Abstract
Urine represents a valuable biofluid for noninvasive measurement of Human Growth Hormone (HGH) secretion. Unfortunately, currently available commercial HGH immunoassays do not achieve the sensitivity needed for urinary HGH measurement in the low picogram per milliliter range, the expected normal concentration range of HGH in urine.
A nanotechnology based sample preprocessing step was used to extract and concentrate HGH in urine so that urinary HGH could be measured with a clinical grade standard immunoassay designed for serum (Immulite 1000, Siemens). We applied the nanoparticle enhanced immunoassay to evaluate the baseline value of urinary HGH in a population of healthy young adults (age 18–30, N=33, median 21, M: F=39%:61%, with no reported medical therapies).
Nanoparticle sample preprocessing effectively improved the lower limit of detection of the Immulite HGH assay by more than 50 fold, shifting the linear range of the assay to encompass the expected value of urinary HGH. The full process between run and within run CV% was 7.9 and 9.0%, respectively. On 33 healthy volunteers, the 95% reference values for hGH in spot urine normalized to specific gravity were 0.64 – 16.85 pg/mL (0.05–5.82 ng/g creatinine).
Nanoparticle preprocessing constitutes a reliable means of measuring urinary HGH with a clinical grade immunoassay, now establishing a normal baseline value for HGH in urine. Nanoparticles can be used to study the kinetics of HGH excretion in urine, and the factors that influence urinary HGH secretion and HGH isoform proportions.
Keywords: Nanoparticles, Human growth hormone, Immunoassay, Reference values, Urine
Introduction
The importance of measuring Human Growth Hormone (HGH) levels in the clinical chemistry laboratory is growing rapidly for several reasons. Firstly, therapeutic administration of hGH has expanded beyond the treatment of short stature to now encompass traumatic brain injury [1], Turner syndrome [2] and aging [3]. Secondly, the abuse of hGH supplementation for enhancement of sport performance is believed to be widely prevalent [4]. While hGH testing is usually done in serum, there is a growing need for the measurement of hGH in urine [5,6]. Urine represents a valuable biofluid for noninvasive measurement of HGH secretion [7]. Unfortunately, currently available commercial HGH immunoassays, not based on radioactive labels, do not achieve the sensitivity needed for urinary HGH measurement. The Lower Limit of Detection (LLD) of the Immulite HGH serum immunoassay, Immulite 1000 (Siemens) is 50 pg/mL, a value similar to the LLD for the current commercial (non-radiometric) immunoassays for HGH or its isoforms in blood [8]. This LLD is 50 fold above the postulated concentration of urinary hGH in healthy individuals, estimated to be in the range of 1 pg/mL, as reported using ultrafiltration concentration and radioimmunoassay measurements conducted in the 1990s [7,9].
Thus, despite the fact that clinical researchers and basic scientists in the field of hGH research urgently need a simple and reliable assay for hGH in urine [6], commercial immunoassays designed for serum cannot be used for urine hGH testing. To address this need, in the present study we have applied hydrogel harvesting nanoparticles as a preprocessing step for HGH measurement in urine. Nanoparticles are added to the urine samples to concentrate the analyte into a small volume, which can then be eluted and measured by a clinical grade standard immunoassay designed for serum (Figure 1). The nanoparticles can rapidly harvest, protect from degradation, and concentrate, low abundance analytes from biologic fluids while actively excluding high abundance proteins such as albumin [10–13].
Figure 1.
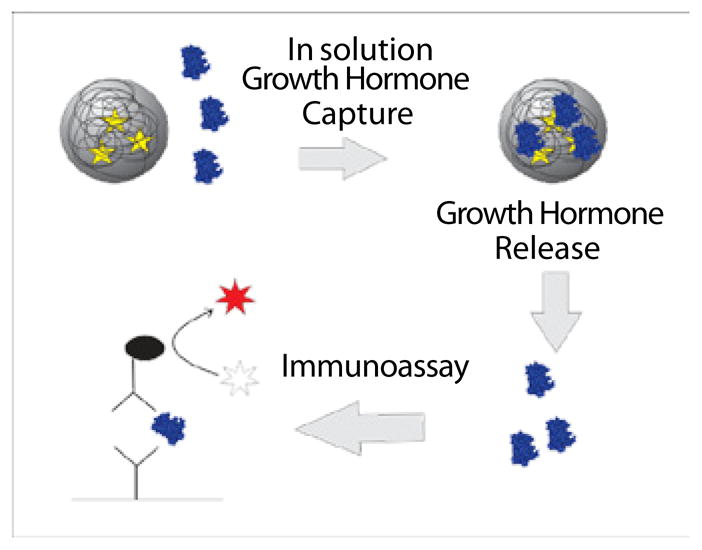
Capturing hydro gel nanoparticles pre-processing step. Well disperse and buoyant particles are introduced in the urine and all the solution phase Human Growth Hormone (HGH) is sequestered. Particles are separated from the urine and captured HGH is eluted and analyzed with a sandwich immunoassay.
Applied as a simple one-step biospecimen pre-processing addition, the nanoparticles can improve the lower limit of analyte detection while preserving the linearity and precision of the analytical platform. In this study, we applied analyte harvesting hydrogel nanoparticles [10,11] to reduce the LLD of the Immulite clinical serum immunoassay for HGH. The Immulite Growth Hormone (HGH) (Recombinant 98/574) is a standard two site sandwich immunoassay which reports a lower of detection of 0.050 ng/mL. The nanoparticle preprocessing as described herein, reduces the LLD by more than 50 fold, shifting the linear range of the assay to encompass the expected value of urinary HGH. Here we report the use of nanoparticle preprocessing to establish an initial reference range value for urinary human growth hormone (hGH) in healthy young adults (age 18–30, N=33, median 21, M:F=39%:61%, with no reported medical therapies), using the Immulite hGH serum immunoassay, Immulite 1000 (Siemens).
Materials and Methods
Subjects
We collected urine and blood from 33 healthy young adults (age 18–30, median 21, M:F=39%:61%, with no reported medical therapies). Spot urine (> 30 mL) collections were done in a standardized time period in the morning (10 to 11 AM). The study was approved by the George Mason University Human Subjects Review Board (protocol GMU # 6081) and conformed to the code of ethics of the world medical association (Declaration of Helsinki), printed in the British Medical Journal (18 July 1964). All subjects gave written informed consent.
Nanoparticle synthesis
Poly N-isopropylacrylamide (NIPAm) co- Acrylic Acid (AAc) nanoparticles were produced by precipitation polymerization and covalently linked to the chemical reactive dyes via zero link amidation reaction [10].
In order to prepare poly (NIPAm-co-AAc) particles, 4.750 grams of NIPAm (Aldrich) and 0.400 grams of N, N′-methylenebisacrylamide (BIS, Aldrich) were dissolved in 500 ml of Milli-Q water. The solution was filtered under vacuum and transferred to a three neck round bottom flask under medium stirring. 500 μl (0.525 gr) of AAc (Sigma Aldrich) was added to the round-bottom flask and the solution was purged with nitrogen for 30 minutes. The temperature of the solution was increased to 70°C and held constant for 15 minutes. 0.276 grams of Potassium persulfate (KPS, Sigma Aldrich) were dissolved in 5 mL of water and added to the round bottom flask. The reaction was help at 70°C for six hours and let cool overnight. Unreacted monomer was washed by centrifugation (19,000 rpm, 50 minute, 20°C) with MilliQ water. Particles were re-suspended in a final volume of 600 mL.
In order to covalently link organic reactive dyes to poly (NIPAm-co-AAc) particles, a preliminary activation of the carboxylic group present in the nanoparticles was performed. Briefly, 20 mL of particle suspension prepared as described before were re-suspended in 0.2 M Monobasic Phosphate Sodium (NaH2PO4) pH 5. The particle suspension was transferred in a round flask and 1 mL of 1% SDS (w/v), 824 mg of N-(3 Dimethylaminopropyl) N′ ethyl carbodiimide hydrochloride (EDC; Fluka Analytical) and 612 of solid N-Hydroxysuccinimide (NHS; Sigma-Aldrich) were added. The reaction was held at room temperature and medium stirring rate for 15 minutes. Meanwhile, organic dye (Remazol Brilliant Blue R, Sigma = 0.151 g, Acid Blue 22, Sigma = 0.178 g, Brilliant Blue R250, Fisher = 0.2 g, Cibacron Blue F3GA, Polysciences = 0.2 g, Rhoda mine 123, Sigma = 0.09 g) was dissolved in 50 mL of 0.2 M Dibasic Phosphate Sodium (Na2HPO4) pH 9 and filtered through a 0.22 μm cellulose acetate membrane (Corning). Due to inferior solubility in aqueous buffers, the dyes Disperse Orange 3, Sigma = 0.058 g, Pararosaniline Base, Sigma =0.073 g were dissolved in 25 ml of ethanol, 5 ml of 1% SDS and 25 ml of 0.2 M Na2HPO4 pH 9 and filtered through a 0.22 μm cellulose acetate membrane (Corning). After 15 minutes of activation reaction, the particle suspension was centrifuged (16.1 rcf, 15 minutes, 25°C), the supernatant was discarded and the particle pellet was re-suspended in the dye solution. The reaction was held at room temperature at medium stirring rate overnight. In order to eliminate the un-reacted dye, particles were washed ten times with water by centrifugation (19,000 rpm, 50 minutes, 25°C). Supernatants were disposed and particles re-suspended in 20 mL of MilliQ water.
Nanoparticle characterization
Particles were characterized by atomic force microscopy (AFM) using an NSCRIPTOR™ DPN® System (NanoInk). Particles solution (1 μg/ml) was deposited on freshly cleaved mica under humid atmosphere for 15 minutes and dried under nitrogen before measurement. Images were acquired under AC mode using a silicon tip with a typical resonance frequency of 300 kHz and a radius smaller than 10 nm. Incorporation of dye in the particles was assessed by weighing the dry particles before and after dye coupling.
Urinalysis, specific gravity and creatinine measurement
Dipstick urinalysis, specific gravity and creatinine measurement were performed on freshly collected urine before particle incubation. Urinalysis was performed with Siemens Multistix 10SG. Specific gravity was measured by means of a digital refractometer (Atago). Creatinine was measured by Creatinine Parameter Assay Kit (R&D systems) according to the manufacturer instructions.
Nanoparticle preprocessing of urine
Aliquots of 30 mL of urine were centrifuged (3,000 rpm, 10 minutes, 20°C) in order to remove cell debris. Urine pH value was measured by and adjusted to 5 with 1 M HCl if necessary. Urine was incubated with 3 mL of poly (NIPAm/RBB) nanoparticles for 30 minutes. The nanoparticles were separated by centrifugation (20,000 rpm, 40 minutes, 25°C). Protein cargo was eluted with 70% acetonitrile 10% ammonium hydroxide and dried under nitrogen flow (Organomation).
Immulite 1000 growth hormone immunoassay
The concentration of HGH eluted from particles was measured using the Immulite 1000 Immulite Growth Hormone (HGH) (Recombinant 98/574) (Siemens Medical Solution Diagnostic). Particle eluates were dried under nitrogen flow, the dried proteins were dissolved in 0.3 mL of Immulite GH sample diluent and hGH was measured according to manufacturer instructions for serum hGH.
Statistical analysis
Reference values and confidence intervals were calculated with the bootstrap method [14] in R Statistical Software [15].
Results
Nanoparticle bait screening
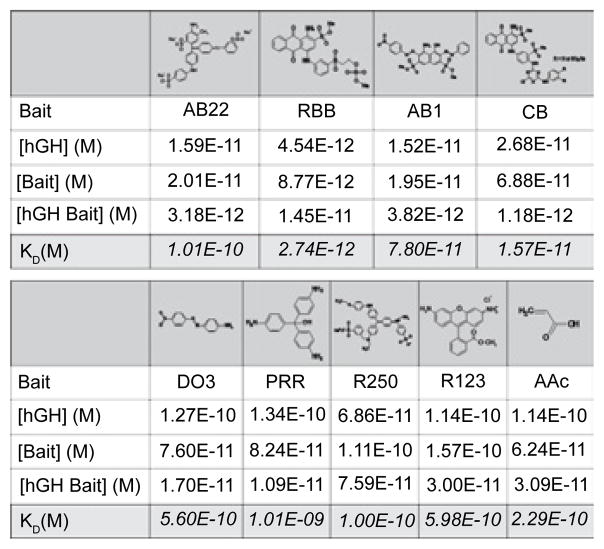
A key element of the hydrogel nanoparticles is the incorporation of the bait [9,10], which captures HGH with high affinity. We screened a series of nine chemical baits for HGH affinity within a urine matrix. The affinity ranges from KD = 10−9 to 10−12 M. We selected the bait that had the highest affinity, which was Remazol Brilliant Blue R (RBB, Figure 1). This previously unpublished bait for HGH had an affinity ten times greater than Cibacron Blue F3GA [12,13] (Figure 2).
Figure 2.
Dissociation constant (KD) values calculated for nine organic chemical baits assuming hGH-bait equimolar binding. AB22 = Acid blue 22, RBB = Remazol brilliant blue R, AB1 = Acid black 1, CB = Cibacron blue F3GA, DO3 = Disperse orange 3, PRR = Pararosaniline base, R250 = Brilliant blue R250, R123 = Rhoda mine 123, AAc = acrylic acid.
Concentration factor calculation
Enhanced detection sensitivity is achieved because the nanoparticles effectively harvest virtually 100% of the HGH from the full 30 mL urine volume, and concentrate the urinary HGH down to a volume of 300 μL. The increase in effective concentration of HGH is:
| (1) |
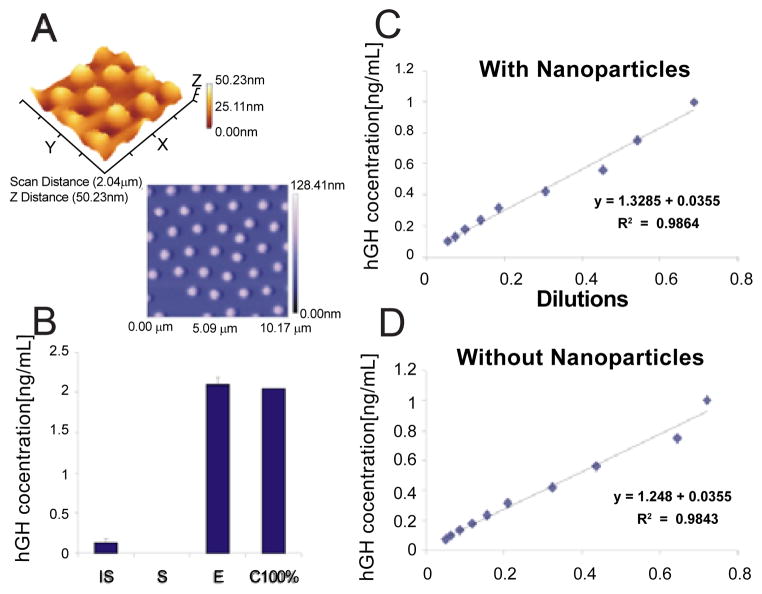
Where vs. = original sample volume, ve = volume of nanoparticle eluate, C = initial concentration of analyte in the sample, and Ci=final increased expected concentration. Consequently a 100 times higher concentration of HGH is introduced into the Immulite assay, compared to the unconcentrated urine. The nanoparticles are buoyant open mesh (>95% solute) hydrogel spheres (Figure 3A) containing a core with an immobilized high affinity chemical bait. When mixed with the urine, the nanoparticles remain in colloid suspension to achieve extremely rapid access [10,11] and sequestration of the HGH target analyte. The bait binds HGH with high affinity (KD<10−12 M, Figure 1), such that no detectable HGH can be measured outside the nanoparticles in the fluid phase.
Figure 3.
A) Atomic force microscope images demonstrate uniformity of the spherical poly (NIPAm/RBB) nanoparticles. B) Nanoparticle-Immulite GH assay has a within run CV = 9% (over ten replicates) and virtually 100% yield. Mean +/− CV. IS = initial solution, S = Supernatant, E = nanoparticle eluate, C100% = calculated GH concentration based on 100% yield. C) And D) Linearity of the immunoassay (9 calibrator values, range 0.750-0.05 ng/mL) with and without nanoparticle preprocessing, respectively. Nanoparticle preprocessing maintains the linearity of the immunoassay (R2=0.986, 0.982, with and without the nanoparticles, respectively).
Concentration method verification
The nanoparticle concentration method was verified with standard samples that were run by trained personnel in a blind fashion, N = 21. The acceptance criteria were percentage difference (Diff %) < 10%, as defined by the following equation:
| (2) |
Where true is the true HGH concentration in the blind standard sample and Meas is the HGH concentration value obtained by nanoparticle Immulite assay performed in a blind fashion.
100% of the samples passed the 10% criteria (Table 1).
Table 1.
Nanoparticle pre-processing step passed the concentration method verification test in our laboratory, N=21. TRUE = true value of HGH concentration in the standard sample, MEASURED = HGH concentration measured in the standard sample in a blind fashion, DIFF% = (MEASURED − TRUE)/TRUE *100. Acceptability criteria = DIFF% < 10%.
| True [Ng/Ml] | Measured [Ng/Ml] | Diff % |
|---|---|---|
| 2.95 | 2.91 | 1.37 |
| 3.61 | 3.6 | 0.28 |
| 1.22 | 1.2 | 1.67 |
| 0.454 | 0.437 | 3.89 |
| 0.15 | 0.154 | −2.60 |
| 0.38 | 0.385 | −1.30 |
| 1.63 | 1.51 | 7.95 |
| 4.35 | 4.52 | −3.76 |
| 6.89 | 6.76 | 1.92 |
| 0.348 | 0.34 | 2.35 |
| 0.354 | 0.389 | −9.00 |
| 10.000 | 10.6 | −5.66 |
| 0.360 | 0.336 | 7.14 |
| 2.200 | 2.14 | 2.80 |
| 0.083 | 0.085 | −2.35 |
| 7.610 | 7.58 | 0.40 |
| 2.130 | 2.140 | −0.47 |
| 1.470 | 1.390 | 5.76 |
| 2.440 | 2.430 | 0.41 |
| 0.891 | 0.879 | 1.37 |
| 0.778 | 0.757 | 2.77 |
Full process precision
The full-process within-run CV for the nanoparticle based measurement of urinary HGH was 9.0% (N=10), with virtually 100% HGH capture efficiency and 100% elution yield (Figure 3B, Initial Solution Average (CV) ng/mL =0.128 (4.0%CV) ng/mL; Supernatant <0.05 ng/mL; Eluate Actual Measurement, Average (CV) =2.095 (9.0%CV) ng/mL; Calculated 100% yield 2.048 ng/mL). The between run precision (%CV) without the particles was 9.5% and the between run precision (%CV) with the nanoparticles was 8.1%. The HGH concentration in the supernatant following nanoparticle incubation was always below the limit of detection for the Immulite. The between run analysis was done over 2 working weeks. (Initial Solution Average (CV) ng/mL = 0.106 (9.5%CV) ng/mL; Supernatant <0.05 ng/mL; Eluate Actual Measurement, Average (CV) =0.576 (8.1%CV) ng/mL; Average total yield (CV) = 91% (13.2%CV).
Full process linearity
Linearity of the immunoassay (9 calibrator values, range 0.750-0.05 ng/mL) was equivalent with and without nanoparticle pre-processing (Figure 3C and 3D, Slope (standard deviation) =0.74 (0.03), 0.79 (0.04); Intercept (standard deviation) =−0.02 (0.02), −0.02 (0.02); R2=0.986, 0.982; Residual standard deviation=0.0286, 0.0347 with and without the nanoparticle processing, respectively).
Reference values for HGH in spot urine
In our cohort of volunteers, the 95% reference values for hGH in spot urine normalized to specific gravity were 0.64 – 16.85 pg/mL (0.05 –5.82 ng/g creatinine) (Table 2). Reference values and confidence intervals were calculated with the bootstrap method [14] in R Statistical Software [15]. These data reveal that there is a one thousand fold difference in the concentration of physiologic HGH in spot urine compared to the value in serum for the same patient, measured at the same point in time (Table 2).
Table 2.
Reference Values of spot urine HGH measured with nanoparticle-Immulite assay and serum HGH measured with Immulite Growth Hormone test.
| Urine hGH concentration, pg/mL | Urine hGH concentration, ng/g creatinine | Serum hGH concentration, ng/mL | |
|---|---|---|---|
| 2.5 percentile (0.90 confidence interval) | 0.64 (0.52–0.84) | 0.05 (0.04 – 0.06) | 0.13 (0.11–0.19) |
| 97.5 percentile (0.90 confidence interval) | 16.85 (9.92–28.369) | 5.82 (2.34 – 9.7) | 16.03 (7.75–23.2) |
| Median | 2.75 | 0.39 | 0.41 |
| Average | 5.01 | 0.98 | 2.42 |
Inter-laboratory proficiency assessment
To verify that this methodology could be validated across multiple laboratories, we did a side by side comparison of nanoparticle preprocessed urine in two different international laboratories using two different immunoassays. The Immulite immunoassay as described above was conducted in George Mason University, USA and a recently developed research grade immunoassay validated for serum samples [16] was performed in IMIM Hospital del Mar, Spain. Four urine proficiency samples spiked with recombinant hGH were prepared and incubated with 1 mL of poly(NIPAm/RBB) nanoparticles. The nanoparticle-processed eluate was measured by the USA and Spain laboratories independently. The correlation, spanning the range of 0.003 – 0.3 ng/mL calculated urine concentration (0.08 – 11 ng/mL measured hGH urine concentration), was y = 0.8454x + 0.2386, R2 = 0.9967, where x = USA results and y = Spain results.
Thus, nanoparticle preprocessing constitutes a reliable means of establishing a normal baseline value for HGH in urine. The important question can now be addressed as to whether the excretion of HGH in the urine has longer time course than the spike of HGH in serum following exogenous administration. Moreover, it will now be possible to study factors that affect urinary HGH excretion and isoform proportions. This is important because urinary HGH measurement may permit the detection of HGH doping that would otherwise be missed with serum testing [6].
Bait functionalized hydrogel nanoparticles [10,11] are a cost effective, user friendly technology that, in this study, permitted the reliable measurement of a low abundance protein analyte (HGH) in urine with a clinical grade immunoassay that until now is used for serum/plasma based analysis. Once the correct bait chemistry is matched to optimal analyte capture, this nanoparticle technology can be applied to the measurement of any analyte in any body fluid using any appropriate measurement platform ranging from immunoassays to mass spectroscopy-based multiple reaction monitoring [10].
Acknowledgments
This work was supported partially by 1) George Mason University, 2) US Anti-Doping Agency, 3) the Italian Istituto Superiore di Sanita’ in the framework of the Italy/USA cooperation agreement between the U.S. Department of Health and Human Services, George Mason University, and the Italian Ministry of Public Health, and 4) Grant 1R21AR061075 – 01 to LAL from NIAMS/NIH. The authors are grateful for fruitful scientific discussions with Simona Pichini, Francesco Facchiano, Ricardo Gutiérrez Gallego, and Jordi Segura.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.High WM, Jr, Briones-Galang M, Clark JA, Gilkison C, Mossberg KA, et al. Effect of growth hormone replacement therapy on cognition after traumatic brain injury. J Neurotrauma. 2010;27:1565–1575. doi: 10.1089/neu.2009.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross JL, Quigley CA, Cao D, Feuillan P, Kowal K, et al. Growth hormone plus childhood low-dose estrogen in Turner’s syndrome. N Engl J Med. 2011;364:1230–1242. doi: 10.1056/NEJMoa1005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone Replacement Therapy and Physical Function in Healthy Older Men. Time to Talk Hormones? Endocr Rev. 2012;33:314–377. doi: 10.1210/er.2012-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt RI, Sönksen PH. Growth hormone, IGF-I and insulin and their abuse in sport. Br J Pharmacol. 2008;154:542–556. doi: 10.1038/bjp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt RI. Detecting growth hormone abuse in athletes. Anal Bioanal Chem. 2011;401:449–462. doi: 10.1007/s00216-011-5068-2. [DOI] [PubMed] [Google Scholar]
- 6.Sönksen PH, Holt RI. GH & IGF Research issue on doping with growth hormone. Growth Horm IGF Res. 2009;19:283–284. doi: 10.1016/j.ghir.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Quade A, Rahn M, Schweikert HU, Bidlingmaier F, Klingmüller D. Urinary excretion of GH in healthy individuals and patients with acromegaly, hypopituitarism and dwarfism. Acta Endocrinol (Copenh) 1993;128:24–28. doi: 10.1530/acta.0.1280024. [DOI] [PubMed] [Google Scholar]
- 8.Hauffa BP, Lehmann N, Bettendorf M, Mehls O, Dörr HG, et al. Central reassessment of GH concentrations measured at local treatment centers in children with impaired growth: consequences for patient management. Eur J Endocrinol. 2004;150:291–297. doi: 10.1530/eje.0.1500291. [DOI] [PubMed] [Google Scholar]
- 9.Baumann G, Abramson EC. Urinary growth hormone in man: evidence for multiple molecular forms. J Clin Endocrinol Metab. 1983;56:305–311. doi: 10.1210/jcem-56-2-305. [DOI] [PubMed] [Google Scholar]
- 10.Tamburro D, Fredolini C, Espina V, Douglas TA, Ranganathan A, et al. Multifunctional Core-Shell Nanoparticles: Discovery of Previously Invisible Biomarkers. J Am Chem Soc. 2011;133:19178–19188. doi: 10.1021/ja207515j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luchini A, Geho DH, Bishop B, Tran D, Xia C, et al. Smart hydrogel particles: biomarker harvesting: one-step affinity purification, size exclusion, and protection against degradation. Nano Lett. 2008;8:350–361. doi: 10.1021/nl072174l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredolini C, Tamburro D, Gambara G, Lepene BS, Espina V, et al. Nanoparticle technology: amplifying the effective sensitivity of biomarker detection to create a urine test for hGH. Drug Test Anal. 2009;1:447–454. doi: 10.1002/dta.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredolini C, Meani F, Reeder KA, Rucker S, Patanarut A, et al. Concentration and Preservation of Very Low Abundance Biomarkers in Urine, such as Human Growth Hormone (hGH), by Cibacron Blue F3G-A Loaded Hydrogel Particles. Nano Res. 2008;1:502–518. doi: 10.1007/s12274-008-8054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solberg HE. Establishment and Use of Reference Values. In: Burtis CA, Ashwood ER, Bruns DE, Tietz NW, editors. Tietz Fundamentals of Clinical Chemistry. WB Saunders; Philadelphia: 2001. pp. 251–261. [Google Scholar]
- 15.R computational team. R: A language and environment for statistical computing. R foundation for statistical computing; Vienna, Austria: 2012. [Google Scholar]
- 16.Bidlingmaier M, Suhr J, Ernst A, Wu Z, Keller A, et al. High-sensitivity chemiluminescence immunoassays for detection of growth hormone doping in sports. Clin Chem. 2009;55:445–453. doi: 10.1373/clinchem.2008.112458. [DOI] [PubMed] [Google Scholar]