SUMMARY
Impaired fetal development is associated with a number of adult chronic diseases and it is believed that these associations arise as a result of the phenomenon of “epigenetic programming”, which involves persisting changes in structure and function of various body organs caused by ambient factors during critical and vulnerable periods of early development. The main goal of the study was to assess the association between lung function in early childhood and prenatal exposure to fine particulate matter (PM2.5 ), which represents a wide range of chemical compounds potentially hazardous for fetal development. Among pregnant women recruited prenatally to the study personal measurements of PM2.5 was performed over 48 hours in the second trimester of pregnancy. After delivery, infants were followed over five years and the interviewers visited participants at their homes to record children’s respiratory symptoms every three months in the child’s first two years of life and every 6 months later. In the fifth year of the follow-up, children were invited for standard lung function testing and quantified by FVC, FEV1 and FEV05 levels. Material consisted of 176 children of nonsmoking mothers, who performed at least two acceptable spirometry measurements. Multivariable linear regression model showed a significant deficit of FVC at the highest quartile of PM2.5 exposure (beta coefficient = − 91.9 , P = 0.008), after adjustment for covariates (age, gender, birth weight, height and wheezing). Also FEV1 level in children was inversely correlated with prenatal exposure to PM2.5, and the average FEV1 deficit amounted to 87.7 ml (P = 0.008) at the higher level of exposure. Although the effect of PM2.5 exposure on FEV05 was proportionally weaker (−72.7, P = 0.026) it was significant as well. The lung function level was inversely and significantly associated with the wheezing recorded over the follow-up. The findings showed that significant lung function deficits in early childhood is associated with prenatal exposure to fine particulate matter, which may affect fetal lung growth.
Keywords: prenatal exposure, air pollution, birth cohort, lung function, preschool children
INTRODUCTION
Although over the last few decades there were many studies on children’s health related to air pollution, they were concerned mainly with morbidity from respiratory diseases in schoolchildren associated with postnatal ambient hazards. There have also been environmental epidemiologic studies investigating prenatal hazards on children’s respiratory health but they were limited to the effects of maternal smoking in pregnancy or postnatal environmental tobacco smoke (ETS).
To date, however, there is a shortage of data on the effect of prenatal ambient air pollution on respiratory health in early childhood, though in the last decade the effect of air pollution on adverse birth outcomes, including low-birth weight, premature births, and intrauterine growth retardation was confirmed by many publications.1–14 It is reasonable to assume that the prenatal exposure to ambient air hazards is not only associated with adverse birth outcomes but also may have repercussion in various fetal body organs and lead to their deficient function in postnatal life.
Development of the fetus proceeds in a sequence of carefully timed events that progress from the cellular level to the formation of tissues and morphologic structures such as lung. Prenatal hazards may permanently change these developmental processes. The issue is of great importance since impaired fetal development and its consequences in postnatal life is associated with a number of adult chronic diseases.15–17 It is believed that these associations arise as a result of the phenomenon of “epigenetic programming”, which involves persistent changes in structure and function of various body organs caused by environmental factors during critical and vulnerable periods of early development.
To our knowledge, up to now there have been no studies on lung function in early childhood and prenatal exposure to ambient fine particulates. The main goal of this study was to test the hypothesis that prenatal exposure to fine particulate matter, which represents a wide range of chemical compounds potentially hazardous for fetal development, may be associated with impaired lung function of children. In contrast to other air pollution studies, we assessed individual exposure to fine particulates (PM2.5) in pregnant women by specially designed personal samplers collecting air pollution particles over 48 hours in the second trimester of pregnancy. The cohort of children is being followed from birth through childhood. This analysis concerns those children whose expiratory lung volumes were assessed at 5 years of age by standard spirometry and quantified by FVC, FEV1 and FEV05 levels. Beside the main exposure variable (PM2.5), the statistical analysis considered a set of covariates including age in months, gender, birthweight, height of children and their wheezing experience as reported by mothers over the follow-up.
METHODS
Subjects
This study uses data from an earlier established birth cohort of children in Krakow which is the result of a collaboration between the Jagiellonian University in Krakow and Columbia University in New York. The design of the study and the detailed selection of the population have been described previously.18 In short, pregnant women were recruited from ambulatory prenatal clinics in their first or second trimesters of pregnancy. Only women 18–35 years of age, who claimed to be non-smokers, with singleton pregnancies, with no history of illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension, and who had resided in Krakow for at least one year prior to pregnancy were eligible for the study. Prior to participation, women read and signed an informed consent. The Ethical Committee of the Jagiellonian University approved the research.
Upon enrollment, a detailed questionnaire was administered to each woman to solicit information on demographic data, house characteristics, medical and reproductive history, occupational hazards, and smoking practices of others present at home. A total of 505 enrolled pregnant women gave birth between January 2001 and February 2004. After delivery, mothers of term babies (>36 weeks of gestation), three months in the first two years of the newborn’s life, and every 6 months later, were submitted to a detailed standardized face-to-face interview on their infant’s health and respiratory symptoms administered by a trained interviewer. The presence of wheezing was defined if the child reported two or more wheezing episodes over the follow-up. During the interviews, mothers were asked whether their children experienced wheezing or whistling in the chest, irrespective of respiratory infection, since the previous interview. The data collected over the course of the 14 follow-up time points was used to identify children with wheezing. Prenatal environmental tobacco smoke defined if the mother declared to be exposed to ETS in pregnancy period was validated by the cord blood cotinine levels. Postnatal ETS exposure was defined if the ETS exposure at home occurred in more than two years over the follow-up. The present analysis was based on data from one hundred seventy six 5-year-olds who were born after 36 weeks of gestation, completed the 5 year follow-up and performed two reliable and acceptable spirometry tests. The study sample in terms of important characteristics did not differ from the group of childern not considered in the present analysis (Table 1).
Table 1.
Characteristics of the study sample in comparison to the total group of children recruited to the study (gestation age > 36)
| Total study sample |
Children not attending |
Total group of children recruited |
P-value for difference between exposure groups |
||
|---|---|---|---|---|---|
| N=176 | N=305 | N=481 | |||
| Gender: Boys Girls |
n (%) n (%) |
87 (49.4) 89 (50.6) |
158 (51.8) 147 (48.2) |
245 (50.9) 236 (49.1) |
0.6844 |
| Gestational age (weeks): > 36 |
mean SD |
39.47 1.166 |
39.58 1.124 |
39.54 1.140 |
0.2894 |
| Birth weight (g): | mean SD |
3432.4 422.7 |
3452.3 444.2 |
3445.0 436.1 |
0.6317 |
| Length at birth (cm): | mean SD |
54.9 2.48 |
54.7 2.70 |
54.8 2.62 |
0.4080 |
| Prenatal PM2.5(µg/m3) > 52.6 | n (%) | 45 (25.6) | 77 (25.2) | 122 (25.4) | 1.0000 |
Dosimetry of cord blood cotinine
The serum cotinine concentration was measured at CDC using the sensitive isotope-dilution high-performance liquid chromatographic/atmospheric pressure ionization tandem spectrometric (LC/MS/MS) procedure The limit of detection (LOD) is below 0.050 ng/mL.19, 20
Dosimetry of prenatal personal exposure to fine particles
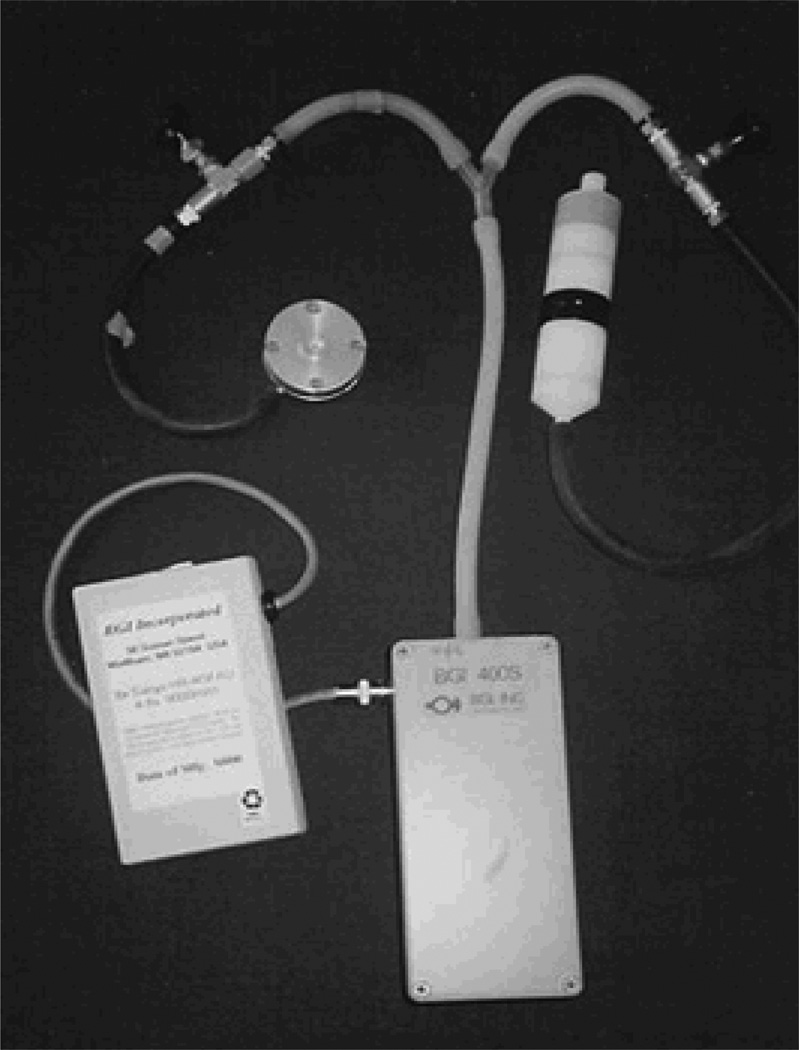
A Personal Environmental Monitoring Sampler (PEMS), designed by the Department of Environmental Health, School of Public Health at Harvard University, was used to measure mass of the particles with size of ≤ 2.5 µm (Figure 1). Flow rates were calibrated (with filters in place) using a bubble meter prior to the monitoring, and were checked again with a change of the battery pack on the second day and at the conclusion of the monitoring. Pumps operated continuously at 2 LPM over the 48-hour period. Particles were collected on Teflon membrane filter (37 mm Teflo™, Gelman Sciences). The combination of low pressure drop (permitting use of a low power sampling pump), low hygroscopicity (minimizing bound water interference in mass measurements), and low trace element background (improving analytical sensitivity) of these filters make them highly appropriate for personal particle sampling.
Figure 1.
Sampling instruments placed in the backpack
During the second trimester, a member of the air monitoring staff instructed the woman in the use of the personal monitor, which was lightweight, quiet and was worn in a backpack (Figure 2). The woman was asked to wear the monitor during the daytime hours for 2 consecutive days and to place the monitor near the bed at night. During the morning of the second day, the air monitoring staff-person and interviewer visited the woman's home to change the battery-pack and administer the full questionnaire. They also checked to see that the monitor has been running continuously and that there have been no technical or operating failures. A staff-member returned to the woman's home on the morning of the third day to pick up the equipment.
Figure 2.
One of the study participants wearing the backpack with personal monitoring sampler for collection of fine particulate matter
Spirometry testing
Children were free of respiratory symptoms on the day of testing and none of the children had any previous experience performing spirometry. Prior to spirometric testing standing height and weight of each child was measured and the children were coached to engage in maximal forced expiratory efforts in a standing position without nose clip. All spirometric measurements were carried out with a computerized PC QRS Card Spirometer with incentive display software by only one staff member (E.Mroz) who was highly experienced in spirometric testing of children. Each day, prior to the lung function examination, the spirometer was calibrated with a one-liter syringe. Each child made at least two good forced exhalation efforts and the primary indicators of lung function, i.e., FVC (Forced Vital Capacity), which is the total amount of air that can forcibly be blown out after full inspiration, FEV1 (Forced Expiratory Volume in 1 second), which is the amount of air that you can forcibly be blown out in 1 second and FEV0.5 (Forced Expiratory Volume in 0.5 second) that is the amount of air that can be blown out in 0.5 second were recorded. Maneuvers were excluded if a sub-maximal expiratory effort was present in which a peak expiratory flow (PEF) was not clearly determined, a slow rise of PEF was apparent, an expiration time was less than 0.5 seconds, cough or an abrupt end of expiration effort appeared in the course of exhalation effort. In accordance with the ATS/ERS guidelines on pulmonary function testing in preschool children,21 expiratory flows were reported from the attempt with the best flow (the greatest sum of FEV1 and FVC) executed by each subject and recorded the spirometric index corrected to BTPS (body temperature, pressure saturated). Spirometry findings were accepted as reliable if the absolute difference between FVCs and the absolute difference between FEV1 of the two best curves were within the range of 100 ml.
Statistical Analysis
The main purpose of the statistical analysis was to assess the relationship between lung function indices (outcome variables) and exposure to fine particulate matter over pregnancy (independent variable) after accounting for covariates (gender, age, height and wheezing). While in all statistical analyses lung function indices, age of children (in months) and height(cm) were treated as continuous variables, the wheezing was treated dummy variable and birthweight divided into ordinal scale by the quartiles of the distribution. As the distribution of PM2.5 was markedly skewed, the level of the exposure was divided into quartiles of the distribution. The preliminary analysis assessed associations between population characteristics and outcome variables in univariate statistical models, where Chi-square statistics (nominal variables), one way of analysis of variance (numerical variables), and nonparametric test for trend tested differences of outcome variables across subgroups with various air pollution level. In the multivariable regression analyses the associations between lung function and air pollution assessed by beta regression coefficients were adjusted for the covariates. All statistical analyses were carried out with STATA 11 version software for Windows. 22, 23
RESULTS
Median PM2.5 concentrations among the pregnant women enrolled in our study was 32.4 µg/m3 (interquartile range: 30.1). Basic characteristics of the children grouped by the level of prenatal exposure to fine particulates (highest quartile of exposure vs. the rest) only differed in terms of spirometric indices (Table 2). Children from the higher exposed group were more likely exposed to postnatal ETS. On average, those exposed had lower FVC by 63 ml (P = 0.059), FEV1 by 54 ml (P = 0.088) and FEV05 by 36 ml (P = 0.225).
Table 2.
Characteristics of children with acceptable spirometry grouped by the prenatal exposure to fine particulate matter (PM2.5)
| Total study sample |
Lower PM2.5 ≤52.6 µg/m3 |
Higher PM2.5 >52.6 µg/m3 |
P-value for difference between exposure groups |
||
|---|---|---|---|---|---|
| N=176 | N=131 | N=45 | |||
| Gender: Boys Girls |
n (%) n (%) |
87 (49.4) 89 (50.6) |
67 (51.1) 64 (48.9) |
21 (44.4) 29 (55.6) |
0.5466 |
| Gestational age (weeks): > 36 |
mean SD |
39.47 1.166 |
39.46 1.152 |
39.49 1.218 |
0.8787 |
| Birth weight (g): | mean SD |
3432.4 422.7 |
3460.8 432.8 |
3349.8 384.6 |
0.1288 |
| Length at birth (cm): | mean SD |
54.9 2.48 |
55.0 2.49 |
54.5 2.42 |
0.1943 |
| Age (in months) | mean SD |
60.7 0.92 |
60.7 0.97 |
60.7 0.75 |
0.9491 |
| Weight (kg) at age of 5 years (kg): |
mean SD |
19.83 2.885 |
19.87 2.840 |
19.73 3.041 |
0.7834 |
| Height (cm) at age of 5 years: | mean SD |
112.8 4.539 |
112.7 4.650 |
113.3 4.218 |
0.4439 |
| FVC (ml): | mean SD |
1124.8 194.1 |
1141.0 200.9 |
1077.7 165.9 |
0.0587 |
| FEV1 (ml): | mean SD |
1070.9 183.8 |
1084.7 187.9 |
1030.5 166.6 |
0.0878 |
| FEV0.5 (ml): | mean SD |
843.1 168.1 |
852.1 168.9 |
816.8 165.0 |
0.2250 |
| Wheezing | n (%) | 47 (26.7) | 34 (26.0) | 13 (28.9) | 0.8504 |
| ETS prenatal exposure | n (%) | 44 (25.0) | 30 (22.9) | 14 (31.1) | 0.3693 |
| ETS postnatal exposure | n (%) | 17 ( 9.7) | 7 (5.3) | 10 (22.2) | 0.0026 |
Pearson correlation coefficients between lung function indices, PM2.5 and potential confounders (birthweight, height of children) indicate that birthweight, but not height of children at the testing time was inversely associated with PM2.5 exposure (r = − 0.163, P = 0.029) (Table 3). The positive trend for unadjusted FVC, FV1 and FEV05 values across the birthweight (in quartiles) was statistically significant (Table 4). The inverse trend for unadjusted FVC, FV1 values across the levels of PM2.5 appeared to be statistically significant (Table 5).
Table 3.
Correlation coefficients between lung function indices, birthweight, height and intrauterine exposure to PM2.5 (in brackets significance level)
| Birthweight | Height | FVC | FEV1 | FEV05 | PM2.5 (log) | |
|---|---|---|---|---|---|---|
| Birthweight | 1.0000 | |||||
| Height | 0.2678 | 1.0000 | ||||
| (0.0003) | ||||||
| FVC | 0.2249 | 0.5475 | 1.0000 | |||
| (0.0024) | (0.0000) | |||||
| FEV1 | 0.1951 | 0.5657 | 0.9238 | 1.0000 | ||
| (0.0087) | (0.0000) | (0.0000) | ||||
| FEV05 | 0.1728 | 0.4779 | 0.6537 | 0.8344 | 1.0000 | |
| (0.0203) | (0.0000) | (0.0000) | (0.0000) | |||
| PM2.5 (log) | −0.1629 | −0.0195 | −0.1605 | −0.1405 | −0.1040 | 1.0000 |
| (0.0289) | (0.7945) | (0.0314) | (0.0600) | (0.1647) |
Table 4.
Unadjusted values of respiratory efforts (FVC, FEV1, FEV05) and the quartiles of birthweight
| FVC | n | mean | SD | P-level |
|---|---|---|---|---|
| Birthweight (quartiles) | ||||
| ≤ 3165 g | 43 | 1070.6 | 202.4 | |
| 3166 – 3425 g | 44 | 1098.0 | 170.4 | z for trend = 3.44 P = 0.0003 |
| 3426 – 3720 g | 44 | 1110.6 | 192.0 | |
| ≥ 3720 g | 45 | 1218.5 | 196.0 | |
| Total | 176 | 1124.8 | 194.1 | |
| FEV1 | ||||
| Birthweight (quartiles) | ||||
| ≤ 3165 g | 43 | 1025.4 | 189.8 | |
| 3166 – 3425 g | 44 | 1040.9 | 179.3 | z for trend = 3.15 P = 0.0005 |
| 3426 – 3720 g | 44 | 1054.5 | 176.8 | |
| ≥ 3720 g | 45 | 1156.9 | 175.9 | |
| Total | 176 | 1070.9 | 183.8 | |
| FEV05 | ||||
| Birthweight (quartiles) | ||||
| ≤ 3165 g | 43 | 861.0 | 158.1 | |
| 3166 – 3425 g | 44 | 858.7 | 179.2 | z for trend = 3.35 P = 0.001 |
| 3426 – 3720 g | 44 | 836.8 | 168.0 | |
| ≥ 3720 g | 45 | 816.8 | 157.0 | |
| Total | 176 | 843.1 | 168.1 | |
Table 5.
Unadjusted values of respiratory efforts (FVC, FEV1, FEV05) and the quartiles of prenatal PM2.5 level
| FVC | n | mean | SD | P-level |
|---|---|---|---|---|
| PM2.5 level (quartiles) | ||||
| < 20.95 µg/m3 | 43 | 1133.9 | 177.0 | |
| 20.95 – 32.42 µg/m3 | 44 | 1182.6 | 213.9 | z for trend = − 2.07 P = 0.039 |
| 32.43 – 52.6 µg/m3 | 44 | 1106.5 | 206.6 | |
| > 52.6 µg/m3 | 45 | 1077.7 | 165.9 | |
| Total | 176 | 1124.8 | 194.1 | |
| FEV1 | ||||
| PM2.5 level (quartiles) | ||||
| < 20.95 µg/m3 | 43 | 1084.0 | 172.5 | |
| 20.95 – 32.42 µg/m3 | 44 | 1108.2 | 189.1 | z for trend = − 2.07 P = 0.039 |
| 32.43 – 52.6 µg/m3 | 44 | 1062.0 | 202.1 | |
| > 52.6 µg/m3 | 45 | 1030.5 | 166.6 | |
| Total | 176 | 1070.9 | 183.8 | |
| FEV05 | ||||
| PM2.5 level (quartiles) | ||||
| < 20.95 µg/m3 | 43 | 861.0 | 165.5 | |
| 20.95 – 32.42 µg/m3 | 44 | 858.7 | 156.6 | z for trend = − 1.72 P = 0.086 |
| 32.43 – 52.6 µg/m3 | 44 | 836.8 | 186.0 | |
| > 52.6 µg/m3 | 45 | 816.8 | 165.0 | |
| Total | 176 | 843.1 | 168.1 | |
In the study sample there was 26.7% of wheezers who declared having two or more episodes of wheezing over the follow-up. There was an inverse trend of unadjusted lung function indices with number of wheezing episodes and the corresponding z values for nonparametric trend of FVC, FEV1 and FEV05 were: −2.66 (P = 0.008), −2.16 (P = 0.081) and −1.89 (P = 0.059).
Multivariable linear regression model (Table 6) showed the very significant deficit of FVC at the highest quartile of PM2.5 exposure (regression coefficient = − 91.9 , P = 0.008), which has been adjusted for covariates (age in months gender of child, birthweight, height, wheezing and prenatal/postnatal ETS). On average, girls had a slightly lower FVC (regression coefficient = − 49.6, P = 0.047) and wheezing was inversely associated with FVC values (beta coefficient = − 73.4, P = 0.011). FVC level was positively associated with height (beta coefficient = 18.9, P <0.0001) and age of children ((beta coefficient = 29.1, P= 0.038) . Positive effect of higher birthweight (highest quartile) was at border significance level (beta coefficient = 61.6, P = 0.094). Neither prenatal nor postnatal ETS were significantly associated with FVC level.
Table 6.
Prenatal PM2.5 exposure (in quartiles) and lung function (FVC) of 5-year-olds adjusted to potential confounders. Multivariable linear regression model
| Predictors | Coef. | P>t | [95% Conf. Interval] | |
|---|---|---|---|---|
| Age (in months) | 29.14 | 0.038 | [1.69, 56.59] | |
| Height (cm) | 18.89 | 0.000 | [13.09, 24.69] | |
| Gender of child (girls) | −49.61 | 0.047 | [−98.60, −0.61] | |
| Prenatal ETS | 22.81 | 0.576 | [−57.67, 103.28] | |
| Postnatal ETS | 43.40 | 0.443 | [−68.01, 154.82] | |
| Wheezing | −73.35 | 0.011 | [−129.59, −17.11] | |
| Birthweight g (quartiles) | ||||
| ≤ 3165 g | Reference | |||
| 3166 – 3425 g | 24.10 | 0.501 | [−46.45, 94.65] | |
| 3426 – 3720 g | 11.71 | 0.739 | [−57.52, 80.94] | |
| ≥ 3720 g | 61.60 | 0.094 | [−10.62, 133.8] | |
| Prenatal PM 2.5 level (quartiles) | ||||
| < 20.95 µg/m3 | Reference | |||
| 20.95 – 32.42 µg/m3 | −10.97 | 0.755 | [−80.37, 58.42] | |
| 32.43 – 52.6 µg/m3 | −42.04 | 0.234 | [−111.58, 27.49] | |
| > 52.6 µg/m3 | −91.92 | 0.008 | [−159.60, −24.24] | |
Adjusted FEV1 level in children was also inversely correlated with PM2.5 prenatal exposure and the average deficit amounted to 87.7 ml (P = 0.008) at the higher level of exposure (Table 7). The estimate of the effect of PM2.5 exposure on FEV05 was proportionally weaker (−72.7, P = 0.026) but significant as well (Table 8). The inverse association between FEV05 and wheezing experienced by children over the follow-up was at the border significance level.
Table 7.
Prenatal PM2.5 exposure (in quartiles) on lung function (FEV1) of 5-year-olds adjusted to potential confounders. Multivariable linear regression model
| Predictors | Coef. | P>t | [95% Conf. Interval] | |
|---|---|---|---|---|
| Age (in months) | 28.97 | 0.029 | [2.96, 54.99] | |
| Height (cm) | 18.44 | 0.000 | [12.94, 23.93] | |
| Gender of child (girls) | −35.42 | 0.134 | [−81.86, 11.01] | |
| Prenatal ETS | −17.15 | 0.657 | [−93.41, 59.12] | |
| Postnatal ETS | 47.50 | 0.375 | [−58.08, 153.09] | |
| Wheezing | −54.65 | 0.045 | [−107.95, −1.36] | |
| Birthweight g (quartiles) | ||||
| ≤ 3165 g | Reference | |||
| 3166 – 3425 g | 12.99 | 0.702 | [−53.87, 79.84] | |
| 3426 – 3720 g | −3.17 | 0.924 | [−68.78, 62.43] | |
| ≥ 3720 g | 54.28 | 0.119 | [−14.16, 122.71] | |
| Prenatal PM 2.5 level (quartiles) | ||||
| < 20.95 µg/m3 | Reference | |||
| 20.95 – 32.42 µg/m3 | −32.84 | 0.325 | [−98.61, 32.92] | |
| 32.43 – 52.6 µg/m3 | −39.79 | 0.235 | [−105.68, 26.10] | |
| > 52.6 µg/m3 | −87.71 | 0.008 | [−151.85, −23.57] | |
Table 8.
Prenatal PM2.5 exposure (in quartiles) and lung function (FEV0.5) of 5-year-olds adjusted to potential confounders. Multivariable linear regression model
| Predictors | Coef. | P>t | [95% Conf. Interval] | |
|---|---|---|---|---|
| Age (in months) | 2.13 | 0.871 | [−23.84, 28.10] | |
| Height (cm) | 14.25 | 0.000 | [8.76, 19.73] | |
| Gender of child (girls) |
−14.18 | 0.546 | [−60.54, 32.19] | |
| Prenatal ETS | −45.74 | 0.237 | [−121.88, 30.41] | |
| Postnatal ETS | 38.54 | 0.471 | [−66.88, 143.96] | |
| Wheezing | −46.63 | 0.085 | [−99.84, 6.58] | |
| Birthweight in g (quartiles) | ||||
| ≤ 3165 g | Reference | |||
| 3166 – 3425 g | 19.50 | 0.565 | [−47.26, 86.25] | |
| 3426 – 3720 g | 1.72 | 0.959 | [−63.79, 67.22] | |
| ≥ 3720 g | 52.64 | 0.130 | [−15.69, 120.97] | |
| Prenatal PM 2.5 level (quartiles) | ||||
| < 20.95 µg/m3 | Reference | |||
| 20.95 – 32.42 µg/m3 | −40.22 | 0.228 | [−105.88, 25.43] | |
| 32.43 – 52.6 µg/m3 | −42.48 | 0.204 | [−108.27, 23.31] | |
| > 52.6 µg/m3 | −72.66 | 0.026 | [−136.70, −8.62] | |
In order to estimate the individual effect of independent variables on the lung size of children the nested regression models has been used (table 9), where the variables (in blocks) were successively introduced in the regression procedures. While the birthweight explained 7.2% (P = 0.0003) of the FVC variability (measured by R square), adding height of children increased R2 by 21.1% (P < 0.00001); other added variables to a lesser degree increased R2 - age (3.1%, P = 0.015), wheezing (2.5%, P = 0.010), gender (2.2%, P =0.019) and PM2.5 (2.2%, P = 0.014).
Table 9.
Nested regression model to establish the effect of various independent variables (blocks) on FVC level in 5 year-olds
| Blocks | Beta standardized coefficients |
R2 | Change in R2 | F test of the change in the R2 |
|---|---|---|---|---|
| Birthweight (in quartiles) | 0.079 | 0.072 | 0.0003 | |
| Age (months) | 0.093 | 0.103 | 0.031 | 0.0149 |
| Height (cm) | 0.475 | 0.314 | 0.211 | 0.0000 |
| Gender of child | −0.137 | 0.335 | 0.022 | 0.0185 |
| Wheezing | −0.164 | 0.361 | 0.025 | 0.0103 |
| PM 2.5 (in quartiles) | −0.151 | 0.383 | 0.022 | 0.0143 |
DISCUSSION
To our knowledge, it is the first epidemiologic study to suggest that prenatal exposure to fine particulate matter may have a negative impact on the development of the fetal lung; and the effect of this exposure may be evidenced in 5-year-olds. The estimates of effect were adjusted for covariates such as birthweight, height, age in months, prenatal/postnatal ETS exposure and wheezing. As the study has been performed only in children whose mothers stated they were not active smokers in pregnancy, the results point to the possibility that prenatal PM2.5 exposure on lung size might not reflect maternal tobacco smoking. In addition, the findings show a negative impact of wheezing on lung function indices.
Fine particles are always present in particle-generating processes, especially combustion processes that generate many toxic agents and PM2.5 may be treated as a proxy measure of a whole complex of toxic agents present in the environment including PAHs (polycyclic aromatic hydrocarbons).24 The biological mechanisms whereby PM2.5 might cause adverse effects on birth outcomes and development of the fetal lung are yet unclear. First, the formation of PAH-DNA adducts may induce the activation of the apoptosis or the binding to receptors of placental growth factors, resulting in the decreased exchange of oxygen and nutrients.25 In this context we should refer to the findings published by Perera et al. who reported that higher PAH-DNA adducts levels measured in cord blood were inversely correlated with birthweight and other birth outcomes compared to infants with lower PAH-DNA adducts.26,27 Since children’s height, being the strongest determinants of the lung size, is associated with birthweight, which inversely correlates with prenatal PM2.5 exposure, then the main potential pathway through which the intrauterine exposure to pollutant may affect lung of children would be the fetal growth restriction resulting from this exposure. Second, other toxic components absorbed in the maternal circulation can cross the placenta and directly affect the fetus,28–30 producing cytotoxic reactive oxygen species that ultimately induce inflammatory and oxidant stress responses.31 Third, high exposure near to the end of gestation may cause disturbances of the pituitary-adrenocortico-placental system with possible anti-estrogenic effects which may also lead to a fetal toxicity.32
Our results documenting the inverse association between wheezing and lung function is in a good agreement with findings in the literature documenting that persistent wheezing or asthma, which begin in early life, are often associated with increased airway responsiveness and reduced infant lung function.33–41 The study is particularly consistent with the recent results of the NAC Manchester Asthma and Allergy Study Group in preschool children, which have shown that both transient and persistent wheezers have reduced lung function compared with nonwheezing children.39 Interestingly, the latter study group was also able to show that diminished lung function in high risk infants at one month of age preceded the subsequent occurrence of wheezing and other respiratory symptoms.41
A strength of our study is the design which enabled us to limit measurement error in estimating prenatal exposure to fine particles by assigning an individual personal exposure level to each child. The personal monitoring of ambient PM2.5 exposure is a highly relevant measure of individual exposure incorporating outdoor and indoor exposures. Good agreement between the personal PM2.5 measurements across all trimesters of pregnancy carried out in the subsample of 85 subjects provided evidence that the measurements of fine particles done in the second trimester was a reflection of exposure level over other pregnancy periods.42 The validation of the questionnaire data on prenatal ETS by the cord blood cotinine measurements has shown a significantly higher cord blood cotinine concentrations in children with confirmed prenatal ETS exposure than in those without it (7.68 vs. 0.86 ng/mL, P = 0.005). Moreover, there was a significant correlation between the average number of cigarettes smoked daily at home and cord blood cotinine level (r = 0.510, p <0.0001) . Previous studies have attempted to quantify the concentration of air pollutants measured in the residence area and assign these exposure values to the study subjects. Estimating individual average exposures during specific gestational months by relying on the ambient air-monitoring stations even close to the maternal residence may result in exposure misclassification. Furthermore, in our study important potential confounders of the relationship between prenatal ambient risk factors and the fetal development of infants, such as chronic diseases of mothers or maternal active tobacco smoking have been removed through entry criteria.
Another strong point of our study on prenatal PM2.5 and lung function in preschool children stems from the fact that we were able to show the effect of wheezing episodes, which in our study were carefully monitored over many regular time points in the course of face-to-face interviews with mothers of children. Other studies usually considered current wheeze (over the last 12 months) and very often used self-administered questionnaires. We believe that quarterly and semiannually collected data on wheeze offered us a good opportunity to explore the importance of early wheeze on lung function.
On the other side, we are aware of the limitations of our study, which are mainly related to relatively small sample size and the lack of repeated comparable measurements of PM2.5 in the postnatal period. It is obvious that the household level of fine particulate matter is the subject of variation over time, which depends to some extent on the number of cigarettes smoked daily at home. Since the mobility of the subjects under study was very moderate and mainly restricted to the same communal air pollution area, this gave us a confidence that the estimates of effects were not biased.
CONCLUSIONS
The findings link prenatal PM2.5 exposure to lung function deficits in childhood, which were not mediated by intrauterine tobacco smoke. Although the main potential pathway through which the intrauterine exposure to pollutant affects the development of lungs would be the fetal growth restriction, the prenatal PM2.5 exposure may lead to additional decrement of fetal lung growth through other pathways. The data presented should help clinicians better understand respiratory health problems in early childhood, and persuade policy makers to consider the effect of prenatal airborne PM2.5 exposure on lung function of young children while setting air pollution guidelines.
Acknowledgements
This is part of an ongoing comparative longitudinal investigation on the health impact of prenatal exposure to outdoor/indoor air pollution in infants and children being conducted in New York City and Krakow. The study received funding from an RO1 grant entitled, “Vulnerability of the Fetus/Infant to PAH, PM2.5 and ETS” (5 RO1 ES10165 NIEHS; 02/01/00 - 01/31/04) and from the NIEHS (RO1 ES010165-0451) the Lundin Foundation and the Gladys T. and Roland Harriman Foundation. Principal investigator: Prof. FP Perera, Co-investigator: Prof. WA Jedrychowski. The sudy was also partly supported by the grant from the International Center for Research in Biommedicine. Luxembourg
REFERENCES
- 1.Xu X, Ding H, Wang X. Acute effects of total suspended particles and sulfur dioxides on preterm delivery: a community-based cohort study. Archives of Environmental Health. 1995;50:407–415. doi: 10.1080/00039896.1995.9935976. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Ding H, Ryan L, Xu X. Association between air pollution and low birth weight: a community-based study. Environmental Health Perspectives. 1997;105:514–520. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perera F, Whyatt R, Jedrychowski W, Rauh V, Manchester D, Santella RM, et al. Recent developments in molecular epidemiology: A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. American Journal of Epidemiology. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- 4.Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH. Air pollution and infant mortality in Mexico City. Epidemiology. 1999;10:118–123. [PubMed] [Google Scholar]
- 5.Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11:502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environmental Health Perspectives. 2000;108:173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejmek J, Solanský I, Benes I, Lenícek J, Srám RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environmental Health Perspectives. 2000;108:1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha EH, Hong YC, Lee BE, Woo BH, Schwartz J, Christiani DC. Is air pollution a risk factor for low birth weight in Seoul? Epidemiology. 2001;12:643–648. doi: 10.1097/00001648-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Maisonet M, Correa A, Misra D, Jaakkola JJ. A review of the literature on the effects of ambient air pollution on fetal growth. Environmental Research. 2004;95:106–115. doi: 10.1016/j.envres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. 2004;15:36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- 11.Lacasaña M, Esplugues A, Ballester F. Exposure to ambient air pollution and prenatal and early childhood health effects. European Journal of Epidemiology. 2005;20:183–199. doi: 10.1007/s10654-004-3005-9. [DOI] [PubMed] [Google Scholar]
- 12.Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, et al. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environmental Health Perspectives. 2004;112:1398–1402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, et al. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environmental Health Perspectives. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh R, Rankin J, Pless-Mulloli T, Glinianaia S. Does the effect of air pollution on pregnancy outcomes differ by gender? A systematic review. Environmental Research. 2007;105:400–408. doi: 10.1016/j.envres.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Byrne CD, Phillips DI. Fetal origins of adult disease: epidemiology and mechanisms. Journal of Clinical Pathology. 2000;53:822–828. doi: 10.1136/jcp.53.11.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutrition. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 17.Barker DJ. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 18.Jedrychowski W, Whyatt RM, Camann DE, Bawle UV, Peki K, Spengler JD, et al. Effect of prenatal PAH exposure on birth outcomes and neurocognitive development in a cohort of newborns in Poland. Study design and preliminary ambient data. International Journal of Occupational Medicine and Environmental Health. 2003;16:21–29. [PubMed] [Google Scholar]
- 19.Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clinical Chemistry. 1997;43:2281–2291. [PubMed] [Google Scholar]
- 20.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. Journal of Analytical Toxicology. 2000;24:333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 21.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An Official American Thoracic Society/European Respiratory Society Statement: pulmonary function testing in preschool children. American Journal of Respiratory and Critical Care Medicine. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 22.STATA software for windows, Release 11. Texas: StaCorp; 2009. [Google Scholar]
- 23.Kohler U, Kreuter F. Data analysis using STATA. Texas: Stata Press Publication, College Station; 2005. [Google Scholar]
- 24.McDonald B, Ouyang M. In: Air cleaning –particles, in Indoor Air Quality Handbook. Spengler JD, Samet JM, McCarthy JF, editors. New York: McGraw-Hill; 2001. pp. 9.1–9.3. [Google Scholar]
- 25.Wood KA, Youle RJ. The role of free radicals and p53 in neuron apoptosis in vivo. Journal of Neuroscience. 1995;15:5851–5857. doi: 10.1523/JNEUROSCI.15-08-05851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in multiethnic population. Environmental Health Perspectives. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera FP, Rauh V, Whyatt RM, Tsai WY, Bernert JT, Tu YH, et al. Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environmental Health Perspectives. 2004;112:626–630. doi: 10.1289/ehp.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyda HJ. Metabolic effects of growth factors and polycyclic aromatic hydrocarbons on cultured human placental cells of early and late gestation. The Journal of Clinical Endocrinology and Metabolism. 1991;72:718–723. doi: 10.1210/jcem-72-3-718. [DOI] [PubMed] [Google Scholar]
- 29.Duvekot JJ, Cheriex EC, Pieters FA, Peeters LL. Severely impaired growth is preceded by maternal hemodynamic maladaptation in very early pregnancy. Acta Obstetrica and Gynaecologica Scandinavica. 1995;74:693–697. doi: 10.3109/00016349509021176. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Connor EE, Chegini N, Shiverick KT. Modulation by benzo[a]pyrene of epidermal growth factor receptors, cell proliferation, and secretion of human chorionic gonadotropin in human placental cell lines. Biochemical Pharmacology. 1995;50:1171–1180. doi: 10.1016/0006-2952(95)00253-v. [DOI] [PubMed] [Google Scholar]
- 31.Donaldson K, Stone V, Borm PJ, Jimenez LA, Glimour PS, Schins RP, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10) Free Radical Biology & Medicine. 2003;34:1369–1382. doi: 10.1016/s0891-5849(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 32.Bui QQ, Tran MB, West WL. A comparative study of the reproductive effects of methadone and benzo[a]pyrene in the pregnant and pseudopregnant rat. Toxicology. 1986;42:195–204. doi: 10.1016/0300-483x(86)90009-0. [DOI] [PubMed] [Google Scholar]
- 33.von Mutius E. Paediatric origins of adult lung disease. Thorax. 2001;56:153–157. doi: 10.1136/thorax.56.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. The New England Journal of Medicine. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 35.Tager IB, Hanrahan JP, Tosteson TD, Castile RG, Brown RW, Weiss ST, et al. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. The American Review of Respiratory Disease. 1993;147:811–817. doi: 10.1164/ajrccm/147.4.811. [DOI] [PubMed] [Google Scholar]
- 36.Strachan D, Gerritsen J. Long-term outcome of early childhood wheezing: population data. The European Respiratory Journal. Supplement. 1996;21:42s–47s. [PubMed] [Google Scholar]
- 37.Dezateux C, Stocks J, Wade AM, Dundas I, Fletcher ME. Airway function at one year: association with premorbid airway function, wheezing, and maternal smoking. Thorax. 2001;56:680–686. doi: 10.1136/thorax.56.9.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Young S, et al. Infants with flow limitation at 4 weeks: outcome at 6 and 11 years. American Journal of Respiratory and Critical Care Medicine. 2002;165:1294–1298. doi: 10.1164/rccm.200110-018OC. [DOI] [PubMed] [Google Scholar]
- 39.Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A. NAC Manchester Asthma and Allergy Study Group. Wheeze phenotypes and lung function in preschool children. American Journal of Respiratory and Critical Care Medicine. 2005;171:231–237. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 40.Borrego LM, Stocks J, Leiria-Pinto P, Peralta I, Romeira AM, Neuparth N, et al. Lung function and clinical risk factors for asthma in infants and young children with recurrent wheeze. Thorax. 2009;64:203–209. doi: 10.1136/thx.2008.099903. [DOI] [PubMed] [Google Scholar]
- 41.Murray CS, Pipis SD, McArdle EC, Lowe LA, Custovic A, Woodcock A. National Asthma Campaign-Manchester Asthma and Allergy Study Group. Lung function at one month of age as a risk factor for infant respiratory symptoms in a high risk group. Thorax. 2002;57:388–392. doi: 10.1136/thorax.57.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jedrychowski W, Perera F, Mrozek-Budzyn D, Mroz E, Flak E, Spengler JD, et al. Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter. Environmental Research. 2009;109:447–456. doi: 10.1016/j.envres.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]