Abstract
The genetic composition of a donor impacts long term allograft survival after kidney transplantation. Effects of the recipient’s genetic make-up, particularly variation in immune response pathway genes are less certain. A report in this issue of Kidney International reveals improved graft survival in transplant recipients with lower copy numbers of the complement 4 gene (C4) after receipt of deceased donor kidneys. Genomics breakthroughs in nephrology and immunology will likely revolutionize the field of transplant medicine.
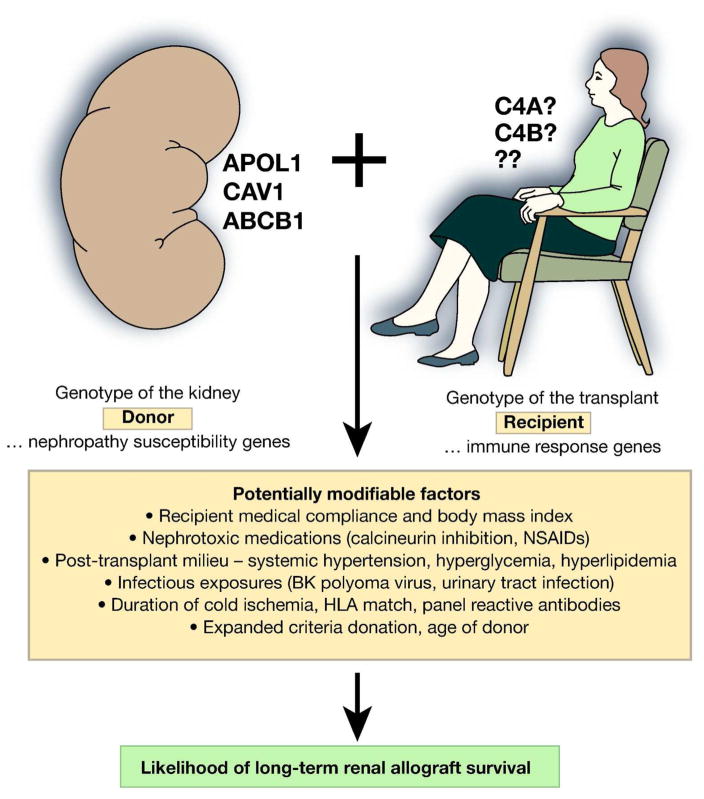
In nephrology, translating advances in molecular genetics into the realm of clinical practice appears closest for transplant medicine. Genome-wide approaches have demonstrated that genes and genomic regions beyond the human chromosome 6 major histocompatibility complex (MHC) impact nephropathy susceptibility. There is mounting evidence supporting the concept that donor genotypes impact long-term allograft survival after deceased donor kidney transplantation. This seems intuitive, as the innate risk for kidney failure due to donor nephropathy risk variants (genetic risk), coupled with the environmental effects of prolonged cold ischemia time (CIT), nephrotoxic immunosuppression (calcineurin inhibition), and opportunistic viral infections is likely to accelerate graft loss. Effects have been observed for allelic variation in the caveolin 1 (CAV1), ATP-binding cassette, sub-family B (MDR/TAP), member 1 (ABCB1; also known as multi-drug resistance 1 encoding P-glycoprotein) and apolipoprotein L1 (APOL1) genes (Figure 1).[1–3] It is tempting to speculate that the CAV1 pathway risk variants influence risk for BK polyoma virus infection of renal parenchyma in transplanted kidneys, while variation in ABCB1 likely influences the cellular metabolism of anti-rejection medications. Although the mechanisms underlying glomerulosclerosis, interstitial fibrosis, and vascular changes in APOL1-associated nephropathy remain unknown,[4] APO1 nephropathy risk variants in deceased kidney donors of African ancestry had stronger effects on subsequent allograft survival than did conventional risk factors such as degree of Human Leukocyte Antigen (HLA) match, CIT, panel reactive antibodies (PRA), and standard (vs. expanded) criteria donation.[3] Variation in APOL1 appeared to fully explain the shorter allograft survival seen in African ancestry donor kidneys, relative to European; and recipient APOL1 genotypes do not impact allograft survival.[5] Some suggest genotyping APOL1 nephropathy risk variants in all potential living donors of African-ancestry.[6]
Figure 1.
Inherited and environmental factors impacting kidney transplant outcomes.
These examples make it apparent that genetic variation in donor kidneys impact transplant outcomes. However, the role of recipient genetic risk is less clear. The concept that recipient immune response genes could impact outcomes after transplantation is as intuitive as the case for intrinsic genetic risk in donor kidneys based on nephropathy susceptibility loci. Determining the degree of recipient immune response could potentially stratify transplant recipients into those necessitating more, versus less powerful and potentially toxic immune-suppression in order to reduce rates of subsequent rejection and graft loss.
A report in this issue of Kidney International explored an important aspect of recipient genetic risk on kidney transplant outcomes, the effect of immune response relating to the complex genetic organization of complement component genes.[7] The complement system, a part of the innate immune system, is active in organ rejection and its effects are potentiated by the acquired immune system, i.e. antibodies.[8] In addition, complement can play a role in non-sensitized patients by influencing damage from ischemia reperfusion injury, regulating the adaptive immune response, and in the well-known role of diagnostics through testing for presence of C4d. Transplantation is a unique medical procedure where all pathways of complement response (classical, alternative, and lectin) can be activated.[9]
Bay et al. prospectively evaluated the effect of copy number variation (CNV) in the C4 gene on five year renal allograft survival in living and deceased donor transplantation.[7] They determined that serum C4 concentrations positively correlated with C4 CNV in the transplant recipients and controls, confirming a biologic effect of this CNV. A survival analysis showed the protective effect of lower C4 copy numbers (<4 copies, relative to ≥4) on graft survival, an effect that was limited to deceased donor transplants. Surprisingly, serum C4 concentration did not correlate with deceased donor kidney transplant outcomes. This report lacked kidney biopsy results or measures of anti-HLA donor-specific antibodies (DSA). Although graft survival is a hard (and ultimately the most important) outcome, it is unclear whether graft survival better assesses complement-mediated processes important in rejection, relative to directly measured complement-derived end products such as C4d or complement-aided rejection factors like DSA.
Unlike the prospective study by Bay et al.[7], Wahrmann and colleagues [10] previously performed a large retrospective analysis using the Collaborative Transplant Study (CTS) database to assess whether CNV in C4, or differences in C4 gene length (short versus long), contributed to changes in allograft outcomes, as had been reported in other infectious and auto-immune diseases. Significant differences were not detected for graft or patient survival up to ten years post-transplant between three groups based upon C4 CNV (<4, 4, or >4 copies). Likewise, there were no apparent differences in patient and graft survival between recipients with varying copy numbers of C4 short or C4 long. These authors acknowledged that the majority of patients from the CTS database were transplanted prior to the introduction of C4d staining and existence of standardized diagnostic criteria to detect antibody mediated rejection.
Ultimately, the retrospective Wahrmann et al.[10] and prospective Bay et al.[7] studies came to opposing conclusions about the role of CNV in the recipient complement 4 gene in renal allograft survival. All clinical reports have shortcomings and precise quantification of CNV can often be difficult. Wahrmann et al. relied upon non-standardized diagnostic criteria in determining antibody mediated rejection and had incomplete results of C4d testing.[10] Bay and colleagues measured a difference in C4 levels based on CNV, but lacked the ability to measure DSA that would be an initiating factor in complement-mediated processes in graft rejection.[7] Without measuring DSA or C4d deposition, these authors could not be certain that the complement cascade was initiated in the grafts that were deemed to have suffered from rejection. As these reports failed to directly assess complement activation in the allograft rejection process, perhaps it is less of a surprise that their results differ.
Bay and colleagues [7] suggest that kidney transplant recipient CNV in C4, an immune response gene impacts long-term allograft survival after deceased donor transplantation. Although it is not clear why their findings differ from those of the prior report, it is abundantly clear that exploring variation in immune response genes and pathways in transplant recipients is likely to impact transplant outcomes and assist in optimizing the choice of anti-rejection therapy. In the case of donor nephropathy risk variants, kidney donors can rapidly be typed for risk variants in APOL1, CAV1, ABCB1, and other soon to be detected genes at the time of organ harvest to stratify the likelihood for long-term graft survival. Once the effects of donor nephropathy genes on transplant outcomes are validated, it is conceivable that patients who would benefit the most from long-term graft survival with their first transplant may appreciate the option of declining kidneys based on presence of multiple nephropathy risk variants. This is particularly true in pediatric patients and young adults with renal-limited diseases. In this way, kidney donor and recipient risk variants may alter our understanding of the suitability of transplanting a given organ into a given recipient. In addition, it may prove beneficial to maximize transplant utility (increase overall allograft survival years) based on “age to age” matching of donors to recipients. This policy could reduce numbers of deaths with functioning grafts by aligning younger donors with younger recipients. Effects of recipient immunologic risk based on genetic composition in conjunction with age matching donors to recipients are worthy of additional study.
We believe the genetics revolution will dramatically impact the field of kidney transplantation. To further this important field of research, we suggest that DNA libraries be created from large numbers of deceased kidney donors and kidney transplant recipients. This genetic information can be linked to outcomes in the Scientific Registry of Transplant Recipients (SRTR) and United Network for Organ Sharing (UNOS) and may ultimately clarify the discordant observations of Bay et al.[7] and Wahrmann et al.[10] Typing gene variants in potential kidney donors and recipients will soon transform the field of kidney transplantation.
Acknowledgments
The authors thank Drs. Amudha Palanisamy and Amber Reeves-Daniel for their critical review and input. This work was supported by NIH grants R01 HL56266, RO1 DK070941 and RO1 DK084149 (BIF).
Footnotes
These authors report no conflicts of interest relating to this article.
Disclosure: Dr. Gautreaux was an ad hoc consultant for Transplant Management Group. Dr. Freedman has nothing to declare.
Reference List
- 1.Moore J, McKnight AJ, Simmonds MJ, et al. Association of caveolin-1 gene polymorphism with kidney transplant fibrosis and allograft failure. JAMA. 2010;303:1282–1287. doi: 10.1001/jama.2010.356. [DOI] [PubMed] [Google Scholar]
- 2.Moore J, McKnight AJ, Dohler B, et al. Donor ABCB1 variant associates with increased risk for kidney allograft failure. J Am Soc Nephrol. 2012;23:1891–1899. doi: 10.1681/ASN.2012030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves-Daniel AM, Depalma JA, Bleyer AJ, et al. The APOL1 Gene and Allograft Survival after Kidney Transplantation. Am J Transplant. 2011;11:1025–1030. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BT, Kumar V, Williams TA, et al. The APOL1 Genotype of African American Kidney Transplant Recipients Does Not Impact 5-Year Allograft Survival. Am J Transplant. 2012;12:1924–1928. doi: 10.1111/j.1600-6143.2012.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen DM, Mittalhenkle A, Scott DL, et al. African American living-kidney donors should be screened for APOL1 risk alleles. Transplantation. 2011;92:722–725. doi: 10.1097/TP.0b013e31822eec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bay JT, Schejbel L, Madsen HO, et al. Low C4 gene copy numbers are associated with superior graft survival in patients transplanted with a deceased donor kidney. Kidney International. 2013 doi: 10.1038/ki.2013.195. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA, Traves P, Walport M. Immunobiology. 5. New York: Garland Science; 2001. The immune system in health and disease. [Google Scholar]
- 9.Sacks SH, Chowdhury P, Zhou W. Role of the complement system in rejection. Curr Opin Immunol. 2003;15:487–492. doi: 10.1016/s0952-7915(03)00100-6. [DOI] [PubMed] [Google Scholar]
- 10.Wahrmann M, Dohler B, Ruhenstroth A, et al. Genotypic diversity of complement component C4 does not predict kidney transplant outcome. J Am Soc Nephrol. 2011;22:367–376. doi: 10.1681/ASN.2010050513. [DOI] [PMC free article] [PubMed] [Google Scholar]