Abstract
Background
Ketamine is widely-used for procedural sedation but there is limited knowledge whether ketamine use is associated with elevated intraocular pressure (IOP).
Objective
To examine whether there is an association of ketamine use with IOP during pediatric procedural sedation.
Methods
We prospectively enrolled children without ocular abnormalities undergoing procedural sedation that included ketamine for non-periorbital injuries. We measured IOP for each eye before and at 1,3,5,15, and 30 minutes after initial IV ketamine administration. We performed Bland-Altman plots (BAP) to determine if IOP measurements in both eyes were in agreement. Linear regression was used to model the mean IOP of both eyes as a function of time, dose, gender, and age, with a robust sandwich estimator to account for repeated measures.
Results
Among 25 participants, median [IQR] age was 11 [9,12] years and 18 (72%) were male. Median ketamine dose was 1.88mg/kg (IQR 1.43, 2.03, range 0.96, 4). BAP demonstrated a mean difference of IOP between eyes near zero at all time points. The largest predicted difference from baseline IOP occurred at 15 minutes with an estimated change of 1.09 mm Hg (95% CI: −0.37,2.55). The association between ketamine dose and mean IOP was not statistically significant or clinically meaningful [p= 0.90, estimated slope 0.119 (95% CI −1.71, 1.95)]. There were no clinically meaningful levels of measured average IOP reached at any time point.
Conclusions
At dosages ≤ 4 mg/kg, there are not clinically meaningful associations of ketamine with elevation of IOP.
Keywords: Ketamine, Intraocular Pressure, Ocular Tonometry, Deep Sedation
Introduction
Children frequently present to the emergency department with fractures, lacerations, or other injuries that require evaluation and interventions that are both painful and frightening. Since its introduction in the late 1960s, ketamine has become a widely-used and effective agent for pediatric procedural sedation because it provides amnesia, analgesia, anxiolysis, and sedation without respiratory depression or hypotension. Based on reports in the literature from the 1960s-1970s, there has been concern that ketamine may elevate intraocular presssure (IOP). These reports had several limitations and reached conflicting conclusions whether there is an association of ketamine use with increased IOP. However, it has become a widely-accepted practice to avoid using ketamine for procedural sedation of all patients with suspected eye injuries. Because of this concern, emergency medicine providers frequently choose other agents for sedation that may expose patients to other adverse effects (e.g., myocardial and respiratory depression).
Normal intraocular pressure is approximately 15 – 20 mmHg based on population studies (1) (Stamper and Tanaka). The largest study used a Schiøtz tonometer and found a mean intraocular pressure of 15.5 mmHg (SD 2.57 mmHg), although the normal limits of diurnal variations were 5 to 25 mm Hg (2) (Leydhecker). Studies using the criterion-standard Goldmann applanation tonometer have found similar values and a right-skewed distribution (Davanger)(3). Studies comparing the Tonopen with the Goldmann applanation tonometer have revealed a correlation between the two methods (Minckler, Stamper et al, Christoffersen et al). Although there is excellent agreement regarding the normal range for mean intraocular pressure, determining the lower and particularly the upper limits of ‘normal’ is much more difficult as the distribution tends to be right-skewed. Less than 1% of the normal population is considered abnormal if 2.5 standard deviations above the mean is designated as as the upper limit of normal (about 24 mmHg). Ocular hypertension, which we take as a our definition of clinical meaningful elevation of IOP, has been most commonly defined as sustained elevation in IOP at or above 22mmHg (Kim et al, Henderson et al, Tavares et al)
The initial work on ketamine and its effects on intraocular pressure was done by Corssen and Hoy in 1967 and described an increase of intraocular pressure, but had several limitations. The study looked at patients of different ages undergoing general anesthesia for surgical operations of various kinds. Their data included fifteen pediatric cases, several of whom had incomplete data. The premedications used in the study were not standardized and included agents not generally used in the PED for this purpose (e.g., barbiturates). The investigators studied the the patients for three minutes after drug administration, a time point which alone is not appropriate for the pharmacokinetics of this drug (Evers). In almost half of the subjects the measurements either did not change or decreased in one or both eyes. A lesser proportion of IOP elevation was noted in pediatric cases, and these subjects yielded a pooled mean increase of less than 3mmHg from control readings. Further, pressure readings (done by Schiøtz tonometer) varied considerably between eyes, within subjects, and between subjects.
Yoshikawa and Murai assessed 15 children for a thirty minute period and reported a peak increase from baseline IOP at 15 minutes of 37% , but used no premedication and a ketamine dose greater than is customary (5mg/kg IM). Other limitations of this study were that recordings were done during various types of ophthalmalogic surgery. Although some descriptive data were reported, there was no mention of statistical tests of inference used. In 1973, Aileen Adams reported increased IOP measurement with general halothane induction followed by ketamine administration in 15 children. However, the ketamine dose was again greater than generally used (10mg/kg IM), and 9 of the children already had known glaucoma changes. The mean IOP increased from 11 to 15.5mmHg at 3-5 minutes and in no child did the pressure rise or stay above the normal upper limit of 22mmHg within the 8 minute measurement window.
In contrast, Peuler and Glass conducted a study of 20 adult patients and concluded that ketamine in clinically-used doses, administered to a premedicated patient, had no significant effect on IOP. Ausinsch and Rayburn studied 10 children and found no effects of ketamine on IOP despite IM doses of 8mg/kg. An 1977 animal study of rabbits receiving large doses of ketamine (10mg/kg IM and also 50mg/kg in conjuction with pentobarbitol) reported increases in IOP (Schutten 1977). More recently, three more human-subject studies (Badrinath 1986 Cugini 1997 Frey 1999) showed decreases in IOP following ketamine administration, but were limited by potential confounders involving endotracheal intubation of patients, relatively low doses of ketamine given, short duration of IOP monitoring, and procedures involving the orbit with retrobulbar nerve blocks. A more recent study looking at IOP effects of different doses of ketamine in children reported a 2mmHg increase in IOP 5 minutes after receiving 6 mg/kg of ketamine IM and no change in children receiving 3mg/kg ketamine IM. However, the study did not include repeated measures analysis and included patients undergoing general anesthesia for ophthalmologic surgery. In addition, no patient's IOP exceeded 20 mmHg (Nagdeve 2006).
Overall, a review of literature in both pediatric and emergency medicine journals indicates conflicting descriptions of the effect ketamine on IOP. Our objective was to examine the association of ketamine with IOP in children with healthy eyes undergoing procedural sedation for non-periorbital injuries.
Methods
We enrolled a prospective convenience sample of patients who were to undergo procedural sedation that included ketamine for non-periorbital injuries in our pediatric emergency department. Eligible patients were 7 to 17 years of age, and exclusion criteria were ASA class 3 or 4, prior eye surgery, ocular abnormality, or periorbital injury. Two investigators were trained to use a Tono-pen® XL applanation tonometer (Medtronic Solan, Jacksonville, USA) by an ophthalmologist prior to study initiation. Following proparacaine anesthetic eye drops, IOP was measured for each eye using a Tonopen and the average value for both eyes was calculated before and at 1,3,5,15, and 30 minutes after the first dose of IV ketamine. We recorded total doses of ketamine and other medications administered.
We performed Bland-Altman plots (BAP) to assess whether measurements of IOP in both eyes were in agreement. Histograms of the mean IOP by time point indicated that the normality assumptions of linear regression were satisfied. A linear regression model was used to assess the time trend of mean IOP, adjusting for dose, age and gender. This model incorporated a linear spline function on time to allow a non-linear relationship between time and mean IOP. The covariance variance matrix of the model was adjusted by Huber's robust sandwich estimator to account for correlated observations. All analyses were performed by the statistical software R 2.10.0.
This study protocol (#031054) was reviewed and approved by the Vanderbilt University Human Protection Program. Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University Medical Center (citation).
Results
Among 25 enrolled participants, median[IQR] age was 11 [9, 12] years and 18 (72%) were male. Median ketamine dose administered was 1.88 mg/kg [1.43, 2.03] with a range of 0.96 to 4 mg/kg. Other medications given at the beginning of the procedural sedation included glycopyrrolate, midazolam, and ondansetron. Procedural sedation was provided for fracture reduction, abscess incision and drainage, laceration repair, foreign body removal, and hip arthrocentesis. Subject characteristics are presented in Table 1.
Table 1.
Characteristics of 25 study participants without orbital or peri-orbital disease or injury who received ketamine sedation.
| Range | ||
|---|---|---|
| Age, yrs* | 11 [9 – 12] | 7-16 |
| Male | 18 /25 (72%) | |
| Total ketamine dose (mg/kg)* | 1.88 [1.43 – 2.03] | 0.96-4.00 |
| Other medications given | ||
| Glycopyrrolate | 11/25 (40%) | |
| Median glycopyrrolate dose (mg/kg) | 0.002 | 0.001-0.005 |
| Ondansetron | 25/25 (100%) | |
| Median ondansetron dose (mg/kg) | 0.10 | 0.05-0.11 |
| Midazolam | 19/25 (76%) | |
| Median midazolam dose (mg/kg) | 0.03 | 0.01-0.05 |
| Procedure | ||
| Fracture reduction | 17/25 (68%) | |
| Abscess Incision and Drainage | 3/25 (12%) | |
| Laceration repair | 2/25 (8%) | |
| Foreign body removal | 2/25 (8%) | |
| Hip arthrocentesis | 1/25 (4%) |
Values are median [IQR]
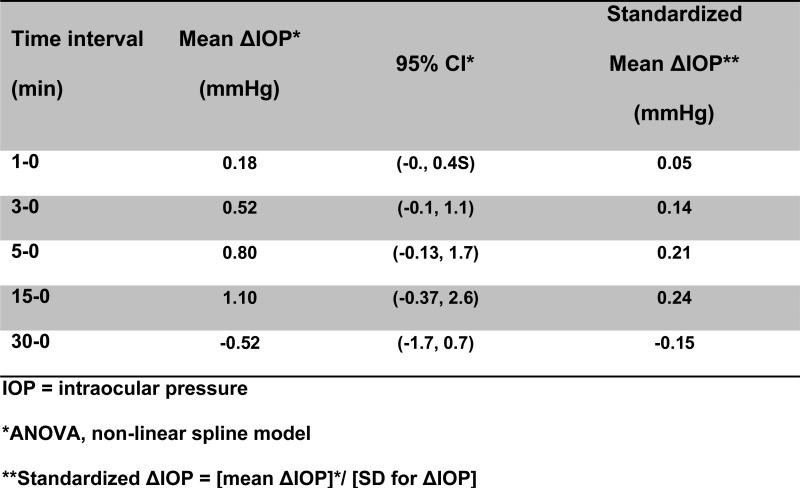
BAP demonstrated a high level of agreement of IOP measurement performed in each eye at all time points. After adjusting for age, gender, and dose, there were no statistically significant or clinically meaningful changes of predicted mean IOP between baseline and any of the time points in our sample (p=0.15, Table 2). All 95% confidence intervals for predicted mean change from baseline IOP crossed zero. The largest predicted difference from baseline IOP occurred at 15 minutes with an estimated change of 1.09 mm Hg (95% CI: −0.37, 2.55). In addition, the association between ketamine dose and mean IOP was not statistically significant or clinically meaningful, with an estimated slope of 0.119 (95% CI: −1.714, 1.951, p=0.90) which, in clinical terms, means a greatest plausible 1.951 mmHg change in IOP for every 1 mg/kg unit increase in ketamine dose.
Table 2.
Model-based predicted intraocular pressure (IOP) does not change (Δ) after administration of IV ketamine at dosages ≤ 4mg/kg in a statistically significant or clinically meaningful way.
| Time (min) | Predicted mean IOP* (mmHg) | 95% CI | Predicted Mean ΔIOP* (mmHg) | 95% CI |
|---|---|---|---|---|
| 0 | 14.66 | (12.46, 16.86) | - | - |
| 1 | 14.84 | (12.65, 17.04) | 0.181 | (−0.03, 0.39) |
| 3 | 15.18 | (12.95, 17.42) | 0.521 | (−0.08, 1.12) |
| 5 | 15.46 | (13.14, 17.78) | 0.799 | (−0.13, 1.73) |
| 15 | 15.75 | (13.23, 18.28) | 1.092 | (−0.37, 2.55) |
| 30 | 14.14 | (11.75, 16.54) | −0.519 | (−1.70, 0.66) |
Linear regression model with restricted cubic spline function, mean ketamine dose 1.88 mg/kg, mean age 11 years, Huber's robust sandwich estimator for repeated measures analysis
There were no clinically meaningful levels (≥22mmHg) of measured average IOP reached at any time point. (Figure 1). Three of the 25 patients had an isolated elevation of IOP ≥ 22mHg at a single time point. One of these three patients was redosed with ketamine with a subsequent decrease in IOP and the other two patients were given single-dose ketamine. One of the 25 patients had elevation of IOP ≥ 22mmHg at two consecutive time points.
Figure 1.
Intraocular pressure change after administration of Ketamine IV.
Discussion
The results of this investigation suggest that at dosages ≤ 4 mg/kg, there are not clinically meaningful associations of ketamine with IOP. The known pharmacokinetics of ketamine are such that associated change of IOP would be unlikely after this interval (Evers). The absence of a clinically meaningful dose-response relationship in this sample between ketamine and IOP further suggests that ketamine use is not associated with elevation in IOP.
These findings question the widely-held concern that ketamine increases intraocular pressure, a concern that appears to be informed by limited evidence. Only one of 25 subjects had a clinically meaningful elevation of IOP during the study period, as defined by an IOP ≥ 22mmHg at two consecutive measurements. It was noted that this patient had a baseline average IOP of 18mmHg, was given single initial dose ketamine 1.5mg/kg for closed reduction of a distal radius fracture, and reached an IOP of 23 and 25mmHg at five and fifteen minutes, respectively. Five subjects had sustained decreases of average IOP at all time points during the 30 minute study period, and three other subjects had decreases of average IOP at four of the five time points. In addition, we observed that in eight of the eleven patients who needed additional doses of ketamine mean IOP decreased at the next IOP measurement. We believe this was a result of ketamine treatment alleviating pain, agitation and anxiety, and does not support the concern that ketamine elevates IOP.
Our study has limitations. Sedation was performed for a variety of procedures, and a cohort undergoing a single type of procedure may eliminate the type of procedure as a potential confounding variable. Also, the study investigator did not participate in decisions for medication administration, and the clinical team determined whether to give glycopyrrolate and/or midazolam during the procedural sedation, as well as other other analgesic medications which, when given, were given prior to the procedural sedation either by EMS providers and/or the clinical team. These ancillary medications may have had independent associations with IOP that are not fully adjusted for in our analyses. However, one could argue that providing anxiolysis and analgesia prior to the procedural sedation would, if anything, work to prevent artificial elevation of IOP due to agitation and pain.
In summary, at dosages ≤ 4 mg/kg, there are not clinically meaningful associations of ketamine with IOP. Further study is warranted to determine if ketamine can be used safely for procedural sedation when elevated IOP or globe injury is a concern.
Acknowledgments
Funding sources: Vanderbilt Institute for Clinical and Translational Research grant support (1 UL1 RR024975 from NCRR/NIH)
References
- 1.Stamper RL, Tanaka GH. Chapter 7 Vol. 2: Intraocular Pressure: Measurement, Regulation, and Flow Relationships. In: Tasman W, Jaeger EA, editors. Duane's Ophthalmology. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. [Google Scholar]
- 2.Leydhecker W. The intraocular pressure: Clinical aspects. Ann Ophthalmol. 1976;8(4):389–392, 395-399. [PubMed] [Google Scholar]
- 3.Davanger M, Holter O. The statistical distribution of intraocular pressure in the population. Acta Ophthalmol (Copenh) 1965;43:314–322. doi: 10.1111/j.1755-3768.1965.tb05455.x. [DOI] [PubMed] [Google Scholar]
- 4.Minckler DS, Baerveldt G, Heuer DK, et al. Clinical evaluation of the Oculab Tono-Pen. Am J Ophthalmol. 1987;104:168. doi: 10.1016/0002-9394(87)90010-9. [DOI] [PubMed] [Google Scholar]
- 5.Adams A. Ketamine in Paediatric ophthalmic practice. Anaesthesia. 1973;28:212–213. doi: 10.1111/j.1365-2044.1973.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 6.Ausinsch B, Rayburn RL, Munson eS, Levy NS. Ketamine and intraocular pressure in children. Anesth Anal. 1976;55(6):773–775. doi: 10.1213/00000539-197611000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Badrinath SK, et al. The effect of different methods of inducing anesthesia on intraocular pressure. Anesthesiology. 1986;65:431–435. doi: 10.1097/00000542-198610000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Corssen G, Hoy JE. A New Parenteral Anesthetic CI-581: its effect on intraocular pressure. J Pediat Ophthal. 1967;4:20–23. [Google Scholar]
- 9.Cugini U, et al. Sedation with Ketamine during cataract surgery. J Cataract Refract Surg. Jun. 1997;23(5):784–6. doi: 10.1016/s0886-3350(97)80291-x. [DOI] [PubMed] [Google Scholar]
- 10.Frey K, et al. Propofol versus propofol-ketamine sedation for retrobulbar nerve block: Comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anest Analg. 89:317–21. doi: 10.1097/00000539-199908000-00013. 199. [DOI] [PubMed] [Google Scholar]
- 11.Peuler M, Glass DD, Arens JF. Ketamine and intraocular pressure. Anesthesiology. 1975;43(5):575–578. doi: 10.1097/00000542-197511000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa K, Murai Y. The effect of ketamine on intraocular pressure in children. Anesth Analg. 1971;50(2):199–202. [PubMed] [Google Scholar]
- 13.Bar-Joseph Gad, et al. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. Neurosurg Pediatrics. 2009;4:000–000. doi: 10.3171/2009.1.PEDS08319. [DOI] [PubMed] [Google Scholar]
- 14.Stamper RL, Gardner ID, Bron AJ. Evaluation of a new, portable tonometer with digital display. Ophthalmol. 1987;94:133. [Google Scholar]
- 15.Christoffersen T, Fors T, Ringberg U, et al. Tonometry in the general practice setting: Tono-Pen compared to Goldmann applanation tonometry. Acta Ophthalmol. 1993;71:103. doi: 10.1111/j.1755-3768.1993.tb04970.x. [DOI] [PubMed] [Google Scholar]
- 16.Schutten WH, Van Horn DL. The effects of ketamine sedation and ketaminepentobarbital anesthesia upon intraocular pressure of the rabbit. Invest Ophthalmol Vis Sci. 1977 Jun;16(6):531–534. [PubMed] [Google Scholar]
- 17.Kim NR, Kim CY, Kim H, et al. Comparison of Goldmann Applanation Tonometer, Noncontact Tonometer, and TonoPen XL for Intraocular Pressure Measurement in Different Types of Glaucomatous, Ocular Hypertensive, and Normal Eyes. Curr Eye Res. 2011 Feb 1; doi: 10.3109/02713683.2010.542865. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Henderson P, Labbe T, Kass MA. Ocular Hypertension. 3rd ed. Mosby; 2008. Yanoff & Duker: Ophthalmology. Chapter 10.9. [Google Scholar]
- 19.Tavares IM, Medeiros FA, Weinreb RN. Inconsistency of the published definition of ocular hypertension. J Glaucoma. 2006 Dec;15(6):529–33. doi: 10.1097/01.ijg.0000212279.03595.70. Review. [DOI] [PubMed] [Google Scholar]
- 20.Harris Paul A., Taylor Robert, Thielke Robert, Payne Jonathon, Gonzalez Nathaniel, Conde Jose G. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evers AS, Crowder CM, Balser JR. Chapter 13. General Anesthetics. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11ed. McGraw-Hill; USA: 2006. [Google Scholar]