Abstract
Primary suture repair of the anterior cruciate ligament (ACL) has been used clinically in an attempt to heal the ruptured ACL. The results, however, were not satisfactory, which in retrospect can be attributed to the used suturing technique and the suboptimal healing conditions. These constraining conditions can be improved by introducing a new suturing technique and by using small intestinal submucosa (SIS) as a bioscaffold. It is hypothesized that the suturing technique keep the torn ends together and that SIS enhance and promote the healing of the ACL. The goat was used as the study model. In the Suture group, the left ACL was transected and suture repaired with a new locking suture repair technique (n=5) allowing approximation and fixation under tension. The Suture-SIS group underwent the same procedure with the addition of SIS (n=5). The right ACL served as control. After 12 weeks of healing, anterior–posterior translation and in situ force of the healing ACL were measured, followed by the measurement of the cross-sectional area and structural stiffness. Routine histology was performed on tissue samples. Gross morphology showed that the healing ACL was continuous with collagenous tissue in both groups. The cross-sectional area of the Suture and the Suture-SIS group was 35% and 50% of the intact control, respectively. The anterior–posterior translations at different flexion angles were statistically not different between the Suture group and the Suture-SIS group. Only the in situ force at 30° in the Suture-SIS group was higher than in the Suture group. Tensile tests showed that the stiffness for the Suture group was not different from the Suture-SIS group (31.1±8.1 N/mm vs. 41.9±18.0 N/mm [p>0.05]). Histology showed longitudinally aligned collagen fibers from origo to insertion. More fibroblasts were present in the healing tissue than in the control intact tissue. The study demonstrated the proof of concept of ACL repair in a goat model with a new suture technique and SIS. The mechanical outcome is not worse than previously reported for ACL reconstruction. In conclusion, the approach of using a new suture technique, with or without a bioscaffold to heal the ACL is promising.
Introduction
The anterior cruciate ligament (ACL) plays an important role in stabilizing the knee joint. It has a complex collagen fiber architecture, which makes it well suited to guide the knee joint and prevent excessive translations and rotations during functional activities. However, during daily living and sports activities the forces on the ACL occasionally exceed their limit, leading to a rupture. Previously, the ACL ruptures were suture repaired like the medial collateral ligament (MCL) and Achilles' tendon ruptures. In contrast to the MCL and Achilles' tendon, which can heal relatively well without surgical intervention, the ACL seldom heals spontaneously. The reported success rates after suture repair of the ACL varied between 0% and 60%.1–3 Due to this inconsistent outcome, suture repair was abandoned in favor of ACL reconstruction with tendon grafts. In retrospect, these low success rates could be attributed to the previously used suture techniques.4
The current ACL reconstruction with tendon grafts is a rather successful procedure. However, there are drawbacks related to ACL reconstruction surgeries.5–7 Recent studies have also shown that 70%–80% of the patients develop radiographic signs of osteoarthritis in the ACL reconstructed knee after 10 to 15 years.8,9 Therefore, clinicians and researchers are aiming to improve the current treatment for ACL ruptures.10 This quest is likewise stimulated by the gained basic knowledge of the ACL and the recent advances in functional tissue engineering. Previous studies have found and hypothesized that many factors such as the local environment and biological characteristics of the ACL have a profound effect on ACL healing. The fluidal environment and retraction of the ACL ends, immediately after rupture, limit the chance for healing.11 Additionally, it can be imagined that by the movement of the ACL stumps during knee motion and the volume within the joint space lead to a low probability for direct or indirect contact to allow for contact healing between the torn ends. The fluidal environment also limits the chance for the formation of a hematoma because of the diluting effect of the synovial fluid.12 A stable hematoma would normally bridge the gap and provide a provisional scaffold for reparative cells at the injury site. Biologically, it is also found that the ACL is a hypocellular tissue and that the properties of fibroblasts are different from those derived from other ligaments that do heal.13 ACL fibroblasts have comparatively low mobility, low proliferation, and metabolic activities as well as a low potential for matrix production.14–16 All these constraining factors are contributing to the failure of spontaneous functional healing of the ACL.
This study is aimed at addressing two major constraining factors by using a new suture technique in combination with a bioscaffold. The first objective is to apply a new suture technique that has a higher tensile and pull-out strength then previously used suture techniques such as the grasping U-loops or multiple depth suture. It is hypothesized that the new suture technique approximate and keep the ACL torn ends together and thus lead to healing. The second objective is to evaluate the effect of a porcine-derived small intestinal submucosa (SIS) bioscaffold (Cook Biotech, Inc.) as an initiator and enhancer on the healing of the ACL. SIS is mainly composed of collagen type 1 and contains endogenous growth factors such as FGF and TGF-β and other chemoattractants.17–19 Several studies have shown that SIS can provide a collagenous matrix to promote cell migration into the healing site to enhance revascularization and repair.20–24 It is hypothesized that SIS enhance and promote the healing of the ACL. Instead of using the SIS as a load-bearing 10-layered bioscaffold, like in the rotator cuff repair, only a single layer of SIS was used to recruit sufficient healing cells to the hypocellular ACL. To test these hypotheses, we used 10 goats and evaluated the effects of the sutures and SIS application on the healing of the ACL in terms of its contribution to the knee laxity, ACL stiffness, in situ ACL forces, and histologic appearance.
Materials and Methods
Locking suture technique
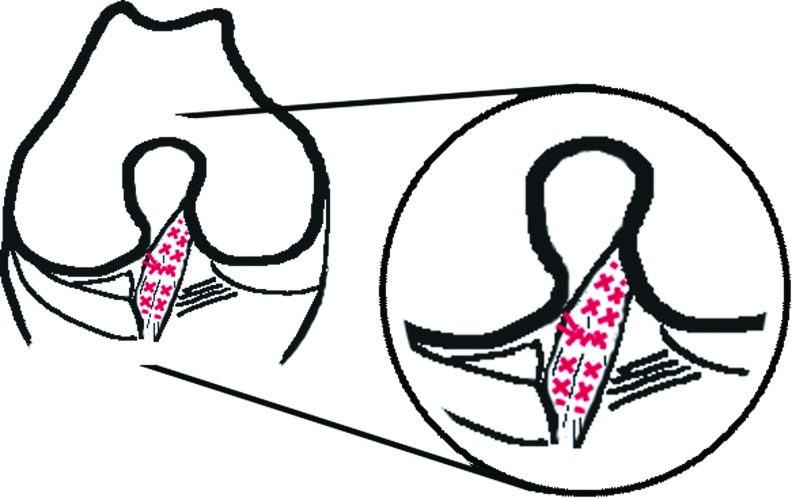
The suture technique used in this study is a modification based on the Becker locking suture. Due to its locking configuration, the suture tightens around the collagen fibers when tensile forces are applied, thus providing approximation under tension, greater ultimate tensile strength, and resistance to gapping.25,26 The suture configuration resembled three X's and for ease it was designated as triple X analogous to multiple U-loops (Fig. 1). Absorbable Vicryl 2-0 (Ethicon, Inc.) was used as suture material. The needle was modified to a smaller needle (±8 mm in length and 1/2 circle form) with round-nose pliers and a side cutter to accustom the narrow notch and the small ACL. To anatomically approximate the ACL's torn ends, we used eight sutures according to the triple X. The sutures were placed on the four corners of the distal part of the ACL stump: anteromedial, anterolateral, posteromedial, and posterolateral. The same was done for the proximal part of the ACL stump. Subsequently, the ACL stumps were approximated by knotting each suture to its opposite counterpart.
FIG. 1.
Sutured ACL according to the customized Becker-suture technique (triple X), red denotes suture threads. ACL, anterior cruciate ligament. Color images available online at www.liebertpub.com/tea
Animal model
Ten skeletally mature female Dutch milk goats (body weight: 78.0±9.5 kg) were used in this study. Surgery and maintenance of the animals followed the study protocol approved by the institutional Animal Ethics Committee at the University of Amsterdam.
All surgical procedures were performed by one surgeon (DTN) using sterile conditions under general endotracheal isoflurane anesthesia. In the left knee, a medial parapatellar incision was made to expose the ACL. The ACL was hooked and transected in its midsubstance with a No. 11 scalpel. The transection was made with three consecutive cuts, which resulted in a total transection with frayed edges, mimicking a clinical mop-end tear. Because of the viscoelastic nature of the tissue, the tissue will retract over time after cutting. This did not appear to affect the suturing in our goat model because the time was short. The transverse meniscal ligament was partially incised to expose the whole tibial ACL attachment. In the Suture repair group (N=5), the suture repair was performed using absorbable Vicryl 2-0 sutures, according to the technique described above. Subsequently, the medial patellofemoral ligament was repaired and the joint capsule, fascia, subcutaneous tissue, and skin were closed as separate layers. In the Suture-SIS group (N=4), the same procedures were performed as in the Suture group with the addition of SIS (Cook Biotech, Inc.). The material was used from the package as supplied. No additional treatment was required before use. Six small pieces of SIS (2 mm×2 mm×200 μm) were placed within the midsubstance of the injury site. These six pieces would be too small to act as a spacer. The rationale behind placing these small pieces of SIS was that these pieces would act as a wick wire to recruit cells to the injury site. A hydrated sheet of SIS (5 cm×2.5 cm×200 μm) was wrapped around the injury site and affixed with Vicryl 6-0 to the ACL. The ACL in the right hind limb was unoperated in both groups and served as an internal intact control.
Buprenex 0.01 mg/kg intramuscular once and a fentanyl patch transdermal were given for postoperative analgesia. Postoperatively, the animals were not immobilized and were allowed free cage activity, food and water ad libitum. After 4 weeks, the goats were transferred to a pasture. At 12 weeks postsurgery, all animals were euthanized with an overdose of sodium pentobarbital. Both hind limbs were disarticulated at the hip joint, wrapped in saline-soaked plaid, sealed in double plastic bags, and immediately stored at −20°C until testing.
Robotic testing
To examine the effects of suture repair of the ACL with the application of SIS on knee laxity and in situ forces, all knees were tested using a robotic universal force-moment sensor (UFS) testing system.27,28 The robotic manipulator (KR 125; KUKA Robots) is capable of achieving position control in six degrees of freedom (DOF) of motion. The UFS (FTI Theta 1500-240; Schunk) can measure three orthogonal forces and three orthogonal moments. The robotic/UFS testing system was used in a force-control mode via the force feedback from the UFS to the robot. For human and goat cadaveric knees, this system has been used successfully to apply external loads to the joint at preselected angles of knee flexion, while the kinematics of the remaining five DOF (medial–lateral, proximal–distal, anterior–posterior [AP] translations, internal–external and varus–valgus rotations) joint motions are determined by minimizing the constraint forces and moments measured by the UFS. By repeating these positions with a high level of accuracy after removing all soft tissue except the ACL, the in situ forces in the ACL can be determined.29,30 Before biomechanical testing, each specimen was thawed for 20 h at room temperature. The specimens were kept moist with 0.9% saline during dissection and biomechanical testing. The femur and tibia were cut at 15 cm from the joint line, the surrounding skin and muscles were removed leaving the capsule intact. Before potting the bones with polyurethane (VossChemie) within thick walled aluminum cylinders, nails were inserted into two predrilled holes to secure the potting. The femoral cylinder was rigidly fixed relative to the base of the robotic manipulator and the tibial cylinder was mounted to the end-actuator of the robot distal to the UFS. The center of the knee (defined as the midpoint between the femoral insertions of the MCL and LCL) was measured relative to the UFS coordinate system.
To identify the neutral flexion motion path, the joint was moved in 1° increments from full extension to 90° of flexion, while the positions with minimized forces and moments within the joint, were recorded. These positions served as the reference locations for the remainder of the protocol. The robotic-UFS testing system was then operated in a force control mode. An anterior and posterior tibial load of 67 N was applied to determine the anterior and posterior laxity at 30°, 60°, and 90°. The flexion angle of 30° corresponds to extension in the human knee. Subsequently, to determine the in situ force in the healing ACL, all soft tissue structures in and around the knee except the ACL were dissected. The articulating surfaces of the femoral condyles were also removed to prevent possible bone-to-bone contact. The recorded kinematics was repeated by the robotic manipulator and the UFS directly recorded the in situ force that was in the ACL since it is the only structure attaching the femur to the tibia.
Uniaxial tensile testing
The specimen was removed from the robotic/UFS testing system and was further prepared for uniaxial tensile testing to determine the stiffness of the ACL after healing. The cross-sectional area of the ACL was measured by means of a mechanical area micrometer.31,32 The protocol followed was similar to that of Lyon et al., whereby the joint was positioned in such a fashion that the ACL was along the direction of loading.33 For this, each specimen was mounted in custom-made clamps that enabled the orientation of the ACL to be adjusted and aligned. The specimen was tested on a uniaxial tensile testing machine (Zwick/Roell Z005; Zwick). After a preload of 2 N, the gauge length reference position of the Zwick's crosshead was reset to 0 mm. Each femur-ACL-tibia complex (FATC) underwent preconditioning by cyclical elongation between 0 and 1 mm for 10 cycles at 20 mm/min. Thereafter, the ACL was loaded to 5 N and elongated from 0 to 2 mm. The load–elongation curves of each complex were recorded and the linear stiffness was calculated from the linear section of the load–elongation curves. By loading the FATC before subfailure (from 0 to 2 mm), the ACL could be kept intact for histologic evaluation, while obtaining the stiffness values.
Histology
The whole ACL was removed from the FATC by cutting the whole ACL from its tibial and femoral bone insertion. Subsequently, the ACL was embedded in the O.C.T. compound (Sakura Finetek), frozen, and stored at −80°C. The tissue blocks were cut with a cryostat into sections of 5 μm. The sections were stained with Masson's trichrome. The sections were fixed in and stained sequentially in the Weigert's Iron Hematoxylin solution, Biebrich Scarlet-Acid Fucshin, and Aniline Blue solution according to the manufacturer's instructions (Sigma). The collagen was stained in blue, the cytoplasm in red, and the nuclei in black. The slides were observed under a Nikon light microscope and pictures were taken with a low magnification (10×) to show the whole ACL and with a higher magnification (200×) obtained from the midsubstance to show the healing areas.
Statistical tests
A paired or unpaired Student's t-test was used for statistical analysis, where appropiate. A probability of p<0.05 was considered statistically significant.
Results
Gross observations
One suture repair in the Suture-SIS group failed peroperatively due to knot slippage, while testing the suture repair by passive flexion and extension of the knee. This specimen was excluded from follow-up and analysis (N=4). The approved protocol did not allow the replacement of this goat. All animals were weight bearing on both hind limbs within a few hours after surgery. Within an hour, recovering goats were challenged for new herd hierarchy through head butting, while standing on their hind limbs. This was concerning since head butting and standing on their hind limb may put unnecessary load to the suture repair. Normal food intake was resumed within 24 h. Normal gait, as determined by visual inspection, was observed in all animals within 2 weeks after surgery.
Robotic testing
The anterior–posterior translation of the tibia with 67 N anterior and posterior force at 30°, 60°, and 90° was not different between the Suture and Suture-SIS groups, (p=0.92, p=0.24, p=0.14), but considerably higher than in the control knees (Table 1).
Table 1.
Anterior–Posterior Translation of the Joint and In Situ Forces in Response to 67 N Anterior–Posterior Load at 30°, 60°, and 90° Flexion, (Mean±SD)
| |
Anterior–posterior tibial translation (mm) |
||
|---|---|---|---|
| 30° | 60° | 90° | |
| I. Suture | 12.4±3.2 | 11.3±1.7 | 6.4±1.3 |
| II. Suture-SIS | 12.2±3.9 | 13.3±2.9 | 8.2±1.8 |
| III. Control | 2.8±0.7a | 3.2±0.8a | 2.2±0.8a |
| |
In situ forces (N) |
||
|---|---|---|---|
| 30° | 60° | 90° | |
| I. Suture | 21.5±17.5 | 4.1±1.6 | 5.3±0.6 |
| II. Suture-SIS | 45.9±11.3b | 11.3±6.9 | 4.8±0.7 |
| III. Control | 61.2±4.4a | 54.9±6.5a | 46.2±10.1a |
For all parameters, the differences between Suture and control and between Suture-SIS and control were statistically significant (paired t-test, p<0.05).
Statistical difference between Suture and Suture & SIS (unpaired t-test, p<0.05).
SIS, small intestinal submucosa.
The in situ forces of the healing ACLs in response to a 67 N anterior tibial load was not different between the Suture and Suture-SIS groups at 60° and 90° flexion, (p=0.06, p=0.26) and lower than control (Table 1). The in situ force at 30° in the Suture-SIS group was about double the ACL force in the Suture group and neared the forces of the control ACL (Table 1), p<0.05).
Visual inspection
The menisci and cartilage showed no evidence of tears or degeneration. The Vicryl 2-0 sutures were fully resorbed and gross inspection revealed healing tissue formation in all knees. In the Suture group, the healing ACL was a continuous band of collagenous tissue with its attachment to the tibia and femur (Fig. 2B). In the Suture-SIS group, the healing ACL was more opaque and had more visible fiber bundles (Fig. 2C).
FIG. 2.
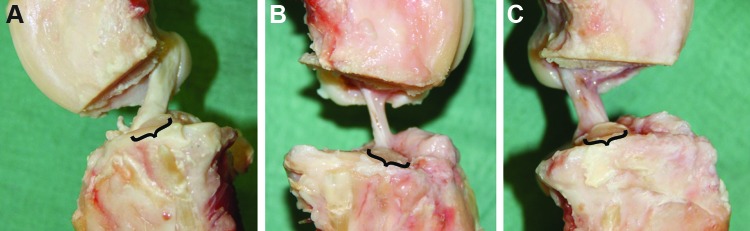
(A) Medial side view of a normal ACL. (B) A healing ACL after suture repair only. (C) A healing ACL after suture repair and SIS. Brackets indicate area of normal insertion site. It can be seen that the treated ACLs do not cover the whole area of normal insertion site. SIS, small intestinal submucosa. Color images available online at www.liebertpub.com/tea
Another interesting observation was that the geometry of the tibial insertion of the healing ACLs in both experimental groups was different from that of the normal ACL. The brackets in Figure 2 indicate the area of the normal tibial ACL insertion site, which spans over the eminentia intercondylaris. In the healing ACLs, it was observed that a part of the anteromedial fiber bundles was missing.
The cross-sectional areas of the healing ACLs were about half to one third of the control ACLs (Table 2). This partly accounted for the lower structural stiffness of the healing ACLs. The ratio of structural stiffnesses over the cross-sectional area of the healing ACLs was 38% and 51% of that of the control ACLs for the Sutured and Sutured-SIS, respectively (Table 2). There were no statistically significant differences between the Suture and Suture-SIS groups as regard to the cross-sectional area, stiffness, and the ratio stiffness over the cross-sectional area (Table 2).
Table 2.
Cross-Sectional Area, Stiffness, and Stiffness/Cross-Sectional Area of the Specimens
| I. Suture | II. Suture-SIS | III. Control | |
|---|---|---|---|
| Cross-sectional area (mm2) | 7.6±4.0 | 10.8±7.4 | 21.7±6.1 |
| Stiffness (N/mm) | 31.1±8.1 | 41.9±18.0 | 179.5±32.7 |
| Stiffness/cross-sectional area (N/mm3) | 3.6±0.5 | 4.9±2.1 | 9.6±4.7 |
Histology
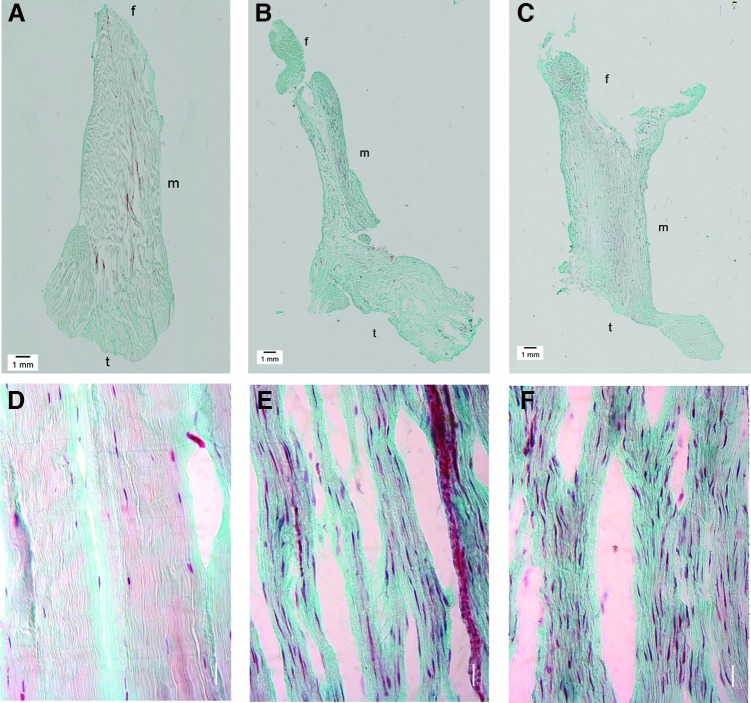
The Masson's trichrome staining showed that the collagen fibers (stained in blue) of the normal ACLs, were compact, regularly aligned, and had a clear crimp pattern (Fig. 3A, D). The fibroblasts (stained in red) were ovoid/spindle-shaped and aligned along the collagen fibers (Fig. 3D). The overview pictures from the ACLs in the Suture group and the Suture-SIS group showed that the ACLs healed or were at least in a process of healing and were continuous from the distal part to the proximal part (Fig. 3B, C). The original transection site could no longer be recognized and the tissue had remodeled over the entire length of the ligament. The tissue was hypercellular (Fig. 3E, F). The healing ACLs from the sutured group had aligned collagen fibers, but were less compact and had no discernible crimp pattern (Fig. 3B), that was also not found with polarized light (not shown in the figure). The fibroblasts appeared long and spindle shaped (Fig. 3E). With SIS, the collagen fibers in the healing ACLs were also aligned and oriented in the longitudinal direction and the fiber arrangement appeared to be more compact (Fig. 3C). The fibroblasts were also spindle shaped and oriented along the collagen fibers (Fig. 3F). Interestingly, when comparing the morphology of the fibroblast, it can be observed that the cells in the Suture- and Suture-SIS samples are more elongated, while those in the control sample are more ovoid shaped. In addition, there were also more fibroblasts in the healing ACLs than in the control ACLs.
FIG. 3.
Histologic sections of a normal ACL (A, D), sutured ACL (B, E), and sutured ACL with SIS (C, F). The sections are stained with Masson's trichrome; the collagen fibers are stained in blue, the fibroblasts are stained in red, and the nuclei in black; m denotes midsubstance, f denotes femoral insertion site, and t denotes tibial insertion site. The transection site could not be recognized and the tissue was remodeled over the entire length of the ACL. Note that the tissue is hypercellular in both the sutured and Suture-SIS ACL and that the fibroblasts are more spindle shaped than the intact ACL. Color images available online at www.liebertpub.com/tea
Discussion
The present study demonstrated that suturing the transected ACL with the new suture repair technique and an absorbable suture resulted in healing of the ACL in all goats. The reason for the failure in one goat was purely technical. If the suturing is successful at the time of surgery, the tissue would heal. The ACL was continuous with collagenous healing tissue with a cross-sectional area that was 35% of the intact control. In terms of knee stability, the AP translation was 4.4 times higher and the stiffness was 17% compared to the intact control. In the Suture-SIS group, the ACL was also continuous with collagenous healing tissue with a cross-sectional area that was 50% of the intact control. The AP translation was not different from the Suture group and the Suture-SIS group. The stiffness was 23% compared to the intact control. When normalizing the stiffness to its cross-sectional area, thereby taking the amount of tissue into account, the normalized stiffness of the healing ACLs was about half of the control ACLs. Histologic evaluation confirmed that the healing tissue in the ACLs was indeed collagenous and consisted of continuous and aligned collagen fibers. It can be concluded that the new suture technique was able to provide contact between the ligament ends for healing. Contact is a requirement for healing of the intra-articular situated ACL. This conclusion is also further supported by several animal studies that have shown that transected ACLs do not heal, but are rather resorbed.12,34 Previously, Zantop et al., have shown in the Achilles tendon model that the SIS is repopulated by bone marrow-derived cells for the regeneration process. Adding SIS to the suture repair could have recruited more reparative cells to the ACL injury site by its chemoattractant properties, leading to more mature collagen fibers and an in situ force that is closer to the intact ACL at 30°. However, this is not sufficient to claim a positive effect of the SIS on the mechanical outcome of the repair. That the in situ force was not closer to the intact ACL at 60 and 90 degrees flexion might be due to the fact that a part of the anteromedial (AM) bundles of both experimental groups was missing and therefore could not be recruited. This absence was reflected in the smaller cross-sectional area and could in turn also explain the lower stiffness and higher values for the anterior tibial translation (ATT). A recent study by Tischer et al., showed that transection of the AM bundle of an intact goat ACL lead to an 88% increase in the ATT.35 A possible explanation for the absence of the AM bundles might be the high forces during the postoperative rehabilitation period. The goats were not immobilized and, unexpectedly, the goats were head butting within hours after the operation. In the following days and weeks, they resumed their normal gait, which also included hyperextension and hyperflexion when lying down. These movements could have led to repetitive and excessive forces on the sutures and could have led to suboptimal healing during the initial healing phase. If overloading actually occurs and the ACL-suture construct is too weak, then the goats should be rehabilitated such that excessive loads on the ACL can be prevented, especially during the initial healing phase, by for example, (short term) casting with a limited range of flexion and extension.
Compared to recent research that focused on suture repair of the ACL in combination with platelet-rich plasma delivery and scaffolds,36–39 the results in the current study are not far off in terms of the functional outcomes. The total AP translation of the repaired ACL was 290% to 440% of the intact control under 67N anterior and posterior tibial load (AP load). Fisher et al. found a 302% increase with the same AP load, and Fleming, Murray et al., found a 350% increase in ATT with a 35 N anterior tibial load.40,41 In terms of stiffnesses of the healing ACL, these were, respectively, 23% in our study, 42% for Fisher et al., and 25% for Fleming, Murray et al. Besides similarities, there are also differences between these studies. For example, the other studies used a mechanical augmentation, where the current study was relying only on the locking suture technique with absorbable sutures.
The laxity and stiffness data are comparable to those for an ACL reconstruction with a bone–patellar tendon–bone graft in goats in terms of the AP laxity and stiffness at 6 weeks postsurgery. The result of ACL healing for the AP laxity was 13.3±2.9 mm, where Abramowitch et al. reported 17.2±3.5 mm, and Spindler et al. reported 19.0±4.5 mm. The stiffness of the healing ACL was 41.9±18 N/mm, which compared well with the reported 41.3±26.1 and 22±14 N/mm of Abramowitch et al. and Spindler et al., respectively.29,42 These ACL reconstruction studies evaluated the outcome at 6 weeks instead of 12 weeks. However, Zantop et al. showed that the outcome of the ACL reconstruction in goats at 12 weeks was not significantly different from 6 weeks.43 This supports the conclusion that the outcome with respect to AP laxity and ligament stiffness is not worse or better than the ACL reconstruction in the goat.
The study is of an exploratory nature, demonstrating the proof of concept of ACL repair in a goat model. No failure testing was performed because the study was aimed at the functional outcome, in terms of stiffness and laxity; thus, not aimed at the strength of the construct. With this, the repaired ACLs could be preserved for histologic evaluation. The power of the study was low to draw definitive conclusions on the beneficial effects of the SIS scaffold. Still, the finding that the ACL has a healing potential and that the mechanical outcome is not worse than the previously reported outcomes of ACL reconstruction in the goat by using a tendon graft is exciting. This study needs to be followed up by more extensive animal studies, addressing the questions that are raised and by the development of arthroscopic tools, which are required for suturing the torn ACL in humans. Furthermore, an evaluation should be conducted of the type of ACL ruptures and the characteristics to decide which ruptures can be considered as good candidates for primary repair. In addition, the timing of the surgery and the postoperative rehabilitation protocol are to be determined. Hence, there are many challenges ahead on the road to primary repair as the alternative to reconstruction with a tendon. This study is one of the first steps on that road.
In conclusion, the positive results in the present study suggest that the approach of using the new suture technique, with or without a bioscaffold to heal the ACL is promising.
Acknowledgment
The authors are grateful for the technical assistance of Maarten van den Berg, Mahyar Foumani, Mikel Reilingh, and Sietske Dellbrügge. The study was sponsored by Mozaïek PhD-training grant 017.004.076 from The Netherlands Organization for Scientific Research (NWO).
Disclosure Statement
The SIS material was provided by Cook Biotech, Inc., West Lafayette, IN, under a Material Use Agreement, without any monetary compensation and without any condition.
None of the authors had any relation with any other third party, commercial or otherwise, that has an interest in the study or could have influenced the study.
References
- 1.Sherman M.F. Lieber L. Bonamo J.R. Podesta L. Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19:243. doi: 10.1177/036354659101900307. [DOI] [PubMed] [Google Scholar]
- 2.Feagin J.A., Jr. Curl W.W. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan N. Wickiewicz T.L. Warren R.F. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18:354. doi: 10.1177/036354659001800404. [DOI] [PubMed] [Google Scholar]
- 4.Radford W.J. Amis A.A. Heatley F.W. Immediate strength after suture of a torn anterior cruciate ligament. J Bone Joint Surg Br. 1994;76:480. [PubMed] [Google Scholar]
- 5.Kartus J., et al. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2–5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:2. doi: 10.1007/s001670050112. [DOI] [PubMed] [Google Scholar]
- 6.Meyers A.B. Haims A.H. Menn K. Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194:476. doi: 10.2214/AJR.09.3200. [DOI] [PubMed] [Google Scholar]
- 7.Busam M.L. Provencher M.T. Bach B.R., Jr Complications of anterior cruciate ligament reconstruction with bone-patellar tendon-bone constructs: care and prevention. Am J Sports Med. 2008;36:379. doi: 10.1177/0363546507313498. [DOI] [PubMed] [Google Scholar]
- 8.von Porat A. Roos E.M. Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinczewski L.A., et al. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35:564. doi: 10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 10.Beynnon B.D. Johnson R.J. Abate J.A. Fleming B.C. Nichols C.E. Treatment of anterior cruciate ligament injuries, part 2. Am J Sports Med. 2005;33:1751. doi: 10.1177/0363546505279922. [DOI] [PubMed] [Google Scholar]
- 11.Woo S.L. Abramowitch S.D. Kilger R. Liang R. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech. 2006;39:1. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Murray M.M. Martin S.D. Martin T.L. Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A:1387. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hsu S.L. Liang R. Woo S.L. Functional tissue engineering of ligament healing. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:12. doi: 10.1186/1758-2555-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagineni C.N. Amiel D. Green M.H. Berchuck M. Akeson W.H. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10:465. doi: 10.1002/jor.1100100402. [DOI] [PubMed] [Google Scholar]
- 15.McKean J.M. Hsieh A.H. Sung K.L. Epidermal growth factor differentially affects integrin-mediated adhesion and proliferation of ACL and MCL fibroblasts. Biorheology. 2004;41:139. [PubMed] [Google Scholar]
- 16.Ge Z. Goh J.C. Lee E.H. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573. doi: 10.3727/000000005783982819. [DOI] [PubMed] [Google Scholar]
- 17.Hodde J.P. Ernst D.M. Hiles M.C. An investigation of the long-term bioactivity of endogenous growth factor in OASIS Wound Matrix. J Wound Care. 2005;14:23. doi: 10.12968/jowc.2005.14.1.26721. [DOI] [PubMed] [Google Scholar]
- 18.Voytik-Harbin S.L. Brightman A.O. Kraine M.R. Waisner B. Badylak S.F. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478. [PubMed] [Google Scholar]
- 19.Liang R. Fisher M. Yang G. Hall C. Woo S.L. Alpha1,3-galactosyltransferase knockout does not alter the properties of porcine extracellular matrix bioscaffolds. Acta Biomater. 2011;7:1719. doi: 10.1016/j.actbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Zantop T. Gilbert T.W. Yoder M.C. Badylak S.F. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J Orthop Res. 2006;24:1299. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 21.Liang R., et al. Long-term effects of porcine small intestine submucosa on the healing of medial collateral ligament: a functional tissue engineering study. J Orthop Res. 2006;24:811. doi: 10.1002/jor.20080. [DOI] [PubMed] [Google Scholar]
- 22.Liang R. Woo S.L. Nguyen T.D. Liu P.C. Almarza A. Effects of a bioscaffold on collagen fibrillogenesis in healing medial collateral ligament in rabbits. J Orthop Res. 2008;26:1098. doi: 10.1002/jor.20616. [DOI] [PubMed] [Google Scholar]
- 23.Raeder R.H. Badylak S.F. Sheehan C. Kallakury B. Metzger D.W. Natural anti-galactose alpha1,3 galactose antibodies delay, but do not prevent the acceptance of extracellular matrix xenografts. Transplant Immunol. 2002;10:15. doi: 10.1016/s0966-3274(01)00044-2. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert T.W. Stewart-Akers A.M. Simmons-Byrd A. Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 25.Hatanaka H. Zhang J. Manske P.R. An in vivo study of locking and grasping techniques using a passive mobilization protocol in experimental animals. J Hand Surg Am. 2000;25:260. doi: 10.1053/jhsu.2000.jhsu25a0260. [DOI] [PubMed] [Google Scholar]
- 26.Becker H. Davidoff M. Eliminating the gap in flexor tendon surgery. A new method of suture. Hand. 1977;9:306. doi: 10.1016/s0072-968x(77)80122-8. [DOI] [PubMed] [Google Scholar]
- 27.Woo S.L. Debski R.E. Wong E.K. Yagi M. Tarinelli D. Use of robotic technology for diathrodial joint research. J Sci Med Sport. 1999;2:283. doi: 10.1016/s1440-2440(99)80002-4. [DOI] [PubMed] [Google Scholar]
- 28.Fujie H. Livesay G.A. Woo S.L. Kashiwaguchi S. Blomstrom G. The use of a universal force-moment sensor to determine in-situ forces in ligaments: a new methodology. J Biomech Eng. 1995;117:1. doi: 10.1115/1.2792266. [DOI] [PubMed] [Google Scholar]
- 29.Abramowitch S.D. Papageorgiou C.D. Withrow J.D. Gilbert T.W. Woo S.L. The effect of initial graft tension on the biomechanical properties of a healing ACL replacement graft: a study in goats. J Orthop Res. 2003;21:708. doi: 10.1016/S0736-0266(02)00265-6. [DOI] [PubMed] [Google Scholar]
- 30.Sakane M., et al. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15:285. doi: 10.1002/jor.1100150219. [DOI] [PubMed] [Google Scholar]
- 31.Ellis D.G. Cross-sectional area measurements for tendon specimens: a comparison of several methods. J Biomech. 1969;2:175. doi: 10.1016/0021-9290(69)90029-3. [DOI] [PubMed] [Google Scholar]
- 32.Noyes F.R. Grood E.S. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am. 1976;58:1074. [PubMed] [Google Scholar]
- 33.Lyon R.M. Woo S.L. Hollis J.M. Marcin J.P. Lee E.B. A new device to measure the structural properties of the femur-anterior cruciate ligament-tibia complex. J Biomech Eng. 1989;111:350. doi: 10.1115/1.3168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter M., et al. Acutely repaired proximal anterior cruciate ligament ruptures in sheep—by augmentation improved stability and reduction of cartilage damage. J Mater Sci Mater Med. 1997;8:855. doi: 10.1023/a:1018597604034. [DOI] [PubMed] [Google Scholar]
- 35.Tischer T., et al. Biomechanics of the goat three bundle anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2009;17:935. doi: 10.1007/s00167-009-0784-2. [DOI] [PubMed] [Google Scholar]
- 36.Fleming B.C. Spindler K.P. Palmer M.P. Magarian E.M. Murray M.M. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vavken P. Murray M.M. Translational Studies in ACL repair. Tissue Eng Part B Rev. 2010;16:5. doi: 10.1089/ten.teb.2009.0147. [DOI] [PubMed] [Google Scholar]
- 38.Murray M.M., et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 39.Murray M.M., et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 40.Fleming B.C. Magarian E.M. Harrison S.L. Paller D.J. Murray M.M. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28:703. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher M.B., et al. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc. 2011;20:1357. doi: 10.1007/s00167-011-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spindler K.P. Murray M.M. Carey J.L. Zurakowski D. Fleming B.C. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27:631. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zantop T., et al. Effect of tunnel-graft length on the biomechanics of anterior cruciate ligament-reconstructed knees: intra-articular study in a goat model. Am J Sports Med. 2008;36:2158. doi: 10.1177/0363546508320572. [DOI] [PubMed] [Google Scholar]