Abstract
Chronic inflammation in lung diseases contributes to lung tissue destruction leading to the formation of chemotactic collagen fragments such as N-acetylated Proline–Glycine–Proline (N-ac-PGP). In this study, we investigated in more detail the mechanism of action of N-ac-PGP in neutrophilic inflammation. N-ac-PGP was chemotactic for human neutrophils via pertussis toxin sensitive G protein-coupled receptors in vitro and directly activated this cell type, which led to cytosolic calcium mobilization and release of CXCL8. Furthermore, using a selective CXCR2 antagonist confirmed that N-ac-PGP-induced neutrophil chemotaxis is mediated through CXCR2 activation. To determine whether N-ac-PGP was solely responsible for the migration and activation of human neutrophils in vitro and not the released CXCL8 upon stimulation with N-ac-PGP, an antibody directed against CXCL8 was used. Performing chemotaxis and calcium influx assays in the presence of this antibody did not alter the effects of N-ac-PGP whereas effects of CXCL8 were attenuated. These experiments indicate that N-ac-PGP, in addition to the direct induction of chemotaxis, also directly activates neutrophils to release CXCL8. In vivo, this may lead in the long term to a self-maintaining situation enhanced by both N-ac-PGP and CXCL8, leading to a further increase in neutrophil infiltration and chronic inflammation.
Keywords: N-ac-PGP, Chemotaxis, Neutrophil, Chronic airway inflammation, CXCL8
1. Introduction
Neutrophils play a pivotal role in pulmonary inflammatory diseases, such as chronic obstructive pulmonary disease (COPD) (Barnes, 2004; Downey et al., 2009; Quint and Wedzicha, 2007). These front line defensive cells are the first to be recruited to the site of inflammation. During inflammation, the major attractants for neutrophils are ELR+ CXC-chemokines, such as CXCL8 (Kobayashi, 2008). However, it has long been known that fragments of the extracellular matrix, such as collagen fragments, have chemotactic properties (Chang and Houck, 1970; Laskin et al., 1986). One of these fragments is N-acetylated Proline–Glycine–Proline (N-ac-PGP), which was first identified by Pfister and colleagues. They described a rabbit model in which it was demonstrated that alkali degradation of whole corneal protein generated a tripeptide, N-ac-PGP (Pfister et al., 1995). Injecting N-ac-PGP in normal rabbit corneas resulted in a rapid and severe neutrophil invasion leading to corneal ulceration and perforation, resembling the neutrophil infiltration in the alkali-injured eye (Pfister et al., 1998). Interestingly, N-ac-PGP has been found in the sputum of COPD (O'Reilly et al., 2009; Weathington et al., 2006) and cystic fibrosis patients (Gaggar et al., 2008).
In collaboration with Weathington et al., we investigated the possible molecular mechanisms of N-ac-PGP's activity in the lung. Intratracheal administration of the collagen fragment in mice resulted in a direct neutrophil influx into the airways. Furthermore, chronic intra-airway exposure of N-ac-PGP resulted in COPD-like characteristics, such as enlargement of alveoli and right ventricular hypertrophy (Weathington et al., 2006). It was proposed that the N-ac-PGP activity is mediated via CXCR1/2, since the tripeptide shares sequence homology with the highly conserved GP motif in ELR+ CXC-chemokines, which is an essential motif for neutrophil cell binding and cell activation (Clark-Lewis et al., 1994), and blocking these chemokine receptors resulted in inhibition of the chemotactic N-ac-PGP response (Weathington et al., 2006). However, in collaboration with De Kruijf et al., we recently reported that N-ac-PGP does not directly activate or interact with these receptors, since the peptide was unable to activate G protein (in)dependent signaling and was unsuccessful in displacing the radioligands [125I]CXCL8 and [3H]-SB265610 from CXCR1- and CXCR2-expressing HEK293T cells or neutrophils. These observations led to the hypothesis that N-ac-PGP interacts indirectly with CXCR1/2 via the release of chemokines known to bind these receptors, or through activation of other receptors on the neutrophil to induce chemotaxis (de Kruijf et al., 2010).
Therefore, the aim of our study was to investigate the mechanism of N-ac-PGP-induced chemotaxis in more detail. In this report, we demonstrated that the collagen fragment N-ac-PGP dose dependently induces pertussis toxin-sensitive chemotaxis of neutrophils, which is associated with a calcium influx and that N-ac-PGP activation induces release of CXCL8. However, using an antibody against CXCL8 (α-CXCL8), we were able to demonstrate that the released CXCL8 is not responsible for N-ac-PGP's directly induced chemotaxis and calcium influx. Yet, the CXCR2 antagonist Compound 1 was able to attenuate the N-ac-PGP induced neutrophilic chemotactic response. These findings indicate that N-ac-PGP is a chemo-attractant for neutrophils in which CXCR2 but not CXCL8 plays a role.
2. Material and methods
2.1. Chemicals and reagents
N-ac-PGP was purchased from AnaSpec (San Jose, CA, USA) and checked for purity by high-performance liquid chromatography and mass spectrometry. Proline–Glycine–Glycine (PGG) was purchased from Bachem AG (Budendorf, Germany). CXCR2 antagonist Compound 1 ((S)-2-(2-(1 H-imidazol-1-yl)-6-(octylthio)pyrimidin-4-ylamino)-N-(3-ethoxypropyl)-4-methylpentanamide) was synthesized at the Schering-Plough Research Institute (Oss, The Netherlands; for molecular structure, see Ho et al., 2006 and de Kruijf et al., 2009). Recombinant human CXCL8 and human α-CXCL8 (antibody directed against CXCL8) were supplied by R&D Systems Europe Ltd. (Abingdon, United Kingdom). LPS, fMLP, pertussis toxin (PTX) and propidium iodide (PI) were purchased from Sigma Aldrich Chemie BV (Zwijndrecht, the Netherlands). The human CXCL8 ELISA kit was from BD Biosciences (Alphen a/d Rijn, the Netherlands). NH4Cl, KHCO3, EDTA (Triplex III) and trisodium citrate dihydrate were purchased from Merck KGaA (Darmstadt, Germany). Fluo-3AM (1 mM solution in DMSO) and probenecid (water soluble) were obtained from Molecular Probes, Invitrogen (Breda, the Netherlands). Ficoll-Paque™ PLUS was purchased from GE Healthcare (Eindhoven, the Netherlands). Heparin was purchased from LEO-Pharma (5,000 IU/ml; Weesp, the Netherlands). PBS and Roswell Park Memorial Institute (RPMI) 1640 medium (without l-glutamine and phenol red) were obtained from Lonza Verviers SPRL (Verviers, Belgium). FBS was from Perbio Science Nederland BV (Etten-Leur, the Netherlands).
2.2. Isolation of human polymorphonuclear leukocytes
Human polymorphonuclear leukocytes (PMNs) were isolated from fresh whole blood, for which donors signed written consent forms, or from buffy coats, which were purchased from Sanquin Blood Bank (Amsterdam, The Netherlands). The PMNs were obtained by centrifugation on Ficoll-Paque™ PLUS (density: 1.077 g/ml), followed by hypotonic lysis of erythrocytes with sterile lysis buffer (0.15 M NH4Cl, 0.01 M KHCO3 and 0.1 mM EDTA, pH at 4 °C is 7.4). After lysis, the PMNs were washed with PBS and finally resuspended in RPMI 1640 medium (without l-glutamine and phenol red) supplemented with 1% heat-inactivated FBS.
Resulting PMN preparations consisted of approximately 95–97% PMNs, based on PMNs physical parameters analyzed by flow cytometry and CD16 expression. The preparations were negative for CD14, meaning that the preparations did not contain monocytes.
2.3. Cell viability
Cell viability was determined by monitoring the fluorescence enhancement of PI in PMNs. This fluorescent probe can only cross the plasma membrane of non-viable cells, after which the fluorescence of this probe is enhanced 20- to 30-fold due to nucleic acid binding. PMNs were incubated for several time points with indicated reagents. After incubation, cells were washed with PBS and resuspended in 300 µl RPMI 1640 medium (without l-glutamine and phenol red) supplemented 1% heat-inactivated FBS and loaded with 3 µl PI (0.5 mg/ml). PI fluorescence was monitored in the FL2 channel using a BD FACSCalibur Flow Cytometer with CellQuest Pro Software (version 5.2.1.).
2.4. CXCL8 ELISA
Buffy coat isolated PMNs (105 cells/well) were incubated for several time points with indicated reagents. After incubation, the supernatants were collected and CXCL8 levels were measured using a human CXCL8 ELISA kit according to manufacturer's instructions.
2.5. Chemotaxis assay
The chemotaxis assay was performed as previously described by Weathington et al. (2006). Briefly, indicated reagents were placed in the bottom wells of a 3-µm 96-well polycarbonate filter plate (Millipore BV, Amsterdam, the Netherlands) in RPMI 1640 medium (without l-glutamine and phenol red) supplemented with 1% heat-inactivated FBS. 2 × 105 PMNs, isolated from fresh whole blood, were added to the top portion. The plate was incubated for one hour at 37 °C in 5% CO2. After removing the upper portion, the cells in each bottom well were counted for 30 s using a BD FACSCalibur Flow Cytometer with CellQuest Pro Software (version 5.2.1.). Data were standardized to a chemotactic index (= cells per well migrating to chemo-attractant/cells per well migrating to medium).
2.6. Calcium mobilization assay
PMNs, isolated from fresh whole blood, were resuspended in RPMI 1640 medium (without l-glutamine and phenol red) supplemented with 1% heat-inactivated FBS. The cells were loaded with the fluorescent dye FLUO-3AM (1 µM) by incubating the cells for 20 min at room temperature in the presence of 2.5 mM probenecid under gentle agitation. After washing with PBS, the cells were resuspended to a concentration of 106 cells/ml. For each measurement, a baseline was obtained, after which the cells were stimulated with the indicated reagents. The calcium mobilization was measured using a BD FACSCalibur Flow Cytometer with CellQuest Pro Software (version 5.2.1.) and analyzed using the program FCS Press (version 1.4 b).
2.7. Statistical analyses
For all statistical analyses, GraphPad Prism version 4.0 was used. One-tailed Student t-tests were used for comparing two groups. For comparing three or more groups, the data were analyzed using a one-way ANOVA followed by Tukey post hoc analysis. Data were considered significant at P<0.05. All results are expressed as means±S.E.M.
3. Results
3.1. N-ac-PGP dose dependently induces chemotaxis of PMNs via a PTX sensitive G protein-coupled receptor and a calcium influx in vitro
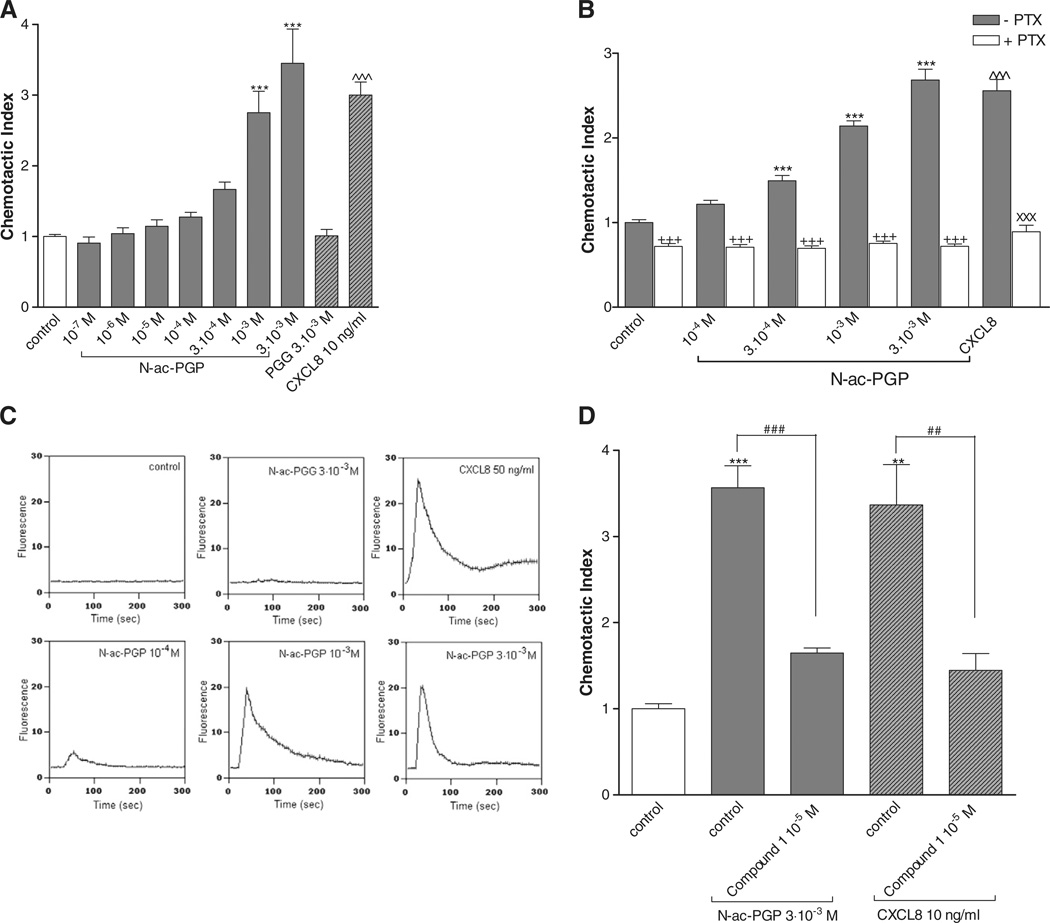
A transwell chemotaxis system was used to evaluate the chemotactic effect of N-ac-PGP on PMNs from fresh human whole blood. After one hour incubation, the migration of PMNs to the lower chamber was quantified and standardized to a chemotactic index. Collagen fragment N-ac-PGP (10−7–3 × 10−3 M) was dose dependently chemotactic for PMNs (Fig. 1A), whereas PGG (3 × 10−3 M) was not.
Fig. 1.
N-ac-PGP induces chemotaxis of PMNs via PTX sensitive G protein-coupled receptor CXCR2 and a calcium influx in vitro. (A) N-ac-PGP (10−7–3 × 10−3 M) induces chemotaxis in PMNs (***, P<0.001 N-ac-PGP vs. control), whereas PGG (3 × 10−3 M) is not chemotactic. CXCL8 (10 ng/ml) was tested as a positive control (^^^, P<0.001 CXCL8 vs. control). (B) Freshly isolated PMNs were incubated with medium or pertussis toxin (PTX; 500 ng/ml) for 90 minutes at 37 °C in 5% CO2. Subsequently, the chemotaxis assay was performed. N-ac-PGP (10−4–3 × 10−3 M) induces chemotaxis in PMNs pre-incubated with medium (black bars; ***, P<0.001 N-ac-PGP vs. control). This migration is completely inhibited in PMNs pre-incubated with PTX (white bars; +++, P<0.001 N-ac-PGP vs. PTX N-ac-PGP), which is not significantly different from PTX control level. Chemotaxis induced by CXCL8 (10 ng/ml; ^^^, P<0.001 CXCL8 vs. control) can be decreased by pre-incubating PMNs with PTX (xxx, P<0.001 CXCL8 vs. PTX CXCL8). (C) 2.25 × 105 freshly isolated PMNs were loaded with FLUO-3AM. For each measurement, a baseline for 15 seconds was obtained, after which the cells were stimulated with medium, N-ac-PGP (10−4–3 × 10−3 M), PGG (3 × 10−3 M), or CXCL8 (50 ng/ml). N-ac-PGP dose dependently induces an instant calcium influx (lower panel Fig. 1C). Medium control and PGG were used as negative controls and CXCL8 was used as positive controls (upper panel Fig. 1C). (D) Prior to chemotaxis, cells were pre-incubated with medium or CXCR2-antagonist Compound 1 for 45 min at 37 °C. N-ac-PGP and CXCL8 induce chemotaxis in PMNs pre-incubated with medium (**, P<0.01; ***, P<0.001 control vs. N-ac-PGP control or CXCL8 control). For both chemo-attractants, this migration is decreased in PMNs pre-incubated with CXCR2-antagonist Compound 1 (##, P<0.001 N-ac-PGP control vs. Compound 1 N-ac-PGP; ##, P<0.01; CXCL8 control vs. Compound 1 CXCL8).
The chemo-attractant CXCL8 initiates its biological activity by binding to G protein-coupled receptors CXCR1 and CXCR2, leading to the mobilization of cytosolic calcium and ultimately to the migration of the cell to the site of inflammation (Henson and Vandivier, 2006; Weinberger et al., 2005). Therefore, we hypothesized that N-ac-PGP, resembling CXCL8, was able to induce neutrophilic migration via G protein-coupled receptors and instantly increase cytosolic calcium in PMNs, which is a measure of cell activation.
To assess the role of G protein-coupled receptors in N-ac-PGP induced chemotaxis, the effect of pertussis toxin (PTX) was investigated. Fig. 1B shows that pre-incubation of PMNs for 1.5 h with 500 ng/ml PTX completely abolished neutrophil migration by N-ac-PGP and CXCL8. Fig. 1C shows that N-ac-PGP (10−4 M–3 × 10−3 M) dose dependently induced an instant calcium influx. PMN stimulation with 3 × 10−3 M PGG did not lead to calcium mobilization, whereas 50 ng/ml CXCL8 stimulation resulted in an increase in cytosolic calcium.
To evaluate the role of G protein-coupled CXCR2 in N-ac-PGP induced neutrophilic chemotaxis, PMNs were pre-incubated with the CXCR2 antagonist Compound 1 for 45 min after which the chemotaxis assay was performed. As shown in Fig. 1D, N-ac-PGP (3 × 10−3 M) and CXCL8 (10 ng/ml) induced a chemotactic response, which could be attenuated for both chemo-attractants due to incubation with Compound 1.
These data demonstrate that N-ac-PGP instantly activates the PMNs via PTX sensitive G protein-coupled receptors and that blockade of CXCR2 receptors resulted in a complete inhibition of the chemotactic response to N-ac-PGP.
3.2. PMNs release CXCL8 upon activation with collagen fragment N-ac-PGP
Early in inflammation, neutrophils migrate from the capillary into the interstitial space, following a chemotactic gradient of CXCL8 (Henson and Vandivier, 2006). At the site of inflammation, neutrophils are activated by stimulants released by macrophages, such as CXCL8 and leukotriene B4 (Barnes, 2004; Barnes et al., 2003). Upon activation, neutrophils release CXCL8, leading to a self-perpetuating inflammatory state where neutrophils attract more neutrophils via CXCR1 and CXCR2 (Bazzoni et al., 1991; Kobayashi, 2008; Mukaida, 2003). This led to the hypothesis that N-ac-PGP, formed after collagen breakdown and working via the same mechanism as CXCL8, activates the neutrophil to synthesize CXCL8, acting in an autocrine/paracrine fashion.
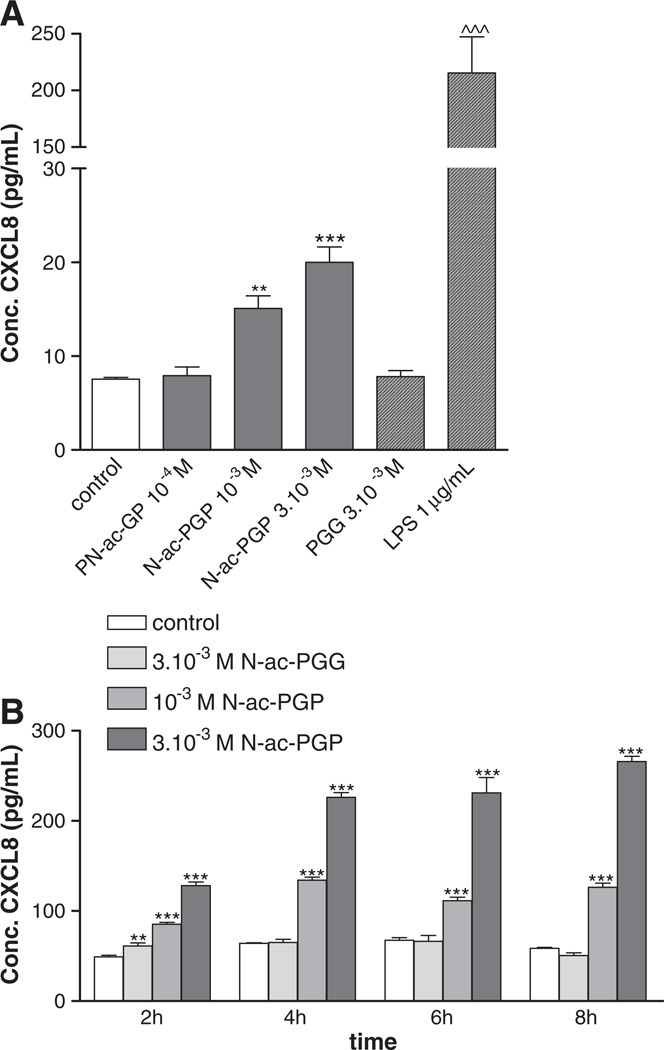
To examine this hypothesis, PMNs were incubated with N-ac-PGP for different time points from one to eight hours. Fig. 2A and B show that stimulation of PMNs with increasing concentrations of N-ac-PGP results in CXCL8 release by these cells at all time points. Significant amounts were produced after stimulation of the cells with 10−3 M–3 × 10−3 M N-ac-PGP. Incubation with 3 × 10−3 M PGG did not lead to a CXCL8 release, whereas 1 µg/ml LPS exposure did.
Fig. 2.
N-ac-PGP induces the release of CXCL8. (A) 105 freshly isolated PMNs were stimulated for 1 hour with N-ac-PGP (10−4–3 × 10−3 M), PGG (3 × 10−3 M) or LPS (1 µg/mL). A CXCL8 ELISA was performed on the supernatants. N-ac-PGP induced the release of CXCL8 from fresh cells within an hour (**P<0.01; ***/^^^, P<0.001 vs. control). (B) 105 buffy coat isolated neutrophils were stimulated for 2, 4, 6 and 8 h with N-ac-PGP (10−7–3 × 10−3 M), PGG (3 × 10−3 M) or LPS (1 µg/mL). N-ac-PGP (10−3 M and 3 × 10−3 M) induced the release of CXCL8 from fresh cells for all measured time points (**, P<0.01; ***, P<0.001 N-ac-PGP vs. control). PGG was negative for all time points, whereas LPS was positive for all time points. Only the concentrations 3 × 10−4–3 × 10−3 M are shown here; the cells stimulated with lower concentrations N-ac-PGP did not release more CXCL8 than the control cells.
Cell viability was assessed by flow cytometry by staining the cells with propidium iodide after different incubation time periods with N-ac-PGP, PGG or LPS (Table 1). At all time points, there were no significant differences between cells incubated with medium or cells incubated with 3 × 10−3 M PGG or 10−7 M–3 × 10−3 M N-ac-PGP. These results indicate that N-ac-PGP treatment does not negatively influence cell viability.
Table 1.
Cell viability of PMNs after N-ac-PGP, PGG and LPS incubation.
| 1 h | 2 h | 4 h | 6 h | 8 h | 19 h | 24 h | |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| %PMNs | 95.7 | 95.6 | 95.5 | 94.3 | 93.0 | 86.2 | 76.4 |
| % PI+ PMNs | 2.7 | 1.9 | 2.3 | 2.8 | 3.9 | 21.5 | 41.6 |
| N-ac-PGP 3 × 10−4 M | |||||||
| %PMNs | 96.7 | 96.6 | 95.6 | 95.0 | 94.7 | 87.3 | 78.7 |
| % PI+ PMNs | 2.2 | 2.0 | 2.7 | 2.5 | 3.5 | 24.9 | 49.4 |
| N-ac-PGP 10−3 M | |||||||
| %PMNs | 97.0 | 96.6 | 95.9 | 94.8 | 94.9 | 89.8 | 84.1 |
| % PI+ PMNs | 2.0 | 1.9 | 2.4 | 2.4 | 3.9 | 19.5 | 45.8 |
| N-ac-PGP 3 × 10−3 M | |||||||
| %PMNs | 96.9 | 96.5 | 95.7 | 95.1 | 95.1 | 91.5 | 87.4 |
| % PI+ PMNs | 2.1 | 2.0 | 2.1 | 2.6 | 3.3 | 13.2 | 34.2 |
| PGG 3 × 10−3 M | |||||||
| %PMNs | 97.1 | 96.5 | 96.3 | 94.5 | 93.7 | 86.7 | 76.6 |
| % PI+ PMNs | 2.0 | 2.0 | 2.1 | 2.6 | 3.0 | 22.9 | 45.4 |
| LPS 1 µg/ml | |||||||
| %PMNs | 86.0 | 94.2 | 90.7 | 88.3 | 86.9 | 60.5 | 47.4 |
| % PI+ PMNs | 9.1 | 4.2 | 5.5 | 6.0 | 9.2 | 49.8 | 74.4 |
7 × 105 buffy coat isolated PMNs resuspended in 300 µl RPMI medium with 1% FCS were loaded with 3 µl PI (5 µg/ml). A gate based on PMNs physical parameters (forward and side scatter) was set to exclude debris. Within this gate, the PI positive population was monitored in the FL2 channel.
N-ac-PGP activated the neutrophil to release CXCL8 and CXCL8 itself works via CXCR1 and CXCR2 on the neutrophil. This led to the suggestion that not N-ac-PGP but CXCL8 released after N-ac-PGP exposure was responsible for effects seen on neutrophil chemotaxis and calcium mobilization. To determine whether N-ac-PGP exerts these effects directly or indirectly on the neutrophil, calcium influx assays and chemotaxis studies in the presence an antibody against CXCL8, α-CXCL8, were conducted.
3.3. Antibodies directed against CXCL8 do not inhibit N-ac-PGP-induced calcium mobilization in PMNs
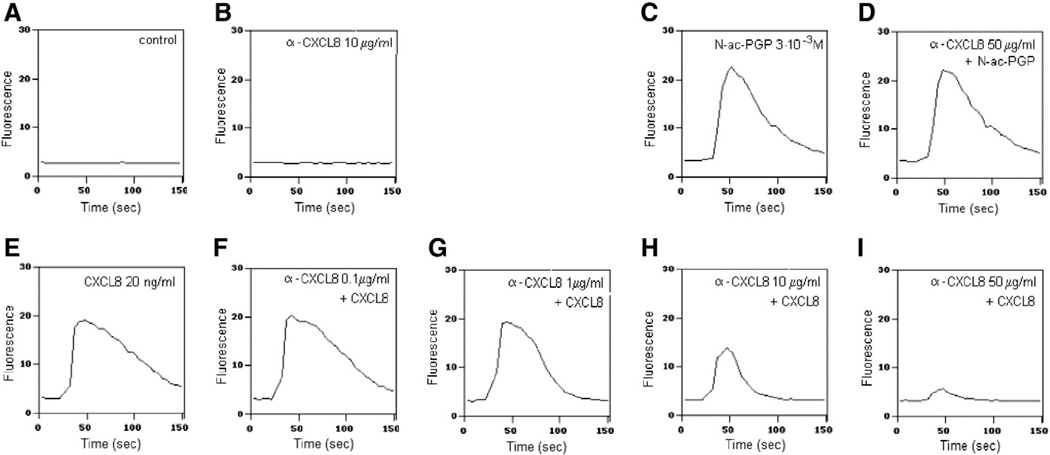
To investigate if N-ac-PGP directly activates PMNs with or without intervention of released CXCL8, a calcium mobilization assay was performed. Prior to stimulation with the indicated reagents, α-CXCL8 was added to the PMNs to capture spontaneously released CXCL8. The cells were stimulated with 20 ng/ml CXCL8 or 3 × 10−3 M N-ac-PGP (Fig. 3). At lower concentrations, the α-CXCL8 antibody blocked the CXCL8-induced calcium influx partially, whereas at higher concentrations of the antibody, it blocked the mobilization completely. However, the antibody did not inhibit the calcium response generated upon N-ac-PGP stimulation, indicating that CXCL8 released by PMNs after N-ac-PGP exposure was not responsible for the observed instant calcium mobilization in PMNs.
Fig. 3.
Antibody directed against CXCL8 does not block N-ac-PGP-induced calcium mobilization in PMNs. 2.25 × 105 freshly isolated PMNs were loaded with FLUO-3AM. Before measuring, medium (A–C and E) or α-CXCL8 (D and F–I) was added to the cells. After 20 s baseline measurement, the cells were stimulated with medium (A), N-ac-PGP (3 × 10−3 M; C and D) CXCL8 (20 ng/ml; E–I) or α-CXCL8 (10 µg/ml; B). The antibody α-CXCL8 does not stimulate the PMN to release calcium into the cytosol (B). N-ac-PGP (C) and CXCL8 (E) induce calcium mobilization. The N-ac-PGP induced calcium mobilization was not influenced by α-CXCL8 incubation (D), whereas the influx induced by CXCL8 could be attenuated dose dependently by α-CXCL8 incubation (0.1–50 µg/ml; F–I).
3.4. CXCL8 is not responsible for N-ac-PGP induced chemotaxis in vitro
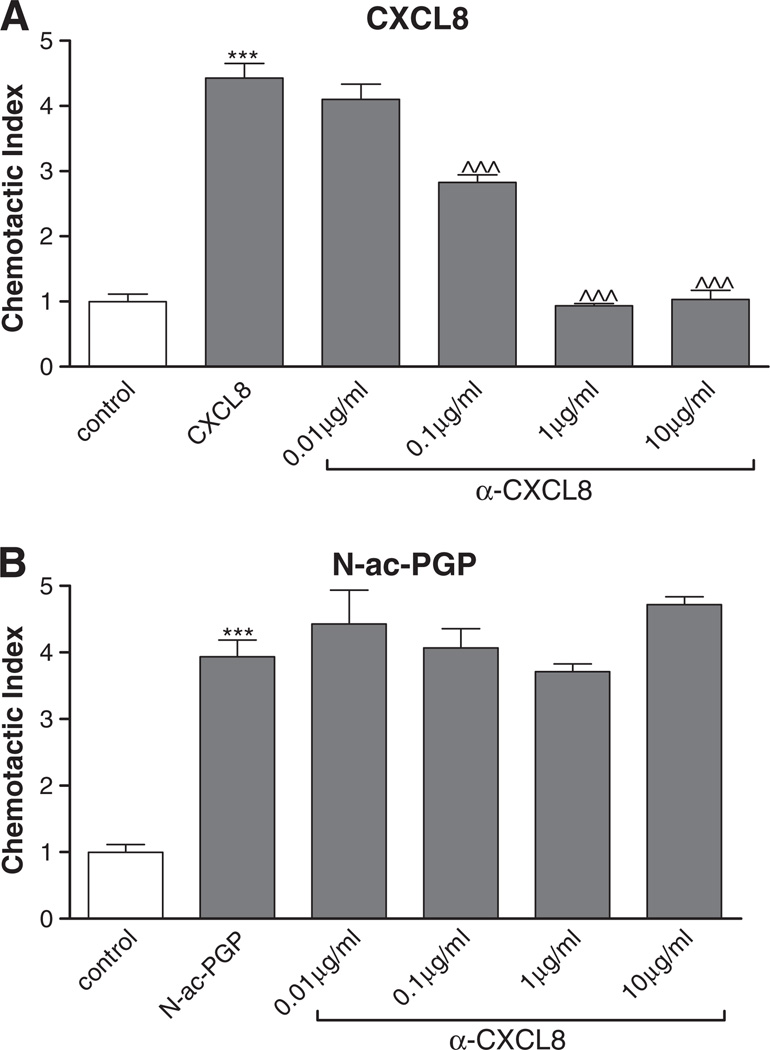
To determine if N-ac-PGP was solely responsible for the migration of neutrophils or whether CXCL8 released by these cells upon stimulation was contributing to migratory signals, cells were incubated with α-CXCL8 antibody for one hour at 37 °C in 5% CO2. Subsequently, the chemotaxis assay was performed. N-ac-PGP (3 × 10−3 M) and CXCL8 (10 ng/ml) induced a chemotactic response, as shown in Fig. 4. The CXCL8 induced chemotaxis was attenuated after α-CXCL8 antibody incubation, whereas the chemotactic effect of N-ac-PGP was not affected.
Fig. 4.
CXCL8 is not responsible for N-ac-PGP induced chemotaxis in vitro. 2 × 105 freshly isolated neutrophils were incubated for one hour at 37 °C with various concentrations of α-CXCL8 (0.01–10 µg/ml). The cells were placed in the top well and the chemo-attractant (N-ac-PGP; 3 × 10−3 M or CXCL8; 10 ng/ml) was present in the bottom well of a 96-wells Millipore Filtration Plate System. After one hour incubation at 37 °C, the cells were counted with FACS. CXCL8 (A) and N-ac-PGP (B) induce chemotaxis in PMNs (***, P<0.001 vs. control). The chemotaxis induced by N-ac-PGP is not inhibited by α-CXCL8 (B), whereas incubation of the PMNs with the antibody resulted in a complete inhibition of the CXCL8 induced chemotaxis (A) (^^^, P<0.001 α-CXCL vs. CXCL8).
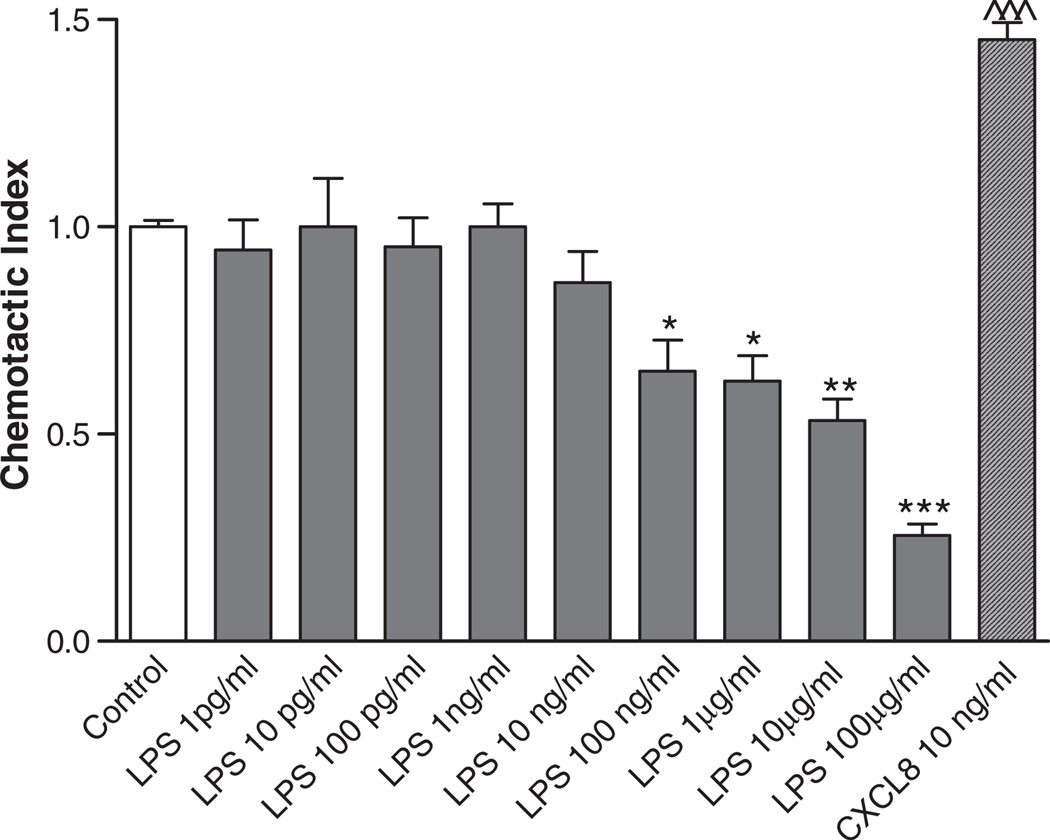
As shown in Fig. 2A, LPS activation led to a significant CXCL8 release from PMNs. To determine whether LPS was able to induce chemotaxis via CXCL8 release, the chemotaxis assay was performed. Fig. 5 shows no chemotactic effect for LPS on PMNs. At higher concentrations of LPS, there was even a significant reduction in basal chemotactic activity, possibly due to overstimulation of the cell, leading to cell death (Table 1). These data support the findings that N-ac-PGP directly activates the PMN, without intervention of released CXCL8.
Fig. 5.
LPS is not chemotactic for PMNs in vitro. 2 × 105 freshly isolated neutrophils were placed in the top well and the chemo-attractant was present in the bottom well of a 96-wells Millipore Filtration Plate System. After one hour incubation at 37 °C in 5% CO2, the cells from each bottom well were counted. Data were standardized to a chemotactic index. LPS did not induce chemotaxis in PMNs. Concentrations from 100 ng/ml to 100 µg/ml led to a significantly lower chemotactic index, possibly due to cell death by overstimulation (*, P<0.05; **, P<0.01; ***/^^^, P<0.001 LPS/CXCL8 vs. control).
4. Discussion
In this report, we showed that N-ac-PGP is chemotactic for neutrophils via pertussis toxin sensitive Gi/o proteins and that this tripeptide can directly activate this cell type, leading to cytosolic calcium mobilization and the release of CXCL8. However, this CXCL8 release is not involved in the observed chemotactic effects of N-ac-PGP in vitro, because studies using an antibody directed against CXCL8 demonstrated that the released CXCL8 is not responsible for the N-ac-PGP induced chemotaxis and calcium influx. CXCL8 is one of the most prominent chemokines found in neutrophilic inflammatory diseases, such as COPD. The levels of this chemokine are increased in sputum of COPD patients and correlate with the increased number of neutrophils found in the lungs (Barnes, 2004). These levels further increase during exacerbation of this disease, leading to increased numbers of neutrophils in the lungs (Barnes, 2004). However, antagonizing CXCL8 with an α-CXCL8 antibody, and blocking leukotrienes, such as leukotriene B4, with an antagonist incompletely prevents neutrophil chemotaxis in COPD patients (Beeh et al., 2003). This suggests that other unidentified chemo-attractants are involved in neutrophil migration in COPD.
During inflammation, mediator release from inflammatory cells such as neutrophils and macrophages leads to extracellular matrix break-down and subsequently, to the formation of collagen fragments (Henson and Vandivier, 2006; Stamenkovic, 2003). Some of these fragments can have chemotactic properties, as described in several reports (Haddox et al., 1999; Laskin et al., 1986; Pfister et al., 1993; Weinberger et al., 2005). One of the most potent collagen fragments with chemotactic properties is N-ac-PGP. This tripeptide was first identified in a rabbit model investigating alkali injury to the cornea (Pfister et al., 1995). Moreover, this collagen fragment can attract neutrophils to the lung, leading to lung damage (Weathington et al., 2006), and has been found in patients suffering from COPD (O'Reilly et al., 2009; Weathington et al., 2006) and cystic fibrosis (Gaggar et al., 2008). In these diseases, neutrophils secrete proteases, including neutrophil elastase and matrix metalloproteinases, such as MMP-8 and MMP-9 (Barnes, 2004; Downey et al., 2009). Proteolytic cleavage of alveolar collagen by MMPs and prolyl endopeptidase and subsequent acetylation, generates N-ac-PGP (Gaggar et al., 2008; O'Reilly et al., 2009), thereby presumably prolonging the influx of neutrophils (Henson and Vandivier, 2006).
In the present study, we demonstrated that N-ac-PGP has pro-inflammatory properties. Consistent with previous studies (Pfister et al., 1995; Weathington et al., 2006), we found that N-ac-PGP was chemotactic for the neutrophil in vitro. Concentrations in the millimolar range of N-ac-PGP were used, which were in a similar range as the concentrations used for chemotaxis and polarization assays by other groups (Haddox et al., 1999; Pfister et al., 1995). Moreover, as shown in Table 1, these concentrations did not induce cell death.
The concentrations of N-ac-PGP and CXCL8 have been measured separately in several COPD and cystic fibrosis patient studies and, to our knowledge, there are no reports in which both CXCL8 and N-ac-PGP were measured using human disease material. However, the levels of both N-ac-PGP and CXCL1 (KC; keratinocyte cell-derived chemokine, a CXCL8 homologue in mice) have been measured in a murine model where A/J mice were exposed to cigarette smoke for five months (Braber et al., 2011). After this exposure, emphysema-like characteristics such as alveolar enlargement and heart hypertrophy were detected. This lung tissue degradation was also reflected by the levels of N-ac-PGP in the bronchoalveolar lavage fluid, which increased after 5 months smoke from 0.06 ng/ml to 12.4 ng/ml (Braber et al., 2011), whereas the concentration of CXCL1 was approximately 0.02 ng/ml (Braber, personal communication). Moreover, Weathington et al. showed in a murine study using BALB/c mice that the N-ac-PGP level was more than 30 times higher than the level of CXCL1 12 hours after LPS aerosolization (Weathington et al., 2006). From these murine studies it can be concluded that the concentrations of N-ac-PGP and the CXCL8 homologue are physiologically relevant and that both chemo-attractants play an important role in the pathology of neutrophilic diseases, such as COPD. In addition, the N-ac-PGP concentrations measured in bronchoalveolar lavage fluid may not be representative for the possible higher concentrations in the tissues, which was not assessed in these reports.
N-ac-PGP induced chemotaxis via pertussis toxin sensitive G proteins and mobilization of cytosolic calcium by a similar mechanism as other chemo-attractants such as fMLP (Krause et al., 1990; Moreno et al., 2005; Weinberger et al., 2005) and CXCL8 (Moreno et al., 2005; Weinberger et al., 2005; Zeilhofer and Schorr, 2000). Pre-incubation of the PMNs with 500 ng/ml PTX, a specific inhibitor of Gi/o type G proteins, completely abolished the N-ac-PGP induced migration, without affecting the cell viability. As expected, chemotaxis induced by CXCL8 was also decreased by pre-incubating PMNs with PTX. Moreover, pre-incubation of neutrophils with CXCR2 antagonist Compound 1 attenuated the chemotactic effect of N-ac-PGP and CXCL8, demonstrating a role for CXCR2 in the chemotaxis induced by these chemo-attractants. N-ac-PGP may resemble CXCL8 in initiating the classical pathway in which its binding to G protein-coupled receptors or other membrane-bound proteins ultimately leads to the release of calcium from the endoplasmatic reticulum into the cytosol. This calcium mobilization is required for processes such as chemotaxis (Malbon, 2005; Stillie et al., 2009; Zeilhofer and Schorr, 2000).
Previously, we have proposed in collaboration with De Kruijf and colleagues that N-ac-PGP may interact indirectly with CXCR1 and CXCR2 via the release of chemokines, known to bind these receptors, or through activation of other receptors on the neutrophil to induce chemotaxis, since the peptide was unable to activate G-protein dependent and G-protein independent signaling and unsuccessful in displacing the radioligands [125I]CXCL8 and [3H]-SB265610 from CXCR1 and CXCR2 expressing HEK293T cells or neutrophils (de Kruijf et al., 2010).
In this report we demonstrated that N-ac-PGP driven chemotaxis for neutrophils is pertussis toxin sensitive. In addition, we showed that N-ac-PGP directly induces calcium mobilization. Moreover, we performed a chemotaxis assay using L1.2 cells transiently expressing CXCR2. These L1.2 cells did not show any chemotactic response upon exposure to N-ac-PGP, while CXCL8 did (Suppl. Fig. 1). These data indicate that N-ac-PGP is able to activate signaling in neutrophils, which is not apparent in HEK293T or L1.2 B cells. The effect of N-ac-PGP appears therefore cell type dependent and the results of CXCR signaling studies performed on HEK293T cells, L1.2 B cells or neutrophils may therefore differ in outcome.
Moreover, it is possible that N-ac-PGP-induced chemotaxis is mediated through a phosphatidylinositol 3-kinase-γ-independent pathway (Stillie et al., 2009). CXCL8-induced chemotaxis is mediated through phosphatidylinositol 3-kinase-γ-independent and -dependent pathways. In the phosphatidylinositol 3-kinase-γ-independent pathway, phospholipase C is activated, which in turn produces inositol trisphosphate, leading ultimately to calcium mobilization from non-mitochondrial stores (Stillie et al., 2009). However, calcium mobilization can also be induced via inositol trisphosphate independent signal transduction pathways, such as via the activation of store-operated channels. These channels are activated by depletion of calcium stores through the calcium influx factor-calcium-independent phospholipase A2 mechanism (Bolotina, 2004). It may be possible that N-ac-PGP and CXCL8 induce chemotaxis through different signal transduction pathways.
Here we report that neutrophils release CXCL8 upon activation with N-ac-PGP. In order to establish whether the released CXCL8 were responsible for the observed N-ac-PGP effects in vitro, PMNs were incubated with α-CXCL8 antibody prior to calcium mobilization measurement and chemotaxis. This incubation with the antibody did not result in a change in N-ac-PGP induced calcium mobilization or chemotaxis, even when high concentrations of α-CXCL8 antibody were used. In contrast, α-CXCL8 antibody attenuated the CXCL8-induced calcium influx and chemotactic effect. Therefore, it can be concluded that the released CXCL8 by neutrophils was not responsible for the observed N-ac-PGP activating and chemotactic effects. Although N-ac-PGP induces CXCL8-independent chemotaxis of neutrophils, it can be hypothesized that N-ac-PGP may interact with other membrane bound proteins as is described for MIF and CD74-CXCR2 and CD74-CXCR4 (Schwartz et al., 2009) or that N-ac-PGP induces the release of other chemokines known to bind to CXCRs (Barnes, 2010). In addition, N-ac-PGP may act indirectly on the CXCR by activating the growth factor receptor tyrosine kinase, thereby inducing the transactivation of G protein-coupled receptors (Delcourt et al., 2007).
Neutrophilic CXCL8 release induced by N-ac-PGP may lead in the long term to a self-perpetuating situation where neutrophils can attract and activate more neutrophils via CXCR1 and CXCR2. In our studies, differences in CXCL8 release and chemotactic properties between N-ac-PGP-stimulated and unstimulated PMNs were measured within one hour. Although CXCL8 is not stored in neutrophils (Mukaida, 2003), we presume that isolation from blood already activates the neutrophil (Glasser and Fiederlein, 1990) to synthesize CXCL8. After stimulation with an activator, such as N-ac-PGP or LPS, CXCL8 release can then be induced from PMNs.
Finally, our data indicate that besides chemotaxis, N-ac-PGP stimulates the neutrophils to release CXCL8, which in vivo may lead to a self-perpetuating situation where N-ac-PGP and CXCL8 work in concert, leading to enhanced neutrophil inflammation and lung inflammation.
Supplementary Material
Acknowledgements
This study was performed within the framework of Dutch Top Institute Pharma (project number T1.103).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.ejphar.2011.03.022.
References
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Medicine. Neutrophils find smoke attractive. Science. 2010;330:40–41. doi: 10.1126/science.1196017. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur. Respir. J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- Bazzoni F, Cassatella MA, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J. Exp. Med. 1991;173:771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeh KM, Kornmann O, Buhl R, Culpitt SV, Giembycz MA, Barnes PJ. Neutrophil chemotactic activity of sputum from patients with COPD: role of interleukin 8 and leukotriene B4. Chest. 2003;123:1240–1247. doi: 10.1378/chest.123.4.1240. [DOI] [PubMed] [Google Scholar]
- Bolotina VM. Store-operated channels: diversity and activation mechanisms. Sci. STKE. 2004;2004:34. doi: 10.1126/stke.2432004pe34. [DOI] [PubMed] [Google Scholar]
- Braber S, Koelink PJ, Henricks PAJ, Jackson PL, Nijkamp FP, Garssen J, Kraneveld AD, Blalock JE, Folkerts G. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol. 2011s;300:L255–L265. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Houck JC. Demonstration of the chemotactic properties of collagen. Proc. Soc. Exp. Biol. Med. 1970;134:22–26. doi: 10.3181/00379727-134-34719. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I, Dewald B, Loetscher M, Moser B, Baggiolini M. Structural requirements for interleukin-8 function identified by design of analogs and CXC chemokine hybrids. J. Biol. Chem. 1994;269:16075–16081. [PubMed] [Google Scholar]
- de Kruijf P, Lim HD, Overbeek SA, Zaman GJ, Kraneveld AD, Folkerts G, Leurs R, Smit MJ. The collagen-breakdown product N-acetyl-Proline–Glycine–Proline (N-alpha-PGP) does not interact directly with human CXCR1 and CXCR2. Eur. J. Pharmacol. 2010;643:29–33. doi: 10.1016/j.ejphar.2010.06.017. [DOI] [PubMed] [Google Scholar]
- de Kruijf P, van Heteren J, Lim HD, Conti PG, van der Lee MM, Bosch L, Ho KK, Auld D, Ohlmeyer M, Smit MJ, Wijkmans JC, Zaman GJ, Smit MJ, Leurs R. Nonpeptidergic allosteric antagonists differentially bind to the CXCR2 chemokine receptor. J. Pharmacol. Exp. Ther. 2009;329:783–790. doi: 10.1124/jpet.108.148387. [DOI] [PubMed] [Google Scholar]
- Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol. Sci. 2007;28:602–607. doi: 10.1016/j.tips.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser L, Fiederlein RL. The effect of various cell separation procedures on assays of neutrophil function. A critical appraisal. Am. J. Clin. Pathol. 1990;93:662–669. doi: 10.1093/ajcp/93.5.662. [DOI] [PubMed] [Google Scholar]
- Haddox JL, Pfister RR, Muccio DD, Villain M, Sommers CI, Chaddha M, Anantharamaiah GM, Brouillette WJ, DeLucas LJ. Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl–Proline–Glycine–Proline. Invest. Ophthalmol. Vis. Sci. 1999;40:2427–2429. [PubMed] [Google Scholar]
- Henson PM, Vandivier RW. The matrix degrades, neutrophils invade. Nat. Med. 2006;12:280–281. doi: 10.1038/nm0306-280. [DOI] [PubMed] [Google Scholar]
- Ho KK, Auld DS, Bohnstedt AC, Conti P, Dokter W, Erickson S, Feng D, Inglese J, Kingsbury C, Kultgen SG, Liu RQ, Masterson CM, Ohlmeyer M, Rong Y, Rooseboom M, Roughton A, Samama P, Smit MJ, Son E, van der Louw J, Vogel G, Webb M, Wijkmans J, You M. Imidazolylpyrimidine based CXCR2 chemokine receptor antagonists. Bioorg. Med. Chem. Lett. 2006;16:2724–2728. doi: 10.1016/j.bmcl.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Krause KH, Campbell KP, Welsh MJ, Lew DP. The calcium signal and neutrophil activation. Clin. Biochem. 1990;23:159–166. doi: 10.1016/0009-9120(90)80030-m. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Kimura T, Sakakibara S, Riley DJ, Berg RA. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J. Leukoc. Biol. 1986;39:255–266. doi: 10.1002/jlb.39.3.255. [DOI] [PubMed] [Google Scholar]
- Malbon CC. G proteins in development. Nat. Rev. Mol. Cell Biol. 2005;6:689–701. doi: 10.1038/nrm1716. [DOI] [PubMed] [Google Scholar]
- Moreno AN, Pereira-da-Silva G, Oliver C, Jamur MC, Panunto-Castelo A, Roque-Barreira MC. The macrophage-derived lectin, MNCF, activates neutrophil migration through a pertussis toxin-sensitive pathway. J. Histochem. Cytochem. 2005;53:715–723. doi: 10.1369/jhc.4A6562.2005. [DOI] [PubMed] [Google Scholar]
- Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L566–L577. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- O'Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir. Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Sommers CI. Alkali-degraded cornea generates a low molecular weight chemoattractant for polymorphonuclear leukocytes. Invest. Ophthalmol. Vis. Sci. 1993;34:2297–2304. [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest. Ophthalmol. Vis. Sci. 1998;39:1744–1750. [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Sommers CI, Lam KW. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-Proline–Glycine–Proline and N-methyl–Proline–Glycine–Proline. Invest. Ophthalmol. Vis. Sci. 1995;36:1306–1316. [PubMed] [Google Scholar]
- Quint JK, Wedzicha JA. The neutrophil in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2007;119:1065–1071. doi: 10.1016/j.jaci.2006.12.640. [DOI] [PubMed] [Google Scholar]
- Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K, Bucala R, Weber C, Bernhagen J. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009;583:2749–2757. doi: 10.1016/j.febslet.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J. Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMNJ. Leukoc. Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Hanna N, Laskin JD, Heck DE, Gardner CR, Gerecke DR, Laskin DL. Mechanisms mediating the biologic activity of synthetic proline, glycine, and hydroxyproline polypeptides in human neutrophils. Mediators Inflamm. 2005;2005:31–38. doi: 10.1155/MI.2005.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Schorr W. Role of interleukin-8 in neutrophil signaling. Curr. Opin. Hematol. 2000;7:178–182. doi: 10.1097/00062752-200005000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.