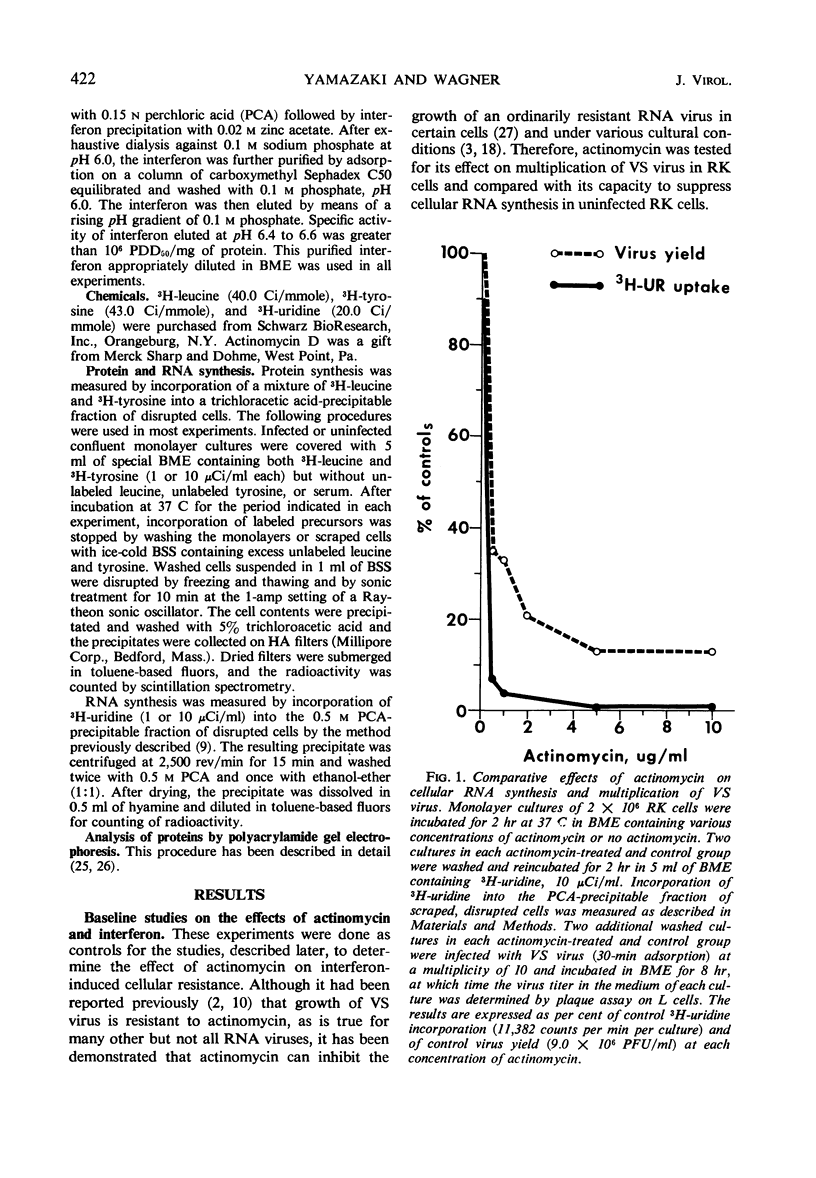
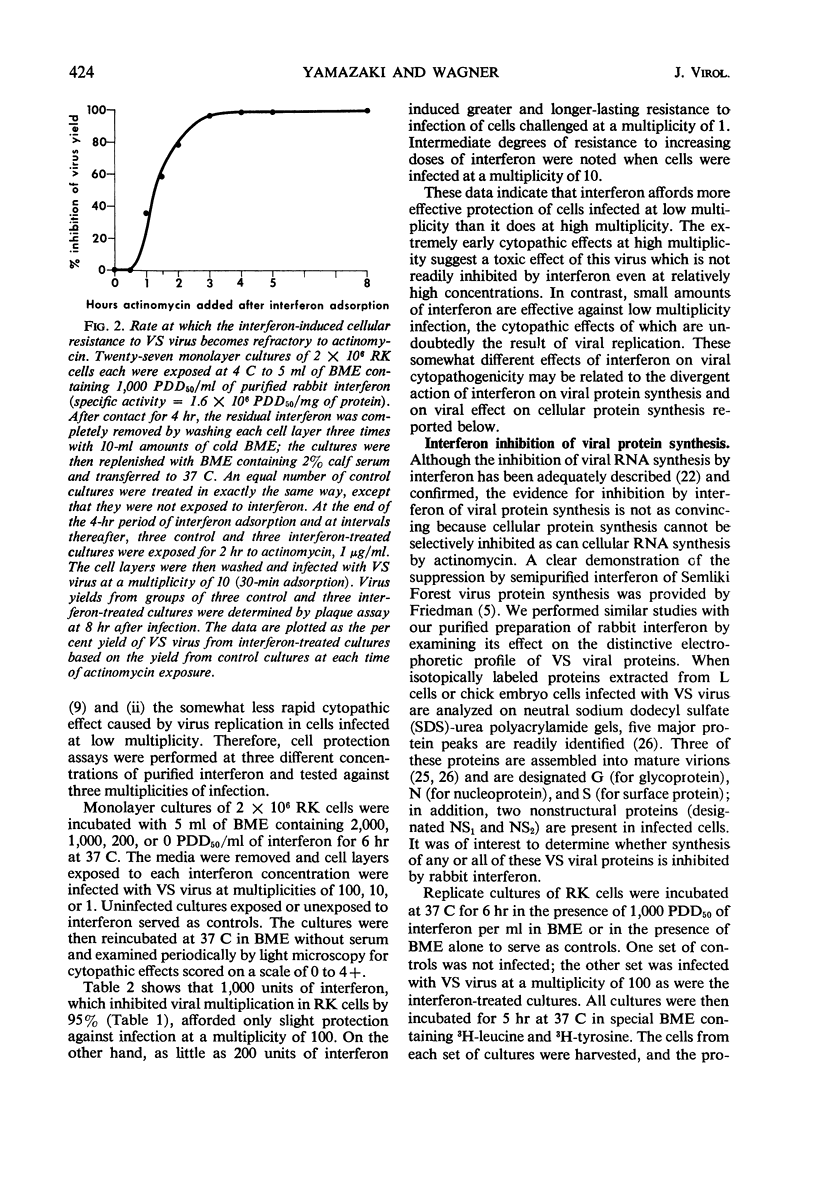
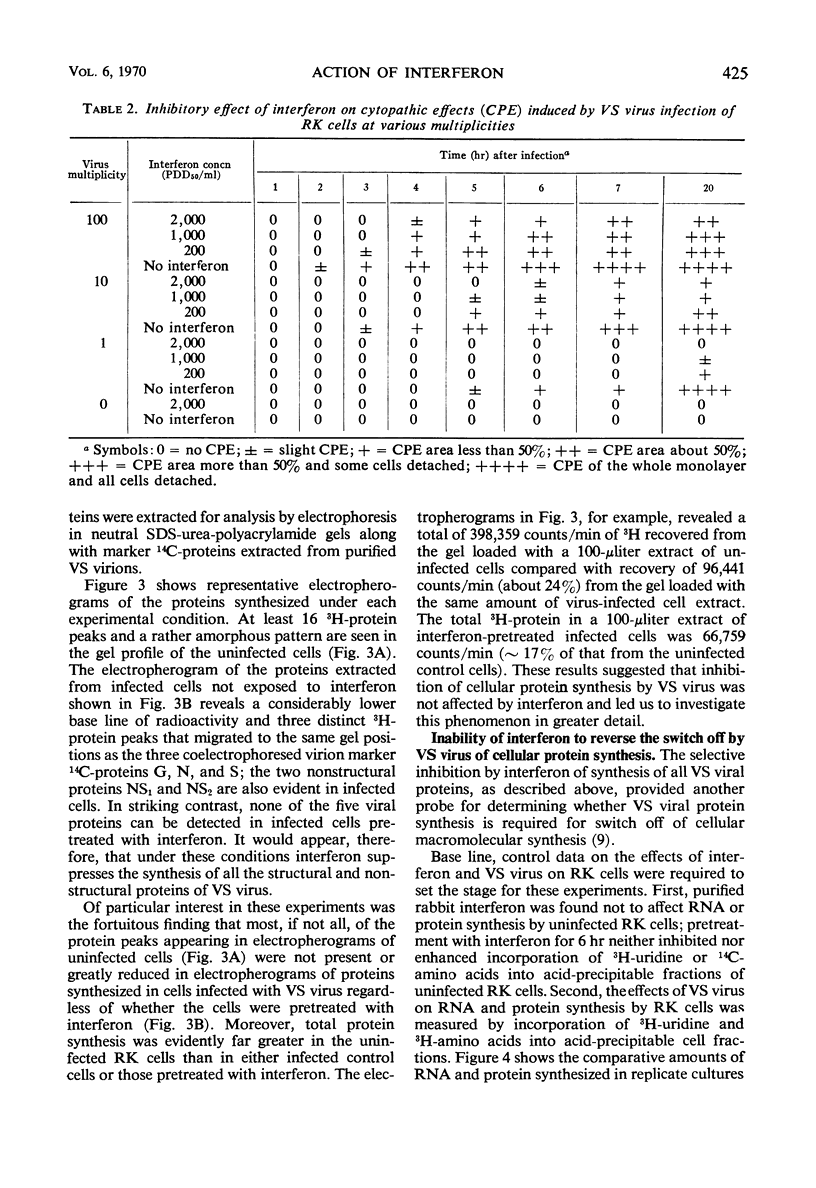
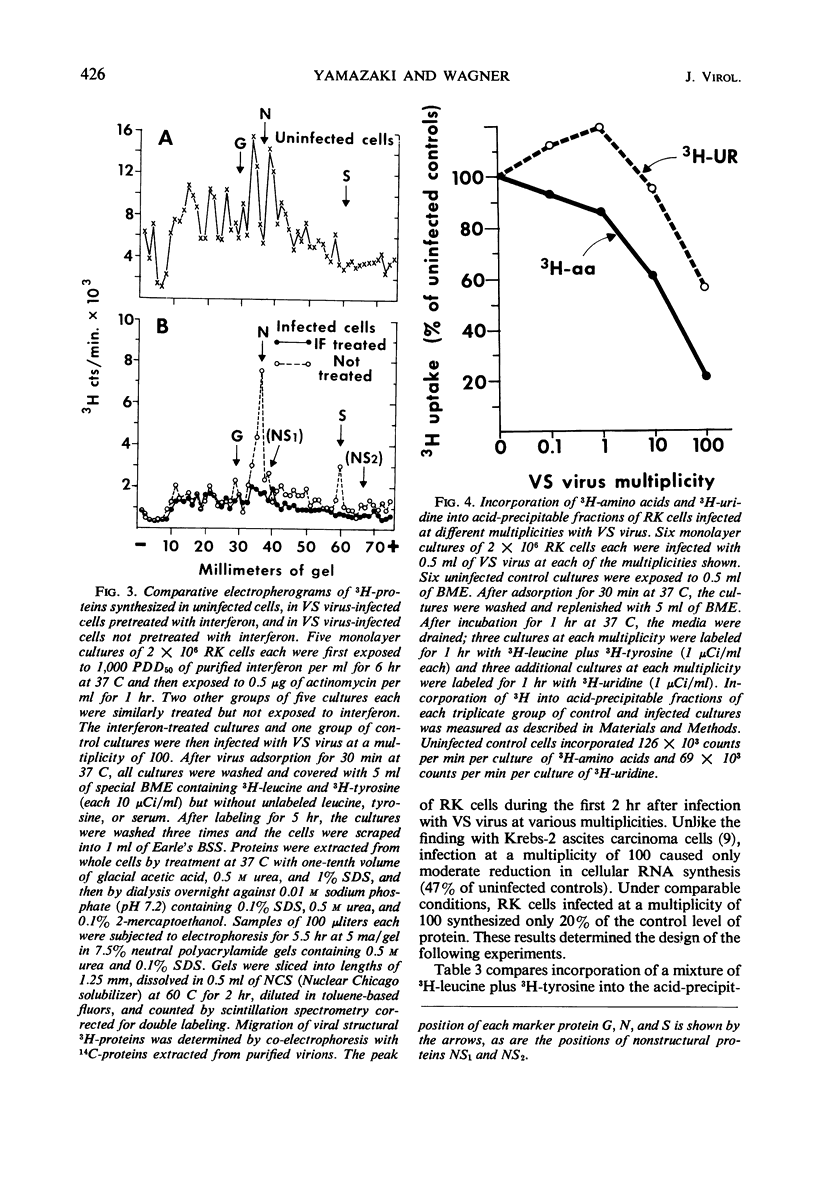
Abstract
A highly purified rabbit interferon was tested for its capacity to inhibit various manifestations of infection of primary rabbit kidney (RK) cells with vesicular stomatitis (VS) virus. A kinetic analysis of the actinomycin-sensitive phase of interferon-induced cellular resistance revealed that RK cells could transcribe virtually all of the hypothetical antiviral messenger ribonucleic acid (mRNA) within 3 hr. Similar exposure to interferon reduced virus yield by 95 to 99% and markedly inhibited cytopathic effect on RK cells infected at a multiplicity of 10 or less. Interferon was less effective in blocking cytopathic effects when RK cells were infected at a multiplicity of 100. However, RK cells pretreated with the same amount of interferon and infected at a multiplicity of 100 failed to incorporate 3H-amino acids into structural or nonstructural proteins of VS virus identified by polyacrylamide gel electrophoresis. Despite this inhibition of viral protein synthesis, interferon did not prevent the switch off by VS virus of cellular protein synthesis. The rapidity with which a high multiplicity of VS virus switched off cellular protein synthesis, even in cells rendered resistant to viral infection by interferon, is further evidence that this reaction is caused by an infecting virion component rather than by a newly synthesized viral product.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., FRANKLIN R. M., CALLENDER J. MENGOVIRUS-INDUCED INHIBITION OF HOST RIBONUCLEIC ACID AND PROTEIN SYNTHESIS. Biochim Biophys Acta. 1963 Nov 22;76:425–430. [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. N., Brown F. The influence of mitomycin C, actinomycin D and ultraviolet light on the replication of the viruses of foot-and-mouth disease and vesicular stomatitis. J Gen Virol. 1968 Dec;3(3):453–457. doi: 10.1099/0022-1317-3-3-453. [DOI] [PubMed] [Google Scholar]
- Cooper P. D. The inhibition of poliovirus growth by actinomycin D and the prevention of the inhibition by pretreatment of the cells with serum or insulin. Virology. 1966 Apr;28(4):663–678. doi: 10.1016/0042-6822(66)90251-0. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Buckler C. E., Baron S. Kinetics of the development of factors responsible for inerferon-induced resistance. Proc Soc Exp Biol Med. 1968 Nov;129(2):535–538. doi: 10.3181/00379727-129-33363. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Inhibition of arbovirus protein synthesis by interferon. J Virol. 1968 Oct;2(10):1081–1085. doi: 10.1128/jvi.2.10.1081-1085.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition of interferon action by puromycin. J Immunol. 1965 Oct;95(4):696–703. [PubMed] [Google Scholar]
- Haase A. T., Levy H., Baron S., Kasel J. A. Mengovirus-induced cytopathic effect in L-cells: protective effect of interferon. J Virol. 1969 Oct;4(4):490–495. doi: 10.1128/jvi.4.4.490-495.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Schnaitman C. A. Fusion of vesicular stomatitis virus with the cytoplasmic membrane of L cells. J Virol. 1969 Jun;3(6):619–622. doi: 10.1128/jvi.3.6.619-622.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Sonnabendja, Martin E. M. Proein-synthetic activity of ribosomes from interferon-treated cells. J Virol. 1970 Feb;5(2):132–144. doi: 10.1128/jvi.5.2.132-144.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. B. STUDIES ON THE MECHANISM OF INTERFERON ACTION. II. THE EFFECT OF INTERFERON ON SOME EARLY EVENTS IN MENGO VIRUS INFECTION IN L CELLS. Virology. 1964 Apr;22:575–579. doi: 10.1016/0042-6822(64)90079-0. [DOI] [PubMed] [Google Scholar]
- Levine S. Persistence of active interferon in cells washed after treatment with interferon. Proc Soc Exp Biol Med. 1966 Apr;121(4):1041–1045. doi: 10.3181/00379727-121-30959. [DOI] [PubMed] [Google Scholar]
- Levy H. B., Carter W. A. Molecular basis of the action of interferon. J Mol Biol. 1968 Feb 14;31(3):561–577. doi: 10.1016/0022-2836(68)90428-2. [DOI] [PubMed] [Google Scholar]
- Long W. F., Burke D. C. The effect of infection with fowl plague virus on protein synthesis in chick embryo cells. J Gen Virol. 1970 Jan;6(1):1–14. doi: 10.1099/0022-1317-6-1-1. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Gordon M. Differential inhibitory effects of actinomycin D among strains of poliovirus. J Bacteriol. 1966 Jun;91(6):2309–2316. doi: 10.1128/jb.91.6.2309-2316.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Wagner R. R. Rabbit macrophage interferons. I. Conditions for biosynthesis by virus-infected and uninfected cells. J Exp Med. 1967 Apr 1;125(4):559–577. doi: 10.1084/jem.125.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnabend J. A., Martin E. M., Mécs E., Fantes K. H. The effect of interferon on the synthesis and activity of an RNA polymerase isolated from chick cells infected with Semliki forest virus. J Gen Virol. 1967 Jan;1(1):41–48. doi: 10.1099/0022-1317-1-1-41. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Scott W. D., Sulkin S. E. Relative sensitivities of viruses to different species of interferon. J Virol. 1969 Aug;4(2):147–153. doi: 10.1128/jvi.4.2.147-153.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Huang A. S. Reversible inhibition of interferon synthesis by puromycin: evidence for an interferon-specific messenger RNA. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1112–1118. doi: 10.1073/pnas.54.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S. Effect of endogenous interferon and actinomycin D on growth of Japanese encephalitis virus in chick embryo cells. Jpn J Microbiol. 1968 Jun;12(2):171–178. doi: 10.1111/j.1348-0421.1968.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Wagner R. R. Purified rabbit interferon: attempts to demonstrate interferon-specific 3H-protein. J Virol. 1970 Feb;5(2):270–273. doi: 10.1128/jvi.5.2.270-273.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



