Abstract
Antibody responses are initiated by the binding of antigens to clonally distributed cell surface B cell receptors (BCRs) that trigger signaling cascades resulting in B cell activation. Using conventional biochemical approaches, the components of the downstream BCR signaling pathways have been described in considerable detail. However, far less is known about the early molecular events by which the binding of antigens to the BCRs initiates BCR signaling. With the recent advent of high-resolution, high-speed, live-cell and single-molecule imaging technologies, these events are just beginning to be elucidated. Understanding the molecular mechanisms underlying the initiation of BCR signaling may provide new targets for therapeutics to block dysregulated BCR signaling in systemic autoimmune diseases and in B cell tumors and to aid in the design of protein subunit vaccines. In this chapter we describe the general procedures for using these new imaging techniques to investigate the early events in the initiation of BCR signaling.
1. INTRODUCTION
B cells play an essential role in the adaptive immune response to many infections by producing highly specific antibodies (Abs) to antigens (Ags) expressed by invading pathogens. Ab responses are initiated by the binding of Ags to cell surface B cell receptors (BCRs) that trigger signaling cascades that lead to the proliferation and differentiation of B cells into antibody secreting cells (Rajewsky, 1996). The BCR is a trans-membrane protein complex composed of a membrane form of Ab and a nocovalently associated disulphide-linked Igα and Igβ heterodimer. The BCR has no inherent kinase activity but rather upon Ag binding the first kinase in the signaling cascade, Lyn, is recruited to the cytoplasmic domains of Igα and Igβ that contain immunotyrosine activation motifs (ITAMs) that are phosphorylated (Reth and Wienands, 1997). The question is: by what molecular mechanisms is the information that Ag has bound to the ectodomain of the BCR translated across the membrane to trigger the recruitment of Lyn? Over the last several decades, biochemical studies have resulted in the description of the components of the downstream BCR signaling pathways in great detail (Dal Porto et al., 2004; DeFranco, 1997). The contribution of biochemical approaches to the understanding of the nature of BCR signaling pathways has been significant. However, as the old parable instructs, if you want to know the number of teeth in a horse's mouth you have to look. In the context of B cell biology, if you want to understand how BCRs trigger signaling you need to look at the BCR as it triggers signaling. The problem is that conventional biochemical approaches cannot provide the spatial and temporal resolution of the events that initiate BCR signaling that are predicted to occur within seconds of Ag binding and to be highly dynamic. Recently, with the advent of high-resolution, high-speed, live-cell and single molecule imaging techniques, it is now possible for the first time to view this central event in B cell activation (Pierce and Liu, 2010). The technological improvements in the fluorescence microscope over the last decade, especially in the cameras and photomultipliers that detect fluorescent signals, coupled with the relative ease of creating fluorescently-tagged proteins, have brought to reality the ability to capture the movement and behavior of proteins down to the level of single molecules in live cells at video or faster frame rates (Bajenoff and Germain, 2007; Balagopalan et al.). Just as spectacular is the fact that much of this technology can be quickly taught to the novice on instruments that can be maintained in most laboratories. The barriers to obtain spatial and temporal information for the molecules in the BCR signaling pathways are continually being chipped away, making it possible to correlate biochemical phenomenology with real-time, live-cell biology.
One imaging technique that is especially applicable for questions aimed at understanding the early events near or on the plasma membrane in the initiation of B cell activation is total internal reflection fluorescence microscopy (TIRFM) (Groves et al., 2008). TIRFM is a spatially limited technique in which the fluorescent signal of a specimen is confined to only ~ 100 nm from the coverslip. TIRFM takes advantage of a long-understood, photo-physical phenomenon in which light incident from a media of higher refractive index (immersion oil/glass) is totally internally reflected when it meets a media of lower refractive index (cell/sample media of specimen chamber). TIRFM uses a laser source to illuminate fluorophores. At the interface where the beam is reflected, an electromagnetic evanescent wave is produced that has the same properties as that of the incident excitation beam. The strength of the beam decays exponentially, penetrating to a depth of about 100 nm into the sample media; thus, fluorophores and autofluorescent molecules beyond the penetration depth are not excited, creating images with extremely high contrast (Groves et al., 2008).
TIRFM is applicable to cells that flatten as they interact with surfaces that recent evidence suggest is highly relevant to B cell's encounter with Ag. Indeed, current evidence indicates that B cells likely encounter Ag, not in solution but on the surface of Ag presenting cells (APCs) in vivo (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2007; Qi et al., 2006). B cells were first shown to avidly response to Ag expressed on the surfaces of APC in vitro and to form a highly organized contact area called the immunological synapse. Subsequently, B cells were shown to form immune synapses following activation with Ags anchored to planar lipid bilayers (PLB) (Fleire et al., 2006). Confocal microscopy provides the ability to image the BCR in the interface between a B cell and an APC in 3D, however, the temporal and spatial resolution is significantly limited compared to TIRFM (Groves et al., 2008). In imaging with TIRFM, the APC is replaced by coating a coverslip either directly with Ag or with a fluid PLB to which Ag is anchored. For our studies, we have chosen a fluid PLB to present Ag to understand the spatio-temporal dynamics of the BCR as the B cell binds to and transduces signals from Ag in the PLB (Sohn et al., 2010). This model system can easily be modified to include the study of BCR coreceptors following interactions with their ligands in PLBs.
Here we describe methods to measure the early events in the initiation of BCR signaling following Ag binding in PLB. As detailed in a recent review (Pierce and Liu, 2010), studies from our lab and others, indicate that a large percentage of BCRs (80%) are freely diffusing and highly mobile in the resting B cell membrane. Upon Ag binding, the BCRs first form submicroscopic oligomers through a mechanism dependent on the extracellular membrane proximal constant domain (Cμ4 or Cγ3) of the membrane Ig of the BCR resulting in a sharp decrease in the long-range diffusion coefficient of the BCRs. This drop in diffusion fits the theoretical predictions of oligomerization-induced trapping by the current picket and fence model of the plasma membrane as proposed by Kusumi and colleagues (Kusumi et al., 2005; Kusumi et al., 2010). Next the immobile BCR oligomers are trapped into microscopic BCR microclusters that grow in area and in the number of BCRs as more and more oligomers accumulate. Oligomerized BCRs perturb the local membrane lipid microenvironment, leading to a transient association of the BCRs with raft lipids and then a more stable protein-protein interaction between the BCR and Lyn kinase. BCR signaling is then initiated from the microclusters via phosphorylation of the ITAMs of Igα and Igβ by Lyn kinase, and the subsequent recruitment of Syk kinase.
In this chapter we describe how to prepare B cells and PBLs for imaging by TIRFM, provide a detailed description of the components necessary to build a in-house TIRFM system and detail several live cell imaging protocols that we have developed in our lab to capture and analyze the early events in the initiation of BCR signaling. Since the BCR is a member of the multichain immune-recognition receptor (MIRR) family that also includes the T cell receptor (TCR) and the high-affinity receptor for IgE (FcεR1), the methodologies described in this chapter for the BCR may also be applied to other MIRR family members with appropriate modifications.
2. EXPERIMENT PREPARATION
2.1 Cell preparation
In this section, we provide the procedures for preparing B cells for live cell imaging experiments. We highlight both human and mouse B cell preparations since both have been extensively used to study B cell signaling. We also address the use of primary cells versus cell lines, as each offer certain advantages depending on the biological question at hand.
2.1.1 Human or mouse primary B cells
Human PBMCs are isolated by Ficoll density-gradient centrifugation from lymphopack samples obtained from healthy donors under the appropriate institutional IRB. B cells are purified from PBMCs via negative selection magnetic cell separation using a human B cell isolation kit II from Miltenyi (Germany). Primary mouse B cells are similarly isolated by negative selection magnetic cell separation from single cell suspensions of spleens of mice. Ideally, for the study of Ag-specific responses spleens should be obtained from transgenic (Tg) mice expressing Ag-specific BCRs, such as IgHB1-8/B1-8 Igк−/− transgenic mice in which the B cells all express BCRs specific for the small hapten, NIP (Tolar et al., 2009). We have imaged mouse primary B cells either directly from spleens or after overnight culture with CpG and LPS (Calbiochem) and gene transfection (Liu et al., 2010b; Liu et al., 2010c).
2.1.2 B cell lines
Most of the commonly used B cell lines are amenable for live cell optical imaging studies. Linked with standard transfection procedures, B cell lines have proven to be useful tools in studies of B cell biology. We have established and characterized by live-cell imaging a variety of B cell lines. To address the role of BCR affinity and isotype in BCR oligomerization and microcluster growth we generated J558L B cell lines stably expressing Igα-YFP, Igβ, light chain Igλ1 and different versions B1-8-IgH-CFP, an IgM and IgG version (Liu et al., 2010b), a high and low affinity version (Liu et al., 2010a), and mutant versions targeting the constant region (Tolar et al., 2009), the trans-membrane region or the cytoplasmic domains (Liu et al., 2010b). To study the perturbations in local lipid environments following BCR oligomerization, we generated CH27 B cell lines stably expressing Igα-YFP and the lipid raft probe Lyn16-CFP, or the non-lipid raft probe CFP-Ger or the Lyn kinase full length protein LynFL-CFP (Sohn et al., 2006; Sohn et al., 2008). To study the FcγRIIB molecule function, the human B cell line, ST486, and the mouse B cell line, A20II1.6, both negative for endogenous FcγRIIB receptor expression, were stably transfected wild type FcγRIIB-YFP (Liu et al., 2010c).
2.1.3 Labeling B cell surface receptors
Prior to imaging experiments, it is essential to appropriately label the BCRs and other membrane receptors of interest using specific Abs conjugated to an appropriate fluorophore. Due to the variation of Ab affinity and Ab fluorophore conjugation stoichiometries, it is necessary to determine by titration the appropriate concentration for each Ab necessary to label the BCR. Generally, cell surface proteins of interest are labeled by incubating approximately 1 × 106 cells with specific, fluorescently labeled Abs at 100–200 nM in 200 μL PBS for 10 min at 4°C followed by washing twice in PBS. It is important to use Ab Fabs for labeling cell surface proteins to avoid receptor crosslinking and internalization and inadvertent engagement of Fc receptors. The fluorophore-conjugated Fabs of specific Abs can be purchased from a number of vendors. If not commercially available, Fabs can be prepared from fluorescently-labeled, intact Ab using a Fab micro preparation kit (Pierce, Rockford, IL), following the manufacturer's protocol. Prior to Ab fragmentation, Abs are conjugated with the desired fluorophore using AlexaFluor mAb labeling kits (Molecular Probes) following manufacturer's protocols. It is critical to select the proper Fab micro preparation kit for the subclass of the Ab to be fragmented. Ficin is commonly used to cleave IgG1 Abs, whereas papain is used for Abs of other IgG subclasses. In addition, Protein A, G or A/G-mediated purification of Fcs should be selected to provide the best binding to the Ab's Fc based on the Abs species and subclass. The Fab micro preparation kit protocols may yield Fab fragments in concentrations that are impractically low. The Fab fragment solution can be concentrated using a 10 kDa MWCO ultracentrifugation filtration device (Millipore), for example. All Ab fragmentations should be confirmed by SDS-PAGE where Fab migrates to 45–50 kDa and 25 kDa under non-reducing and reducing conditions, respectively. Appropriate SDS-PAGE controls are discussed in the Fab micro preparation kit protocol. Relatively low amounts of Fab are generated from the starting material, so silver staining offers a highly sensitive way to develop SDS-PAGE gels, detecting as little as 1 ng/band, such that it is reasonable to load only 100–200 ng of sample per lane for analysis.
2.2. Preparation of antigen-presenting planar lipid bilayers
Supported membrane bilayers have been commonly used in various functional biological studies. In B cell activation studies, Ag-containing PLBs have been used to mimic APCs. The Ags are tethered to the lipids in the PLBs and when prepared appropriately the Ag is completely mobile and freely diffusing in the PLB. To make fluid PLBs, an ultra-clean support system is essential. For live cell imaging glass chamber slides are generally used and this section describes the procedures for cleaning the glass supports. We also describe making the small unilamellar vesicle (SUV) stock solution used to prepare the PLBs and preparing Ag-containing PLBs.
2.2.1 Cleaning of glass items
The required materials include:
KOH/EtOH cleaning solution: 2.1 M KOH, 85% EtOH;
5 ml clear glass vials with a V shaped bottom (NextGenTM V vial, Wheaton Science Products, Millville, NJ);
Coplin jars with lid;
Rinsing solution: 95% EtOH (Grain Alcohol 190PROOF-USP Grade);
EZ-spread plating glass beads (MP Biomedicals, Solon, OH);
Deionized ultrapure water, which has a resistivity of 18.2 MΩ-cm and total organic contents less than 5 parts per billion (ppb);
100% EtOH (Ethyl Alcohol 200 Proof-USP Grade).
The glass items are cleaned as follows:
Immerse all glass items in freshly prepared KOH/EtOH cleaning solution in a 2L beaker for 10 min. Make sure that the vials are completely filled with the solution without air bubbles.
Transfer and similarly immerse the items in the rinsing solution (95% EtOH) in a 2 L beaker and moderately swirl several times.
Rinse the items thoroughly under a flow of ultrapure deionized water with vigorous shaking.
Put all the glass items in a detergent-cleaned beaker and bake at 160°C for 1 h.
For vial cap washing, immerse all caps in 100% EtOH, vigorously shake for a few minutes, and then rinse three times with a copious amount of ultrapure deionized water.
Put the caps in a detergent-cleaned beaker and completely dry at 60°C.
2.2.2. Preparing SUVs
The required materials include:
25 mM of 1,2-Dioleoyl-sn-Glycero-3-phosphocholine (DOPC) (Avanti Polar Lipids, Alabaster, AL), 10 mM of 1,2-Dioleoyl-sn-Glycero-3-phosphoethanolamine-cap-biotin (DOPE-cap-biotin) for the use of biotinylated Ags or 10 mM of 1,2-Dioleoyl-sn-Glycero-3-[N(5-Amino-1-Carboxypentyl) Imino-diacetic Acid]-Succinyl (Nickel Salt) (DOGS-Ni-NTA; Avanti Polar Lipids, Alabaster, AL) for the use of His-tagged Ags;
Hamilton syringes (Hamilton, Reno, NV);
High purity chloroform (Burdick & Jackson, Muskegon, MI),
Compressed argon gas;
Nanostrip (OM Group Ultra Pure Chemicals Ltd, Derbyshire, UK);
Water-bath-type sonicator (Model: G112SP1G, Laboratory Supplies, Hicksville, NY);
Ultra-Clear 13 × 51 mm ultracentrifuge tubes (Beckman Instrument Inc, Palo Alto, CA) and Beckman rotor, SW55Ti (Beckman Instruments Inc., Palo Alto, CA);
SUVs are prepared as follows:
During SUV preparation, glass items cleaned as described above must be used. Clean the Hamilton syringe using chloroform and 100% EtOH. In a fume hood, for each lipid to be measured fill three vials with EtOH and three vials with chloroform. In an EtOH vial, move the syringe piston up and down several times and repeat the process sequentially in the next two EtOH vials. Repeat the process in the chloroform vials. Air-dry the syringes on Kimwipes.
To make SUVs of DOPC and DOPE-cap-biotin to which biotinylated Ags or other molecules can be tethered, mix in a vial 1 mL of 25 mM DOPC and 25 μl of 10 mM DOPE-cap-biotin, resulting in a 100:1 ratio of DOPC:DOPE-cap-biotin in a 5 mM final concentration of DOPC solution. For tethering His-tagged Ags or other molecules to SUVs, make SUVs of DOPC and DOGS-NiNTA by mixing 250 μl of DOGS-Ni-NTA with 1 mL of 25 mM DOPC, giving a 10:1 molar ratio of DOPC:DOGS-Ni-NTA. Tighten the cap and mix by briefly shaking. Immediately fill the vials containing the stock lipid solutions with argon gas to avoid exposure to air and store the lipid stocks at −20°C until proceeding with the next step
Dry the lipid mixture with a gentle stream of argon gas (~5 psi) while slowly rotating the vial until the solution is dry and a thin film of lipid forms on the walls of the vial. Be careful not to splash the mixture up the walls of the vial. For the drying process, a Pasteur pipette can be connected to the argon tank regulator via plastic tubing. After completely evaporating the residual chloroform, dry the vial in a vacuum at room temperature (RT) overnight.
Hydrate the lipid film by adding 5 ml of degassed PBS freshly prepared at RT. Degass the PBS in a Nanostrip-cleaned and oven-dried side arm flask. Immediately after degassing, fill the vial with argon gas, cap tightly and vortex for about 30 s until the lipid film is completely dissociated from the wall and makes an opaque solution. From this step forward, keep this 5 mM SUV stock solution on ice.
Sonicate the lipid mixture using a water bath-type sonicator, maintaining a bath temperature of < 4°C. Perform the sonication in 10 min rounds until the lipid mixture becomes clear; this indicates the formation of SUVs. In between rounds of sonication, pause for 10 min to avoid overheating the lipid solution.
To clarify the SUV solution ultracentrifuge it using a Beckman SW55Ti rotor. First spin at 4°C for 1 h at 46,800× g and then transfer the supernatant containing the SUVs into a new tube using a sterile pipette and ultracentrifuge at 4°C for 8 h at 54,700× g. Note: Save aliquots of the SUV solution before each stage of ultracentrifugation to determine the concentration of the final SUV stock solution as described in Step 7.
The SUV mixture is filtered through a 0.2 μm-syringe-type Whatman filter for further purification; the resulting concentration of lipid solution is about half of the original concentration as determined by measuring the O.D. at 234 nm using a UV-Vis spectrophotometer. Carefully transfer the supernatant into a syringe tube and filter through to a new 15 ml polypropylene conical tube, fill the tube with argon gas, cap, and seal with parafilm. The SUVs stock solution is ready for use. Note: Be sure to save an aliquot of the SUV mixture before filtration so that the O.D. can be compared with the O.D. of the mixture after filtration. The SUVs are stable for several months at 4°C under argon gas. To minimize repeated exposure of the SUV stock to the air and to prolong stability, make aliquots in 15 mL tubes under argon and seal with parafilm
2.2.3 Making PLBs and tethering antigens to PLBs
Here we describe tethering the model Ag, NIP, or a surrogate Ag, anti-IgG to PLBs. The method can be generalized to attach any biotinylated or His-tagged molecule to biotin- or nickel-containing PLBs.
The required materials include:
24 × 50 mm #1.5 cover glass;
Lab-Tek chamber #1.0 Borosilicate coverglass (Cat No. 155411, Nalge Nunc International, Rochester, NY); Sylgard 164 Silicone Elastomer adhesive (Dow Corning Corp., Midland, MI);
Biotinylated goat anti-mouse (or human) IgM (or IgG) F(ab’)2, hapten 4-Hydroxy-3-iodo-5-nitrophenyl (NIP)-conjugated peptide NIP1-ASTGKTASACTSGASSTGS-His12 (NIP1-His12) or NIP1-His12 peptide coupled to Hylight647 through its cysteine residue (NIP1-His12-Hylight647) (Anaspec, San Francisco, CA). The hapten-peptide conjugates are HPLC purified and verified by mass spectroscopy with >95% purity;
Imaging buffer: 1× Hank's Balanced Salt Solution (HBSS) containing 0.1% FBS (Gibco/BRL).
Ag is tethered to PLBs as follows:
Clean three Coplin jars with Nanostrip. Fill one with Nanostrip, one with deionized ultrapure water and one with 100% EtOH.
Place 24 × 50 #1.5 coverslips into the jar containing Nanostrip for at least 1 h and then transfer the coverslips into the jar containing deionized ultrapure water. Shake the jar several times and discard, fill with water, and repeat the process 10 times with fresh ultrapure water. Then transfer the coverslips to the jar containing 100% EtOH for several minutes. Take out a coverslip and blow dry completely with argon gas. Place the coverslip on top of a monolayer of KOH/EtOH-cleaned glass beads (see Section 2.2.1).
Tear off the bottom coverslip of an 8 well Lab-Tek chamber and replace with a Nanostrip-cleaned coverslip using Sylgard 164. Fill the channels at the base of each well with Sylgard using an 18 G needle and syringe, place the Nanostrip-cleaned coverslip over the bottom of the chamber, and apply gentle pressure to the channels, allowing 30 min for adhesion and drying. Prepare chambers on the day of use as dust will re-accumulate and prevent the formation of good quality PLBs.
To make PLBs on the chamber coverglass, make 0.1 mM final concentration of SUV working solution in PBS, dispense 200 μl into each of the chamber wells, and wait for 10 min. Rinse the planar lipid bilayer with about 20 ml of PBS per well, keeping the bilayer under the solution at all times. Fill the chamber to the top with PBS after the last rinse (each chamber holds approximately 700 μL). Note: It is important to maintain PLBs under hydrated conditions to avoid the oxidation induced modifications to the PLBs as described (Plochberger et al.).
For tethering the biotinylated Ag to the PLBs, remove 400 μl of PBS from the filled wells, add 250 μl of streptavidin in PBS at 0.1 μM, mix twice by gently pipetting up and down with a micropipette and repeat the washing step described in Step 4. Add biotinylated Ag at a final concentration of 100 nM, incubate for 20 min, and wash unbound Ag by repeating Step 4. Note: To remove aggregated Ags, spin the antigen solution at maximum speed for at least 10 min prior to adding it to the chambers.
For tethering His-tagged Ag to the PLBs, skip the step of adding streptavidin and instead directly add His-tagged Ag as described in Step 5.
The mobility of Ag-tethered PLBs should be assessed before imaging experiments. We check the quality of PLBs by examining the mobility of PLB-tethered fluorophore-conjugated Ags, such as NIP1-His12-Hylight647. The mobility fluorophore-conjugated Ag molecules on PLBs is quantified using single particle tracking-based analysis tools as detailed in Section 3.1 or in our previous publications.
Immediately before imaging, exchange PBS with imaging buffer by washing the chamber with 10 mL of imaging buffer. Note: PLBs are less stable in the buffer containing serum, so use within 1-2 h if possible.
2.3 TIRF Microscope Design
The popularity of TIRFM over recent years has encouraged major microscope manufactures to introduce sophisticated “turn-key” TIRFM systems. Such systems are a tremendous boon to core microscopy facilities but offer little room for in-house modifications in laboratories with unique requirements. Implementation of a through-lens TIRFM system to an existing inverted microscope base is relatively straightforward; it requires access to the rear excitation port (typically where the mercury arc lamp is attached), knowledge of the focal length of microscope base's tube lens, which, in turn, allows the user to match an external lens to focus the laser beam at the back focal plane of the objective lens.
Here, we discuss some general considerations and list the components for our two modified, inverted Olympus IX81 microscope systems that were used perform the live-cell experiments described in this chapter. The components are discussed in order of excitation to image detection. The principles of TIRFM and detailed instructions on the assembly of a TIRF microscope are beyond the scope of this chapter; however, useful information can be found within the following references (Axelrod, 1981; Axelrod, 1989; Axelrod, 2001; Axelrod, 2003; Axelrod, 2008; Mattheyses et al.).
2.3.1 Excitation light source
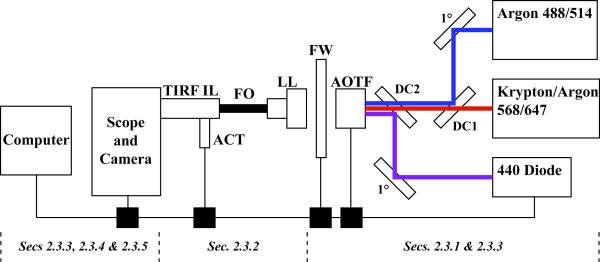
Continuous wave lasers provide the illumination source for our TIRFM systems. Typical laser types include gas, diode-pumped and straight diode; all can be used in combination. The lasers are mounted on an optical breadboard with their polarity and vertical beam heights matched so that they can be combined into a single-mode fiber optic cable, which carries the light to the TIRFM illuminator on the microscope as schematically illustrated in Figure 1.
Figure 1.
System Design. Shown is a generalized diagram one of the TIRFM rigs used in the experiments described. The pertinent sections that discuss the specific components are noted beneath the figure. All optical components to the right of the fiber optic cable (FO) are mounted on a metal breadboard with ¼” screws on standard post mounts. Three lasers (boxes on right) are used to provide five usable excitation lines (wavelengths in nm indicated). After reflection on a primary mirror (1°), the argon laser lines are directed to the AOTF via a dichroic mirror (DC1). The krypton-argon lines pass through both DC1 and DC2. After reflection on a primary mirror (1°), the 440 nm diode laser line is directed to the AOTF via DC2. Power at the 440 nm laser head is computer controlled. Control boxes are required to interface with the computer software and are depicted as black boxes. Thin black lines at the bottom of the figure indicate computer connections. The AOTF and excitation filter wheel (FW) are linked to their respective control boxes, which in turn, are coupled to the PC workstation and are controlled by MetaMorph acquisition software. The user selects the laser line via the AOTF and blocks unneeded lines with the filter wheel. The selected line is directed to the laser launch (LL) lens to allow entry into the fiber optic cable. The fiber optic cable is coupled to the TIRF illuminator (TIRF IL). The TIRF angle is controlled through the software via a motorized actuator (ACT).
Mixed gas lasers provide an economic means to obtain multiple laser lines from a single device. Wavelength selection is accomplished using a software-controlled, acousto-optical tunable filter (AOTF). Because of the inadequate blocking power of the AOTF and the fact that gas lasers produce multiple usable (and unusable) wavelengths, extraneous excitation light must be blocked from reaching the extremely sensitive cameras used in TIRFM with “clean up” excitation filters. On our systems, the clean up filters are placed in a software-controlled wheel on the breadboard after the AOTF to provide versatility and to avoid reflection artifacts that occur if placed in the traditional location within the dichroic beamsplitter housing (Figure 1) (see section 2.3.3).
Straight diode and diode-pumped solid-state lasers can be up to ten times smaller in size and are a noise-and heat-free alternative relative to their gas-driven equivalents. Straight diode lasers have a longer life expectancy relative to gas lasers whose tubes must be replaced (usually at a third of the full cost) every two to three years. Straight diodes can be modulated by software control, bypassing the need for an AOTF and thus can be directly linked to a fiber optic cable either at the laser head or, as in the case of our system, mirrored directly into fiber optic coupler. It is important to note that the square beam shape of a typical diode laser usually results in an unavoidable and significant loss of power throughput at the point of fiber optic coupling. Diode-pumped lasers use a different technology to produce their monochromatic lines and their output must be modulated through an AOTF.
Component list (also shown in Figure 1)
Optical table components and design (Solamere Technology Group, Salt Lake City, UT)
440 nm, 50 mW, straight diode (Blue Sky Research, Milpitas, CA)
488 and 514 nm, 300 mW Argon gas laser, ~70 mW each line (Dynamic Laser, Salt Lake City, UT)
568 and 647, 80 mW Krypton/Argon gas laser ~20 mW each line (Dynamic Laser, Salt Lake City, UT)
Filter wheel and control box (Applied Scientific Instrumentation, Eugene, OR)
AOTF and control box (NEOS Technologies, Melbourne, FL)
Fiber optic launch (OZ Optics, Ottawa, Ontario)
2.3.2 TIRFM illuminator port
The heart of the TIRF microscope is the illuminator port. While illuminator port designs can be complex, all serve the same fundamental purpose: to direct and focus the diverging beam that exits the fiber optic at the back focal plane of the objective lens. A simple two-lens configuration is used in the Olympus illuminator port and is also found in many systems designed in-house (Figure 1). An internal 200 mm focal length tube lens is positioned just before the dichroic beam splitter turret and an external 100 mm lens is present in the TIRFM illuminator. The lenses are held at a fixed distance to one another. For a simplified in-house system, the external illuminator lens can be placed on a simple post mount in line with the rear microscope port. At the entrance of the illuminator, the fiber optic cable is coupled to an adjustable mount that centers the beam in the light path. The mount is manually translated horizontally along a slider to focus the beam at the objective lens's back focal plane. The beam is collimated as it exits the objective and, if projected on the ceiling, will produce a circular spot when of good quality (see Section 2.3.3). To achieve TIR, the mount's height is lowered vertically with a manual micrometer to translate the excitation beam from the center to the side of the objective lens to angle the beam as it exits the lens. In the simplified system, a fiber optic coupling mount can be assembled on a manual axis positioner mounted to the microscope table to move the assembly in the x, y or z plane.
The disadvantage of a single port illuminator system is that beam focus and optimal TIR angle can be set for only one excitation wavelength at a time. More sophisticated TIRFM illuminators now provide independent coupling mounts for multiple fibers that are motorized. To increase the versatility of our system, we replaced the manual TIRF angle micrometer with a motorized micrometer and replaced the internal tube and external illuminator lens with achromatic lenses of the same focal length. The motor enables rapid, software controlled angle switches that are necessary for experiments that require a specific penetration depth for all excitation wavelengths and the achromatic lenses abrogate the need to compromise focus between different excitation wavelengths. However, it is important to recognize that while small motor movements are relatively fast in multi-channel imaging experiments, extremely rapid, stream acquisitions can only be achieved by TIRFM illuminators that have multiple fiber coupling mounts if the TIR angle must be optimized between wavelengths.
Component list
Fiber optic cable, type FC/APC (OZ Optics, Ottawa, Ontario)
Achromatic lens FL100 mm, JML 78903; FL200 mm, JML 32701A (JML Optical, Rochester, NY)
Motorized actuator (Act) to control TIRF angle (Thor Labs, Newton, NJ)
Actuator controller (Applied Scientific Instrumentation, Eugene, OR)
2.3.3 Filters, mirrors and lenses
Quality dichroic beam splitters and excitation, emission and notch filters are essential for successful TIRFM and are available from a variety of manufacturers. Of note, newer, so-called sputter designs are brighter and more durable than traditional coated glass surfaces and when available, should be the glass of choice when assembling a new system. Reflection artifacts unique to TIRFM can be avoided at the outset by directly communicating with the filter manufacturer because specialty matched sets and less common designs are not always available for self-choice on websites or catalogues.
Component list
Excitation, emission, dichroic beam splitters (Chroma Technology, Bellows Falls, VT)
Notch filters (Semrock, Rochester, NY)
Objective lenses: 60x/1.45 NA PlanApoN; 100x/1.45 NA PlanApo; 150x/1.45 NA UApo (Olympus, Center Valley, PA)
Excitation/clean up filters
As noted in Section 2.3.1, the excitation/clean up filters are located on a software-controlled, motorized wheel located on the laser breadboard. Because the AOTF only blocks light to an O.D. of 104, these filters are necessary to completely block all unwanted excitation wavelengths from gas lasers and side bands from some diodes. Note that using clean up filters comes at a cost of losing up to 5% of the excitation power before the laser is launched into the fiber optic cable.
Dichroic beam splitters
For single particle imaging experiments where photons on both the excitation and emission side are at a premium, dichroic beam splitters that reflect at a single wavelength are optimal. However, for multicolor imaging, it is best to use beam splitters that are designed to reflect all excitation wavelengths required for the experiment. Switching between beam splitters to reflect the necessary wavelength to the specimen is costly in time, will likely result in a pixel shift in the image overlay, and will most likely change the TIR angle as explained below.
Reflection artifacts are a challenge in TIRFM and it is necessary to ensure that the beam splitter rests at a 45° angle in the housing cube. Standard Olympus cubes are equipped with a clip under which the rectangular beam splitter is placed. The beam splitter is fixed in place with setscrews. If the setscrews are over-torqued (even very slightly), bending of the beam splitter's surface will occur, which, in turn, disrupts the reflection of the excitation beam; this is readily observed by viewing the projected collimated beam on the ceiling. Bowed beam splitters distort the circular spot. Certain filter manufacturers who are aware of these issues now glue the beam splitter into its housing and use thicker glass substrates to compensate for stress.
Emission filters, notch filters and emission splitters
Emission filters serve two important functions: to provide the desired band or long pass emission spectra and to block the excitation light to the camera. Under conditions where signal from the specimen is extremely low and the excitation power is high, we found it necessary to use notch filters to provide additional blocking against the excitation source to raise the signal to background ratio. Notch filters are designed to block with an extremely narrow stopband (~10 nm in width) yet allow >95% of the desired wavelengths to the camera. In our system, the notch filters are placed in the emission filter port of the beam splitter housing. The filters can be purchased without metal collars to fit multiple filters in one port.
To accommodate rapid channel switching, emission filters are best placed in a software-controlled filter wheel. If extremely fast (stream imaging is required for multiple channels), the emission spectra from the specimen can be split using a dichroic beam splitter in the emission light path to simultaneously project each channel to the respective right and left half of the camera's CCD. Emission splitting devices available on the market can be threaded between a camera and filter wheel via a C-mount. The splitters provide a cassette that houses a beam splitter and ports for emission filters. Optical alignment of separated channels on the CCD takes great care and is never perfect throughout an entire field of view due to spherical aberration. Post-acquisition alignment is frequently necessary and, therefore, a good practice is to capture reference images of fixed fluorescent beads to calibrate the image analysis software. If a third-party objective lens is used on the microscope system, it is also important to check for a focus shift due to chromatic aberration by acquiring a Z-series of beads that are fluorescent in both channels. If the beads do not peak in intensity in the same Z-slice, the chromatic shift is calculated by distance between peaks and is corrected with a lens placed in either one of the emission filter ports of the splitter. If the microscope comes equipped with a focus motor, an alternative would be to offset the focus for one channel to adjust for the chromatic shift during image acquisition.
Objective lenses
Depending on the manufacturer, objective lens magnification ranges from 60X to 150X for TIRFM. All TIRFM lenses have an extremely high numerical aperture (greater than 1.4), that is necessary to achieve the critical angle required for TIR. The larger the numerical aperture, the greater the working range for critical angle adjustment.
2.3.4 Ancillary equipment
Component list
ZDC infrared focus control laser (Olympus, Center Valley, PA)
LiveCell environmental chamber (Semrock, Rochester, NY)
Isolation table (Technical Manufacturing Corporation, Peabody, MA)
Focus control
Because the focal plane in TIRFM is extremely narrow (~100 nm), focus control becomes a key issue for time-lapse acquisitions. Now most microscope manufacturers provide a peripheral infrared laser system to provide continuous focus using the coverslip as a point of reference. The latest generation of these autofocus laser systems can even be used during a continuous stream acquisition.
Temperature control
Additional challenges are faced if the live specimen must be maintained above ambient temperature during imaging. The specimen chamber can be heated directly in a closed-chamber system, but changes (stimulation, alteration in media, and addition of cells) to the system must be made by perfusion. Open chambers with coverslip bottoms offer more versatility because changes can be made with a pipetter. Open chambers are generally heated in two ways: by enclosing the microscope in a heating chamber or by using a stage-top heating chamber in conjunction with an objective lens heater. Enclosed microscopes are better at maintaining consistent temperature but are slow to heat and cool. Stage-top devices heat rapidly but a lid must be removed to gain access to the specimen, making an autofocus system very useful for time-lapse experiments. In addition, stage-top devices require the lens to be heated separately using a collar that transfers heat to the lens barrel. It is critical that the temperature of the objective lens be equilibrated to the specimen chamber; otherwise, the specimen under observation will quickly drop to the temperature of the lens (an oil objective is assumed here).
Vibration
An isolation table is a necessity. Building and equipment vibration can cause significant blur in TIRFM applications, especially when imaging particles in motion at fast frame rates that are below the optical resolution of the microscope. In addition, care must be taken not to translate vibration from cooling fans present in the microscope's electronic control equipment to the imaging platform by hanging wire harnesses. In some cases, satellite cooling may be required to replace an on board camera fan.
2.3.5 Image acquisition hardware and software
Component list
EMCCD Cameras: Cascade IIB 512 and Cascade II 1024 (Princeton Instruments, Trenton, NJ)
Acquisition software: MetaMorph (Molecular Devices, Downingtown, PA); Analysis software: MetaMorph; MatLab (Mathworks, Natick, MA); ImagePro (MediaCybernetics, Bethesda, MD); ImageJ (rsbweb.nih.gov/ij/)
Acquisition: PC workstations assembled by Molecular Devices and Solarmere Technologies; Analysis workstations (Optiplex, Dell, USA)
Camera
The advent of high sensitivity/low noise electron-multiplying (EM) CCD technology has revolutionized wide-field fluorescence imaging to allow for video rate image acquisition and detection of single fluorophores in live biological specimens. The drawbacks of an EMCCD are the chip's large pixel size (13 to 16 microns), reducing its resolving power for certain biological structures, and its costs. Worthy of attention is the rapidly improving, significantly less expensive sCMOS technology for scientific imaging, with chip pixel sizes ~2.5 times smaller than a typical EMCCD.
Software
There are a several software companies that sell image acquisition packages that can operate just about any microscope and its peripheral components and some of the microscope manufacturers offer their own software solutions. In addition, a well-supported, open-source option to consider is Micro-Manager (http://valelab.ucsf.edu/~MM/MMwiki/index.php/Micro-Manager).
Computer and data storage
Because of Moore's law (http://en.wikipedia.org/wiki/Moore's_law), it is futile to make recommendations for computer specifications. However, investing in a workstation that maximizes useful RAM, processor speed and video card capability is paramount. While the cost of hard drive space has dropped significantly, the temptation to store data on the acquisition workstation should be avoided. Acquisition programs are just beginning to take advantage of the multi-threading capability of modern 64-bit systems. If the software is designed for a 32-bit system, it is unnecessary to go beyond 4 GB of RAM.
3. IMAGE ACQUISITION AND ANALYSIS
In this section we describe the methods used to acquire and analyze TIRF imaging data pertaining to the very early events in B cell signaling initiation, including BCR oligomerization, growth of BCR microclusters and the subsequent activation of various BCR signaling-associated molecules.
3.1 Imaging B cell receptor oligomerization by single molecule tracking
To investigate the changes in the BCR upon Ag binding that result in oligomerization and microcluster formation we tracked single BCRs in TIRFM. Our published results from these analyses indicated that on resting cells, the majority of BCRs exist as highly mobile, freely diffusing monomers that are not receptive to oligomerization (Tolar et al., 2009). With Ag binding, a force is exerted that brings the BCRs into an oligomerization-receptive form such that random bumping of Ag-bound BCRs results in oligomerization through the exposed membrane-proximal constant domain (Cμ4 or Cγ3) of membrane Ig. Upon oligomerization, the diffusion of the BCR drops dramatically such that the BCRs essentially becomes immobilized as they are trapped within areas of confinement within the plasma membrane (Tolar et al., 2009). Thus, BCR oligomerization can be measured as a change in the diffusion behavior of the BCRs, as measured by single molecule TIRF imaging.
B cells are labeled for single molecule TIRF imaging by incubating 1 × 106 cells with fluorescently-labeled BCR-specific Fab at sub-nanomolar concentration (25–250 pM, or 1.25–12.5 ng/mL range) in 200 μL PBS for 10 min at 4°C. The concentration of labeling Ab should be low enough that single BCR molecules can be visualized without the need for photobleaching (Tolar et al., 2009). Single molecules show single-step fluorescence bleaching profile, providing a diagnostic test for the single molecule images.
Cells are washed three times in PBS after labeling, resuspended in 200 μL PBS, incubated with Ag-presenting lipid bilayers for 5–10 min (50 μL cells per bilayer chamber) and imaged with 5 mW of laser (at the objective lens in epifluorescence mode).
To acquire images of single BCR molecules a 100 × 100 pixel region of interest is imaged for an exposure time of 35 ms using a streamline acquisition mode for 300 frames over approximately 10 s. A typical movie showing the mobility of single BCR molecules is provided as Supplementary Movie 1.
The processing of single molecule TIRF videos was detailed previously (Tolar et al., 2009), with tracking and analysis performed using Matlab (The Mathworks, Natick, MA) code based on available tracking algorithms (Douglass and Vale, 2005; Douglass and Vale, 2008, available at http://physics.georgetown.edu/).
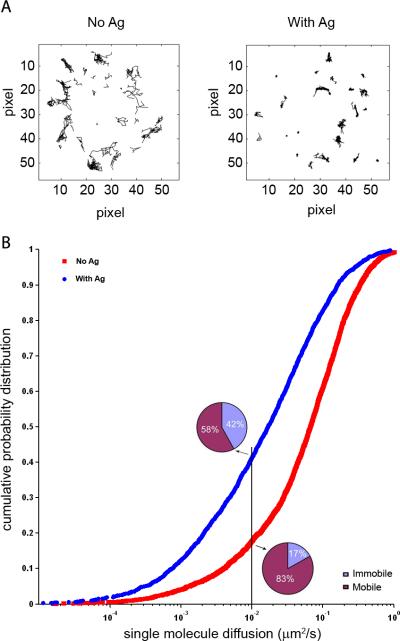
Trajectories are visually inspected and occasional mis-tracking is corrected manually. The trajectories of the single BCR molecules imaged in Supplementary Movie 1 are shown in Figure 2A. A two dimensional-Gaussian fit is used to refine the positions of the diffraction-limited spots in the trajectories. Mean square displacement (MSD) and instant diffusion coefficients for each BCR trajectory are calculated from positional coordinates as described by Douglass and Vale (2005; 2008).
Short-range diffusion coefficients are calculated from linear fits to MSD data of individual molecules for the time intervals 35–140 ms and plotted as cumulative probability distribution graphs, as shown in Figure 2B for BCR molecules on human peripheral blood B cells placed on bilayers with or without Ag. BCR molecules with a diffusion coefficient ≤ 0.01 μm2/s are considered immobile.
Figure 2.
Single molecule TIRF imaging. (A) Trajectories of individual BCR molecules accumulated over the entire time course of Supplementary Movie 1. (B) Cumulative probability plots of the diffusion coefficients of individual BCR molecules were obtained from time-lapse TIRF movies (such as those shown in Supplementary Movie 1) of human peripheral blood B cells labeled with DyLight 649-Fab anti-IgM and placed on bilayers with (blue curve) or without (red curve) goat anti-human IgM F(ab’)2 Ag. The trajectories used to construct each probability curve were collected from two independent experiments (n = 1871 with Ag; n = 3622 without Ag). Also given are the percent of mobile and immobile BCRs for cells with and without Ag..
3.2 Imaging B cell receptor cluster dynamics
Following Ag binding-induced BCR oligomerization, a microscopic structure termed the BCR microcluster is formed. BCR microclusters are believed to be the fundamental platform for initiating BCR signaling. To have a better understanding of B cell signaling activation, it is critical to image the dynamics of BCR microclusters and their coordination with downstream BCR signaling proteins. Here we described the general protocols for imaging the dynamic growth of BCR microclusters by two-color TIRFM using the example of NIP-specific J558L B cells expressing B1-8-High BCR, which is composed of Igα-YFP, Igβ and B1-8-High-IgM-CFP (IgM-High J558L B cells). We summarize the fluorophore commonly used in combination for multiple-color TIRF imaging of B cell activation (Table 1).
To prepare enough B cells for four imaging chambers, 1 × 106 IgM-High J558L B cells are labeled with 200 nM AlexaFluor 568-Fab anti-IgM in a total volume of 200 μL for 10 min at 4°C. Cells are washed twice in 200 μL PBS and resuspended in 200 μL PBS for imaging.
50 μL of IgM-High J558L B cells from Step 1 (about 0.25 × 106 B cells) are loaded onto NIP1-His12 Ag-containing planar lipid bilayers, the preparation of which is detailed in Section 2.2
Beginning with initial cell contact with the PLBs, the cells are examined every 2 s by collecting TIRF images of Igα-YFP and AlexaFluor 568-Fab anti-IgM over a total of 2 min (as shown in Figure 3). Note: The acquisition is controlled by Metamorph software using its multiple dimensional acquisition mode (Molecular Devices), with a 100 ms exposure time for both images. It is also important to consider minimizing bleaching effects by titrating the output laser power.
- The acquired TIRF image movies are analyzed and processed using a combination of Image Pro Plus (Media Cybernetics) and Matlab (Mathworks) software as described below. Similar to the analyses of single BCR molecules, as introduced in Section 3.1, Igα-YFP or AlexaFluor568-Fab anti-IgM TIRF image movies are processed by a Matlab code based on available 2D-Gaussian positional fitting and tracking algorithms (http://physics.georgetown.edu/matlab/) as shown in Figure 3. Briefly, individual BCR microclusters in each TIRF image are fit to a typical 2D Gaussian function by means of least squares. In case the profiles of some microclusters are not perfectly circular, the Gaussian function was allowed to adopt an elliptical shape (Holtzer et al., 2007).
Table I.
Fluorophores used in two-color TIRFM for imaging B cell activation
| Probes | Ex (nm) | Em (nm) |
|---|---|---|
| CFP | 433 | 475 |
| eGFP | 488 | 507 |
| YFP | 514 | 527 |
| Alexa Fluor 488 | 499 | 519 |
| Alexa Fluor 568 | 579 | 603 |
| Alexa Fluor 647 | 652 | 668 |
This is a table of some commonly used fluorophores for TIRFM imaging. Peak excitation and emission wavelengths of each fluorophore are given. A combination of two or three colors can be used in TIRFM imaging depending on the availability of an appropriate dichroic beamsplitter and excitation and emission filters. Note: There are some analogous fluorophores exist in market. For example, AlexaFluor 647 is an analogous fluorophore to Dylight 649 and Hylight 647. These analogous fluorophores have comparable excitation and emission spectrum and thus can be substituted depending on availability in imaging experiments.
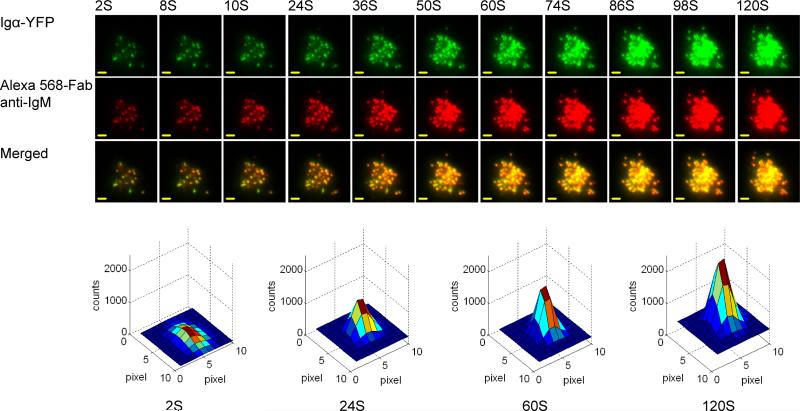
Figure 3.
Two-color time-lapse TIRF images capture the response of B cells upon initial contact with Ag-containing PLBs. Upper Panel: IgM-High J558L B cells labeled with AlexaFluor 568-Fab anti-IgM were placed on PLBs containing NIP1-His12 Ag and were examined by TIRFM over 120 s by imaging Igα-YFP (green) and AlexaFluor 568-Fab anti-IgM (red). Bars, 1.5 μm. Bottom panel: pseudo-color 2.5D Gaussian images of one representative Alexa Fluor 568–Fab anti-IgM microcluster are shown at the indicated times.
For each microcluster the fit will yield the parameters including local background fluorescence intensity (FI) (Z0), position (Xc, Yc), integrated FI (I) indicating the brightness of the cluster, and generalized full width at half maximum peak height (σr) of the intensity distribution indicating the size of the cluster. The tracking function of the Matlab code is able to link both the FI and size information to each individual BCR microcluster trajectory.
Note: In our hands, only the first 120 s of each track of the BCR microclusters from IgM-High J558L B cells or only the first 40 s of each track from B1-8 primary B cell microclusters are selected for full analyses. This is necessary to avoid microcluster tracking and 2D Gaussian fitting errors, both of which arise from BCR microclusters merging and/or overlapping at later time points of the observed processes. Tracking of BCR microclusters that are in close proximity is not feasible and 2D Gaussian fitting is reliable only for well-separated BCR microclusters. For the FI and size values of each BCR microcluster trajectory, values belonging to the same track are normalized to the first position of each track. Subsequently, the arithmetic means and standard errors of the values are calculated and plotted over the imaging time. The statistical test used to compare the kinetics of microcluster growth is performed as previously described (Elso et al., 2003; T. Baldwin, 2007) or is available through the online server at http://bioinf.wehi.edu.au/software/compareCurves/index.html.
Note: A second live cell imaging technique to observe BCR microcluster dynamics is FRET-based TIRF imaging as we have reported previously (Sohn et al., 2008; Tolar et al., 2005). Briefly, FRET donor CFP and acceptor YFP are coupled to the cytoplasmic domain of Igα or mIgH within the BCR complex. By examining the FRET changes upon BCR recognition of Ag using live cell time-lapse imaging, we are able to demonstrate the dynamic umbrella opening-like conformational changes within the cytoplasmic domains of BCRs comprising microclusters. We refer the reader of interest to our early publications for additional details (Sohn et al., 2008; Tolar et al., 2005).
3.3 Imaging B cell receptor signaling
BCR signaling is initiated from the BCR microclusters. In this section, we describe how to quantify the recruitment into BCR microclusters of intracellular kinases, adaptors and phosphatases in the BCR signaling pathway.
B cells are prepared as described in Section 2.1, including the labeling of any cell surface proteins of interest with specific, fluorescently-tagged antibodies.
2.5 × 106 cells in 50 μL of Hank's balanced salt solution with Ca2+ and Mg2+ (HBSS) are added to each chamber containing prepared bilayers in 200 μL imaging buffer (see Section 2.2) and incubated for the desired length of time at 37°C. Low buffer volumes for cells and bilayers facilitate faster cell settling, that is essential for achieving B cell interacting with the PLB over short incubation times (e.g., 2 min, which is the lower limit for stimulation by this method).
The chambers are washed with 2-3 mL HBSS using cut, 1 mL pipet tips for adding and removing fluid and the cells are fixed in 4% paraformaldehyde for 10 min at 37°C, as described by Depoil et al.(2008).
Cells are washed, permeabilized with 0.1% Triton X-100 for 5 min at 20°C, washed again and blocked with 1% BSA, 1% FCS, 1% goat serum and 0.05% Tween-20 in PBS (blocking solution) for 30 min at RT.
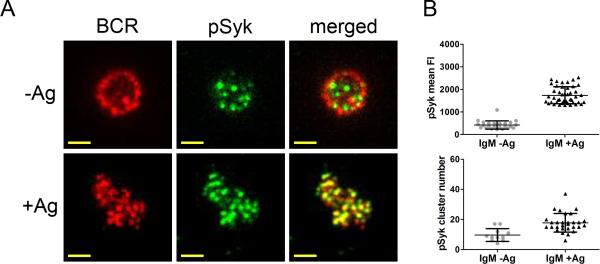
Cells are stained in blocking solution using Abs specific to the signaling molecule of interest for 1 h at RT. Washing and blocking are repeated and the primary Abs are detected by adding an AlexaFluor-conjugated secondary Abs for 30 min at RT and then washing once more before imaging. Figure 4A shows representative two-color TIRF imaging of the BCR with the signaling molecule pSyk on human peripheral blood B cells that were placed on bilayers without or with Ag for 10 min.
As shown for pSyk in Figure 4B, the parameters used to describe the levels of BCR signaling-associated molecules include the mean fluorescence intensity (mean FI) of the signaling molecule within the contact area that the B cell makes with the Ag-presenting membrane (i.e., per unit area) and the number of signaling molecule microclusters per unit area.
The mean FI of the signaling molecule is measured using ImageJ software (National Institutes of Health, available at http://rsbweb.nih.gov/ij/). The image threshold should be adjusted to cover only the precise cell area (Image menu → Adjust Threshold). If there is more than one cell per image, select one at a time using a Region of Interest. To measure cell contact area and mean FI data, from the Analyze menu, select Measure to open the Results window, choose Edit → Set Measurements and check the boxes next to Area, Mean Gray Value and Limit to Threshold. The Area is output as pixel area, so it must be converted to μm2 or nm2 using the dimensions per pixel for your specific microscope objective. Also, acquire the Mean Gray Value from a non-cell region of each image to subtract as background from the cell values.
To count microclusters in Image J, from the Analyze menu, select Analyze Particles. The size should range from 0–infinity and circularity from 0–1, and check the box next to Display Results.
Unpaired two-tailed t-tests are performed for statistical comparisons (95% confidence interval). Linear regression analyses are conducted to assess the relationship between signaling molecule cluster number or mean FI and B cell contact area or BCR mean FI from fixed cell images using Prism software (GraphPad, LaJolla, CA).
Colocalization between the BCR and signaling molecules of interest is quantified from background-subtracted images via intensity correlation analysis as described by Li et al. (2004), using the WCIF plugin of ImageJ to obtain the Pearson's correlation index (Liu et al., 2010c). After installing the plugin, under the Plugins menu select Colocalization Analysis→ Intensity Correlation Analysis. If the image file is a stack of images, be sure to check the box next to Current Slice Only. If there is more than one cell per image, select one at a time using a Region of Interest. The output value ‘Rr’ is the Pearson's correlation coefficient that should range from – 1 to 1. Unpaired two-tailed t-tests are again performed for statistical comparisons.
Figure 4.
Imaging BCR signaling. (A) Two-color TIRF images show the distribution of the BCR and accumulation of pSyk on the membrane of human peripheral blood B cells that were placed on bilayers without (top panels) or with (bottom panels) goat anti-human IgM F(ab’)2 Ag for 10 min, fixed and labeled as described in Section 3.3. Specifically, BCRs and pSyk are visualized using TIRFM by imaging DyLight 649-Fab anti-IgM (red) and AlexaFluor 488-labeled pSyk, respectively. Bars, 1.5 μm. (B) pSyk mean FI and pSyk cluster number quantified from several TIRF images of IgM-expressing B cells placed on bilayers without (gray circles) or with Ag (black triangles) as shown in (A). Each data point represents one cell analyzed in one of three independent experiments and the bars indicate the mean ± SD.
Note: It is also feasible to image the dynamics of downstream BCR signaling molecules through high speed live cell imaging. For example, we are able to visualize the immobilization and recruitment of Syk molecules to the plasma membrane proximal and to BCR microclusters upon BCR recognition of Ag by imaging B cells transfected with GFP-Syk (Tolar et al., 2009).
Supplementary Material
Supplementary Movie 1: Time-lapse TIRF movies show the mobility of individual BCR molecules. Human peripheral blood B cells labeled with DyLight 649-Fab anti-IgM were placed on bilayers without (IgM –Ag; left panel) or with (IgM +Ag; right panel) goat anti-human IgM F(ab’)2 Ag and the labeled BCR molecules were monitored by TIRFM over approximately 7 s (200 frames with a 35 ms interval). (Movies are shown at 30 frames/s.)
Acknowledgements
We thank Dr. Rajat Varma at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) for expert advice on TIRF optics. This work has been supported by the Intramural Research Program of the NIAID-NIH.
References
- Axelrod D. Cell-substrate contacts illuminated by total internal reflection fluorescence. J Cell Biol. 1981;89:141–5. doi: 10.1083/jcb.89.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy. Methods Cell Biol. 1989;30:245–70. doi: 10.1016/s0091-679x(08)60982-6. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Traffic. 2001;2:764–74. doi: 10.1034/j.1600-0854.2001.21104.x. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 2003;361:1–33. doi: 10.1016/s0076-6879(03)61003-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Chapter 7: Total internal reflection fluorescence microscopy. Methods Cell Biol. 2008;89:169–221. doi: 10.1016/S0091-679X(08)00607-9. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Germain RN. Seeing is believing: a focus on the contribution of microscopic imaging to our understanding of immune system function. Eur J Immunol. 2007;37(Suppl 1):S18–33. doi: 10.1002/eji.200737663. [DOI] [PubMed] [Google Scholar]
- Balagopalan L, Sherman E, Barr VA, Samelson LE. Imaging techniques for assaying lymphocyte activation in action. Nat Rev Immunol. 11:21–33. doi: 10.1038/nri2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–71. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol. Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr. Opin. Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat. Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AD, Vale RD. Single-molecule imaging of fluorescent proteins. Method Cell Biol. 2008;85:113–25. doi: 10.1016/S0091-679X(08)85006-6. [DOI] [PubMed] [Google Scholar]
- Elso CM, Roberts LJ, Smyth GK, Thomson RJ, Baldwin TM, Foote SJ, Handman E. Leishmaniasis host response loci (lmr1-3) modify disease severity through a Th1//Th2-independent pathway. Genes Immun. 2003;5:93–100. doi: 10.1038/sj.gene.6364042. [DOI] [PubMed] [Google Scholar]
- Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D, Batista FD. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–741. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- Groves JT, Parthasarathy R, Forstner MB. Fluorescence imaging of membrane dynamics. Annu Rev Biomed Eng. 2008;10:311–38. doi: 10.1146/annurev.bioeng.10.061807.160431. [DOI] [PubMed] [Google Scholar]
- Holtzer L, Meckel T, Schmidt T. Nanometric three-dimensional tracking of individual quantum dots in cells. Applied Physics Letters. 2007;90 [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–4. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–78. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Shirai YM, Koyama-Honda I, Suzuki KG, Fujiwara TK. Hierarchical organization of the plasma membrane: investigations by single-molecule tracking vs. fluorescence correlation spectroscopy. FEBS Lett. 2010;584:1814–23. doi: 10.1016/j.febslet.2010.02.047. [DOI] [PubMed] [Google Scholar]
- Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, G alpha (o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J. Neurosci. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Antigen affinity discrimination is an intrinsic function of the B cell receptor. J. Exp. Med. 2010a;207:1095–1111. doi: 10.1084/jem.20092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity. 2010b;32:778–789. doi: 10.1016/j.immuni.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Won Sohn H, Tolar P, Meckel T, Pierce SK. Antigen-induced oligomerization of the B cell receptor is an early target of FcgRIIB inhibition. J. Immunol. 2010c;184:1977–89. doi: 10.4049/jimmunol.0902334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheyses AL, Simon SM, Rappoport JZ. Imaging with total internal reflection fluorescence microscopy for the cell biologist. J Cell Sci. 123:3621–8. doi: 10.1242/jcs.056218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Pierce S, Liu W. The tipping points in the initiation of B cell signalling: how small changes make big differences. Nat. Rev. Immunol. 2010;10:767–777. doi: 10.1038/nri2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plochberger B, Stockner T, Chiantia S, Brameshuber M, Weghuber J, Hermetter A, Schwille P, Schutz GJ. Cholesterol slows down the lateral mobility of an oxidized phospholipid in a supported lipid bilayer. Langmuir. 26:17322–9. doi: 10.1021/la1026202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–6. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor B Annual Review of Immunology. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- Sohn HW, Tolar P, Brzostowski J, Pierce SK. A method for analyzing protein-protein interactions in the plasma membrane of live B cells by fluorescence resonance energy transfer imaging as acquired by total internal reflection fluorescence microscopy. Methods Mol Biol. 2010;591:159–83. doi: 10.1007/978-1-60761-404-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn HW, Tolar P, Jin T, Pierce SK. Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc. Natl. Acad. Sci. U S A. 2006;103:8143–8. doi: 10.1073/pnas.0509858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn HW, Tolar P, Pierce SK. Membrane heterogeneities in the formation of B cell receptor-Lyn kinase microclusters and the immune synapse. J. Cell Biol. 2008;182:367–79. doi: 10.1083/jcb.200802007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T, A. S. J. M. C. B. K. G. K. S. S. J. F. E. H. Wound healing response is a major contributor to the severity of cutaneous leishmaniasis in the ear model of infection. Parasite Immunology. 2007;29:501–513. doi: 10.1111/j.1365-3024.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity. 2009;30:44–55. doi: 10.1016/j.immuni.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar P, Sohn HW, Pierce SK. The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat. Immunol. 2005;6:1168–76. doi: 10.1038/ni1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1: Time-lapse TIRF movies show the mobility of individual BCR molecules. Human peripheral blood B cells labeled with DyLight 649-Fab anti-IgM were placed on bilayers without (IgM –Ag; left panel) or with (IgM +Ag; right panel) goat anti-human IgM F(ab’)2 Ag and the labeled BCR molecules were monitored by TIRFM over approximately 7 s (200 frames with a 35 ms interval). (Movies are shown at 30 frames/s.)