Summary
Intense focus has been centered around how the primary cilia transduces the hedgehog signal from Smoothened to the Gli transcription factors. New data indicates that ligand and signaling lipids help regulate small GTPase-dependent accumulation and activity of signaling components.
Introduction
Across the metazoan phyla, hedgehog (Hh) signaling plays a crucial role in organogenesis as it promotes proliferation and migration of stem cells in the body. Inappropriate Hh signaling results in a panoply of developmental defects and cancers and is implicated in the induction, maintenance and/or metastasis of up to 25% of human tumors [1]. While the pathway has been studied intensively, particular focus has been centered recently around how the serpentine membrane receptor smoothened (Smo) interprets the Hh signal to the Gli/Cubitus interruptus (Ci) zinc finger family of transcription factors, as this interpretation dictates the magnitude and quality of the resultant Hh-dependent target gene induction. Ongoing pathway studies in Drosophila have identified conserved components and pathway logic. However, studies of the pathway in higher vertebrates resulted in a major paradigm shift in our understanding of the pathway with the discovery that Hh signaling in normal development requires components of the primary cilia. Genetic studies in mice and humans uncovered a set of mutants resembling hedgehog mutants, but which contain mutations in components of the small, antenna-shaped organelle [2–4]. Resulting questions have emerged as to how the cilium accomplishes it’s signaling responsibilities, and whether all or part of hedgehog signal transduction requires an intact cilium. This review will detail recent genetic and biochemical studies of the hedgehog pathway that give a glimpse of the inner workings of this novel organelle.
Primary cilia are cellular sensory transducers
Primary cilia are small microtubule-based organelles that use intraflagellar transport (IFT) of membrane-bound cargo along microtubules for regulating cell division, signal processing and cellular movement [3,5–8]. The ciliium can be found on most vertebrate and many lower eukaryotic cells from Chlamydomonas to human and emerges from the older of the two centrioles after mitosis. The best example of how cilia transduce environmental signals comes from adhesion-based gamete selection in Chlamydomonas reinhardi, where the tip of the cilium adheres to an appropriate gamete and induces tyrosine kinase-dependent cascades within the cilium [9]. These signals depend not on coated pits or caveolae, but on ciliary IFT to induce the resulting gamete fusion and zygote formation. Experiments using a temperature-sensitive mutant in Kinesin II, an integral component of IFT, show that IFT helps transmit the adhesion signal, although the molecular details of regulating gamete fusion and other cilia-dependent signaling events remains poorly understood.
The logic of the hedgehog pathway
In both the invertebrate and vertebrate pathways, Hh ligand binds and inhibits the Patched (Ptch) receptor, allowing Smo to tip the balance of activity of the Gli/Ci proteins from repression to activation [10]. Smo does this both by inhibiting Ci repressor and inducing the accumulation of the Ci activator. First, Smo blocks protein kinase-dependent Gli3/Ci repressor formation that occurs through the cleavage of full-length Gli3/Ci into a smaller transcriptional repressor. Blocking this cleavage allows the expression of derepressed Hh target genes. For higher levels of target gene induction, Smo also enhances the full-length, activator forms of Gli/Ci. In flies, the atypical kinesin protein costal2 (Cos2) is a key interpreter of the signal from Smo. It does so by scaffolding Ci on vesicles with cleavage-promoting kinases, resulting in Ci phosphorylation and subsequent repressor formation [11]. With the addition of Hh binding to Ptch, Smo relocalizes to the plasma membrane and forms a distinct signaling complex with Cos2 away from the kinases and preventing efficient Ci repressor formation. Cos2 plays an additional role in stimulating activator forms by scaffolding the kinase Fused, which acts positively to increase the transcriptional activity of full-length Ci [11,12]. How Cos2 tethers Smo in the cytoplasm and what regulates Smo movement has not been identified.
Distinct Regulatory Pathways within the Cilia
Increasing data suggests the primary cilium serves an analogous function as Cos2 in higher vertebrates in forming repressor and activating transcriptional activators. Ci transcriptional activator and repressor functions have been subsumed by the activator functions of Gli1 and Gli2 while the repressor functions are conferred on Gli3 and to a lesser extent Gli2. In contrast, Cos2 homologs KIF7 and KIF27 do not have a strict hedgehog phenotype, suggesting greater functional redundancy than exists in Drosophila [13,14]. Initial observations of murine primary cilia mutants indicate that they phenocopy aspects of the Sonic hedgehog (Shh) mutant phenotype in the neural tube [2], have an impaired ability to cleave Gli3 into a repressor, and fail to stimulate Gli2 into an activator. Further support for a direct role of the organelle in processing comes from the colocalization of all three Gli proteins and Smo with acetylated tubulin, a cilium marker [15,16]. While suggestive of a role for the cilium in regulating the pathway, the critical question remained how pathway components were regulated.
Two new studies indicate that Ptch and Smo entry and exit from the cilium is a critical step in pathway regulation [17]. Previous studies performed with overexpressed proteins at single time points lacked an accurate appreciation of the kinetics of signaling [15]. Using newly made antibodies that detected endogeous Ptch and Smo, the authors found that Ptch accumulates in a skirt-like distribution at the base of the cilia, near the ciliary necklace [18]. By contrast, Smo is excluded from the cilia. Fluorescently-labeled Shh added to cultured cells accumulates at the primary cilium with Ptch. Surprisingly, Shh addition caused Ptch levels to decrease in the skirt area while Smo levels accumulate as signaling begins. These observations support the idea that the cilium senses Shh levels and that Ptch-containing vesicles indirectly inhibit the movement and or accumulation of Smo in the cilia.
New genetic mutations in ciliary components also now separate the processes of Gli activator and repressor creation in the cilium. Strong cilia mutations that affect core IFT functions result in both the inability of the Gli3 repressor and the Gli1 and Gli2 activators to be formed at high levels and fail to provide additional insight into the individual pathways. A recent mouse mutation hennin, also identified by a forward genetic screen, encodes a small GTPase (see below). Hennin mutants are weaker than other mutations previously identified in the same screen and retain partial cilium structure [19]. Interestingly, Gli3 repressor functions of hennin-mutant cilia are retained, while accumulation of the Gli2 activator is defective. These data argue that the cilium contains two distinct hedgehog-dependent signaling processes, one for hennin-dependent activator formation and one for repressor formation. Given that pathway members assemble on vesicles, this data argues for the existence of two separable populations of Gli-associated vesicles within the cilia.
A “Rab-id” Transit System controls membrane shape and movement
What controls the precise trafficking and accumulation of Smo and Gli proteins in the cilium? Kinetic studies demonstrate that intraflagellar transport (IFT) maintains ciliary structure and coordinates rapid, bidirectional transport of vesicles between the cytoplasm and the distal tip of the cilium. While the role of these proteins is well-documented and has been the subject of many reviews [2–4], how the bidirectional transport regulates distinct signaling complexes remains unknown. Moreover, due to a lack of a conditional IFT mutant in hedgehog-responsive cells, direct evidence for how IFT regulates Gli activator and repressor accumulation remains elusive.
However, several recent studies suggest movement of signaling components within the cilium is critical and that Gli protein modification is accomplished by an army of small GTPases that move and tether distinct populations of vesicles. Small GTPases are related to the oncogene Ras and subdivided into several subfamilies including the Rab, ADP-Ribosylation (Arf) and Arf-like (Arl) groups. These proteins function as molecular switches that toggle between GTP (on) and GDP (off) states through the actions of GEFs (on) and GAPs (off). They perform the work in moving or tethering a vesicle from one subcompartment to another through the assembly of large compartment-specific effector complexes onto cytoplasmic motors [20,21].
Recent studies now bring the total to seven the small GTPases that play a role in the structure and/or function of the organelle in hedgehog signaling. That the number and phenotypes of these mutants come from this class of proteins speaks strongly for the important role for small GTPase both in the general function of the cilia and more specifically in regulating components of Hh signaling.
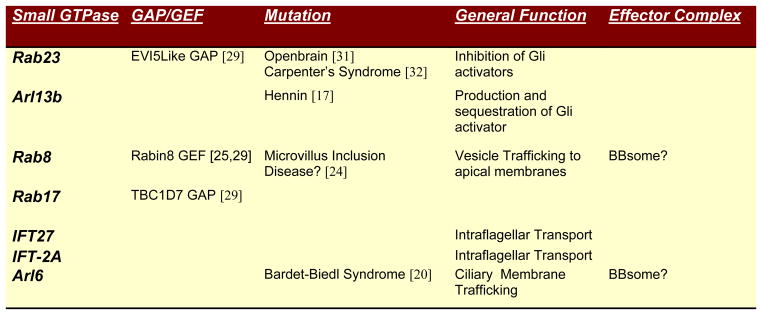
Previous studies examining the components of the primary cilia had noted several key structural components that were small GTPases[3,4,22]. Joining other GTPases Arl 6 /BBS3 [23], Rablike5 / IFTA-2 [24], and Rablike 4/IFT27 [25] in the general control of cilia membrane biogenesis is Rab8. Previous studies with Rab8 support its role in directing the trafficking of post-golgi vesicle to polarized apical membranes [26,27]. Biochemical analysis found that the eight core proteins of the Bardet-Biedl Syndrome (BBS) formed a tight complex called the BBsome [28]. Previous genetic analysis had implicated these proteins in this pleiotropic syndrome with patterning and polarity defects, obesity, and photoreceptor abnormalities not unlike those seen in primary cilia and Shh mutations [29–31]. In complex with the core BBsome was Rab8 and the Rab8GEF, Rabin8, which were shown [28], and confirmed independently [32], to be required for ciliary structure. These studies link the proteins of the BBsome with general effectors of ciliary function and suggests that Rabin8 stabilizes the Rab8GTP form to direct vesicular movement from the peripheral satellites into the cilia. In vivo results suggest some functional redundancy exists. While overexpressed GDP-locked Rab8 has a mild BBS-like phenotype in the retina [28], Rab8A-deficient mice do not display the classic polarity phenotypes seen in IFT mutants [27]. Additional experimentation will be required to determine if Rab8 isoforms or other small GTPases such as Rab17 [32] that also functions in ciliary formation, may compensate for Rab8A.
More specific to the regulation of the Hh signaling pathway are two small GTPases, Rab23 and Arl13B, that appear to balance the levels of Gli2 activator. Rab23, identified by the Anderson lab with other cilia mutants affecting Shh signaling, renders the Shh pathway constitutively active when lost [33,34]. Consistent with this mouse phenotype, human Rab23 mutations have also been found in Carpenter syndrome that causes craniosynostosis, polysyndactyly, obesity, and cardiac defects [35]. Shh pathway analysis demonstrates that Rab23 plays a minor role in promoting Gli3 cleavage to form Gli3 repressor but has a critical role in preventing the accumulation of the Gli1 and Gli2 activators [33].
Mutant Arl13b has a complementary phenotype to that of Rab23 mutants [19]. Arl, or Arf-like small GTPases function, in part, to regulate the tethering of endosome-derived transport vesicles [36]. Mutants of the hennin locus that encodes mouse Arl13b, have low levels of Gli2 activator function as indicated by the low Shh target gene induction in the ventral and middle of mutant neural tubes. In contrast, biochemical analysis supports the fact that like Rab23 mutants, Gli3 repressor can still form in Arl13b mutants. With its localization to the base of the cilia, Arl13B may function to antagonize Rab23-dependent inhibition of Gli2 activator accumulation. An intriguing possibility is that Arl13B effector complexes assemble, while Rab23 effector complexes dissolve, the Gli2 activator complex. Of further interest is the fact that newly identified mutations Fantom, a protein associated with a GTPase regulator [37], and two novel transmembrane proteins Evc [38], and Tectonic [39], each localize to the cilia and affect Shh signaling. The phenotypes of these mutants suggest that these the three proteins may function with Arl13b and in opposition to Rab23-dependent inhibition of Gli2 activator accumulation.
Signaling Lipids and Ligands Direct Traffic
While movement and targeting of membrane-associated signaling complexes seems likely to constitute the key step in determining the strength and duration of signaling, the intriguing question remains what regulates the traffic flow of the vesicles to their particular compartments? Studies over the past few decades have demonstrated that the distribution and composition of lipids possessing signaling properties accumulate differentially in different subcellular compartments [40]. The best known signaling lipid is inositol phosphate, whose metabolites play a well-established role in regulating vesicle trafficking during endocytosis through promotion of lipid -protein interactions [41,42]. Interestingly, the BBS5 component of the BBsome, contains two pleckstrin homology domains and binds to specific phospoinositides [28], supporting a role for ciliary vesicle trafficking.
Several clues implicate other lipid signaling molecules and ligands as traffic directors through Ptch in regulating Smo activity. Ptch is a member of the 12 pass transmembrane proteins containing sterol sensing domains (SSD). The best characterized member of the family is the Sterol response element binding protein (SREBP)-cleavage activating protein (SCAP) which controls the subcellular localization and activity of SREBP. High levels of cholesterol bind to the SSD-containing SCAP and allow the trafficking of SREBP from the endoplasmic reticulum to the golgi where golgi-specific enzymes release SREBP to enter the nuclease and downregulate cholesterol biosynthetic genes [43,44]. Screening of lipid ligands led to the discovery that oxysterols such as 25-hydroxycholesterol could activate Shh signaling in the absence of Shh [45,46]. With antibodies to endogenous Smo and Ptch it was also demonstrated that oxysterols could also allow accumulation of Smo in the cilia [17]. However, unlike Shh that moves Ptch out of the cilia, oxysterols function to allow Smo trafficking despite the presence of the inhibitory effects of Ptch. This argues that oxysterols induce a conformational change in Smo to make it Ptch-resistant. The observation that oxysterols do not bind to Smo but regulate Smo trafficking much like that of the synthetic Smo agonist SAG [45] suggest that oxysterols affect Smo function either by altering membrane dynamics or act indirectly through an as yet unidentified oxysterol-binding protein.
In summary, intense study of how the primary cilia transduces signals from Smo to the Gli transcription factors have led to enormous insight into the organization and regulation of vesicle-based signaling. The overall pathway logic has survived the divergence between Drosophila and higher vertebrates, but the use of the primary cilia small GTPase transit system to partition signaling complexes seems to afford greater control and potential cross talk opportunities with other signaling pathways. Uncovering how the Rab and Arl effector complexes partition signaling in response to ligands and signaling lipids are likely to uncover additional ways to intervene therapeutically as well.
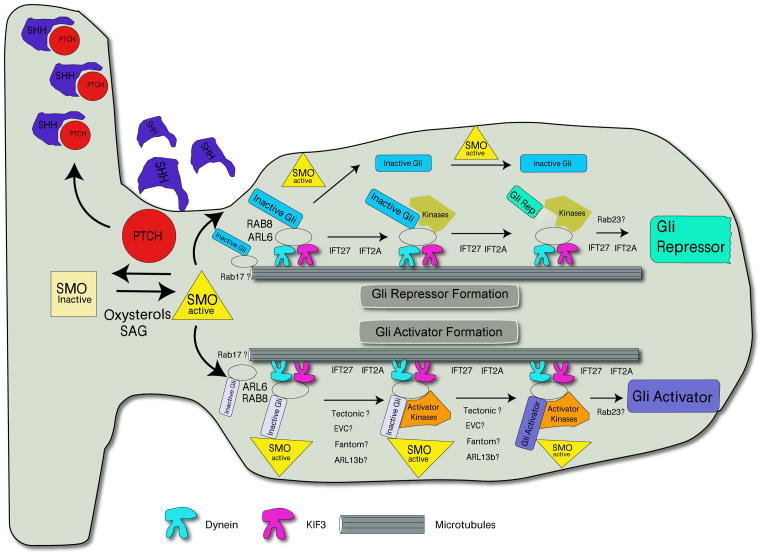
Figure 1. Trafficking Shh signaling components.
Model for the movement of Shh signaling components in the primary cilia. In the absence of Shh, Ptch blocks Smo trafficking to the cilia. Shh binding to Ptch moves Ptch out of the cilia, allowing Smo to become active in the cilia. Oxysterols and the synthetic Smo agonist SAG alter Smo to become Ptch-insensitive and enter the cilia. Distinct Gli dependent protein complexes appear to assemble with the help of small GTPases to allow activator and repressors to form.
Figure 2.
Identified small GTPases and their function in primary cilia
Acknowledgments
The author apologizes in advance for the inadvertent omission of any pertinent references. AEO is funded by the NIH (AR046786 and ARO54780).
Footnotes
Conflicts of Interest
None Perceived
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lum L, Beachy PA. The hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 2.Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- 3.Eggenschwiler JT, Anderson KV. Cilia and Developmental Signaling. Annu Rev Cell Dev Biol. 2006 doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res. 2006;66:6463–6467. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- 5.Inglis PN, Boroevich KA, Leroux MR. Piecing together a ciliome. Trends Genet. 2006;22:491–500. doi: 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 8.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 10.Oro AE. Mammalian variations on a theme: a Smo and Sufu surprise. Dev Cell. 2006;10:156–158. doi: 10.1016/j.devcel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, Jiang J. Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell. 2005;8:267–278. doi: 10.1016/j.devcel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Kalderon D. Hedgehog signaling: Costal-2 bridges the transduction gap. Curr Biol. 2004;14:R67–69. doi: 10.1016/j.cub.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 13•.Varjosalo M, Song-Ping L, Taipale J. Divergence of Hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell. 2006 doi: 10.1016/j.devcel.2005.12.014. The authors demonstrate using both in vitro and in vivo studies that mutations in Cos2 homologs have little unique effect on Shh signaling. This result, in conjunction with mutations in IFT and the primary cilia, suggests the cilia serves an analogous function. [DOI] [PubMed] [Google Scholar]
- 14.Chen MH, Gao N, Kawakami T, Chuang PT. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25:7042–7053. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 16.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. The authors use new antibodies that detect endogenous Smo and Ptch and demonstrate that movement of Smo and Ptch into the cilia is a critical step in pathway regulation. They further show that oxysterols, Shh, and the Smo agonist SAG may effect Ptch and Smo trafficking differently. [DOI] [PubMed] [Google Scholar]
- 18.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. This work identifies the hennin mutation as a defect in the small GTPase Arl13b. Of interest is the relatively normal Gli3 repressor formation with abnormal Gli2 activator accumulation, suggesting that the two processes can be separated. [DOI] [PubMed] [Google Scholar]
- 20.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, et al. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet-Biedl syndrome. Nat Genet. 2004;36:989–993. doi: 10.1038/ng1414. [DOI] [PubMed] [Google Scholar]
- 24.Schafer JC, Winkelbauer ME, Williams CL, Haycraft CJ, Desmond RA, Yoder BK. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J Cell Sci. 2006;119:4088–4100. doi: 10.1242/jcs.03187. [DOI] [PubMed] [Google Scholar]
- 25.Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpaa K, Laakkonen P, Peranen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366–369. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- 28••.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. The authors purify proteins of the Bardet-Biedl syndrome and show they form a tight complex in vitro. Moreover, they show that Rab8 and the Rab8GEF Rabin8 are components of the BBsome and required for ciliary membrane biogenesis. Finally, they identify BBS5 as a phospholipid binding protein, linking the BBsome to the membrane. [DOI] [PubMed] [Google Scholar]
- 29.Blacque OE, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci. 2006;63:2145–2161. doi: 10.1007/s00018-006-6180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badano JL, Mitsuma N, Beales PL, Katsanis N. The Ciliopathies: An Emerging Class of Human Genetic Disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 31.Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. doi: 10.1016/j.devcel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 32•.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. The authors screen all available Rab GTPases for their role in primary cilia and identify Rab8, Rab23, and Rab17 with their cognate RabGAPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev Biol. 2006;290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifiova D, Mathijssen IM, Morton JE, et al. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burd CG, Strochlic TI, Gangi Setty SR. Arf-like GTPases: not so Arf-like after all. Trends Cell Biol. 2004;14:687–694. doi: 10.1016/j.tcb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- 39.Reiter JF, Skarnes WC. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes Dev. 2006;20:22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volpicelli-Daley L, De Camilli P. Phosphoinositides’ link to neurodegeneration. Nat Med. 2007;13:784–786. doi: 10.1038/nm0707-784. [DOI] [PubMed] [Google Scholar]
- 42.Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci U S A. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 45•.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. The authors show that endogenous oxysterols do not bind to Smo but can alter Smo activity in the absence of Shh. [DOI] [PubMed] [Google Scholar]
- 46•.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. The authors show that identified oxysterols can alter Smo activity in the absence of Shh. [DOI] [PMC free article] [PubMed] [Google Scholar]