Abstract
An increased sympathetic drive is an adverse characteristic in chronic heart failure (CHF). The protein expression of neuronal nitric oxide synthase (nNOS)- and hence nitric oxide (NO)-mediated sympathoinhibition is reduced in the paraventricular nucleus (PVN) of rats with CHF. However, the molecular mechanism(s) of nNOS downregulation remain(s) unclear. The aim of the study was to reveal the underlying molecular mechanism for the downregulation of nNOS in the PVN of CHF rats. Sprague-Dawley rats with CHF (6–8 wk after coronary artery ligation) demonstrated decreased nNOS dimer/monomer ratio (42%), with a concomitant increase in the expression of PIN (a protein inhibitor of nNOS known to dissociate nNOS dimers into monomers) by 47% in the PVN. Similarly, PIN expression is increased in a neuronal cell line (NG108) treated with angiotensin II (ANG II). Furthermore, there is an increased accumulation of high-molecular-weight nNOS-ubiquitin (nNOS-Ub) conjugates in the PVN of CHF rats (29%). ANG II treatment in NG108 cells in the presence of a proteasome inhibitor, lactacystin, also leads to a 69% increase in accumulation of nNOS-Ub conjugates immunoprecipitated by an antiubiquitin antibody. There is an ANG II-driven, PIN-mediated decrease in the dimeric catalytically active nNOS in the PVN, due to ubiquitin-dependent proteolytic degradation in CHF. Our results show a novel intermediary mechanism that leads to decreased levels of active nNOS in the PVN, involved in subsequent reduction in sympathoinhibition during CHF, offering a new target for the treatment of CHF and other cardiovascular diseases.
Keywords: sympathetic nerve activity, nNOS, PIN, angiotensin II, paraventricular nucleus
nitric oxide (no) is a gaseous second messenger produced by a family of nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzymes named NO synthases (NOS). NO produced by the neuronal isoform of NOS (nNOS) plays an important role in the peripheral and central nervous system. In the paraventricular nucleus (PVN), NO acts as a retrograde inhibitory neurotransmitter that regulates synaptic efficacy and modulates neuronal activity leading to changes in sympathetic nerve activity. Previously, we reported that rats with chronic heart failure (CHF) have a blunted NO system, specifically, decreased protein expression of nNOS in the PVN (21). However, angiotensin II (ANG II) type 1 (AT1) receptor blocker losartan (Los) treatment significantly ameliorated the decreased nNOS expression, suggesting a role for the AT1 receptor (AT1R) in nNOS downregulation during CHF (37). The regulation details and the possible mechanism(s) of this phenomenon remain largely unknown.
Of the three NOS isoforms, nNOS is the largest, due to a 300-amino acid PDZ (PSD-95/Discs large/zona occludens-1) domain insertion at the NH2 terminus targeting nNOS to the postsynaptic density (PSD) protein in the synaptic spines of neurons (13). The catalytic activity of nNOS is regulated by different mechanisms such as Ca2+-calmodulin binding and phosphorylation (1). Several other scaffolding proteins such as syntrophin, PSD93, and PSD95 have been identified to interact with nNOS in neurons and modify its activity. The NMDAR (N-methyl-d-aspartic acid receptor) and nNOS are linked through intermediary adaptor protein PSD95 for NMDAR-mediated calcium influx and nNOS activation in the PSD of synaptic spines (13). In the brain, carboxy-terminal PDZ ligand of nNOS (CAPON) competes with PSD95 for interaction with nNOS, therefore acting as a negative regulator of NO production and its downstream signaling effects (12, 13). In synaptic spines, another nNOS-interacting protein (NOSIP) interacts with nNOS and inhibits its activity (4, 13).
A small-molecular-weight protein, dynein light chain (DLC), also known as PIN (protein inhibitor of nNOS) (14), was reported to bind to a 17-residue peptide fragment from Met-228 to His-244 of nNOS (7). PIN binding destabilizes the nNOS homodimer (14), a conformation necessary for the catalytic activity of the enzyme (6). It has already been established that the monomeric form of nNOS is actively ubiquitinated and degraded by the proteasomal pathway (2, 6). In addition, nNOS has been found in ubiquitin (Ub) conjugates in HEK 293 cells treated with lactacystin (LC), an inhibitor of proteasomal function, and in rat brain homogenates (2). Similar studies with the use of reticulocyte lysates and purified nNOS indicate that the monomeric form of nNOS is preferentially ubiquitinated (6). These results strongly support a role for the Ub-proteasome pathway in regulating the degradation of nNOS in vivo.
Previously, we have shown that there is a decrease in nNOS expression (21) and an enhanced expression of CAPON within the PVN of rats with CHF (37). Moreover, Los treatment restores sympathetic nerve activity and normalizes the changes in expression of nNOS and CAPON, suggesting that the AT1R activation may be linked to the reciprocal changes in CAPON and nNOS in the PVN. Furthermore, silencing of CAPON in NG108 neuronal cell line increases the expression of nNOS, suggesting a functional interaction between nNOS and CAPON. To further elucidate the underlying mechanism of downregulation of nNOS, here we examined the expression of PIN in the PVN of rats with CHF compared with Sham controls. We also studied the effect of ANG II on the expression of PIN in a neuronal cell line. Therefore, we hypothesize that downregulation of nNOS in the PVN during CHF is due to the Ub-mediated proteolytic degradation involving PIN through an ANG II-mediated signaling pathway(s). Here, we report that CHF increases the protein expression of PIN within the PVN. Additionally, ANG II treatment in a neuronal cell line also increased PIN expression, as well as ubiquitination of nNOS, leading to its degradation via proteasome. This suggests that posttranslational processes, such as protein degradation/stabilization, are involved in ANG II-dependent downregulation of nNOS.
METHODS
Animal Model of Heart Failure
Male Sprague-Dawley rats weighing 180–200 g (Sasco Breeding Laboratories, Omaha, NE) were fed and housed according to institutional guidelines. The University of Nebraska Medical Center Institutional Animal Care and Use Committee approved all protocols. Coronary artery ligation produces a model of heart failure in the rat similar to the most common cause of human heart failure (17, 18, 41). Our lab, as well as others, has used this model extensively (17, 28, 29, 40, 41). Rats were randomly assigned in two groups, CHF and Sham, and were ventilated at a rate of 60 breaths/min with 2–3% isoflurane during the surgical procedure. A ligature was placed on the coronary artery of each CHF rat, between the pulmonary artery outflow tract and the left atrium as described previously (17, 18, 28, 41). Sham rats underwent the same surgery without ligation. All rats were allowed to recover for 6–8 wk prior to experimentation. Hemodynamic and anatomic parameters were used to assess left ventricular dysfunction and failure at the end of each experiment (37). Echocardiograms were performed to measure left ventricular end-systolic and left ventricular end-diastolic dimensions, fractional shortening (FS), and ejection fraction (EF). Left ventricular end-diastolic pressures (LVEDP) were measured using a Mikro-Tip catheter (Millar Instruments, Houston, TX) inserted into the left ventricle via the right carotid artery. To measure infarct size, the heart was dissected free of adjacent tissues, and atria and the right ventricle were removed. The left ventricle was opened with a lengthwise incision such that the heart was opened and laid flat. A digital image of the left ventricle was captured with a Kodak DC290 digital camera (Kodak, Rochester, NY), and the infarcted area and total left ventricle area were quantified with Sigma Scan Pro. Infarct size (%) was determined by dividing the size of the infarcted area by the total size of the left ventricle. Rats with elevated LVEDP (≥15 mmHg) and infarct size (>30% of total left ventricle wall) were considered to be in CHF. The total number of rats used in this study was 87, 53 in CHF group and 34 in Sham group. We excluded 14 rats with coronary artery surgery because they did not fit the criteria (listed above) for inclusion in the study.
Expression of PIN Within the PVN
PIN expression in the PVN of Sham and CHF rats was studied by Western blotting and immunofluorescence staining.
Western blotting was performed from PVN punches. After the animal was euthanized by overdose of pentobarbital (65 mg/kg ip), the brain was removed and quickly frozen on dry ice. The PVN was punched bilaterally as described previously (37). Briefly, punched tissues were incubated in 100 μl lysis buffer A (10 mM Tris, 1 mM EDTA, 1% SDS, 0.1% Triton X-100 containing complete protease inhibitor cocktail). PVN lysates were subjected to a protein extraction procedure (18). The protein lysates (20–30 μg), mixed with SDS-PAGE buffer, were fractionated on 7.5% polyacrylamide gel and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). Non-fat dry milk (5% wt/vol) in TBST (10 mM Tris, 150 mM NaCl, 0.05% Tween-20) was used to block membrane at ambient temperature for 1 h. Then the membrane was incubated with the appropriate primary antibody overnight (anti-nNOS mouse) (Santa Cruz, sc-302), anti-PIN (rabbit) (Santa Cruz, sc-13969), and anti-actin (rabbit) (Sigma, A-2066), followed by the corresponding peroxidase-conjugated secondary antibody for 1 h. An enhanced chemiluminescence substrate (Pierce Chemical, Rockford, IL) was used to visualize the signals, which were detected by Worklab digital image system. Image J (NIH) was used to quantify the signal. Actin was used as housekeeping genes.
Immunofluorescence staining was performed for nNOS and PIN in brain coronal sections including the PVN from Sham and CHF groups. The rats were anesthetized with pentobarbital (65 mg/kg) and perfused transcardially with 150 ml of heparinized saline followed by 250 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer (37). The brain was postfixed at 4°C for 4 h in 4% paraformaldehyde solution and then placed in 30% sucrose for 72 h. The free-floating 30 μm sections were incubated with 10% normal goat serum, 0.02% Triton X-100 in phosphate-buffered saline (PBS) for 1 h at room temperature and then incubated with PIN (sc-13969) and nNOS(sc-302) primary antibody for 4–6 h at 4°C. After being washed with PBS, the sections were incubated with Cy3-conjugated goat anti-rabbit (1:500) and Cy2-conjugated goat anti-mouse secondary antibody (1:500) for 4 h in the dark at room temperature. The nuclei were stained with DAPI (Molecular Probes). After being washed with PBS, the sections were mounted on slides with fluoromount-G (Southern Biotech). The slides were observed under a Leica-DMR microscope with corresponding filters and scaled and quantified using Image J software (NIH). Three alternate sections (2.0 ± 0.2 mm posterior to bregma) representing the PVN were analyzed.
Assessment of Dimer-Monomer Ratio of nNOS in the PVN
Low-temperature polyacrylamide gel electrophoresis (LT-PAGE) was performed in the PVN punches to quantify catalytically active nNOS dimers and catalytically inactive monomers in the native state as described previously by Klatt et al. (16). For the LT-PAGE, 40 μg of protein in standard Laemmli buffer was incubated at 4°C for 30 min before fractionation with a 5% separating gel. All gels and buffers were pre-equilibrated to 4°C before electrophoresis, and the electrophoresis was done in a cold room at 50 V to maintain the gel temperature <15°C. Transfer was done overnight in a cold room at 30 V. A monoclonal antibody specific to nNOS (sc-302) and anti-mouse IgG conjugated with horseradish peroxidase (Sigma Chemical) were used as the primary and secondary antibodies, respectively.
Knockdown of PIN and Levels of Catalytic Active nNOS in NG108 Cells
Neuronal cell culture.
NG108–15 (neuroblastoma × glioma) hybrid cells were grown in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin G, and streptomycin (37). The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. Cells were seeded in six-well plates and grown until 60–70% confluent before treatment with ANG II or Los for 24 h.
Silencing of PIN.
siRNA targeting PIN and its negative control were purchased from Invitrogen (Carlsbad, CA). Plasmid constructs pSilencer2.1-U6-PIN-shRNA (psi-PIN-shRNA) and pSilencer2.1-U6-randomer-shRNA (psi-null-shRNA) were generously provided by Dr. Thomas R. Magee (UCLA Medical Center, Torrance, CA). Transient transfection was performed in NG108 cells cultured in six-well plates using 2.5 μg of plasmid and 10 μl of Lipofectamine 2000 (Invitrogen) according to the manufacturer instructions.
LT-PAGE was performed to quantify active nNOS dimers and inactive monomers as described above in psi-null-shRNA and psi-PIN-shRNA transfected NG108 cells.
Intracellular NO.
Intracellular NO concentration was measured in ANG II-treated as well as in psi-null-shRNA and psi-PIN-shRNA transfected NG108 cells using 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) fluorescence dye. Subconfluent NG108 cells were grown on a Nunc Lab-Tek chamber and transfected with psi-PIN-shRNA or psi-null-shRNA 48 h before experimentation. In a different set of cultures, cells were treated with ANG II 24 h before the final experiment. Briefly, cells were loaded with DAF-AM (5 μM) for 30 min at 37°C followed by additional incubation after washing for 20 min to complete de-esterification of dye. NO concentration was measured by DAF-FM fluorescence with a Zeiss LSM 510 META confocal microscope with a ×63 oil immersion objective and 488 nm wavelength excitation. DAF-FM has a detection limit of 3 nM and does not react with other stable oxidized forms of NO˙, such as NO2, or reactive oxygen species (19).
Effects of ANG II Treatment on PIN Expression in NG108 Cells
NG108 cells were treated with increasing doses of ANG II (0–100 μM), and Western blotting was performed for PIN. In a separate experiment Western blotting was done in ANG II (100 μM), Los (1 μM), and ANG II + Los-treated NG108 cell lysates.
Immunofluorescence microscopy of NG108 cells was performed in NG108 cells treated with ANG II. Adherent NG108 cells were grown on laminin-coated 6 mm Transwell-Clear inserts (Corning, Costar). Cells were fixed in 4% paraformaldehyde for 10 min and then permeabilized with 0.2% Triton X-100 for 20–30 min. The remaining steps were the same as mentioned in the above section for staining the PVN sections. Coverslips were then mounted onto frosted glass microscope slides using Fluoromount G (Southern Biotechnology) and visualized under a Leica-DMR microscope.
Coimmunoprecipitation of PIN and nNOS
NG108 cell lysates in lysis buffer A were cleared by centrifugation, and the supernatants normalized for protein content were subjected to preclearing with protein A/G agarose (Santa Cruz Biotechnology) for 30 min. Cleared supernatants were incubated with nNOS (anti-nNOS rabbit) (Santa Cruz, sc-648) antisera overnight at 4°C. The immune complexes were precipitated after 2 h incubation with protein A/G agarose by centrifugation. The precipitates were washed twice with ice-cold lysis buffer. For Western blotting, samples were dissolved in sample buffer [200 mM Tris·HCl, pH 6.8, 6% SDS, 20% glycerol, 10% dithiothreitol (DTT), and 0.1 mg/ml bromophenol blue], separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes.
Effect of Los Treatment on PIN Expression in the PVN in CHF
Three weeks following coronary artery ligation, Los (10 mg/kg/day) was given to rats (10) in the drinking water for a period of 3 wk. Western blotting for PIN was done in the four groups, namely Sham, Sham + Los, CHF, and CHF + Los.
Ubiquitination of nNOS in the PVN
Western blotting of high-molecular-weight forms of nNOS.
To study the ubiquitination of nNOS in the PVN, we performed Western blotting of high-molecular-weight forms of nNOS. Punched PVN tissues were sonicated in 50 μl of lysis buffer B (10 mM HEPES, pH 7.4, 0.32 M sucrose, 2 mM EDTA), complete protease inhibitor cocktail, 10 mM sodium orthovanadate (Na3VO4), 1% Nonidet P-40, and 5 mM N-ethylmaleimide. The protein (30–40 μg) was resolved on 5% SDS-polyacrylamide gels and transferred to PVDF membranes for 10 h at 4°C. The membranes were probed with 0.1% anti-nNOS (mouse) antibody (sc-302).
Immunofluorescence staining.
We performed immunofluorescence staining for nNOS and Ub in the PVN from Sham and CHF groups by overnight anti-nNOS (mouse) (Santa Cruz; sc-302) and anti-Ub (rabbit) (Dako, Z0458). Distribution of immunofluorescence within the PVN was viewed under a Zeiss LSM 510 META confocal microscope with ×10 and ×63 oil immersion-objective using an Olympus fluorescence microscope equipped with a digital camera.
Ubiquitination of nNOS in NG108 Cells
Immunoprecipitation and Western blotting of high-molecular-weight forms of nNOS from NG108 cells was performed. nNOS was immunoadsorbed from NG108 cell lysate (400 μg) with 2 μg of polyclonal anti-nNOS IgG (rabbit) from Santa Cruz Biotechnology (sc-648) at 4°C overnight followed by 10 μl of protein A/G plus-Agarose (sc-2003) in a total volume of 300 μl of lysis buffer B for 2 h at 4°C. In studies where Ub was immunoadsorbed, 5 μg of anti-Ub IgG (Z0458) was replaced with anti-nNOS IgG. Immune pellets were washed three times with ice-cold lysis buffer B and then boiled in SDS sample buffer containing DTT (6.0 mg/ml). Western blotting of nNOS-Ub species was done as described above.
In vivo substrate-dependent Ub ligase assay.
This protocol was done according to the method of Furukawa (9) using NG108 cells transiently transfected with pCMV-(HA-Ub)8 using Lipofectamine 2000 (Invitrogen) according to the manufacturer instructions. Twenty-four hours posttransfection, cells were treated with 100 μM ANG II for 18 h followed by LC (10 μM) for an additional 6 h to accumulate the high-molecular-weight polyubiquitinated forms. Immunoprecipitation was carried out on total cell lysates (400 μg) using anti-nNOS (rabbit) antibody followed by immunoblotting with anti nNOS (mouse) and hemagglutinin (HA) antibodies to detect nNOS and nNOS-ubiquitinated species [nNOS-(Ub)n] respectively.
Statistical Analyses
Data are expressed as means ± SE and statistical significance was achieved at P ≤ 0.05. Statistical comparisons of the groups were made by a Student's t-test and one-way or two-way ANOVA, followed by the Newman-Keuls test for post hoc analysis.
RESULTS
Characteristics of CHF Model
The baseline characteristics of Sham and CHF rats (6–8 wk after coronary artery ligation) used in this study are summarized in Table 1. The infarcted area in the CHF group was ∼36% of the endocardial surface of the left ventricle. Sham rats had no observable damage to the myocardium. LVEDP was significantly increased in the CHF group compared with Sham. dP/dt maximum and minimum were significantly decreased in the CHF group compared with the Sham group, indicating a decreased contractility and relaxation. EF and FS were greatly reduced in the infarcted group, suggesting that the rats were in CHF. Taken together, the >30% infarct size, increased LVEDP, and decreased dP/dt indicate that rats in CHF group were experiencing cardiac dysfunction.
Table 1.
Morphological and hemodynamic characteristics of Sham and CHF groups
| Sham (n = 10) | CHF (n = 10) | |
|---|---|---|
| Infarct size, % of left ventricle | 0 | 36 ± 0.02* |
| LVEDP, mmHg | 3 ± 0.5 | 26 ± 2.8* |
| dP/dt max, mmHg/s | 8,291 ± 443 | 6,936 ± 291* |
| dP/dt min, mmHg/s | −9504 ± 697 | −6157 ± 416* |
| MAP, mmHg | 112 ± 8.3 | 107 ± 4.0 |
| HR, beats/min | 348 ± 8.3 | 347 ± 7.2 |
| EF, % | 69 ± 0.9 | 41 ± 2.7* |
| FS, % | 40 ± 0.7 | 2.1 ± 1.7* |
Data are represented as means ± SE. CHF, chronic heart failure; LVEDP, left ventricular end-diastolic pressure; dP/dt, delta pressure divided by delta time; EF, ejection fraction; FS, fractional shortening; MAP, mean arterial pressure; HR, heart rate.
P < 0.05 vs. Sham.
Expression of PIN Within the PVN
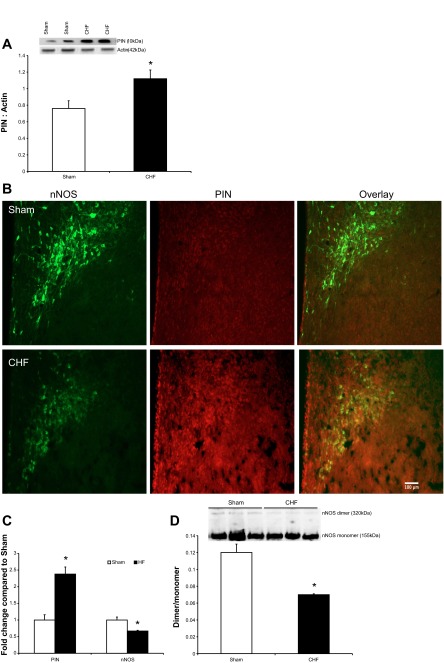
PIN expression was significantly increased (∼47%) in the PVN of CHF rats compared with Sham (Fig. 1A). Consistent with our previous findings of decreased nNOS in the PVN (21, 42), immunofluorescence of nNOS was decreased, while PIN was increased in the PVN of CHF rats compared with Sham (Fig. 1, B and C). PIN has been shown to disrupt nNOS dimerization (14). Therefore, we examined the level of monomeric and dimeric nNOS in PVN lysates by LT-PAGE so that the SDS-resistant dimeric form of nNOS could be measured (Fig. 1D). Previously, we showed a decrease in the nNOS expression in the PVN of CHF rats via denaturating-polyacrylamide gel electrophoresis using SDS and 2-mercaptoethanol (after boiling), which produces an anti-nNOS reactive band at 155 kDa (monomer). In Fig. 1D, we present the dimeric nNOS via LT-PAGE without boiling the samples. To show the functional SDS-resistant dimers we overexposed the gel. Therefore, the saturation of immunodetected monomeric nNOS band at 155 kDa due to the overexposure of gel masks the difference in monomeric nNOS between the two groups reported previously (21, 37). However, at lower exposure periods the difference is still apparent. Nevertheless, the dimer-monomer ratio of nNOS was decreased by 42% in the PVN of CHF compared with Sham rats, suggesting decreased functionally active nNOS.
Fig. 1.
A: Western blot analysis of protein inhibitor of neuronal nitric oxide synthase (PIN) in the paraventricular nucleus (PVN) of Sham and chronic heart failure (CHF) groups. Top: a representative Western blot; bottom: densitometric analyses of PIN level normalized to actin. B: immunofluorescence photomicrographs of the PVN stained for PIN (red) and neuronal nitric oxide synthase (nNOS, green) in Sham and CHF rats. C: composite data of PIN and nNOS immunofluorescence signal in the PVN. D: assessment of monomers and dimers of nNOS in the PVN of Sham and CHF rats. Dimeric and monomeric nNOS were separated by low temperature (LT)-PAGE in a cold room and visualized by immunoblotting using an anti-nNOS antibody. Top: a representative Western blot; bottom: densitometric analyses of monomer and dimer protein levels represented as a ratio of dimer to monomer. Values are means ± SE (n = 4–5 rats per group). *P < 0.05 vs. Sham.
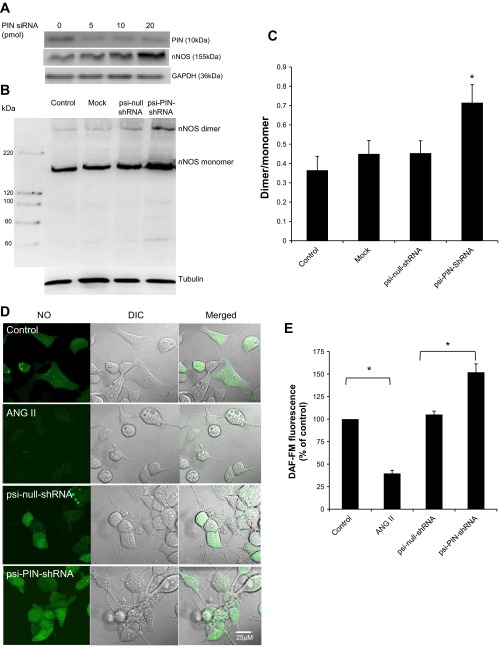
Knockdown of PIN Increases the Levels of Catalytic Active nNOS in NG108 Cells
PIN knockdown using siRNA leads to accumulation of nNOS (Fig. 2A), suggesting a functional role for PIN in regulating the expression of nNOS. Moreover, silencing of PIN using psi-PIN-shRNA leads to accumulation of nNOS as well as a 58% increase in dimer/monomer ratio compared with control vector (psi-null-shRNA) in NG108 cells, suggesting the effect of PIN on the dimerization of catalytically active nNOS (Fig. 2, B and C). To evaluate the functional significance of PIN silencing on nNOS catalytic activity, the changes in NO levels were measured with DAF-FM, a fluorescent green NO dye (Fig. 2, D and E). Quantitative analysis of cell images show that the ANG II treatment decreases the intensity of NO staining compared with control cells (100% ± 1.0 vs. 39.7% ± 0.2), while transient transfection with psi-shRNA-PIN augments the NO staining compared with psi-null-shRNA-transfected cells (105.1% ± 0.5 vs. 151.9% ± 0.3), suggesting a functional role for PIN in regulating the activity of nNOS.
Fig. 2.
PIN decreases the catalytically active form of nNOS in NG108 neurons. A: knockdown of PIN in NG108 cell line using siRNA. B: estimation of monomers and dimers of nNOS in NG108 cells transfected with Lipofectamine alone (Mock) or plasmid constructs: psi-PIN-shRNA and psi-null-shRNA. After 48 h, dimeric and monomeric nNOS were separated by LT-PAGE in a cold room and visualized by immunoblotting using an anti-nNOS antibody. C: densitometry analyses of monomer and dimer protein levels represented as a ratio of dimer to monomer. *P < 0.05 vs. psi-null-shRNA group. D: representative pictures of intracellular nitric oxide (NO) as measured by 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) in NG108 cells of 4 experimental groups (control, ANG II 100 μM, psi-null-shRNA, and psi-PIN-shRNA). E: cumulative data represented as percent change in NO compared with control without any treatment (n = 8–10 cells from 3 coverslips in each group).
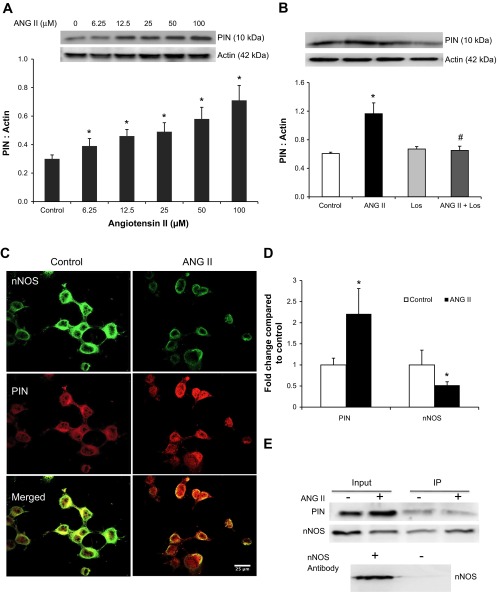
Effects of ANG II Treatment on PIN Expression in NG108 Cells
Previously, we have shown that levels of ANG II are higher in rats with CHF (210 pg/ml) compared with Sham (68 pg/ml) (18). Therefore, we hypothesized that the increased levels of ANG II acting on AT1R are perhaps increasing the expression of PIN. NG108 cells were treated with increasing concentrations of ANG II, and changes in PIN expression were analyzed after 24 h (Fig. 3A). In contrast to the decreased expression of nNOS as we observed previously (37), PIN expression increased in a dose-dependent manner, and the increase was statistically significant even at 50 μM (0.58 ± 0.08) and 100 μM ANG II (0.71 ± 0.10) compared with the untreated control cells (0.31 ± 0.03). Moreover, Los treatment for 1 h prior to ANG II significantly ameliorated the increased PIN expression (Fig. 3B), suggesting the involvement of AT1R in PIN upregulation. Immunofluorescence staining of NG108 cells (Fig. 3, C and D) revealed that intensity of PIN staining was increased, while nNOS staining was decreased after ANG II treatment. These results also suggest that ANG II downregulates the expression of nNOS and upregulates the expression of PIN in NG108 cells.
Fig. 3.
PIN expression with increasing doses of ANG II (A) and in control, ANG II (100 μM), losartan (Los, 1 μM), and ANG II + Los-treated NG108 cells (B). Top: a representative Western blot; bottom: densitometric analyses of PIN normalized to actin. Values are plotted as means ± SE from 4 independent experiments. Immunostaining of PIN (red) and nNOS (green) in control and ANG II-treated cells. C: representative pictures. D: cumulative data. Immunoprecipitation (IP) of PIN from control and ANG II-treated NG108 cell lysates (E). All gels are representative of 3–4 independent experiments. *P < 0.05 vs. Control, #P < 0.05 vs. ANG II.
Furthermore, to assess the interactions between PIN and nNOS, cell homogenates were immunoprecipitated with an anti-PIN bound protein-A/G complex, separated by SDS-PAGE, and probed by PIN and nNOS antibodies. The crude lysate was used as an input as well as the positive control. The results shown in Fig. 3E demonstrate that PIN coimmunoprecipitates with nNOS. As ANG II increased the expression of PIN in the NG108 cells, this increased PIN bound more nNOS in ANG II-treated cells compared with untreated cells. To include a negative control, the NG108 cell lysates were also incubated overnight with the protein-A/G complex without prebound antibody. Western blot showed that nNOS was not present in the eluted precipitates. These findings suggest that nNOS interacts with PIN under in vitro conditions.
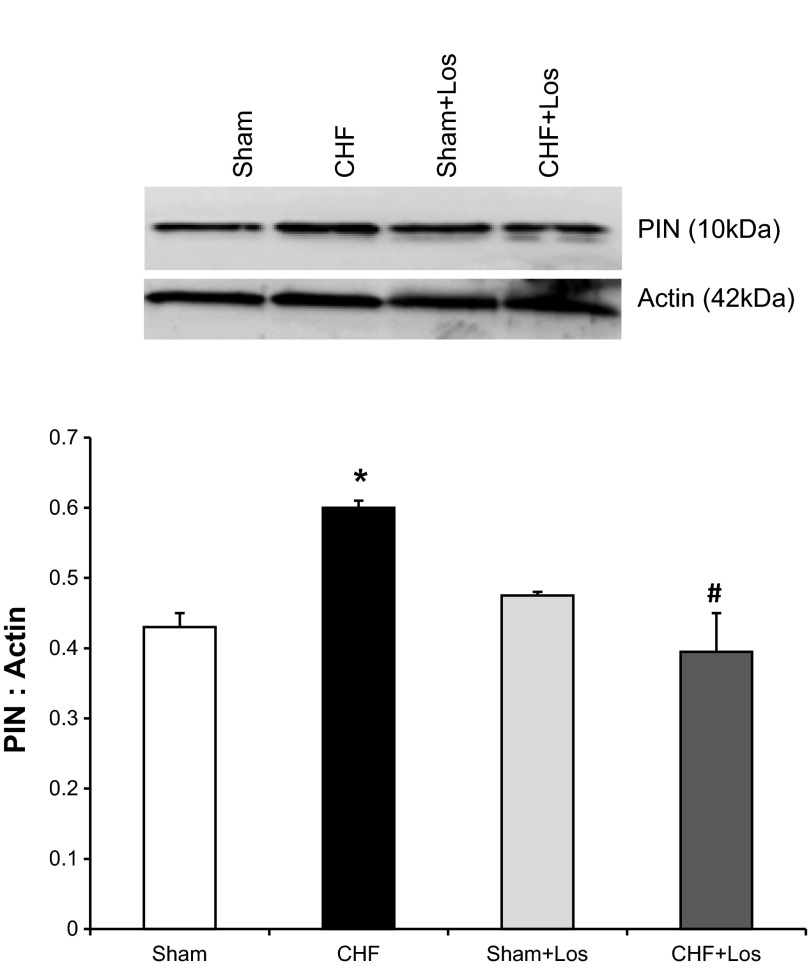
Los Ameliorates the Increased PIN Expression in the PVN in CHF
Figure 4 shows PIN protein expression in the PVN in four groups of rats (Sham, Sham + Los, CHF, CHF + Los). As shown previously PIN expression was increased in the CHF group. However, PIN expression in the CHF + Los group was reduced compared with the CHF group and was not significantly different from Sham and Sham + Los groups. These data indicate that chronic AT1R blockade by Los blocks upregulation of PIN in rats with CHF.
Fig. 4.
PIN expression in the PVN in four groups [Sham + Veh, CHF + vehicle (Veh), Sham + Los, CHF + Los] of rats. Top: a representative Western blot; bottom: densitometric analyses of PIN level normalized to actin. Values are means ± SE (n = 3–5 rats per group). *P < 0.05 vs. Sham, #P < 0.05 vs. CHF.
Ubiquitination of nNOS in the PVN
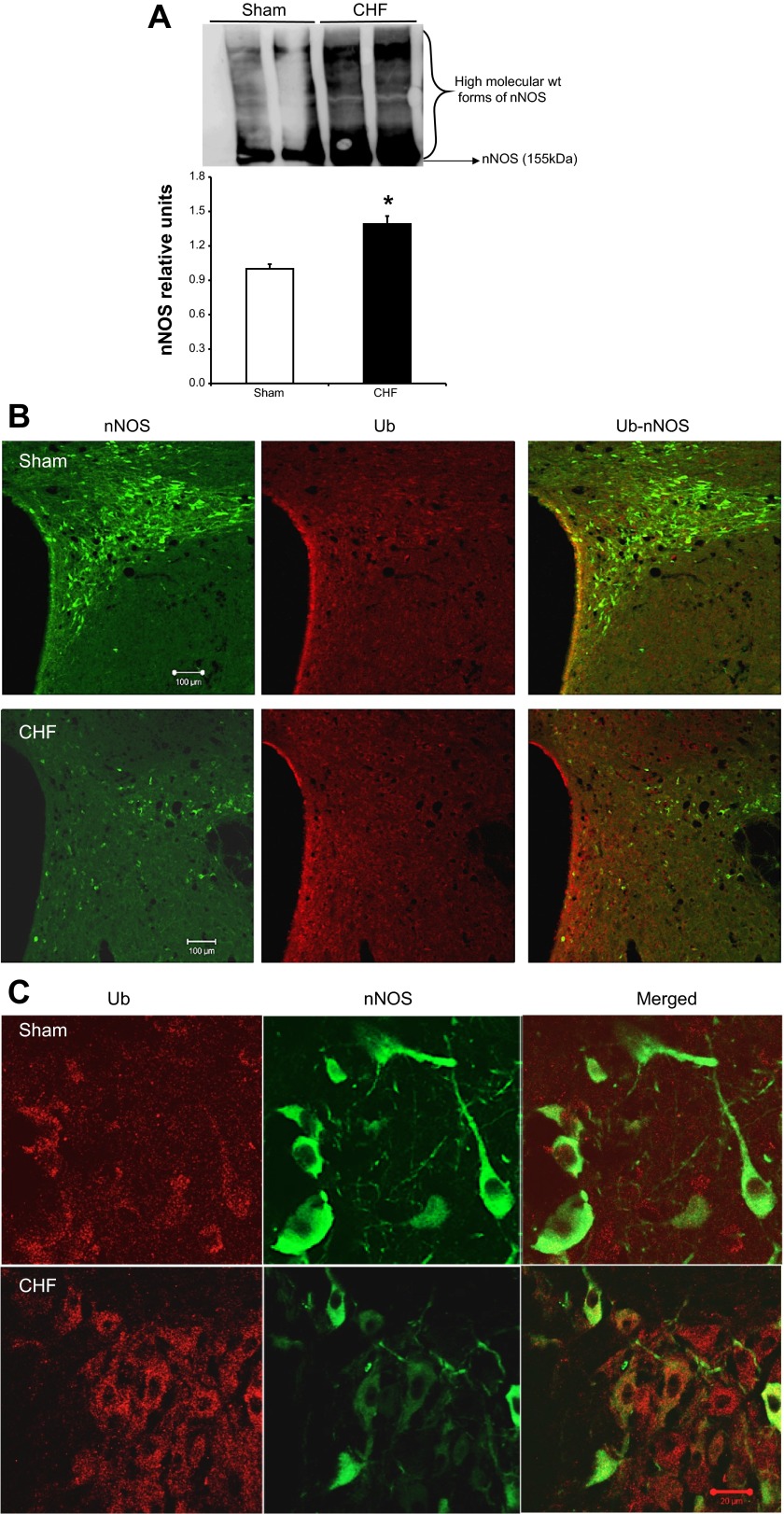
The inactive monomeric nNOS is known to be preferentially ubiquitinated over the dimeric functional nNOS (26). To probe for the ubiquitinated, high-molecular-weight forms of nNOS, PVN lysates from Sham and CHF rats were immunoblotted with a monoclonal antibody against nNOS. As shown in Fig. 5A, there was an increased accumulation of higher-molecular-weight nNOS-ubiquitin (nNOS-Ub) conjugates in the PVN of CHF rats compared with Sham (1.29 ± 0.06 vs. 1.0 ± 0.05). To corroborate the biochemical data, sections of the PVN were immunostained for nNOS and Ub, in situ, in Sham and CHF rats (Fig. 5B). In agreement with our previous studies, nNOS immunostaining was reduced in the ventromedial parvocellular sub nucleus as well as in the magnocellular portions of the PVN in CHF rats compared with Sham. The colocalization of nNOS and Ub demonstrate little overlap in low magnification (×10), likely because these ubiquitinated nNOS forms are immediately degraded in the proteasome. Nevertheless, confocal images of the PVN in CHF rats at higher magnification (×63) revealed colocalization of nNOS and Ub, suggesting that nNOS undergoes ubiquitination in the PVN of CHF rats (Fig. 5C).
Fig. 5.
A: increased accumulation of high-molecular-weight forms of immunodetectable nNOS in the PVN of CHF rats. Top: a representative Western blot; bottom: densitometric analyses of ubiquitin (Ub)-nNOS bands. Values are means ± SE (n = 4–5 rats per group). *P < 0.05 vs. Sham. B: confocal photomicrographs of nNOS (green) and Ub (red) in the PVN of Sham and CHF rats. C: higher-magnification images showing Ub-nNOS colocalization in the PVN of CHF rats. Green, nNOS; red, Ub; orange, nNOS-Ub.
Ubiquitination of nNOS in NG108 Cells
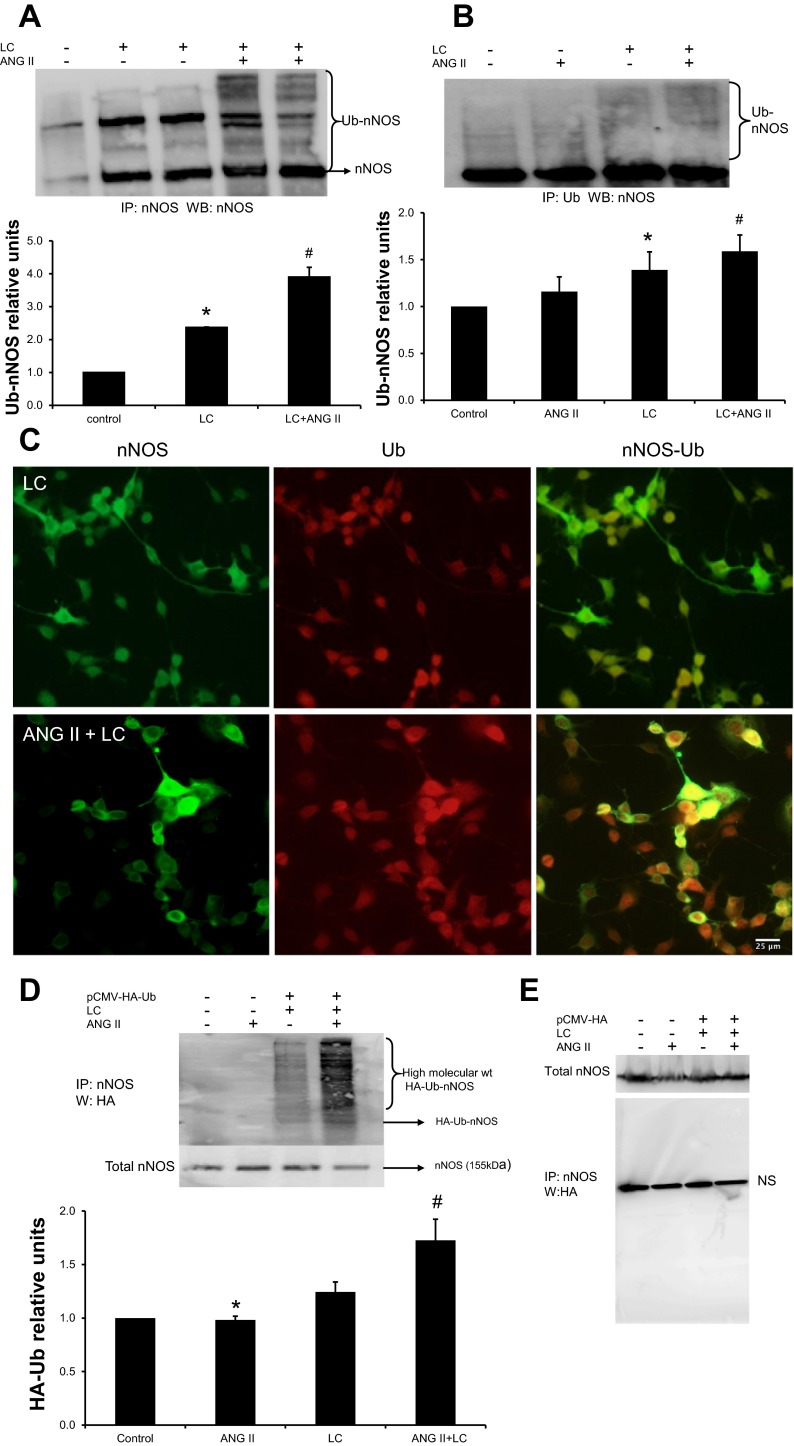
To confirm that ANG II-promoted downregulation of nNOS was Ub dependent, we performed in vitro experiments with NG108 cells in the presence of proteasomal inhibitor LC (a cell-permeable inhibitor that specifically blocks the activity of the 26S proteasome). Thus, LC causes accumulation of ubiquitinated proteins destined to be degraded in the proteasome. Figure 6A demonstrates that proteasomal inhibition of NG108 cells with LC (10 μM) for 4 h prior to harvesting the cells yields more high-molecular-weight bands compared with the native enzyme detected upon probing with the nNOS antibody (cf. lane 2 with lane 1). These intense higher-molecular-weight bands were not detected in control cells that were not treated with LC (lane 1), indicating the inhibition of degradation of these high-molecular-weight forms of nNOS upon LC treatment. As shown in lanes 4 and 5, there is 67% more accumulation of higher-molecular-weight bands of nNOS when cells were treated with ANG II and LC together compared with LC alone. These results suggest that ANG II increases ubiquitination of nNOS in NG108 cells. Moreover, the cumulative data display a significant increase in higher-molecular-weight bands compared with cells without ANG II treatment (2.37 ± 0.04 vs. 3.91 ± 0.04). Furthermore, as shown in Fig. 6B, immunoprecipitation with a Ub antibody and subsequent Western blotting with anti-nNOS IgG increase nNOS-Ub conjugates in ANG II-treated cells compared with the LC-treated control (cf. lane 3 with lane 4) (1.45 ± 0.04 vs. 1.66 ± 0.04). These data suggest that higher-molecular-weight forms of nNOS are Ub bound. Immunocytochemical staining of Ub and nNOS in NG108 cells showed an increased presence of nNOS with Ub in cells treated with ANG II and LC compared with LC alone (Fig. 6C), suggesting that ANG II treatment increases ubiquitination of nNOS.
Fig. 6.
ANG II increases the accumulation of higher-molecular-weight forms of immunodetectable nNOS in NG108 cells. A: immunoprecipitation of nNOS from cells treated with ANG II (100 μM), lactacystin (LC, 10 μM), or both was carried out and immunoblotted with anti-nNOS. Top: a representative Western blot; bottom: densitometric analyses of Ub-nNOS bands. Values are means ± SE from 4 independent experiments. *P < 0.05 vs. control, #P < 0.05 vs. LC. B: IP of nNOS from cells treated with ANG II, LC, or both with anti-Ub antibody followed by Western blot with anti-nNOS. Top: a representative Western blot; bottom: densitometry analyses of Ub-nNOS bands. Values are plotted as means ± SE from 4 independent experiments. *P < 0.05 vs. control, #P < 0.05 vs. LC. C: immunocytochemical localization of nNOS (green) and Ub (red) in LC (control) and ANG II along with LC-treated NG108 cells. IP of nNOS-Ub with an anti-nNOS antibody from NG108 cell lysates transfected with pCMV-HA-Ub (D) or control vector (E) and treated with ANG II followed by a Western blot with anti-HA. Top: a representative Western blot; bottom: densitometry analyses of Ub-nNOS bands. A representative experiment distinctive of 4 independent experiments is shown. Values were means ± SE from 4 independent experiments. *P < 0.05 vs. control, #P < 0.05 vs. LC.
Furthermore, we investigated whether nNOS could be ubiquitinated by exogenously introduced Ub. NG108 cells were transfected with pCMV-(HA-Ub)8 vector. In parallel experiments HA-tagged ubiquitin (HA-Ub) was replaced with an empty vector. Forty-eight hours after transfection, cell lysates were subjected to immunoprecipitation with an anti-nNOS antibody followed by Western blotting with an HA antibody. HA-Ub in transfected cells could be detected in cell lysates immunoblotted with an anti-HA antibody as higher-molecular-weight HA-Ub-nNOS conjugates (Fig. 6D). ANG II treatment along with LC augmented HA-Ub-nNOS levels compared with control (cf. lane 3 with lane 4) (1.24 ± 0.09 vs. 1.72 ± 0.19). In contrast, in cells transfected with an empty vector, higher-molecular-weight HA-Ub-nNOS conjugates could not be detected (Fig. 6E).
DISCUSSION
The novel finding of the present study is that nNOS expression decreases in CHF via posttranslational modification through an ANG II-mediated enhanced expression of PIN. PIN not only colocalizes with nNOS in neurons, but it also interacts physically with nNOS as demonstrated by our coimmunoprecipitation studies. PIN-mediated inactivation of nNOS increases proteolytic degradation by the proteasomal pathway. Furthermore, in vitro studies with the NG108 neuronal cell line demonstrated that ANG II via AT1R upregulates PIN expression with a concomitant decrease in the expression of nNOS. Taken together, these data suggest that elevated levels of ANG II in CHF may upregulate PIN expression in the PVN and inhibit nNOS and its inhibitory influence. These data provide insight into possible molecular mechanisms at the level of the PVN that may be involved in the reduction of the inhibitory NO mechanisms resulting in an overactivation of sympathetic drive commonly observed during CHF.
In the present study, we used the coronary artery ligation model of CHF, which mimics a reliable and consistent simulation of the CHF condition (17, 18, 41, 42) and has advantages over other models of CHF, such as ventricular pacing, because ligation of the coronary artery simulates blockage of an artery, commonly seen in patients with CHF. To further substantiate our in vivo whole animal results, we have used NG108 cell line for in vitro studies. NG108 cells have neuronal properties, are immunoreactive to renin, and have angiotensin, angiotensinogen, angiotensin-converting enzyme as well as AT1 and AT2 receptor subtypes (8, 20, 39). These cells also have endogenous expression of nNOS (23, 37, 38) and NMDAR (3, 18, 25) and therefore serve as a suitable model for studying the interplay between receptors and gene expression at the cellular level in neurons (33, 36).
NO is known to be synthesized in different tissues by NOS and plays a wide variety of physiological roles. NOS enzymes are bidomain, large proteins consisting of an NH2-terminal oxygenase domain and a COOH-terminal reductase domain. These domains are connected by a calmodulin-binding region consisting of 30 amino acids (1). NO biosynthesis depends on the availability of the NOS cofactors: viz. reduced flavins, heme iron (6R)-5,6,7,8-tetrahydrobiopterin (BH4), as well as NADPH as an electron source. Homodimerization and cofactor binding are critical for the enzymatic activity of nNOS. Electrons are transferred from the oxygenase domain of one monomer to the reductase domain of another monomer, thus requiring homodimer formation for functional activity (1). The dimerization of nNOS is promoted by heme incorporation, and BH4 is required to stabilize the nNOS dimer once it is formed (15, 35). nNOS activation without proper BH4 binding uncouples normal electron transfer to produce superoxide. It has been suggested that limited availability of BH4 contributes to eNOS dysfunction in hypercholesterolemia, diabetes, atherosclerosis, hypertension, and heart failure (5). In CHF, BH4 bioavailability is known to be impaired in endothelial cells (34); however, the role of NOS cofactors in neuronal nNOS regulation in CHF remains to be examined.
NO is an important signaling molecule in the nervous, immune, and cardiovascular systems. Despite the beneficial roles of NO as a messenger and in-host defense mechanism, an enhanced unregulated release of NO production can give rise to pathological conditions. Therefore, the regulation of nNOS activity must be highly controlled to perform variety of functions. nNOS activity is subject to transcriptional, translational, and posttranslational regulation, which dictate the specificity of NO signaling and limit NO toxicity (1). The posttranslational controls include lipid modifications, phosphorylation events, and protein-protein interactions. A large number of protein partners for nNOS have been identified. CAPON and PIN interact with nNOS specifically through its PDZ and PIN binding domains, respectively. These two binding regions are unique to nNOS and are located within the specific amino terminal sequence. CAPON binding can restrict NO generation (13). The function of PIN binding is not fully understood, but it was originally reported to destabilize the nNOS dimer (14).
Some other studies suggest its function as a DLC involved in nNOS axonal transport but not an inhibitor of the enzyme (31). This is due to the fact that the PIN recognition sequence in nNOS lies outside the catalytic core of nNOS and is not part of the dimerization region of nNOS (7). The possibility that PIN may have a role even in dimer assembly via interfering with nNOS dimerization remains to be examined. Several other proteins also interact with nNOS, such as PSD95, PSD93, α1-syntrophin, phosphofructokinase-M, Caveolin-3, heat shock protein 90, NOSIP, and plasma membrane calcium/calmodulin-dependent calcium ATPase. All of these can also affect the localization and regulation of nNOS (11).
In our previous studies, we reported that nNOS expression in the PVN is decreased in CHF (21, 42); however, in the present study we observed increased PIN protein expression along with a decrease in the nNOS dimer/monomer ratio in the PVN of rats with CHF. These findings, along with the current understanding of PIN function, suggest that the decrease in nNOS is due to increased PIN expression, which inactivates nNOS via destabilizing dimers. Others have reported a decrease in the dimerization of nNOS and production of total NO in the PVN by expressing dominant-negative nNOS, which suggests that active nNOS dimers are required for catalytic activity (32). On the other hand, some reports have shown that PIN neither inhibits nNOS nor promotes monomerization (31) and therefore concluded that PIN has no effect on nNOS dimerization. On the contrary, in present studies in vitro knockdown of PIN in neuronal cells resulted in an accumulation of nNOS as well as an increase in dimer-monomer ratio with increased NO production, demonstrating that PIN affects nNOS expression and catalytic activity therefore plays an important role in the regulation of nNOS, offering a new target for the treatment of CHF and other cardiovascular diseases. Examining the effect of PIN silencing on NO-mediated sympathoinhibition using PIN-shRNA microinjection in the PVN of CHF rats remains to be explored.
Ubiquitination has emerged as a major regulatory mechanism in controlling the levels and function of several neuronal proteins besides phosphorylation. The stability of many proteins at the postsynaptic density is regulated by the Ub-proteasome system (30). As deduced from the literature, nNOS undergoes enhanced proteolytic degradation on treatment with metabolism-based inactivators (6), antihypertensive agent guanabenz (24), or by the inhibition of the HSP90-based chaperone system with geldanamycin (26). Importantly, the heme-deficient monomeric form of nNOS is preferentially ubiquitinated over that of the heme-bound homodimer form. This suggests that ubiquitination of nNOS contributes to the regulated proteolysis of the nonfunctional enzyme (2). Therefore, conditions like decreased heme availability, BH4 depletion, and addition of a dimerization inhibitor that favors a change in dimer-monomer ratio of nNOS will have an effect on the proteolytic degradation of nNOS. In this study we identified an increase in PIN expression with concomitant decrease in dimer-to-monomer ratio in the PVN during CHF. Therefore, the monomerization of nNOS by PIN leads to Ub-mediated proteolytic degradation and, hence, a decrease in levels of nNOS in CHF. Moreover, not only did we demonstrate an increased accumulation of Ub-nNOS conjugates in the PVN of CHF rats compared with Sham, but we also provided evidence for Ub colocalization in vivo with nNOS via confocal microscopy. Interestingly, the present data show that ANG II treatment in NG108 cells augmented PIN expression with concomitant decrease in nNOS expression and also that there is increased PIN binding to nNOS after ANG II treatment, suggesting an important functional interaction between these two proteins. Furthermore, in vitro we observed an increase in Ub-nNOS conjugates in the neuronal cells treated with ANG II compared with the untreated control emphasizing the role for ANG II as a key player in the regulation of nNOS in CHF.
Previously we have demonstrated an important role for altered GABA and glutamate actions within the PVN of rats with CHF (22, 40, 41). In that context the role of PIN on GABA and glutamate interactions is probably indirect via its actions on nNOS and subsequently NO. Monomerization of nNOS via PIN causes formation of a catalytically impaired enzyme leading to a decreased amount of NO. Decrease levels of NO would be expected to reduce GABA release and its inhibitory actions (27). NO would also be expected to reduce the enhanced glutamatergic actions observed in rats with CHF. Recently we demonstrated that upregulation of nNOS transgenically, significantly decreased the enhanced renal sympathetic activity, arterial blood pressure, and heart rate responses to N-methyl-d-aspartic acid in rats with CHF (43), which suggests that maybe PIN indirectly regulates NMDA receptors via nNOS as well. This possibility remains to be examined in more detail.
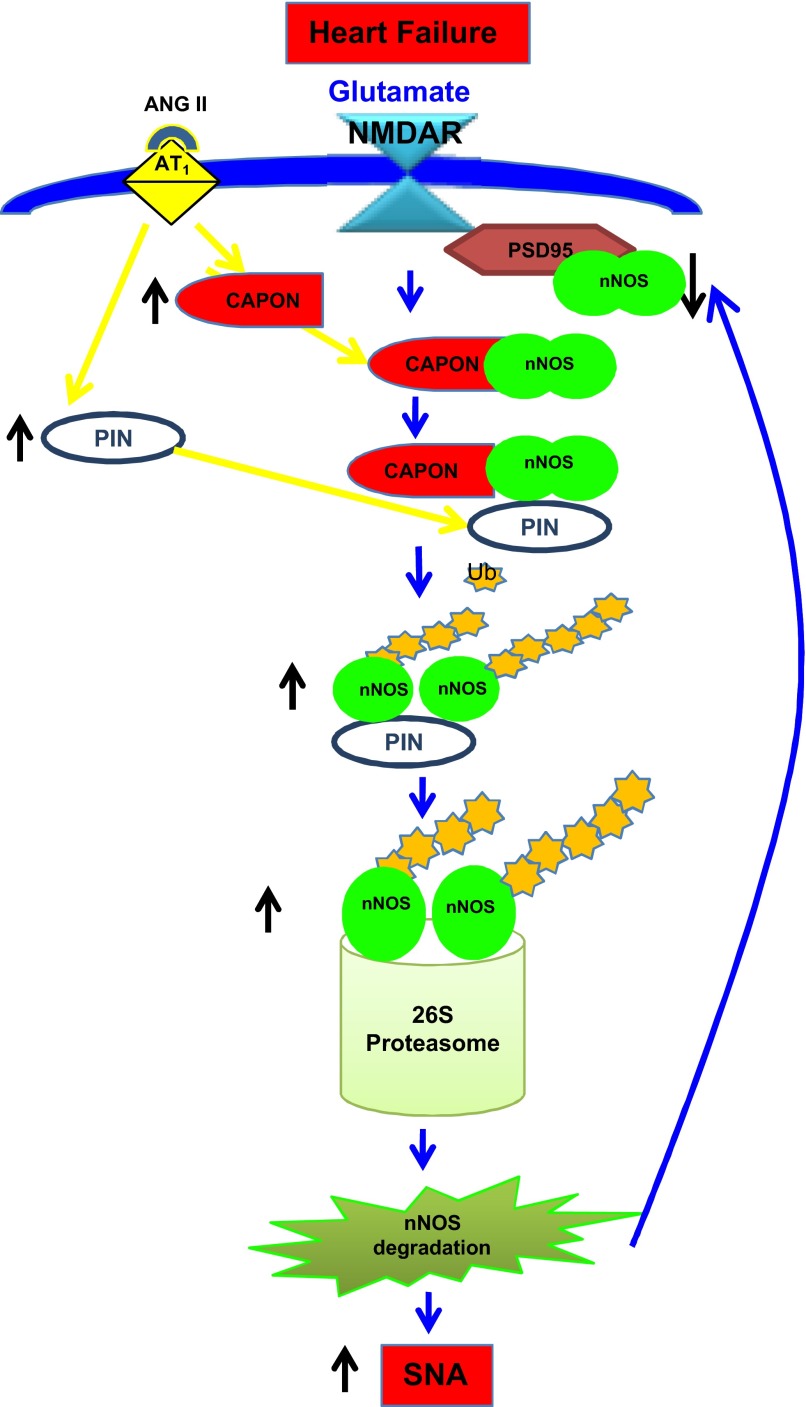
In summary, our previous (37) and present studies have revealed that nNOS in the PVN is regulated posttranslationally by protein-protein interactions as summarized in Fig. 7. The expression of CAPON (37) and PIN are upregulated, likely due to increased ANG II levels in the PVN in CHF. CAPON competes with PSD95 for binding to nNOS, but the augmented levels of CAPON during CHF enhance CAPON binding and sequester nNOS while decreasing NMDAR/PSD95/nNOS complexes on cell membranes. Subsequently, the binding of PIN to nNOS in the cytosol leads to the destabilization of nNOS dimers, a conformation required for the catalytic activity of enzyme. The inactive monomeric state is the trigger that renders nNOS susceptible to ubiquitination and subsequent proteasomal degradation. Reduced levels of nNOS result in a decrease in the production of NO, resulting in reduced inhibitory influences to neurons in the PVN and, thereby, enhanced sympathoexcitation observed in CHF. Taken together, these observations provide a significant insight into the possible mechanism(s) within the PVN that contributes to the overactivation of the sympathetic drive during CHF and offer a new target for treatment of enhanced sympathoexcitation in CHF and other cardiovascular diseases.
Fig. 7.
Proposed model for the downregulation of nNOS by posttranslational regulation in the PVN in CHF. In CHF, carboxy-terminal PDZ ligand of nNOS (CAPON) and PIN are overexpressed due to increased ANG II levels and AT1 receptors in the PVN. Increased CAPON competes with postsynaptic density (PSD)95 for binding to nNOS and sequesters nNOS therefore decreasing N-methyl-d-aspartic acid receptor (NMDAR)/PSD95/nNOS complexes. Binding of PIN to nNOS in CHF destabilizes nNOS dimers, which renders nNOS catalytically inactive, by interfering with either the assembly or dimer stability. Inactive nNOS monomers are susceptible to ubiquitination and subsequent proteasomal degradation. This results in decreased levels of nNOS in the PVN of CHF rats. A decreased level of nNOS reduces NO production in the PVN during CHF causing an increase in sympathetic nerve activity (SNA).
GRANTS
Funding from National Institutes of Health, Heart, Lung, and Blood Institute Grant HL-62222 supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.M.S. and K.P.P. conception and design of research; N.M.S. and T.L. performed experiments; N.M.S., T.L., H.Z., and K.P.P. analyzed data; N.M.S., T.L., H.Z., and K.P.P. interpreted results of experiments; N.M.S. prepared figures; N.M.S. drafted manuscript; N.M.S., T.L., H.Z., and K.P.P. edited and revised manuscript; N.M.S., T.L., H.Z., and K.P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The technical assistance of Kurtis Cornish and Xuefei Liu is deeply appreciated.
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender AT, Demady DR, Osawa Y. Ubiquitination of neuronal nitric oxide synthase in vitro and in vivo. J Biol Chem 275: 17407–17411, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Cai YC, Ma L, Fan GH, Zhao J, Jiang LZ, Pei G. Activation of N-methyl-d-aspartate receptor attenuates acute responsiveness of delta-opioid receptors. Mol Pharmacol 51: 583–587, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Dreyer J, Schleicher M, Tappe A, Schilling K, Kuner T, Kusumawidijaja G, Muller-Esterl W, Oess S, Kuner R. Nitric oxide synthase (NOS)-interacting protein interacts with neuronal NOS and regulates its distribution and activity. J Neurosci 24: 10454–10465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA 104: 15081–15086, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunbar AY, Kamada Y, Jenkins GJ, Lowe ER, Billecke SS, Osawa Y. Ubiquitination and degradation of neuronal nitric-oxide synthase in vitro: dimer stabilization protects the enzyme from proteolysis. Mol Pharmacol 66: 964–969, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Fan JS, Zhang Q, Li M, Tochio H, Yamazaki T, Shimizu M, Zhang M. Protein inhibitor of neuronal nitric-oxide synthase, PIN, binds to a 17-amino acid residue fragment of the enzyme. J Biol Chem 273: 33472–33481, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Fishman MC, Zimmerman EA, Slater EE. Renin and angiotensin: the complete system within the neuroblastoma x glioma cell. Science 214: 921–923, 1981 [DOI] [PubMed] [Google Scholar]
- 9.Furukawa M, Andrews PS, Xiong Y. Ubiquitin proteosome protocols. Methods Mol Biol 301: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gonçalves ARR, Fujihara CK, Matter AL, Malheiros DMAC, Noronha IL, De Nucci G, Zatz R. Renal expression of COX-2, ANG II, and AT1 receptor in remnant kidney: strong renoprotection by therapy with losartan and a nonsteroidal anti-inflammatory. Am J Physiol Renal Physiol 286: F945–F954, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol 49: 99–170, 1977 [DOI] [PubMed] [Google Scholar]
- 12.Jaffrey SR, Benfenati F, Snowman AM, Czernik AJ, Snyder SH. Neuronal nitric-oxide synthase localization mediated by a ternary complex with synapsin and CAPON. Proc Natl Acad Sci USA 99: 3199–3204, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron 20: 115–124, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274: 774–777, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Klatt P, Pfeiffer S, List BM, Lehner D, Glatter O, Bachinger HP, Werner ER, Schmidt K, Mayer B. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J Biol Chem 271: 7336–7342, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and l-arginine in the formation of an SDS-resistant dimer. EMBO J 14: 3687–3695, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol 298: H1546–H1555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Laflamme L, Brechler V, Reudelhuber TL, Gallo-Payet N, Deschepper CF. The renin-angiotensin system in hybrid NG108–15 cells. Renin gene is from mouse neuroblastoma, angiotensinogen and angiotensin-converting enzyme genes are of rat glioma origin. Regul Pept 77: 9–15, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Muller D, Greenland KJ, Speth RC, Middendorff R. Neuronal differentiation of NG108–15 cells has impact on nitric oxide- and membrane (natriuretic peptide receptor-A) cyclic GMP-generating proteins. Mol Cell Endocrinol 320: 118–127, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Noguchi S, Jianmongkol S, Bender AT, Kamada Y, Demady DR, Osawa Y. Guanabenz-mediated inactivation and enhanced proteolytic degradation of neuronal nitric oxide synthase. J Biol Chem 275: 2376–2380, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Ohkuma S, Katsura M, Chen DZ, Chen SH, Kuriyama K. Presence of N-methyl-d-aspartate (NMDA) receptors in neuroblastoma x glioma hybrid NG108–15 cells-analysis using [45Ca2+]influx and [3H]MK-801 binding as functional measures. Brain Res Mol Brain Res 22: 166–172, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Osawa Y, Lowe ER, Everett AC, Dunbar AY, Billecke SS. Proteolytic degradation of nitric oxide synthase: effect of inhibitors and role of Hsp90-based chaperones. J Pharmacol Exp Ther 304: 493–497, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Proc Soc Exp Biol Med 226: 814–824, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol 265: R923–R928, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503–512, 1979 [DOI] [PubMed] [Google Scholar]
- 30.Rinetti GV, Schweizer FE. Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. J Neurosci 30: 3157–3166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Crespo I, Straub W, Gavilanes F, Ortiz de Montellano PR. Binding of dynein light chain (PIN) to neuronal nitric oxide synthase in the absence of inhibition. Arch Biochem Biophys 359: 297–304, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Rossi NF, Maliszewska-Scislo M, Chen H, Black SM, Sharma S, Ravikov R, Augustyniak RA. Neuronal nitric oxide synthase within paraventricular nucleus: blood pressure and baroreflex in two-kidney, one-clip hypertensive rats. Exp Physiol 95: 845–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schelman WR, Kurth JL, Berdeaux RL, Norby SW, Weyhenmeyer JA. Angiotensin II type-2 (AT2) receptor-mediated inhibition of NMDA receptor signalling in neuronal cells. Brain Res Mol Brain Res 48: 197–205, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 113: 47–63, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Scott-Burden T. Regulation of nitric oxide production by tetrahydrobiopterin. Circulation 91: 248–250, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Sharma NM, Zheng H, Li YF, Patel KP. Nitric oxide inhibits the expression of AT1 receptors in neurons. Am J Physiol Cell Physiol 302: C1162–C1173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma NM, Zheng H, Mehta PP, Li YF, Patel KP. Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II. Cardiovasc Res 92: 342–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song T, Hatano N, Kume K, Sugimoto K, Yamaguchi F, Tokuda M, Watanabe Y. Inhibition of neuronal nitric-oxide synthase by phosphorylation at Threonine1296 in NG108–15 neuronal cells. FEBS Lett 579: 5658–5662, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Tallant EA, Diz DI, Khosla MC, Ferrario CM. Identification and regulation of angiotensin II receptor subtypes on NG108–15 cells. Hypertension 17: 1135–1143, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332–H2341, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension 58: 966–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]