Abstract
Congenital diaphragmatic defects (CDDs) are a common group of birth defects, yet we presently know little about their pathogenesis. No systematic study documenting the detailed morphology of CDD has been performed, and current classification schemata of diaphragm phenotypes incompletely capture the location and extent of diaphragmatic involvement. To define the range of CDD anatomy, diaphragmatic pathology was reviewed from an examination of 181 autopsy records of children with CDDs at Children’s Hospital Boston between 1927 and 2006. Defects were classified according to several parameters, including type (communicating versus noncommunicating) and location (anterior, posterior, etc.). The information permitted development of a phenotyping worksheet for prospective use on patients undergoing diaphragmatic repair at Children’s Hospital Boston or MassGeneral Hospital for Children. Fifty-three patients who died between 1990 and 2006 had a total of 63 defects. Thirty-nine had a “classic” CDD phenotype (64% posterolateral, 18% hemidiaphragmatic aplasia, and 18% anterior). The remaining 19 defects, not fitting classical descriptions, were located in the posteromedial, anterolateral, or lateral regions of the diaphragm. Prospective data collected during surgical repair revealed posterolateral defects in 34 of 41 cases that demonstrated wide phenotypic variability in size, location, shape, type, and extent of organ displacement. Congenital diaphragmatic defects display significant phenotypic variation. Because rigorous anatomic evaluation and documentation are important steps towards elucidating the developmental biology of these disorders, we suggest establishment of a new and more precise classification using the model presented herein.
Keywords: Bochdalek, CDH, diaphragm, hernia, pulmonary hypoplasia, sac hernia
INTRODUCTION
Our knowledge of mammalian development has progressed significantly over the past 15 years with the availability of murine and other animal models to study organogenesis. For example, genetic pathways necessary for the normal development of the heart, palate, and skeleton have been investigated in animal models and then translated to the human or vice versa [1–4]. Although the genetic contribution to most birth defects is complex, gene identification facilitates improved understanding of the mechanism and, in turn, opportunities to target pathways with therapeutics [5]. As disease-associated genetic aberrations are identified, accurate phenotypic information for comparison of human disease to animal research models is gathered. Precise phenotypic characterization of birth defects is also likely to improve the ability to associate disease with outcome and to identify those patients who require a nonstandard approach to treatment.
Almost all congenital diaphragmatic defects (CDDs) are presently considered as occurring in one of several “classical” locations, such as posterolateral (Bochdalek), anterior (often named Morgagni, Larrey, or substernal hernias) [6–8], and central and anterior (eg, septum transversum hernia of the Cantrell type) [9]. Congenital diaphragmatic defects originating from the trigonal lumbocostalis, a less muscularized region of the posterior diaphragm, were described by Victor Alexander Bochdalek in 1848 [6,10]. The prevailing assumption that all defects in the posterior location result from the same or similar mechanism of pathogenesis due to a developmental defect in the trigonal lumbocostalis is not proved and in fact may well be untrue. This is supported by the lack of evidence that the trigonal lumbocostalis creates the end point of the pleuroperitoneal canal and by the occurrence of diaphragmatic defects that do not involve this specific region [10].
Achieving clarity in communicating the phenotype of diaphragmatic defects is an important step towards improved translational research. Accordingly, we retrospectively, and then prospectively, characterized the phenotypic range of developmental defects found in the human diaphragm. The retrospective portion of our study involved observations at autopsy from an era when death often occurred without surgical repair. The results from this study provide a framework for standardizing the classification of developmental diaphragmatic abnormalities. The creation of a rigorous phenotyping system will improve the ability to predict the clinical course of human CDDs and will aid in identifying developmental pathways responsible for the disease.
METHODS
A systematic review of written and computerized records at Children’s Hospital Boston was conducted to identify all patients with a diagnosis of diaphragmatic defect or congenital diaphragmatic hernia at autopsy between 1927 and 2006. Autopsy records and photographs from each patient were reviewed to evaluate diaphragmatic anatomy.
Diaphragmatic defects were classified according to location, type, size, degree of organ displacement, sidedness, and number. Defect size was determined consistently only in the prospectively studied surgical cases, because many autopsied patients had their defects repaired.
The location of the diaphragm defect was defined as follows: (1) Hemidiaphragmatic, involving the entire hemidiaphragm except the most anterior aspect. For communicating defects (see below), these are also called aplasia. (2) Posterior, involving the posterior 3rd of the diaphragm. Posterior defects were classified as either posteromedial or posterolateral. (3) Lateral, if the majority of the defect was lateral and the diaphragmatic tissue posterior to the defect was preserved. (4) Anterior, in the retroor parasternal regions. (5) Anterolateral, occurring lateral to the sternal area but anterior to the midaxillary line. (6) Central tendon, the unmuscularized medial region of each hemidiaphragm. Posterior or lateral defects that extended to the central tendon were described as such.
The type of diaphragm defect was defined as follows: (1) Communicating, presence of a direct communication between the peritoneal and thoracic cavities; referred to as “communicating,” or CDD-c. (2) Noncommunicating, absence of a direct communication between the peritoneal and thoracic cavities; referred to as “noncommunicating,” or CDD-nc. Noncommunicating defects were further classified as either focal (well-circumscribed lesions with a distinct edge between the defect and the surrounding tissue) or diffuse (no distinct edges) lesions.
The size of the diaphragm defect was defined by length of the 2 longest axes. Size was also estimated, as previously described [11], by categorizing lesions as aplasia (all with patch repair), nonaplasia with primary repair, or nonaplasia with patch repair. Size and shape were also assessed by inspection of drawings on the phenotyping worksheet (described below). Ideally, total diaphragm size would also be measured; however, this is a difficult measurement to make at the time of surgery. Instead, we advocate using surrogate measures of total diaphragm size, such as patient weight.
The degree of maximal organ displacement was assessed as follows: (1) mild, less than one third of what would typically be expected to constitute the thoracic cavity filled with abdominal organs; (2) moderate, one to two thirds filled; (3) severe, greater than two thirds filled. Organ displacement is not a static parameter and may be affected by respiration, positional shifting, surgical/autopsy prosection techniques, and other factors. For these reasons, we classified based on the maximum amount of displacement observed and defined the “thoracic cavity” as the area that would make up the thoracic cavity if the diaphragm were at the expected height. Acknowledging that precision may remain difficult in certain cases, we accepted the use of mild-to-moderate or moderate-to-severe when exact distinction was difficult.
The sidedness of the diaphragm defect was defined as (1) right, defect occurring predominantly (>95%) in the right hemidiaphragm; (2) left, defect occurring predominantly (>95%) in the left hemidiaphragm; and (3) other, single defect crossing the midline or one that is otherwise difficult to define.
The number of diaphragm defects was indicated as single or multiple. In cases of more than 1 separate CDD, each diaphragmatic defect was classified separately.
Traditional (eg, “classic”) descriptions for various types of CDD are compared with descriptions using the proposed classification system in Table 1.
Table 1. Examples of our proposed descriptive classification system.
| Classical designation | Proposed designation |
|---|---|
| Left Bochdalek CDH without sac membrane, major herniation filling the chest |
Left posterolateral CDD-c, severe organ displacement |
| Right Bochdalek “sac” hernia filling half of right chest | Right posterolateral CDD-nc, focal, moderate organ displacement |
| Left posterior muscularization defect with eventration of kidney | Posterior CDD-nc, focal, mild organ displacement |
| Right anterolateral sac hernia with contents filling three quarters of chest |
Right anterolateral CDD-nc, focal, severe organ displacement |
| Eventration of most of left hemidiaphragm filling half of chest |
Hemidiaphragmatic Left CDD-nc, diffuse, moderate organ displacement |
CDH indicates congenital diaphragmatic hernia; CDD-c, congenital diaphragmatic defect with thoracic-peritoneal communication; CDD-nc, congenital diaphragmatic defect noncommunicating.
To document the scope of normal diaphragmatic anatomy, diaphragms from 90 infants and children without CDD who died between 1984 and 1987 were carefully examined in situ and then removed, photographed, and examined for subtle variations. The evaluation had been done by a single pathologist.
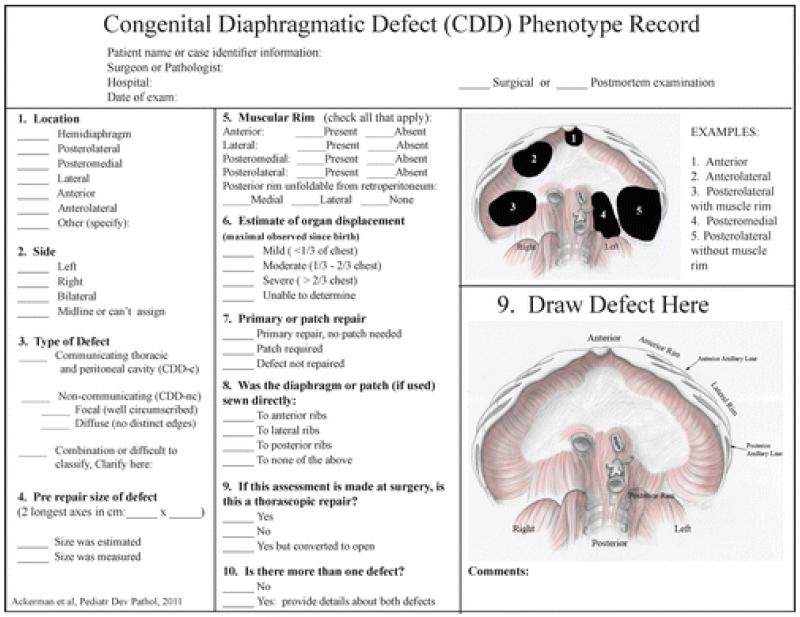
In 2006 we designed a “congenital diaphragmatic defect phenotyping worksheet” to be used prospectively at the time of surgical repair of the diaphragm [12]. This worksheet requested information regarding defect location; sidedness; presence or absence of a contiguous diaphragm (type); size of defect; presence of muscular rims; and method of repair, including whether the diaphragm or prosthetic patch was anchored or sewn directly to the anterior, lateral, and/or posterior ribs. The worksheet included pictorial representations of several types of CDD, plus a schematic of a normal diaphragm on which the operating surgeon was asked to sketch the patient’s actual defect. Implementation of the worksheet resulted in revisions and improvements. Revisions included the addition of more realistic artwork, a precise definition of type (communicating vs noncommunicating), the addition of questions to provide details about the muscular rim surrounding the defect, details on the type of repair (primary vs patch), estimate of organ displacement, and whether the defect was repaired with a thorascope. The current version of the phenotyping worksheet is shown in Figure 1.
Figure 1.
Worksheet for recording detailed phenotypic information for congenital diaphragmatic defects.
The need to document thoroughly the extent, location, and severity of diaphragm defects at the time of autopsy also led to a minor modification of the traditional autopsy procedure (see Discussion section for further details).
Examination of autopsy records and photographs and prospective evaluation of diaphragmatic phenotypes in living patients were approved by the Institutional Review Board at Children’s Hospital, Boston, and at Massachusetts General Hospital.
RESULTS
Autopsied patients
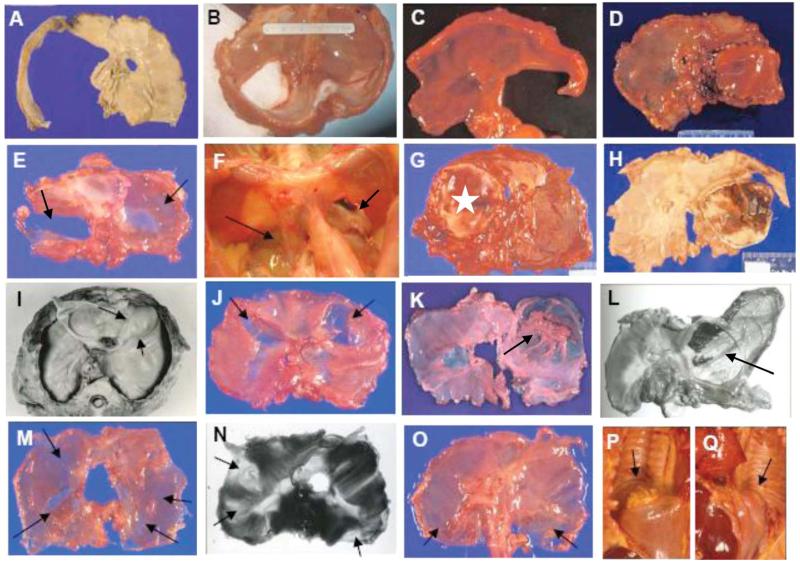
A review of pathology records identified 181 children with a diagnosis of a diaphragmatic defect who were autopsied between 1927 and 2006. Descriptions and available photographs did not allow for precise anatomic localization in the majority of patients prior to 1990, because many were simply described as having Bochdalek hernias. Nevertheless, good-quality photographs did exist of a few of these patients and are used to illustrate the range of diaphragmatic defects (Fig. 2). Patients (n = 7) diagnosed with Pentalogy of Cantrell were excluded because these defects likely represent a different embryologic mechanism.
Figure 2.
Phenotypic range of diaphragmatic defects associated with early childhood lethality. These lesions either involve the entire hemidiaphragm (A, hemidiaphragmatic CDD or aplasia) or the posterolateral regions (B–D). Other defects involve lateral regions but have preserved posterior diaphragmatic tissue (E,G; G is repaired with star marking the patch). Occasionally, defects are posteromedial (F). Both E and F have contralateral noncommunicating defects that mirror the communicating defects (both defects marked with arrows). Some defects are more anterior than posterolateral (G vs D or H), even though they were previously diagnosed as Bochdalek hernias. Noncommunicating defects (arrows) may be focal (I–L), diffuse (M), or both (N,O). They may be associated with mild (I,J,M–Q) or severe (K,L) organ displacement. They may be unilateral (I,K,L), bilateral, and symmetric (J,O–Q), or bilateral and nonsymmetric (M,N). Noncommunicating defects with mild organ displacement are appreciated better on in situ examination. For example, posterior CDD-nc regions in O resulted in mild displacement of the kidneys (arrows: P, right; Q, left). A color version of this figure is available online.
“Normal” diaphragms
The majority (65 of 90) of the diaphragms examined as controls were from infants with a diagnosis of sudden infant death syndrome, and 25 were from children (primarily infants) with other conditions. In addition to examination in situ, photographs of these carefully excised diaphragms were used to reference the range of normal. Some diaphragms showed occasional thinning or apparent posterolateral small triangular amuscular areas consistent with the trigonal lumbocostalis. These occurred bilaterally (n = 9), on the left (n = 8), or on the right (n = 10). The central tendon showed remarkable consistency in size; however, it was larger than expected in 4 cases. There was 1 incidental finding of a minimal right anteromedial noncommunicating defect of the right diaphragm.
Distribution of CDDs
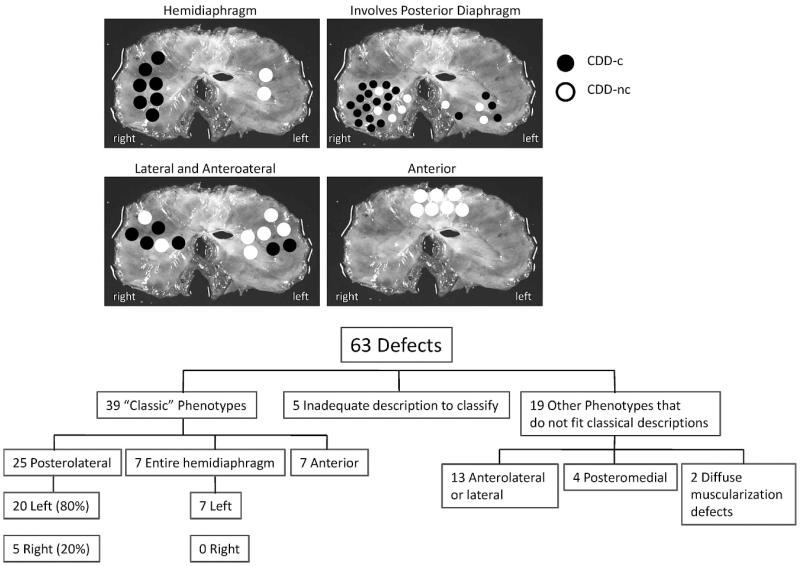
Examination of the 53 patients with CDD autopsied after 1990 identified 10 patients with bilateral defects, resulting in a total of 63 defects. Five defects had inadequate descriptions or photographs and were excluded from further analysis. The distribution of defects is outlined in Figure 3. Although two thirds (67%) of the 58 well-characterized defects fit into a classical phenotypic category, the remainder did not, and these included lateral and anterolateral hernias, posteromedial hernias, and diffuse CDD-nc defects. Anterior defects were always CDD-nc, because they were covered by thin diaphragmatic tissue. These were distributed equally in the middle, left, and right sides. Lateral and anterolateral hernias extended into the central tendon of the diaphragm and were noncommunicating in 7 of 13 (54%) cases. The anterolateral defects always coexisted with pulmonary hypoplasia, suggesting that they are a phenotype distinct from the anterior substernal type.
Figure 3.
Locations of diaphragmatic defects. Each black dot represents the region in which the defect occurred, and each white dot represents a noncommunicating-type defect. Each dot approximates the region of the defect but does not necessarily represent the midpoint. The collection of dots represents the anatomic range of defect in each location. Although most defects involved the posterolateral region, many did not fit the classical location of a Bochdalek hernia.
Posterior CDD-c defects
Representative photographs illustrating the range of phenotypes found in autopsied patients are shown in Figure 2. Each image in the top row (Fig. 2A–D) shows either hemidiaphragmatic CDD-nc (aplasia) or a classic posterolateral CDD-c defect. In the 2nd row, Figure 2E and G shows lateral defects with well-preserved posterior muscular and connective tissue. These are markedly different from the bilateral posteromedial defects depicted in Figure 2F.
Figure 2D, G, and H shows patch repairs of CDDs that were initially diagnosed as Bochdalek hernias; however, the patch in Figure 2G is much more anterior (with preserved posterior region) than those in Figure 2D and H, and this CDD-c is therefore classified in the lateral and anterolateral category.
CDD-nc defects
Both focal and diffuse defects were observed. Photographs shown in Figure 2 (3rd row) depict well-circumscribed lesions. These show variability in location, circumferential size, and degree of organ displacement. The lesions have sharply demarcated rims, spare the posterolateral diaphragm, and are associated with pulmonary hypoplasia. Diffuse noncommunicating defects appear as poorly defined regions of thin diaphragmatic muscle (Fig. 2M) or as multiple distinct focal defects (Fig. 2N). These defects are often difficult to appreciate in a flattened diaphragm, and must be examined in situ (Fig. 2O–Q).
Bilateral CDDs
Ten patients had bilateral defects (Table 2), often discordant between the 2 hemidiaphragms. In other words, each patient’s hemidiaphragm had a different phenotype (eg, communicating with severe organ displacement on 1 side vs noncommunicating with mild displacement on the other side), although defects were often symmetrically located. For example, Figure 2E, F, J, and O–Q shows symmetry in location of the defects with variability in the type.
Table 2. Phenotypes of cases with bilateral defects.
| Left-sided defect | Right-sided defect | Picture |
|---|---|---|
| Posteromedial CDD-nc showing lack of muscle | Posteromedial CDD-nc showing lack of muscle | Not shown |
| Posterolateral CDD-c (Bochdalek) | CDD-nc, exact location unclear | Not shown |
| Hemidiaphragmatic CDD-c | Hemidiaphragmatic CDD-nc, diffusely undermuscularized diaphragm |
Not shown |
| Posterolateral CDD-c | CDD-nc involving much of the central tendon, exact location undefined |
Not shown |
| Lateral CDD-c | Anterolateral CDD-nc | Figure 2E |
| Anterior and lateral (sparing posteromedial region) CDD-nc, diffuse |
Posterior and lateral (sparing anterior region) CDD-nc, diffuse |
Figure 2M |
| Anterolateral CDD-nc, focal | Anterolateral CDD-nc, focal | Figure 2J |
| Posterolateral CDD-nc with mild organ (kidney) displacement |
Posterolateral CDD-nc with mild organ (kidney) displacement |
Figure 2O–Q |
| Anterolateral CDD-nc, focal | Anterolateral CDD-nc, focal | Not shown |
| Posteromedial CDD-c | Posteromedial CDD-nc | Figure 2F |
CDD-nc indicates congenital diaphragmatic defect noncommunicating; CDD-c, congenital diaphragmatic defect with thoracic-peritoneal communication.
Lung findings
Pulmonary hypoplasia was diagnosed in most patients; however, the method of determining the hypoplasia was not standardized. Lung hypoplasia occurred in some patients despite minimal organ displacement, and in several cases lungs showed evidence of abnormal patterning. In the systematically reviewed autopsy cohort, 42 reports contained detailed description of lobar structure and the presence of normal major fissures. Ten patients were missing at least 1 major fissure. Of these, 6 patients were missing 1 fissure (4 total lobes), and an additional 4 were missing 2 or more fissures (including 1 with nonlobated lungs who had a right posterior CDD-c and an atrioventricular canal cardiac defect).
Prospectively evaluated surgical patients
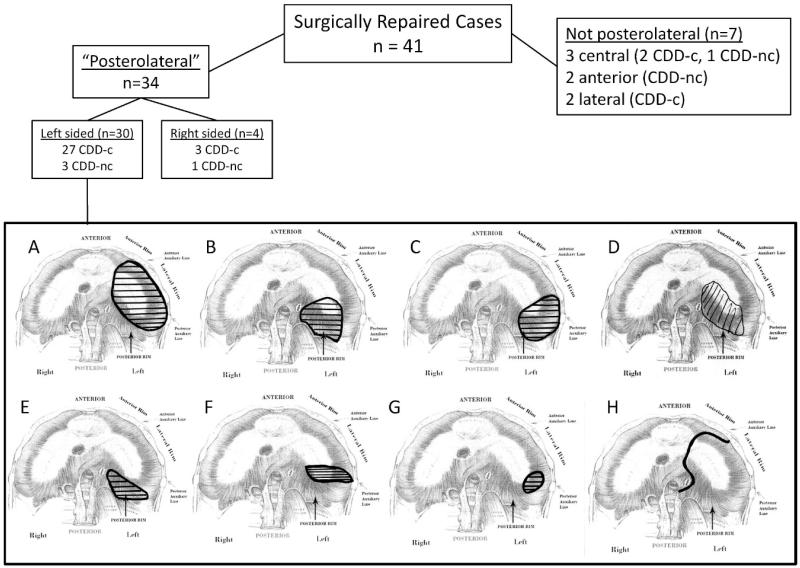
We used the CDD phenotyping worksheet (Fig. 1) to collect information from 41 prospectively identified patients undergoing surgical repair of their diaphragmatic defect. A drawing of the defect, accompanied by descriptive information, was provided by each surgeon. Of these, 34 were defined as posterolateral by the surgeon, and 7 were described as being other than posterolateral. This nonposterolateral group included 2 anterior CDD-nc (so-called “Morgagni hernias”) and 5 that were difficult to categorize, occurring laterally in 2 and more centrally in 3. In 1, the defect was clinically described as central; however, in the sketched representation, it extended posterolaterally, making it difficult to determine whether its location best fit into a central tendon or a posterolateral defect category.
The 34 posterolateral defects showed considerable variability in location, size, and presence of diaphragmatic tissue along the chest wall. Examples are shown in Figure 4. Half of the patients had either hemidiaphragmatic CDD-c (aplasia) or a posterolateral CDD lacking a posterolateral muscular rim (as in Fig. 4C). Five patients had posterior defects that did not involve the lateral diaphragm. Of interest, 3 of these were noncommunicating (covered by a membrane), whereas all of the aplasia and posterolateral defects were communicating lesions. This paralleled the findings in the autopsy series, in which 3 of 4 posteromedial hernias did not show direct communication between the peritoneal and thoracic cavities.
Figure 4.
Left-sided diaphragmatic defect variability captured using the phenotyping worksheet for surgically repaired cases. Congenital diaphragmatic defects traditionally called “posterolateral hernias” or “Bochdalek hernias” vary in their location, preservation of peripheral diaphragmatic tissue, and size. Eight examples of clinically diagnosed posterolateral defects show actual locations including hemidiaphragmatic CDD-c (aplasia) with an intact posteromedial diaphragm (A), posterior CDD-nc with a normal lateral diaphragm (B), posterolateral CDD-c with no lateral tissue (C), posteromedial CDD-nc (D), posterior CDD-c (E), elongated “slit” CDD-c with extension to the lateral wall but well preserved posterior diaphragm (F), posterolateral CDD-c with preserved diaphragmatic tissue on all sides (G), and aplasia (CDD-c) with only anterior diaphragm present (H).
In nearly half the posterolateral defect cases (n = 16, 47%) the surgeons described intact diaphragmatic tissue along the lateral chest wall, whereas in several others (n = 7, 20%) they described intact diaphragmatic tissue posteriorly. Only a few of these lesions localized to the trigonal lumbocostalis, which is the less muscularized region of the posterior lateral diaphragm described by Bochdalek as a predilection spot for hernias. It is currently not possible to determine whether the larger defects could have originated in this region.
Reproduced drawings of 32 additional prospectively collected CDD phenotypes not included in Figure 4 are available in a worksheet provided online (give link to URL). One drawing from the total collected is not presented due to space allocations. These 32 additional drawings include posterolateral CDD-c and hemidiaphragmatic CDD-c, as well as anterior CDD and nonclassical phenotypes, and illustrate the current spectrum of defects presenting to a pediatric surgical practice for repair.
DISCUSSION
Congenital diaphragmatic defects remain a significant cause of morbidity and mortality in the neonate, and prognostication continues to be difficult in many cases. Although comorbid congenital malformations or genetic syndromes significantly affect survival, it is also clear that larger-sized defects of the diaphragm are associated with worse outcome [11,13]. The reasons for size variability are unknown, and the variability also occurs in animal models [14]. Although defect size is important, little attention has been paid to the precise anatomic details of the defect or defect type, in large part due to the absence of a system for capturing and conveying these findings. The development of a method to differentiate diaphragmatic phenotypes both by location and type is important for several reasons. First, identification of specific phenotypic categories might more accurately predict outcomes or associated comorbidities. Second, detailed phenotyping information is needed to compare and contrast the anatomy, mechanisms, and genetics of development being investigated in animal models of CDD. Third, identification of subtle patterns of development in humans may help direct investigation of mechanisms in animal models. Thus, findings in this study better define the range of CDD phenotypes and establish a method for recording these phenotypes for future large scale prospective analysis.
As expected, the “posterolateral” location of defect was the most prevalent CDD identified in our patient cohorts, the majority being the CDD-c type (ie, an actual “hole” in the diaphragm). These posterolateral CDDs were, however, quite varied in their exact location and shape. A subset of “posterolateral” defects did not extend to the body wall. So far, the phenotypes in published mouse models are different than this subset in that they always extend to the body wall and they often involve the posteromedial diaphragm [14–16]. These phenotypic differences are important to consider when extrapolating findings from mice to humans or when predicting outcomes in humans.
The relatively small prospective component of our study, currently based on only 41 patients, does not permit drawing correlations between outcomes and specific CDD phenotypes. It is our goal, however, to accomplish this in the future. Preliminary examination of the data shows that among our 6 surgically repaired patients who died, all had left hemidiaphragmatic aplasia (CDD-c). As a group, patients with hemidiaphragmatic aplasia fared worst, with a survival rate of only 54%. This compares poorly with the 100% survival rate for patients with smaller diaphragmatic defects (eg, smaller than the entire hemidiaphragm), even though this latter group included 8 patients with additional major birth defects above and beyond CDD. Although larger cohorts will need to be studied to confirm whether hemidiaphragmatic aplasia patients have higher mortality compared with patients with smaller diaphragmatic lesions and other congenital anomalies, these findings suggest that future investigations should focus on reasons for outcome differences in patients with large, communicating defects. A prospective study of a larger cohort would also be valuable for identifying associations between specific diaphragmatic phenotypes and morbidity, including the presence of other birth defects and CDD-associated problems, such as sensorineural hearing loss and scoliosis.
A large number of CDD patients had evidence of pulmonary hypoplasia, although the retrospective nature of our analysis precluded consistent definition for this condition. It was evident that some children had small lungs, despite having only small diaphragmatic defects. Furthermore, some had abnormal lung patterning. Although the autopsy cohort was biased towards patients with severe cardiopulmonary failure, it is important to note that diaphragmatic defects without large degrees of organ displacement can be associated with severe pulmonary hypoplasia. These children likely have a primary developmental defect in both organs.
The involvement of muscle precursor cells vs nonmuscular primordial diaphragmatic mesenchyme in the pathogenesis of diaphragmatic hernia is an ongoing area of investigation. The combined presence of different CDD types in the same patient, in different members of the same kindred, and in the setting of specific genetic defects suggests that both muscle and mesenchymal cell types are important for normal development of the diaphragm [4,17,18]. We observed a variety of CDD-nc cases that appeared to have abnormal diaphragmatic muscle patterning. Some of these cases displayed well-circumscribed lesions, while in others the lesions were diffuse. All focal CDD-nc’s had a thickened muscular rim at the periphery. The presence of this may reflect an accumulation of the normal muscle that was destined to populate the affected region [19]. It is possible that the small focal lesions represent milder variants along a spectrum that includes CDD-nc with massive organ displacement (historically called “sac hernias”). Perhaps the size of the defect helps to determine how much stretch occurs in the nonmuscularized connective tissue, or perhaps the diaphragms containing CDD with severe organ displacement have a weak connective tissue component contributing to the defect.
The validity of our conclusions is strengthened by the large number of patients whose diaphragmatic defects were systematically and carefully phenotyped. One limitation, however, is that during surgical repair of CDD, the entire diaphragm is not routinely examined. Although surgical phenotyping will underestimate subtle contralateral defects, the presence of a clinically significant “occult” defect that adversely affects diaphragmatic function would most likely be recognized with routine chest radiography, whereas the associated pulmonary hypoplasia would be appreciated by measurement of lung size (volume) using magnetic resonance imaging [20], as is quickly becoming a standard of care.
Moving forward, we anticipate that a subset of patients for whom a surgical phenotyping worksheet is generated will, unfortunately, not survive. If a careful autopsy is performed using the protocol described below, we can begin to make correlations between descriptive information collected at the time of operation, anatomic data collected at autopsy, lung volume data determined by magnetic resonance imaging while the patient was alive, and survival or morbidity. Refinements in the worksheet and data collection process can then be implemented as needed.
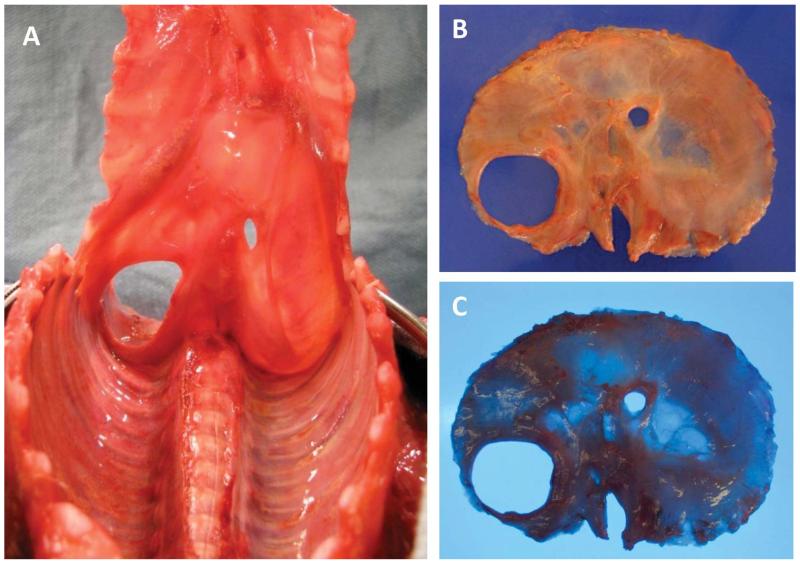
Although observations at autopsy would seem a gold standard for anatomic evaluation of the diaphragm, analysis is made more difficult after repair or if the diaphragm is not evaluated carefully in situ. The traditional method for opening the body cavities during the autopsy disrupts the anterior attachments of the diaphragm, and we therefore instituted some modifications. After the standard Y-shaped incision, the skin and anterior chest wall muscles are reflected and the thoracic cavity is opened by incising the manubrioclavicular joint followed by the uppermost 4 or 5 costal cartilages close to their osseous junctions. The sternum is then reflected anteriorly from its rostral end while remaining attached to the lowermost costal cartilages. This permits full exposure of the thoracic cavity and assessment of the diaphragmatic defect while not disturbing the anterior attachments of the diaphragm. After the thoracic organs are removed en bloc, the entire diaphragm is then fully exposed on its superior aspect, permitting optimal evaluation and facilitating photographic documentation. The diaphragm can be inspected and photographed from its inferior aspect after removal of the abdominal organs and can then be dissected as closely as possible off its costal, sternal, and vertebral attachments. Adipose tissue is removed and the specimen weighed. The diaphragm is photographed with overhead and transilluminated light, the latter facilitating identification of minor defects. This method of dissection is shown in Figure 5.
Figure 5.
Photos from a newborn during autopsy with left congenital diaphragmatic defect with thoracic-peritoneal communication exposed using the modified dissection method recommended in the text, which, by reflecting the sternum from the rostral end, does not disrupt the anterior diaphragm. The entire diaphragm should be visualized, examined, and photographed in situ (A, view from the thoracic side). It should next be carefully dissected off the chest wall, removed, and photographed with overhead light (B) and with transillumination (C). A color version of this figure is available online.
We suggest a departure from the use of eponyms to refer to diaphragmatic defects and a move towards a universal system that communicates more precise anatomic data. The term “CDH” is broad and has historically implied a defect of the Bochdalek type, when our data indicate this often is not the case. The terms “hernia,” “eventration,” “sac hernia,” and “sac membrane” do not consistently convey the same meaning between clinicians or investigators. Accordingly, we support the use of the term “congenital diaphragmatic defect” [21], followed by an anatomic description based on location, size, presence of a membrane contiguous with the diaphragmatic tissue (communicating vs noncommunicating), and degree of maximal organ displacement. In addition, we advocate collecting information about the presence or absence of retained diaphragmatic rim tissue. In patients with multiple defects involving the diaphragm, a determination of phenotype should be made for each lesion. It is likely that the evaluation of a large data set using the type of phenotyping worksheet presented herein will reveal recurring patterns of abnormal development. We are making this worksheet available online (give link to URL) for open use and encourage feedback about how it may be further revised to best reflect the full range of CDD phenotypes.
Our systematic review of CDD and successful implementation of a phenotyping worksheet support the existence of discrete phenotypes that are separable via gross visualization. An improved understanding of the spectrum of developmental anomalies of the diaphragm will lay the groundwork for drawing genotype–phenotype and phenotype–outcome correlations. We anticipate that the availability of a CDD phenotyping worksheet will prompt others to carefully evaluate and record diaphragmatic phenotypes.
ACKNOWLEDGMENTS
The painting of the pediatric diaphragm used for our revised CDD phenotyping worksheet was created in watercolor by Barbara E. Hyams, MA, AMI, AIMI. This painting was made after extensive review of drawings and photographs, as well as consultation with the authors. We thank the following pediatric surgeons who contributed to the prospective phenotyping database: Drs Terry Buch-miller, Catherine Chen, Daniel Doody, Thomas Hamilton, Craig Lillehei, Bradley Linden, Shawn Rangel, Robinson, Daniel Ryan, C. Jason Smithers, and Chris Weldon. Appreciation is also extended to Dr Corine Farewell for German translating assistance and to Anna Frangulov for her technical assistance in preparing this manuscript.
REFERENCES
- 1.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- 2.Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 12:167–178. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldridge D, Shchelochkov O, Kelley B, Lee B. Signaling pathways in human skeletal dysplasias. Annu Rev Genomics Hum Genet. 2010;11:189–217. doi: 10.1146/annurev-genom-082908-150158. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman KG, Herron BJ, Vargas SO, et al. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet. 2005;1:58–65. doi: 10.1371/journal.pgen.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC., 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787–2795. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irish MS, Holm BA, Glick PL. Congenital diaphragmatic hernia: a historical review. Clin Perinatol. 1996;23:625–653. [PubMed] [Google Scholar]
- 7.Al-Salem AH. Bilateral congenital Morgagni-Larrey’s hernia. World J Pediatr. 2010;6:76–80. doi: 10.1007/s12519-010-0011-8. [DOI] [PubMed] [Google Scholar]
- 8.Stokes KB. Unusual varieties of diaphragmatic herniae. Prog Pediatr Surg. 1991;27:127–147. doi: 10.1007/978-3-642-87767-4_8. [DOI] [PubMed] [Google Scholar]
- 9.Wesselhoeft CW, Jr, DeLuca FG. Neonatal septum transversum diaphragmatic defects. Am J Surg. 1984;147:481–485. doi: 10.1016/0002-9610(84)90009-6. [DOI] [PubMed] [Google Scholar]
- 10.Tondury G. [118. On the development and anatomy of the diaphragm in man] Langenbecks Arch Chir. 1967;319:722–729. doi: 10.1007/BF02659365. [DOI] [PubMed] [Google Scholar]
- 11.Lally KP, Lally PA, Lasky RE, et al. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120:e651–e657. doi: 10.1542/peds.2006-3040. [DOI] [PubMed] [Google Scholar]
- 12.Ackerman KG, Pober BR. Congenital diaphragmatic hernia and pulmonary hypoplasia: new insights from developmental biology and genetics. Am J Med Genet C Semin Med Genet. 2007;145:105–108. doi: 10.1002/ajmg.c.30133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rygl M, Pycha K, Stranak Z, et al. Congenital diaphragmatic hernia: onset of respiratory distress and size of the defect: analysis of the outcome in 104 neonates. Pediatr Surg Int. 2007;23:27–31. doi: 10.1007/s00383-006-1788-3. [DOI] [PubMed] [Google Scholar]
- 14.Greer JJ, Cote D, Allan DW, et al. Structure of the primordial diaphragm and defects associated with nitrofen-induced CDH. J Appl Physiol. 2000;89:2123–2129. doi: 10.1152/jappl.2000.89.6.2123. [DOI] [PubMed] [Google Scholar]
- 15.Ackerman KG, Greer JJ. Development of the diaphragm and genetic mouse models of diaphragmatic defects. Am J Med Genet C Semin Med Genet. 2007;145:109–116. doi: 10.1002/ajmg.c.30128. [DOI] [PubMed] [Google Scholar]
- 16.You LR, Takamoto N, Yu CT, et al. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci USA. 2005;102:16351–16356. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleyl SB, Moshrefi A, Shaw GM, et al. Candidate genes for congenital diaphragmatic hernia from animal models: sequencing of FOG2 and PDGFRalpha reveals rare variants in diaphragmatic hernia patients. Eur J Hum Genet. 2007;15:950–958. doi: 10.1038/sj.ejhg.5201872. [DOI] [PubMed] [Google Scholar]
- 18.Slavotinek AM. Fryns syndrome: a review of the phenotype and diagnostic guidelines. Am J Med Genet A. 2004;124:427–433. doi: 10.1002/ajmg.a.20381. [DOI] [PubMed] [Google Scholar]
- 19.Clugston RD, Klattig J, Englert C, et al. Teratogen-induced, dietary and genetic models of congenital diaphragmatic hernia share a common mechanism of pathogenesis. Am J Pathol. 2006;169:1541–1549. doi: 10.2353/ajpath.2006.060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer S, Klaritsch P, Petersen S, et al. The correlation between lung volume and liver herniation measurements by fetal MRI in isolated congenital diaphragmatic hernia: a systematic review and meta-analysis of observational studies. Prenat Diagn. 2011;31:1086–1096. doi: 10.1002/pd.2839. [DOI] [PubMed] [Google Scholar]
- 21.Czeizel A, Kovacs M. A family study of congenital diaphragmatic defects. Am J Med Genet. 1985;21:105–117. doi: 10.1002/ajmg.1320210115. [DOI] [PubMed] [Google Scholar]