nocturnal SBP. Morning HR increase was defined using the same formula.
Arterial stiffness.
We used carotid-to-femoral pulse wave velocity (cfPWV) as an index of large-artery stiffness (14). A foot-to-foot methodology was employed to determine pressure wave transit time at the carotid and femoral artery in relation to the R-wave of the electrocardiogram. Pulse transit length was estimated by subtracting the distance between the sternal notch and the measuring point at the carotid artery from the distance between the sternal notch and the measuring point at the femoral artery. cfPWV was calculated from the transit length divided by the transit time.
Baroreflex sensitivity.
Sympathetic BRS was assessed by using the slope of the linear correlation between MSNA and DBP during spontaneous breathing in each subject (6, 30) after confirming that the r value was >0.5 as described previously (27). MSNA burst incidence was calculated over a 3-mmHg DBP bin (6, 21) and statistically weighted to reduce the impacts of inherent nonbaroreflex variability (e.g., respiration) and minor variation of bin width and position (13). Cardiovagal BRS was assessed by averaging the values of the slope of the linear correlation between R-R interval and beat-by-beat SBP during the two Valsalva maneuvers in each subject after confirming the r value was >0.8 (18). Values for SBP were linearly regressed against the corresponding R-R interval (lag 1) (18) during the hypertensive phase (phase IV), which was in the same direction of the BP change with the morning surge.
Statistical Analysis
Values are expressed as means ± SE. Linear regression analysis was used to evaluate the correlation between morning surge and arterial stiffness and sympathetic and cardiovagal BRS. Data between hypertensive and normotensive subjects were compared using unpaired t-tests. If normality tests and/or equal variance tests failed, we compared the differences between groups using Mann-Whitney Rank Sum Tests. Multiple-regression analysis was used to assess factors related with morning surge in elderly hypertensive and normotensive subjects. A P value of <0.05 was considered statistically significant.
RESULTS
Ambulatory Blood Pressure and Heart Rate
ABPM-BPs were higher in hypertensive than normotensive subjects for all periods: 24 h, awake, and asleep (Table 2). Both the lowest nocturnal SBP and morning SBP were higher in hypertensive than normotensive subjects with no difference in night time dip in SBP. ABPM-HR was not different between groups in any period.
Table 2.
Twenty four-hour ambulatory blood pressure and heart rate
| Variables | Hypertensive Subjects | Normotensive Subjects | P Values |
|---|---|---|---|
| ABPM-SBP, mmHg | |||
| 24 h | 142 ± 1 | 121 ± 1 | <0.001 |
| Awake | 147 ± 2 | 124 ± 1 | <0.001 |
| Asleep | 129 ± 2 | 110 ± 1 | <0.001 |
| ABPM-DBP, mmHg | |||
| 24 h | 79 ± 1 | 70 ± 1 | <0.001 |
| Awake | 81 ± 1 | 73 ± 1 | <0.001 |
| Asleep | 70 ± 1 | 61 ± 1 | <0.001 |
| Morning SBP, mmHg | 147 ± 2 | 121 ± 1 | <0.001 |
| The lowest nocturnal SBP, mmHg | 111 ± 2 | 97 ± 1 | <0.001 |
| Nighttime dip in SBP, % | 12 ± 2 | 11 ± 1 | 0.767 |
| ABPM-HR, beats/min | |||
| 24 h | 69 ± 1 | 71 ± 2 | 0.461 |
| Awake | 72 ± 1 | 74 ± 2 | 0.369 |
| Asleep | 63 ± 1 | 63 ± 1 | 0.918 |
| Morning HR, beats/min | 69 ± 2 | 71 ± 2 | 0.539 |
| The lowest nocturnal HR, beats/min | 56 ± 1 | 56 ± 1 | 0.919 |
Values are means ± SE. ABPM, ambulatory blood pressure monitoring; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Supine Resting Variables
Table 3 depicts hemodynamics and MSNA indexes during supine rest. Supine BP was similar to asleep ABPM-BP in both hypertensive and normotensive subjects, whereas all supine BPs were still higher in hypertensive than normotensive subjects. There were no differences in HR, stroke volume, and cardiac output between groups, whereas TPR was higher in hypertensive than normotensive subjects (P = 0.081). All MSNA indexes were similar between groups.
Table 3.
Hemodynamic variables during supine rest on testing day in the laboratory
| Variables | Hypertensive Subjects | Normotensive Subjects | P Values |
|---|---|---|---|
| SBP, mmHg | 128 ± 2 | 112 ± 3 | <0.001 |
| DBP, mmHg | 72 ± 2 | 66 ± 2 | 0.006 |
| HR, beats/min | 66 ± 1 | 68 ± 2 | 0.374 |
| Stroke volume, ml | 61 ± 2 | 61 ± 3 | 0.744 |
| Cardiac output, l/min | 4.0 ± 0.1 | 4.1 ± 0.2 | 0.653 |
| TPR, dyn·s/cm5 | 1,708 ± 46 | 1,570 ± 66 | 0.081 |
| MSNA burst frequency, bursts/min | 40 ± 1 | 40 ± 2 | 0.986 |
| MSNA burst incidence, bursts/100 beats | 65 ± 2 | 65 ± 2 | 0.974 |
| Total activity, U/min | 573 ± 24 | 591 ± 35 | 0.650 |
Values are means ± SE. TPR, total peripheral resistance; MSNA, muscle sympathetic nerve activity.
Morning Surge, Arterial Stiffness, and Baroreflex Sensitivity
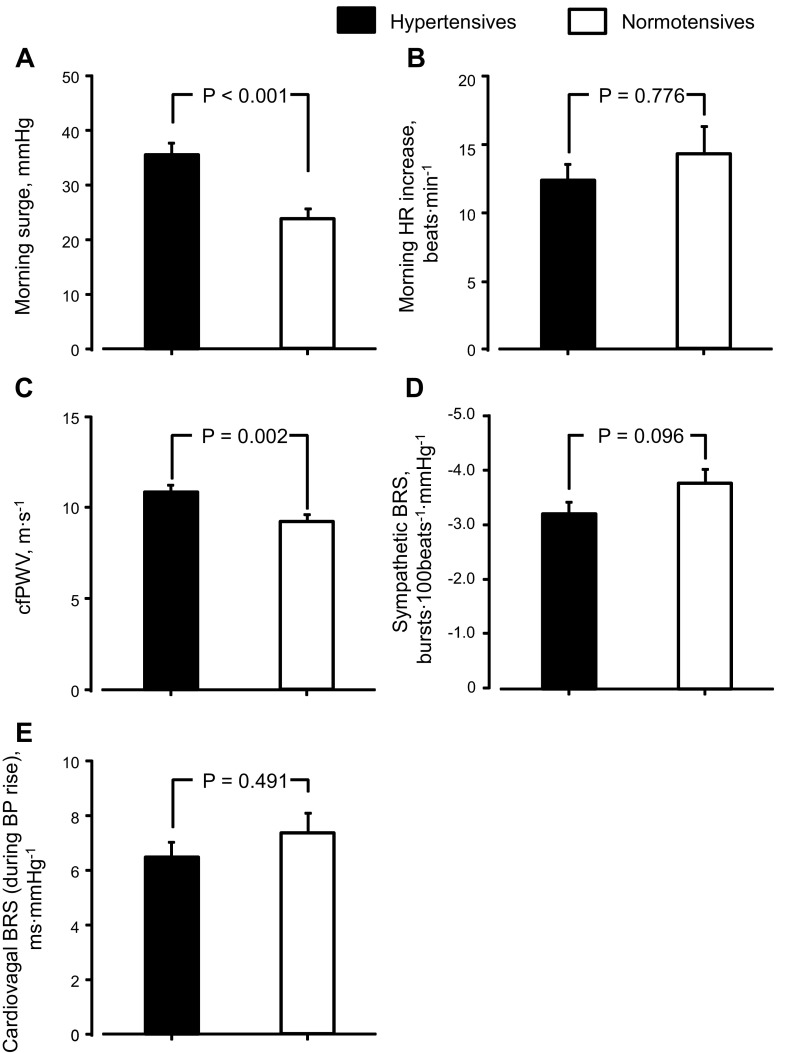
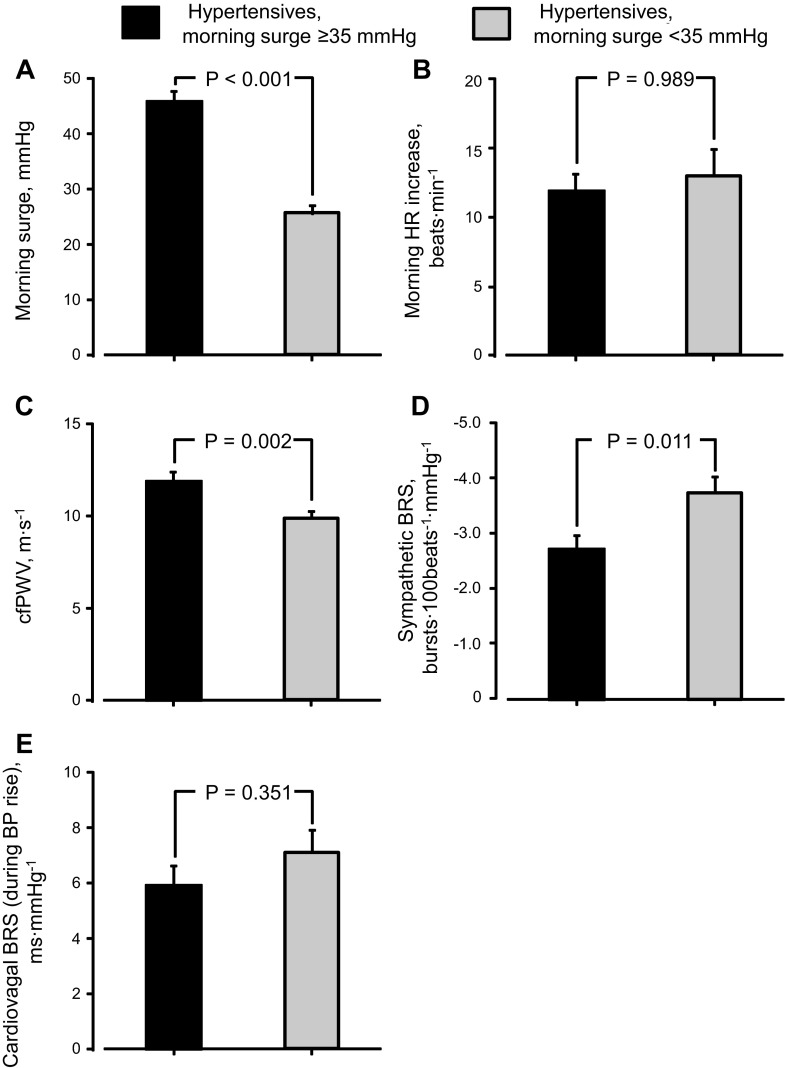
Morning surge was greater in hypertensive than normotensive subjects, but morning HR increase was similar between groups (Fig. 1, A and B). cfPWV was higher in hypertensive than normotensive subjects (Fig. 1C). Sympathetic BRS was lower in hypertensive than normotensive subjects (P = 0.096), but cardiovagal BRS was similar between groups (Fig. 1, D and E). To compare these variables between hypertensive subjects with greater and lesser morning surge, we set the median of morning surge (35 mmHg) as a threshold and divided hypertensive subjects into two subgroups (Fig. 2A): those with morning surge ≥35 mmHg (n = 20) and <35 mmHg (n = 20) [similar age and physical characteristics (all P > 0.050) and the same men-to-women ratio (10:10) between subgroups]. Likewise, between subgroups, morning HR increase and cardiovagal BRS were similar (Fig. 2, B and E), whereas cfPWV was higher and sympathetic BRS was lower in hypertensive subjects with morning surge ≥35 mmHg than those with morning surge <35 mmHg (Fig. 2, C and D).
Fig. 1.
Morning blood pressure surge (A), heart rate (HR) increase in the morning (B), carotid-to-femoral pulse wave velocity (cfPWV, C), sympathetic baroreflex sensitivity (BRS, D), and cardiovagal BRS (E) in elderly hypertensive and normotensive subjects. BP, blood pressure. Values are means ± SE.
Fig. 2.
Morning blood pressure surge (A), HR increase in the morning (B), cfPWV (C), sympathetic BRS (D), and cardiovagal BRS (E) in elderly hypertensive subjects with morning surge ≥35 mmHg and those with morning surge <35 mmHg. Values are means ± SE.
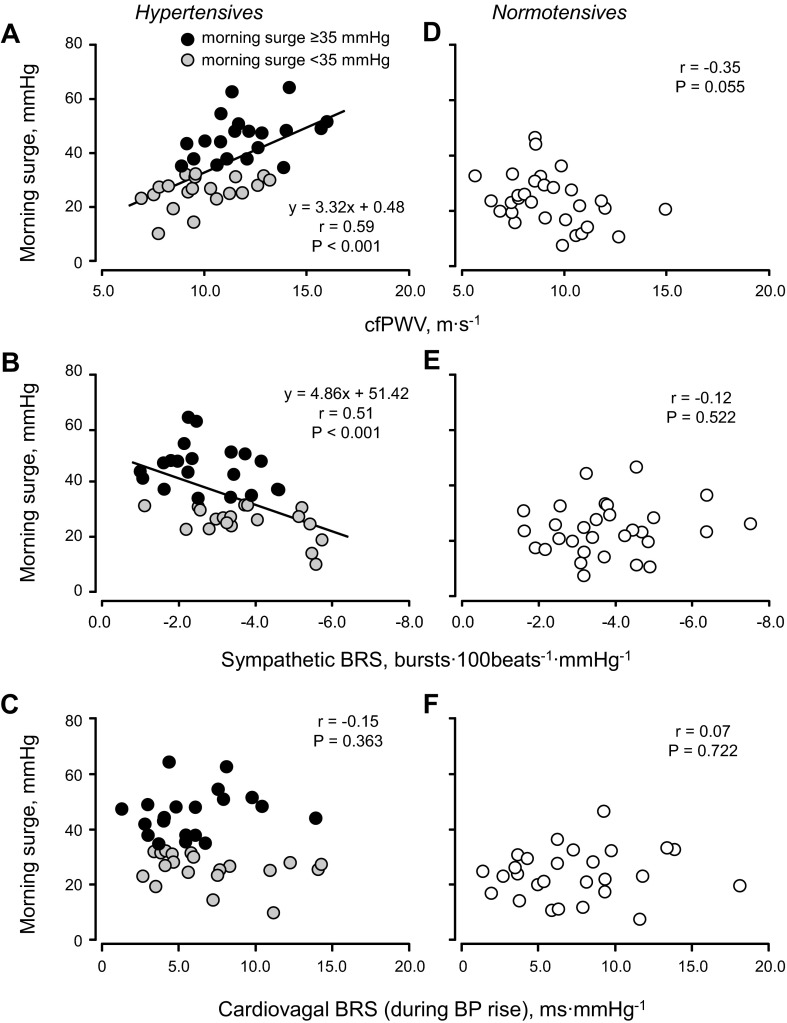
In hypertensive subjects, morning surge was positively correlated with cfPWV (Fig. 3A) and negatively correlated with sympathetic BRS (Fig. 3B). However, morning surge was not correlated with cardiovagal BRS (Fig. 3C), MSNA burst frequency (r = 0.10, P = 0.529), or total activity (r = 0.08, P = 0.609). In normotensive subjects, morning surge was not correlated with cfPWV (Fig. 3D), sympathetic BRS (Fig. 3E), cardiovagal BRS (Fig. 3F), or MSNA indexes (burst frequency: r = −0.29, P = 0.124; burst incidence: r = 0.08, P = 0.689; or total activity: r = −0.14, P = 0.689). Multiple-regression analysis showed that cfPWV and sympathetic BRS were relatively strong factors for morning surge in hypertensive subjects, whereas cardiovagal BRS and morning HR increase tended to be correlated with morning surge in normotensive subjects (Table 4).
Fig. 3.
Linear regression analysis of the interindividual relationships between morning blood pressure surge and cfPWV (A and D), sympathetic BRS (B and E), and cardiovagal BRS (C and F) in elderly hypertensive and normotensive subjects.
Table 4.
Determinant of morning blood pressure surge
| Hypertensive Subjects |
Normotensive Subjects |
|||
|---|---|---|---|---|
| β | P | β | P | |
| Multiple r2 | 0.58 | 0.42 | ||
| cfPWV, mmHg | 0.458 | <0.001 | −0.342 | 0.152 |
| The lowest nocturnal SBP, mmHg | Excluded variable | −0.275 | 0.270 | |
| Morning SBP, mmHg | −0.047 | 0.834 | Excluded variable | |
| Dip of night HR, beats/min | −0.290 | 0.040 | 0.181 | 0.482 |
| Morning HR increase, beats/min | 0.093 | 0.660 | −0.590 | 0.088 |
| Total activity, U/min | 0.036 | 0.799 | −0.163 | 0.540 |
| Sympathetic BRS, bursts · 100 beats−1 · mmHg−1 | 0.332 | 0.023 | 0.198 | 0.467 |
| Cardiovagal BRS phase IV, ms/mmHg | 0.057 | 0.668 | −0.508 | 0.075 |
cfPWV, carotid-to-femoral pulse wave velocity; BRS, baroreflex sensitivity.
Upright Sympathetic Baroreflex Sensitivity and Vasoreactivity
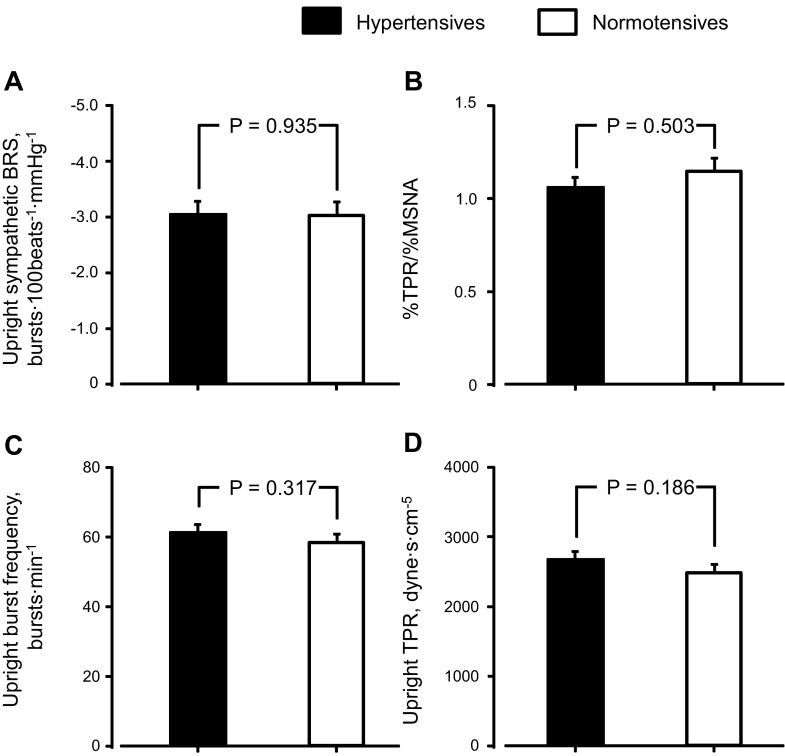
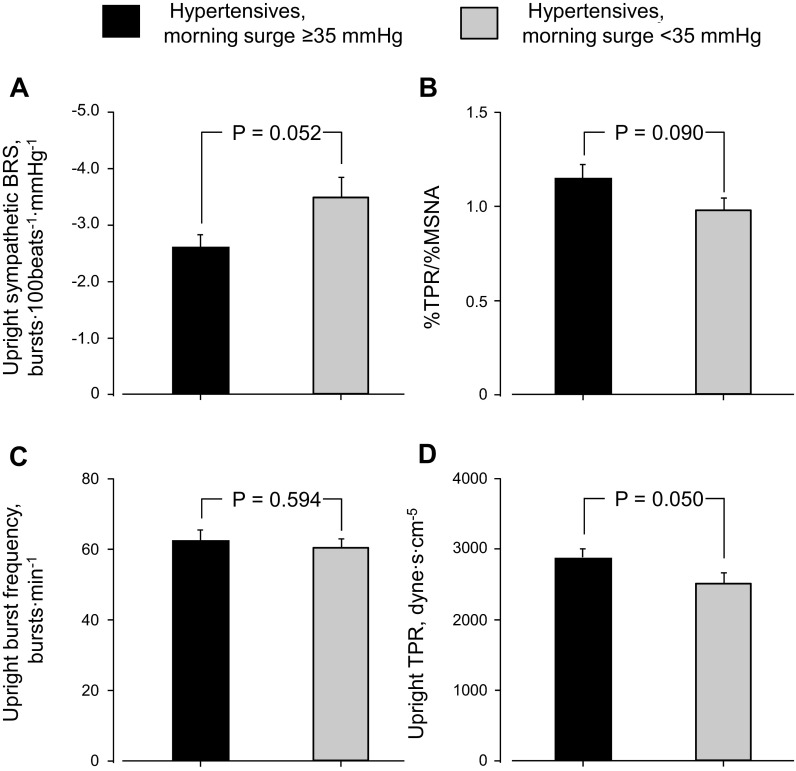
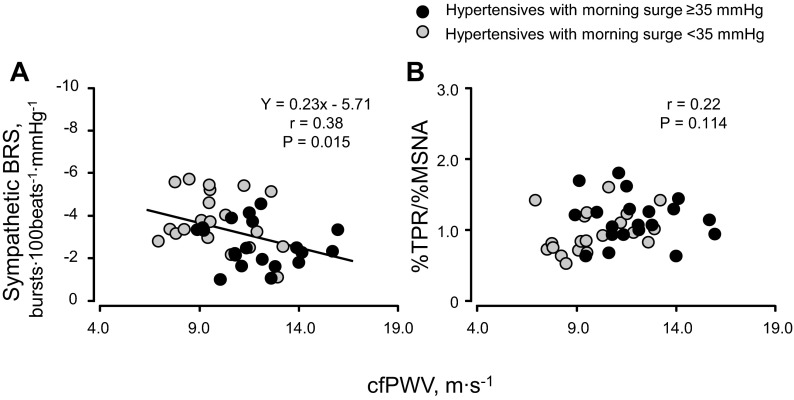
There was no difference in upright sympathetic BRS, %TPR/%MSNA, upright MSNA burst frequency, or TPR between elderly hypertensive and normotensive subjects (Fig. 4). Upright sympathetic BRS was smaller (P = 0.052, Fig. 5A) and %TPR/%MSNA by 60° head-up tilt was higher (P = 0.090, Fig. 5B) in hypertensive subjects with morning surge ≥35 mmHg than those with morning surge <35 mmHg. Although there was no difference in upright MSNA burst frequency between hypertensive subgroups (Fig. 5C), upright TPR was higher in hypertensive subjects with greater morning surge than those with lesser morning surge (Fig. 5D). Sympathetic BRS, but not %TPR/%MSNA, was correlated with cfPWV in hypertensive subjects (Fig. 6).
Fig. 4.
Upright sympathetic BRS (A), efficacy of muscle sympathetic nerve activity (MSNA) on total peripheral resistance (TPR) from supine to upright (B), upright MSNA burst frequency (C), and upright TPR (D) in elderly hypertensive and normotensive subjects. Values are means ± SE.
Fig. 5.
Upright sympathetic BRS (A), efficacy of MSNA on TPR from supine to upright (B), upright MSNA burst frequency (C), and upright TPR (D) in elderly hypertensive subjects with morning surge ≥35 mmHg and those with morning surge <35 mmHg. Values are means ± SE.
Fig. 6.
Linear regression analysis of the interindividual relationships between sympathetic BRS and cfPWV (A) and between efficacy of MSNA on TPR and cfPWV (B) in elderly hypertensive subjects.
DISCUSSION
The major findings from this study are that 1) cfPWV was higher and sympathetic BRS was lower in elderly hypertensive subjects, especially those with greater morning surge than in elderly normotensive subjects; 2) morning surge was correlated with cfPWV and sympathetic BRS in hypertensive subjects only; and 3) there was no difference in supine or upright MSNA indexes between the groups, whereas the change in TPR for a given change in MSNA during head-up tilt was greater in hypertensive subjects with greater morning surge than those with lesser morning surge. These results suggest that morning surge is associated with sympathetic BRS and vasoreactivity to orthostasis, as well as arterial stiffness, in elderly hypertensive subjects.
Sympathetic Neural Control of Blood Pressure in the Morning
Morning surge has been proposed to be related to an excessive increase of sympathetic activity in the morning. This notion is supported by the Hypertension and Lipid Trial (22) indicating that α-adrenergic blockade decreased morning BP. Kario et al. (11) demonstrated that original (baseline) morning surge had a strong correlation with a reduction of the morning surge by α-adrenergic blockade, doxazosin. Conversely, we and others (8) found no correlation between morning surge and MSNA in hypertensive patients. This discrepancy suggests that other sympathetic neural control system(s), rather than absolute MSNA, may be responsible for the morning surge. If sympathetic baroreflex function were maintained in hypertensive subjects with greater morning surge, MSNA should have been reduced when BP increased markedly in the morning, and morning absolute MSNA should have been negatively correlated with morning surge. The absence of a relationship between morning surge and MSNA in hypertensive subjects suggests that sympathetic BRS in the patients with higher morning surge may be impaired and/or the baroreflex curve may be reset to a higher BP level in the morning. Indeed, hypertensive subjects with morning surge ≥35 mmHg, which was similar to the median value for elderly hypertensive subjects in a previous study (39), had a lower sympathetic BRS than hypertensive subjects with morning surge <35 mmHg, whereas the latter had similar sympathetic BRS to that of normotensive subjects. Sympathetic BRS was found to decrease while sleeping and increase while awake (20). Moreover, it was found that the MSNA response was enhanced and the HR response was unchanged or lessened to the change in BP during orthostasis (16), mental stress (1), cold stress (9), and exercise (34) with age, especially in hypertensive subjects (3, 16). Therefore, in elderly hypertensive subjects, baroreflex control of TPR through MSNA seems to be predominant compared with baroreflex control of HR in the BP regulation at the time of awaking. The negative correlation between morning surge and sympathetic BRS and an exaggerated increase in TPR in the morning (36) in hypertensive subjects suggest that impaired sympathetic baroreflex function may result in the lack of a buffer system against morning surge in elderly hypertensive subjects.
There are some studies focusing on the circadian pattern of cardiovagal BRS. All of them demonstrated that a reduction of cardiovagal BRS occurred concomitant with BP increase in the morning, and it was suggested that cardiovagal BRS may be one of the determining factors for morning surge (4, 33, 36). Given the fact that a reduction of cardiovagal BRS and its correlation with morning BP was also observed in healthy normotensive subjects (4, 33) and that this relationship disappeared in hypertensive seniors in the current study, cardiovagal BRS may not be a predominant factor for morning BP increase in elderly hypertensive subjects. Indeed, multiple regression analysis showed that the lower cardiovagal BRS tended to be the factor for the higher morning surge in normotensive subjects, but not in elderly hypertensive subjects whose morning surge was significantly impacted by sympathetic BRS.
Orthostatic Effects
Morning surge includes the pressor response to orthostasis after awakening. The current study showed that upright sympathetic BRS was also lower in hypertensive subjects with morning surge ≥35 than those with morning surge <35 mmHg, suggesting that impaired upright sympathetic baroreflex function may be, at least partially, related to the morning surge evoked by orthostasis. However, the difference of upright sympathetic BRS between hypertensive subgroups categorized by morning surge was weaker than the difference of supine sympathetic BRS between them (P = 0.052 vs. 0.011). This suggests that morning surge during orthostasis may be also affected by other mechanisms. One of the other possible sympathetic systems influencing morning surge may be vasoreactivity to a change in MSNA. In this study, the efficacy of the increase in MSNA by head-up tilt on TPR was greater in hypertensive subjects with greater morning surge than those with lesser morning surge. Therefore, upright TPR was significantly higher in hypertensive subjects with greater morning surge despite upright MSNA being similar to those with lesser morning surge. This greater efficacy of MSNA on TPR during head-up tilt may be attributable to a greater sympathetic vascular transduction via augmented α1-adrenergic responsiveness observed in elderly hypertensive subjects in the previous study (32) and/or the enhanced renin-angiotensin-aldosterone system.
Arterial Stiffness and Morning Surge
To our knowledge, there are only two studies published evaluating the relationship between morning surge and cfPWV. Both studies demonstrated that morning surge was significantly correlated with cfPWV in subjects, including patients with type 2 diabetes and untreated and treated hypertension (23, 28). We found in elderly hypertensive subjects that morning surge was correlated with both sympathetic BRS and cfPWV and that there was a significant correlation between sympathetic BRS and cfPWV. There seems to be a close link between morning surge, arterial stiffening, and impaired sympathetic baroreflex function. Because baroreceptors are stretch receptors, the distortion of the barosensory artery is required to initiate neural firing. Taylor et al. (33) found that the decrease in cardiovagal BRS in the morning was the result of a reduced carotid artery distortion by the change in BP but not a blunted neural response to the distortion. Because the mechanical change of the artery is a common component between the cardiovagal and sympathetic baroreflex, higher arterial stiffness could suppress the day time increase in sympathetic BRS. Conversely, no relationship between the efficacy of change in MSNA on TPR and cfPWV during upright posture was observed in this study, suggesting that vasoreactivity may be independent of arterial stiffness as a factor for the morning surge. Because this study was designed as a descriptive study, we cannot demonstrate cause-effect relationships; however, it is possible that stiffening of the arteries may cause enhanced morning surge via the lower sympathetic BRS.
Perspectives
The synergistic effects of a smaller decrease of MSNA even with a greater BP increase because of impaired supine and upright sympathetic BRS and greater vasoreactivity to MSNA by standing up may result in enhanced morning surge in elderly hypertensive subjects. This could place hypertensive patients at a higher risk for cardiovascular events. This study may provide some insight into the best treatment strategy for elderly hypertensive subjects with greater morning surge. Recently, the large-scale, international, multicenter SURGE study clearly demonstrated that morning BP was higher than lunch time and evening BP in elderly hypertensive subjects whose morning BP control was very poor (control rate 13.1%), even with antihypertensive drugs (26). Pharmacological and nonpharmacological approaches that can decrease large-artery stiffness, increase sympathetic BRS, and attenuate sympathetic vasoconstriction for 24 h may be particularly effective in reducing cardiovascular risks in these patients. Conversely, Verdecchia et al. (38) reported controversial relationships between morning surge and cardiovascular events; blunted morning surge was a predictor of cardiovascular events, whereas increased morning surge did not change the risk. This discrepancy seemed to be because of the interaction of morning BP rise and nocturnal BP dip; a smaller nocturnal dip in BP, another risk factor for cardiovascular events, resulted in a smaller morning surge. It seems to be necessary to distinguish morning surge caused by morning BP rise and that by nocturnal BP dip.
Limitations
First, consistent with the results of a previous study (39), the repeatability coefficient expressed as percentage of maximal variation was 39.7% for hypertensive and 62.9% for normotensive subjects. The relatively low reproducibility of the morning surge, especially in normotensive subjects, may obscure significant relationships between morning surge, cfPWV, and sympathetic BRS. However, even BP conventionally used in clinics exhibited a similar repeatability (∼50%) to the morning surge (35), and many reports have indicated the significance of morning surge as a predictor for cardiovascular events in hypertensive subjects (12, 15). Therefore, we believe that our results are reliable and may provide insight into the underlying mechanisms of the increased morning surge for cardiovascular diseases in elderly hypertensive subjects. Second, we used cfPWV to assess arterial stiffness. It was found that cfPWV was affected by BP (29). However, cfPWV normalized by mean BP showed a similar relationship with morning surge (hypertensive subjects: r = 0.53, P < 0.001; normotensive subjects: r = −0.38, P = 0.040) as that with original cfPWV. Third, sympathetic BRS was evaluated during spontaneous breathing. Thus, the sensitivity was measured without assessing the entire baroreflex curve, which includes nonbaroreflex BP fluctuations. However, we used the binning method with MSNA burst incidence to reduce the impact of nonbaroreflex influence (30). Therefore, we can reveal the physiological modulation of sympathetic control around prevailing and operating point (6). Fourth, although we excluded patients with diagnosed sleep apnea, patients with unknown sleep apnea might have been included, because we did not perform polysomnography. However, it has been reported that patients with and without sleep apnea do not have any difference of the morning surge (24). Fifth, although we evaluated supine sympathetic BRS and cfPWV as factors of morning surge, they were measured at different time points from the period when a part of morning surge occurs, before waking up. Conversely, upright sympathetic BRS and %TPR/%MSNA were measured in a similar situation to when a part of morning surge occurs, orthostasis after waking up. Sixth, our subjects on average were considered overweight because of the difficulty to find elderly individuals who were healthy (except for stage I hypertension in the patient group), sedentary, but not overweight in the Dallas-Fort Worth area. However, there was no difference in weight or body mass index between groups in our study. Finally, we excluded patients with stage II hypertension for safety reasons. Although we found no difference in MSNA between hypertensive and normotensive subjects, which was similar to previous findings in borderline hypertensive patients (25), MSNA in moderate- to severe middle-aged hypertensive subjects was found to be higher than that in normotensive subjects (7). Whether similar results can be obtained in elderly patients with stage II and white-coat hypertension needs to be determined.
In summary, elderly hypertensive subjects with greater morning surge had higher arterial stiffness and smaller sympathetic BRS than those with lesser morning surge. In addition, morning BP surge was positively correlated with arterial stiffness, whereas both variables were negatively correlated with sympathetic BRS in elderly hypertensive subjects. Furthermore, smaller sympathetic BRS and greater TPR with similar MSNA were observed in hypertensive subjects with greater morning surge during upright posture. These results suggest that morning surge may be associated with sympathetic BRS altering concomitantly with large-artery stiffness and vasoconstrictor sensitivity (e.g., sympathetic vascular transduction) during orthostasis in elderly hypertensive subjects.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-091078.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.O., S.S., W.V., B.D.L., and Q.F. conception and design of research; Y.O., M.M.G., S.S., S.S.J., T.B.B., and Q.F. performed experiments; Y.O., M.M.G., S.S., S.S.J., T.B.B., and Q.F. analyzed data; Y.O., M.M.G., S.S., S.S.J., T.B.B., W.V., B.D.L., and Q.F. interpreted results of experiments; Y.O. and Q.F. prepared figures; Y.O. and Q.F. drafted manuscript; Y.O., T.B.B., W.V., B.D.L., and Q.F. edited and revised manuscript; Y.O., M.M.G., S.S., S.S.J., T.B.B., W.V., B.D.L., and Q.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the study volunteers for participation. We also thank Rhonda L. Meier, Peggy Fowler, and Cindi D. Foulk for valuable laboratory assistance. Additionally, we thank Dr. Jeffrey L. Hastings and Dr. Keri M. Schafer for clinical expertise in subject preparation and Beverley Adams-Huet for assistance in statistical evaluation.
REFERENCES
- 1.Barnes RF, Raskind M, Gumbrecht G, Halter JB. The effects of age on the plasma catecholamine response to mental stress in man. J Clin Endocrinol Metab 54: 64–69, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi K, Tomizawa H, Ishikawa J, Hoshide S, Pickering TG, Shimada K, Kario K. Factors associated with baroreflex sensitivity: association with morning blood pressure. Hypertens Res 30: 723–728, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 29: 992–996, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 31: 68–72, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Hering D, Kucharska W, Kara T, Somers VK, Narkiewicz K. Resting sympathetic outflow does not predict the morning blood pressure surge in hypertension. J Hypertens 29: 2381–2386, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol 107: 1076–1082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kario K, Pickering TG, Hoshide S, Eguchi K, Ishikawa J, Morinari M, Hoshide Y, Shimada K. Morning blood pressure surge and hypertensive cerebrovascular disease: role of the alpha adrenergic sympathetic nervous system. Am J Hypertens 17: 668–675, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 107: 1401–1406, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Thijs L, Hansen TW, Kikuya M, Boggia J, Richart T, Metoki H, Ohkubo T, Torp-Pedersen C, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Sandoya E, Kawecka-Jaszcz K, Ibsen H, Imai Y, Wang J, Staessen JA. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 55: 1040–1048, 2010 [DOI] [PubMed] [Google Scholar]
- 16.London GM, Weiss YA, Pannier BP, Laurent SL, Safar ME. Tilt test in essential hypertension. Differential responses in heart rate and vascular resistance. Hypertension 10: 29–34, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Marfella R, Siniscalchi M, Nappo F, Gualdiero P, Esposito K, Sasso FC, Cacciapuoti F, Di Filippo C, Rossi F, D'Amico M, Giugliano D. Regression of carotid atherosclerosis by control of morning blood pressure peak in newly diagnosed hypertensive patients. Am J Hypertens 18: 308–318, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 281: H284–H289, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79: 733–743, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Nakazato T, Shikama T, Toma S, Nakajima Y, Masuda Y. Nocturnal variation in human sympathetic baroreflex sensitivity. J Auton Nerv Syst 70: 32–37, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension 59: 98–104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering TG, Levenstein M, Walmsley P. Differential effects of doxazosin on clinic and ambulatory pressure according to age, gender, and presence of white coat hypertension. Results of the HALT Study Hypertension and Lipid Trial Study Group. Am J Hypertens 7: 848–852, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Polonia J, Amado P, Barbosa L, Nazare J, Silva JA, Bertoquini S, Martins L, Carmona J. Morning rise, morning surge and daytime variability of blood pressure and cardiovascular target organ damage. A cross-sectional study in 743 subjects. Rev Port Cardiol 24: 65–78, 2005 [PubMed] [Google Scholar]
- 24.Prejbisz A, Makowiecka-Ciesla M, Paschalis-Purtak K, Pucilowska-Jankowska B, Bielen P, Kluk M, Kowalewski G, Cendrowska-Demkow I, Florczak E, Buchner T, Sliwinski P, Januszewicz A, Kabat M. Obstructive sleep apnea and morning blood pressure surge in never treated hypertensive patients (Abstract). J Hypertens 28: e249, 2010 [Google Scholar]
- 25.Rea RF, Hamdan M. Baroreflex control of muscle sympathetic nerve activity in borderline hypertension. Circulation 82: 856–862, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Redon J, Bilo G, Parati G. Home blood pressure control is low during the critical morning hours in patients with hypertension: the SURGE observational study. Fam Pract 29: 421–426, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Sanchez Gelos DF, Otero-Losada ME, Azzato F, Milei J. Morning surge, pulse wave velocity, and autonomic function tests in elderly adults. Blood Press Monit 17: 103–109, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 13: 101–107, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272: 383–397, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supiano MA, Hogikyan RV, Sidani MA, Galecki AT, Krueger JL. Sympathetic nervous system activity and alpha-adrenergic responsiveness in older hypertensive humans. Am J Physiol Endocrinol Metab 276: E519–E528, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Taylor CE, Atkinson G, Willie CK, Jones H, Ainslie PN, Tzeng YC. Diurnal variation in the mechanical and neural components of the baroreflex. Hypertension 58: 51–56, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation 86: 1789–1799, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Thijs L, Celis H, Clement D, Gil-Extremera B, Kawecka-Jaszcz K, Mancia G, Parati G, Salvetti A, Sarti C, van den Meiracker AH, O'Brien E, Staessen JA, Fagard R. Conventional and ambulatory blood pressure measurement in older patients with isolated systolic hypertension:second progress report on the ambulatory blood pressure monitoring project in the Syst-Eur trial. Blood Press Monit 1: 95–103, 1996 [PubMed] [Google Scholar]
- 36.Tochikubo O, Kawano Y, Miyajima E, Toshihiro N, Ishii M. Circadian variation of hemodynamics and baroreflex functions in patients with essential hypertension. Hypertens Res 20: 157–166, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Ramundo E, Gentile G, Ambrosio G, Reboldi G. Day-night dip and early-morning surge in blood pressure in hypertension: prognostic implications. Hypertension 60: 34–42, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Wizner B, Dechering DG, Thijs L, Atkins N, Fagard R, O'Brien E, de Leeuw PW, Parati G, Palatini P, Clement D, Grodzicki T, Kario K, Staessen JA. Short-term and long-term repeatability of the morning blood pressure in older patients with isolated systolic hypertension. J Hypertens 26: 1328–1335, 2008 [DOI] [PubMed] [Google Scholar]