Abstract
The modern laboratory mouse has become a central tool for biomedical research with a notable influence in the field of hematopoiesis. Application of retroviral-based gene transfer approaches to mouse hematopoietic stem cells (HSCs) has led to a sophisticated understanding of the hematopoietic hierarchy in this model. However, the assumption that gene transfer methodologies developed in the mouse could be similarly applied to human HSCs for the treatment of human diseases left the field of gene therapy in a decade-long quandary. It is not until more relevant humanized xenograft mouse models and phylogenetically related large animal species were used to optimize gene transfer methodologies that unequivocal clinical successes were achieved. However, the subsequent reporting of severe adverse events in these clinical trials casted doubts on the predictive value of conventional pre-clinical testing, and encouraged the development of new assays for assessing the relative genotoxicity of various vector designs.
Introduction
Hematopoietic stem cells (HSCs) are indispensable for lifelong blood production. HSCs can sustain long-term and functional hematopoiesis due to their ability to both differentiate to produce mature progeny of all myeloid and lymphoid blood lineages or to self-renew to replace the cells that become progressively committed to differentiation. The majority of HSCs, however, are quiescent and do not contribute to daily production of mature blood cells. Our understanding of the nature and properties of HSCs has been greatly influenced by the seminal murine studies of Till and McCulloch1,2 over five decades ago. Since then, the highly standardized and easily accessible laboratory mouse has continued to dominate the field of hematopoiesis because durable, long-term in vivo reconstitution of the hematopoietic system of a recipient animal after transplantation is the only operational means of unequivocally identifying HSCs, raising an obvious impediment to studying human HSCs. The introduction of genetic markers into mouse HSCs and their progeny using retroviral vectors was instrumental in providing both conceptual and methodological insights for the identification and characterization of individual stem cells, leading to a refined understanding of murine stem cell behavior in vivo over time.
The potential of applying similar gene transfer approaches to human HSCs is considerable, as it offers a powerful methodology for the characterization of these cells and an approach to permanent correction of various inherited or acquired hematologic, metabolic and immunologic disorders. Gene transfer of a therapeutic gene into human HSCs is necessary to achieve long-lasting correction; mature cells and committed progenitors do not have the proliferative capacity for long-term reconstitution of the entire hematopoietic system and must be replenished from HSCs. However, direct application of gene transfer techniques developed in the mouse to human HSCs initially met with limited success. Recent efforts have centered on the optimization of existing gene transfer approaches using more predictive models to achieve efficient gene delivery into human HSCs.3 The clinical successes that ensued were tarnished by the development of malignancies linked to insertional genotoxicity, forcing the scientific community to further re-evaluate and refine pre-clinical models to be used for testing of potentially safer approaches for HSC gene therapy. This review summarizes the benefits and drawbacks of the laboratory mouse model in the development and safety evaluation of methodologies used for the genetic manipulation of human HSCs for gene therapy applications.
Development of methodologies for the genetic manipulation of human HSCs: the influence of mouse transplantation models
Gene transfer into mouse HSCs
Murine gene marking studies
Early murine transplantation experiments stressed the importance of genetic markers to follow the progeny of HSCs after reconstitution of an ablated syngeneic recipient.4 The use of donor versus host genetic differences, including enzyme isotypes or polymorphic hemoglobin and immunoglobin markers, led to the demonstration that all mature blood cell types in the reconstituted recipient mouse were donor derived but the limited resolution (only two possible markers) of the donor versus host marker system did not permit a definition of the developmental potential, self-renewal capability and overall proliferative capacity of individual stem cells. An important refinement to the transplantation system was achieved with the use of X-ray induced random chromosomal abnormalities as markers for individual stem cells and the clones derived from them.5–8 Although clonally precise, this strategy suffered from low-efficiency as well as marker visibility limited to actively dividing cells, and could reflect abnormal hematopoiesis linked to major mutational events.
Several groups sought to extend the early in vivo clonal analyses by stably integrating new genetic information into the genomic DNA of murine HSCs via transmissible retroviral vectors.9–12 Gammaretroviral vectors (γ-RV) based on murine leukemia virus (MLV), a well-characterized member of the Retroviridae family of viruses, were used in these initial studies. For successful transduction with MLV-based vectors, the target cells must be replicating at the time of infection in order for the vector to enter the nucleus.13 This requirement for active cell division is a disadvantage of MLV-based gene transfer vectors for use in the quiescent HSC targets. Active growth stimulation ex vivo by 3 or more cytokines (e.g. interleukin-3 (IL3), interleukin-6 (IL6) and stem cell factor (SCF)) for 3–4 days was used to coax HSCs into cycle and allow efficient vector entry and integration.14
Marking with provirus provided a more efficient, quantifiable and less detrimental approach than other means of genetic marking. Because of the largely random nature of integration of retroviruses, each cell in an infected population carries the integrated retrovirus at a unique and permanent location. These identifiable integration sites provide a genetic tag to follow the progeny of HSCs after in vivo reconstitution of an ablated recipient. Initial applications of the retroviral marking strategy yielded results that extended earlier classical studies. The observation of lymphoid and myeloid cells harboring the same proviral integrant 10–20 weeks after transplantation confirmed the existence of pluripotent stem cells capable of contributing to all cell lineages.9,11,12 In addition, the ability to accurately quantitate the extent to which a given tissue was repopulated by progeny of marked stem cells made possible a rigorous demonstration of the ability of a single stem cell to reconstitute the entire murine hematopoietic system.9 Molecular analysis of the spectra of proviral integration sites found in each fractionated cell population also provided evidence for the existence of lineage restricted stem cells, as previously proposed.5,8
Murine gene therapy preclinical models for evaluation of MLV-based vectors
The ability of MLV-based retroviral vectors to permanently introduce marker genes into murine cells with lifelong repopulating ability led investigators to test whether this approach was feasible when clinically relevant genes were used. Genetic disorders with high morbidity and mortality and with no effective conventional treatments were considered prime candidates for preclinical evaluation.
Severe combined immunodeficiency disease (SCID)
SCID resulting from adenosine deaminase (ADA) deficiency was initially chosen and stable expression of functional human ADA was demonstrated in all hematopoietic lineages at levels near endogenous murine levels in reconstituted murine transplants.15–20 The efficiencies of gene transfer and ADA levels achieved in these preclinical models, together with the anticipated in vivo selective survival advantage of transduced T cells, were thought to be predictive of successful correction of lymphoid dysfunction in patients with ADA deficiency.21 Gamma-retroviral vector-mediated gene transfer has also been used successfully to evaluate gene therapy strategies in several murine models of human immunodeficiencies, including those cause by deficiency of the common γ-chain expressed in T-cell cytokine receptors (X-SCID),22–24 Jak3 kinase deficiency,25 deficiency in the zap70 protein,26 and recombination-activating gene-2 (RAG-2) deficiency.27
Gaucher disease
Gaucher disease is an inherited deficiency of the lysosomal enzyme glucocerebrosidase (GC) and the associated accumulation of glucocerebroside in the lysosomes of macrophages results in multisystem damage, including hepatosplenomegaly, gradual replacement of bone marrow (BM), skeletal deterioration and neuropathology in some cases of Gaucher disease. Correction of enzyme deficiency in macrophages by HSC gene therapy has been considered an attractive therapeutic option for these patients. A transgenic GC-deficient mouse model has been created but, due to alternative physiological pathways of lysosomal metabolism in rodents, the resulting phenotype was significantly different from that observed in humans.28 The profoundly severe phenotype observed in this model resulted in perinatal lethality that precluded its use in most gene transfer studies. However, normal mouse BM transplant models have been invaluable for initial evaluation of MLV-based gene transfer vectors developed for Gaucher disease.29–34 These studies established the feasibility of efficient transfer of the GC gene to normal mouse HSCs and long-term expression in their progeny after reconstitution, strengthening the rationale for gene therapy as a treatment option for Gaucher disease.
Hemoglobinopathies
Hemoglobinopathies, including β-thalassemia and sickle cell disease, were also among the first diseases selected as targets for genetically based therapeutic approaches. Transfer of the human β-globin gene into murine HSCs proved more challenging due to the requirement to include specific endogenous regulatory elements from the β-globin locus that were obligatory to achieve clinically relevant expression. Early preclinical studies demonstrated that MLV vectors containing the β-globin gene and its promoter could transduce murine HSCs. However, human β-globin gene expression was either absent or very low, usually varying between 0% and 2% of mouse β-globin RNA levels.35–41 As outlined below, it is not until new vector designs became available that successful genetic correction of β-thalassemia was first demonstrated in murine preclinical models, laying the foundation for a recent clinical trial of gene therapy for β-thalassemia.42
Chronic granulomatous disease (CGD)
CGD results from mutations in any one of four genes encoding the essential subunits of the phagocytic antimicrobial system NADPH phagocyte oxidase (phox)43, including gp91phox, p22phox, p47phox, and p67phox, rendering individuals born with CGD particularly susceptible to bacterial and fungal microorganisms. MLV-based gene transfer vectors have been tested in two CGD knockout mouse models. In studies utilizing the gp91phox−/− (X-CGD) mouse, BM cell were transduced with a γ-RV harboring the murine gp91phox cDNA and transplanted into lethally irradiated syngeneic X-CGD recipients.44,45 Long-term (≥18 months) restoration of NADPH oxidase activity was demonstrated in 50–80% of peripheral blood neutrophils and gene corrected neutrophils were detected in secondary transplants, indicating successful transduction of long-term reconstituting murine HSCs. Improved resistance to respiratory challenge with Aspergillus fumigates was achieved in all mice with >10% gene-corrected neutrophils, consistent with observations in human X-CGD carriers in which women with low levels of functional neutrophils (5–20%) were usually resistant to fungal infections.46,47 In another study, recipient p47phox−/− animals received a sublethal dose of radiation prior to transplantation of p47phox−/− BM transduced with a MLV-based retroviral vector carrying the p47phox gene.48 The percentage of superoxide-generating peripheral blood neutrophils increased up to 17% in individual mice but long-term expression was not detected.48 Nevertheless, mice challenged with B. cepatia at a time when ~3% oxidase-positive neutrophils were present in the peripheral blood had significantly prolonged survival compared to untreated mice. Taken together, these studies suggested that gene transfer into murine HSCs is feasible and that partial reconstitution of NADPH oxidase activity achieved after retroviral gene transfer can improve host defense if an adequate number of phagocytes exhibit enzyme activity.
Wiskott-aldrich syndrome (WAS)
WAS is an X-linked complex primary immunodeficiency disorder caused by mutations in the gene that encodes the WAS protein (WASP)49. It is characterized by an increased susceptibility to recurrent infections associated with adaptive and innate immune deficiency, thrombocytopenia, eczema and autoimmunity.50,51 The generation of two WASP-deficient mice have facilitated preclinical safety and efficacy studies of MLV-based vectors for the treatment of WAS. In one study,52 the WAS gene was inserted into hemizygous WASP-deficient animals BM cells using a γ-RV vector, followed by transplantation into lethally irradiated WASP− recipients. Vector-mediated WASP expression was shown to correct the T-cell proliferative and cytokine secretory defects, as well as the defective secondary T-cell response to influenza virus infection in WASP− mice. In a similar study, investigators also demonstrated rescue of T-cell signaling and amelioration of colitis upon transplantation of transduced WAS HSCs in mice, supporting the development of gene therapy approaches for WAS.53
Gene transfer into human HSCs: lessons from the first generation gene transfer trials
Because of the absence of in vivo assays that measure the repopulating capacity of human HSCs, gene transfer protocols employed in early clinical trials were adapted from the murine studies described above, and initially tested using human in vitro colony forming cell (CFC) assays that detect committed progenitor cells, and long-term bone marrow culture (LTBMC) assays that detect a cell capable of maintaining production of CFC for at least 5 weeks on a layer of stromal cells. Notwithstanding the fact that progenitors do not have the same biological properties as stem cells, investigators were initially encouraged when high gene transfer efficiency (up to 100%) was observed in these in vitro progenitor assays using gene transfer vectors and transduction conditions comparable to those employed in the mouse. 54–56 Demonstration of correction of GC and ADA deficiency after retroviral vector-mediated gene transfer into progenitors from patients with Gaucher disease57 and ADA-SCID,58 respectively, provided even further impetus to regulators and investigators to initiate a first generation of human HSC gene transfer clinical trials, including gene marking studies and gene therapy trials with therapeutic intent (Table 1-First generation HSC gene therapy clinical trials). However, it was not long before the drawbacks of relying on murine studies and in vitro assays as surrogates for optimization of gene transfer in human HSCs became overtly apparent to investigators anxious to see patients in need benefit from this procedure.
Table 1.
First, Second and Third Generation HSC Gene Therapy Clinical Trials
| FIRST GENERATION HSC GENE THERAPY CLINICAL TRIALS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Indication | Gene | CD34+ cells | Transduction | Conditioning | Outcome | |||
| Cytokines and FN |
Duration (MOI) |
Vector | Gene marking (# patients) |
Genotoxicity (# patients) |
|||||
| Brenner MK et al. (1993)59 | Gene marking (AML) |
NeoR | BM | No cytokines No FN | P: 0hr T: 6hrs (MOI=10) |
γ-RV Ampho env MLV-LTR promoter |
Busulfan Cyclophosphamide |
Low long-term gene marking | No |
| Brenner MK et al. (1993)60 | Gene marking (AML, neuroblastoma) |
NeoR | BM | No cytokines No FN |
P: 0hr T: 6hrs (MOI=10) |
γ-RV Ampho env MLV-LTR promoter |
Bu/Cy (AML) Carboplatin/Etoposide (neuroblastoma) |
Low long-term gene marking | No |
| Rill DR et al. (1994)62 | Gene marking (Neuroblastoma) |
NeoR | BM MNC | No cytokines No FN |
P: 0hr T: 6hrs (MOI=10) |
γ-RV Ampho env MLV-LTR promoter |
Carboplatin Etoposide |
Low long-term gene marking | No |
| Deisseroth AB et al. (1994)64 | Gene marking (CML) |
NeoR | BM | No cytokines No FN |
P: 0hr T: 6hrs (MOI=10) |
γ-RV Ampho env MLV-LTR promoter |
TBI Cyclophosphamide Etoposide |
Low long-term gene marking | No |
| Dunbar CE et al. (1995)66 | Gene marking (MM and BC) |
NeoR | BM MPBC |
IL3, SCF +/− IL6 No FN |
P: 0hr T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
Melphalan/TBI (MM) or ICE chemotherapy (BC) |
Low long-term gene marking | No |
| Cornetta K et al (1996)69 | Gene marking (AML and ALL) |
NeoR | BM | No cytokines No FN |
P: 0hr T: 4hrs |
γ-RV Ampho env MLV-LTR promoter |
Busulfan Cyclophosphamide |
Low long-term gene marking | No |
| Emmons RV et al. (1997)67 | Gene marking (MM and BC) |
NeoR | BM MPBC |
No cytokines +/− Stroma No FN |
P: 0hr T: 6–72hrs |
γ-RV Ampho env MLV-LTR promoter |
Melphalan/TBI (MM) or ICE chemotherapy (BC) |
Low long-term gene marking | No |
| Bachier CR et al. (1999)68 | Gene marking (NHL) |
NeoR | BM MPBC |
No cytokines No FN |
P: 0hr T: 6hrs (MOI=10) |
γ-RV Ampho env MLV-LTR promoter |
TBI Cyclophosphamide Etoposide |
Low long-term gene marking | No |
| Alici E et al. (2007)65 | Gene marking (MM) |
NeoR | BM MPBC |
IL3, IL6, SCF, bFGF No FN |
P: 0hr T: 12hrs (MOI=5) |
γ-RV Ampho env MLV-LTR promoter |
Melphalan | Low long-term gene marking | No |
| Bordignon C et al. (1995)72 | ADA-SCID | ADA | BM | Co-culture No cytokines No FN |
P: 0hr T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Low long-term gene marking | No |
| Kohn DB et al. (1995, 1998)74,302 | ADA-SCID | ADA | CB | IL3, IL6, SCF FN |
P: 0hr T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Low long-term gene marking | No |
| Malech HL et al. (1997)76 | CGD | P47phox | MPBC | PIXY321, G-CSF No FN |
P: 18hrs T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Low long-term gene marking | No |
| Hesdorffer C et al. (1998)303 | Breast CA Ovarian CA Glioblastoma |
MDR1 | BM MPBC |
IL3, IL6, SCF FN |
P: 48hrs T: 24hrs |
γ-RV Ampho env MLV-LTR promoter |
High dose chemotherapy |
Low long-term gene marking | No |
| Dunbar CE et al. (1998)75 | Gaucher | GC | BM MPBC |
IL3, IL6, SCF No FN |
P: 0hr T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Low long-term gene marking | No |
| Kohn DB et al. (1999)304 | HIV | RRED | BM | IL3, IL6, SCF +/− FN |
P: 0hr T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Low long-term gene marking | No |
| Cowan KH et al. (1999)305 | Breast CA | MDR1 | BM MPBC |
IL3, IL6, SCF No FN |
P: 0hr T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
ICE chemotherapy | Low long-term gene marking | No |
| Liu JM et al. (1999)306 | FA | FANCC | BM MPBC |
IL3, IL6, SCF No FN |
P: 0hr T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Low long-term gene marking | No |
| SECOND GENERATION HSC GENE THERAPY CLINICAL TRIALS | |||||||||
| Reference | Indication | Gene | CD34+ cells | Transduction | Conditioning | Outcome | |||
| Cytokines and FN | Duration (MOI) |
Vector | Gene marking (# patients) |
Genotoxicity (# patients) |
|||||
| Cavazzana-Calvo M et al. (2000)240,243,244 | X-SCID | IL2Rγ | BM | SCF, Flt3L, MGDF, IL3 FN |
P: 24hrs T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Correction of X-SCID (9/10) |
T-ALL (4/9) LMO2, CCND2, BMI1 gene insertions248–250 |
| Abonour R et al. (2000)211 | Germ cell tumors | MDR1 | MPBC | SCF, IL6 or SCF,MGDF, G-CSF FN |
P: 48hrs T: 48hrs |
γ-RV Ampho env HaMSV-LTR promoter |
Etoposide 2250mg/m2 Carboplatin 2100 mg/m2 |
Low long-term gene marking (6/11) | No |
| Aiuti A et al. (2002)253 | ADA-SCID | ADA | BM | SCF, Flt3L, TPO, IL3 FN |
P: 24hrs T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
Busulfan 4mg/Kg | Correction of ADA- SCID (2/2) |
No |
| Amado RG et al. (2004)307,308 | HIV (Phase I) | Anti- HIV1 ribozyme |
MPBC | MGDF, SCF +/− FN |
P: 18hrs T: 72hrs (MOI=5) |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Low long-term gene marking (10/10) | No |
| Gaspar HB et al. (2004)232,242,251 | X-SCID | IL2Rγ | BM | SCF, Flt3L, TPO, IL3 FN |
P: 40hrs T: 56hrs |
γ-RV GALV env MLV-LTR promoter |
No conditioning | Correction of X- SCID (10/10) |
T-ALL (1/10) LMO2 gene insertion252 |
| Thrasher AJ et al. (2005)245 | X-SCID | IL2Rγ | BM | SCF, Flt3L, MGDF, IL3 FN |
P: 24hrs T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Failure to correct X- SCID in older patients (2/2) |
No |
| Ott MG et al. (2006)236 | X-CGD | gp91phox | MPBC | SCF, Flt3L, TPO, IL3 FN |
P: 36hrs T: 72hrs |
γ-RV Ampho env SFFV-LTR promoter |
Busulfan 8mg/Kg | Correction of X-CGD (2/2) |
MDS (2/2) MDS1-EVI1, PRDM16, SETBP1 gene insertions239,246 |
| Gaspar HB et al. (2006)233 | ADA-SCID | ADA | BM | SCF, Flt3L, TPO, IL3 FN |
P: 40hrs T: 56hrs |
γ-RV GALV env SFFV-LTR promoter |
Melphalan 140mg/m2 | Correction of ADA- SCID (1/1) |
No |
| Chinen J et al. (2007)241 | X-SCID | IL2Rγ | MPBC | SCF, Flt3L, TPO, IL3 FN |
P: 16hrs T: 96hrs (MOI=1–2) |
γ-RV GALV env MLV-LTR promoter |
No conditioning | Clinical improvement (3/3) |
No |
| Mitsuyasu RT et al. (2009)308,309 | HIV (Phase II) |
Anti- HIV1 ribozyme |
MPBC | MGDF, SCF FN |
P: 36hrs T: 48hrs (MOI=5) |
γ-RV Ampho env MLV-LTR promoter |
No conditioning | Lower HIV-1 viral load (10/10) |
No |
| Aiuti A et al. (2009)230 | ADA-SCID | ADA | BM | SCF, Flt3L, TPO, IL3 FN |
P: 24hrs T: 72hrs |
γ-RV Ampho env MLV-LTR promoter |
Busulfan 4mg/Kg | Correction of ADA- SCID (9/10) |
No |
| Boztug K et al. (2010)143,247 | WAS | WAS | MPBC | SCF, Flt3L, TPO, IL3 No FN |
P: 48hrs T: 48hrs (MOI=5) |
γ-RV GALV env MPSV promoter |
Busulfan 8mg/Kg | Correction of WAS (9/10) |
T-ALL (4/9) LMO2 +/− other gene insertions247 |
| Kang EM et al. (2010)234 | X-CGD | gp91phox | MPBC | SCF, Flt3L, TPO, IL3 FN |
P: 18hrs T: 96hrs (MOI=2) |
γ-RV Ampho env MLV-LTR promoter |
Busulfan 10mg/Kg | Long-term clinical benefits (2/3) |
No |
| Kang HJ et al. (2011)235 | X-CGD | gp91phox | MPBC | SCF, Flt3L, TPO, IL3 FN |
P: 40hrs T: 40hrs (MOI=1-2) |
γ-RV Ampho env MLV-LTR promoter |
Fludarabine 120mg/m2 + Busulfan 1.6mg/Kg |
Short-term clinical benefit (2/2) |
No |
| Gaspar et al. (2011)231 | ADA-SCID | ADA | BM | SCF, Flt3L, TPO, IL3 FN |
P: 40hrs T: 56hrs |
γ-RV GALV env SFFV-LTR promoter |
Melphalan 140mg/Kg or Busulfan 4mg/Kg |
Correction ADA- SCID (4/6) |
No |
| THIRD GENERATION HSC GENE THERAPY CLINICAL TRIALS | |||||||||
| Reference | Indication | Gene | CD34+ cells | Transduction | Conditioning | Outcome | |||
| Cytokines and FN |
Duration (MOI) |
Vector | Gene marking | Genotoxicity | |||||
| Cartier N et al. (2009)142 | X-ALD | ABCD1 | MPBC | SCF, Flt3L, MGDF, IL3, FN |
P: 19hrs T: 17hrs (MOI=25) |
SIN-HIV-1 VSV-G env MND promoter |
Busulfan 16mg/Kg + Cyclophos 200mg/Kg |
Corrrection of X- ALD (2/2) |
No |
| Cavazzana-Calvo et al. (2010)42 | β-thalassemia | β-globin | BM | SCF, Flt3L, TPO, IL3 FN |
P: 34hrs T: 18hrs |
SIN-HIV-1 VSV-G env β-globin promoter β-LCR |
Busulfex 12.8mg/Kg | Correction of β- thalassemia (1/1) |
Clonal dominance (1/1) HMGA2 insertion42 |
ADA (adenosine deaminase); ALD (adrenoleukodystrophy); ALL (acute lymphoblastic leukemia); AML (acute myelogenous leukemia); Ampho (amphotropic) BC (breast cancer); bFGF (basic fibroblast growth factor); BM (bone marrow); BMI1 (B lymphoma MLV insertion region 1 homolog); Bu (busulfan); CB (cord blood); CCND2 (cyclin D2); CGD (chronic granulomatous disease); CA (cancer); CML (chronic myelogenous leukemia); Cy (cyclophosphamide); Env (envelope); EVI1 (ecotropic virus integration site 1 protein homolog); FA (Fanconi anemia); FANCC (Fanconi anemia complementation group C); Flt3L (fms-related tyrosine kinase 3 ligand); FN (fibronectin); GALV (gibbon-ape-leukemia-virus); GC (glucocerebrosidase); G-CSF (granulocyte-colony stimulating factor); HaMSV (Harvey murine sarcoma virus); HIV (human immunodeficiency virus); HMGA2 (high mobility group AT-hook 2); ICE (ifosfamide, carboplatin, etoposide); IL2Rγ (interleukin 2 receptor γ chain); ABCD1 (ATP binding cassette subfamily D, member 1); IL3 (interleukin 3); IL6 (interleukin 6); LCR (locus control region); LMO2 (LIM domain only 2); LTR (long-terminal repeat); MDR1 (multidrug resistance gene 1); MDS (myelodysplastic syndrome); MGDF (megakaryocyte growth and development factor); MLV (murine leukemia virus); MM (multiple myeloma); MNC (mononuclear cells); MND (myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer binding site substituted); MOI (multiplicity of infection); MPBC (mobilized peripheral blood cells); MPSV (myeloproliferative sarcoma virus); NeoR (neomycin resistance); NHL (non-Hodgkin’s lymphoma); P (prestimulation); Phox (phagocyte oxidase); PRDM16 (PR domain containing 16); RRED (rev-responsive element decoy); RV (retroviruses); SCF (stem cell factor); SCID (severe combined immunodeficiency disease); SETBP1 (SET binding protein 1); SFFV (spleen focus forming virus); SIN (self-inactivating); T (transduction); T-ALL (T-lineage acute lymphoblastic lekemia); TBI (total body irradiation); TPO (thrombopoietin); VSV-G (vesicular stomatitis virus-G protein); WAS (Wiskott Aldrich syndrome).
Human gene marking studies
As previously done in the mouse BM transplantation model, Brenner and colleagues utilized MLV-based retroviral vectors as a tool for marking human HSCs in patients with acute myeloid leukemia (AML) or neuroblastoma undergoing autologous BM transplant after consolidation therapy to determine whether contaminating tumor cells in the transplant contributed to tumor relapse and to establish if transduced HSCs could contribute to long-term hematopoietic reconstitution.59–63 Several patients were shown to relapse with genetically marked cells, implying that the “remission” BM contained tumor cells that contributed to relapse. The presence of the marker gene was also followed in non-malignant hematopoietic cells. Gene-marked cells contributed for only 0.1–1% of the total BM but detection of the marker gene in T cells and B cells for as long as 18 months after transplantation was consistent with low level transduction of primitive hematopoietic cells with multilineage capacity. In a similar marking study, Deisseroth et al. determined that autologous BM used for transplantation in patients with chronic myelogenous leukemia (CML) following intensive therapy also contained cells that contributed to relapse.64 Other gene marking trials involving patients with multiple myeloma65–67, breast cancer,66,67 follicular lymphoma,68 and AML69 failed to show stable levels of marked cells of more than 0.1% after transplantation with autologous gene marked grafts, contrasting with the high levels marking observed in preclinical mouse models.
Gene therapy trials with therapeutic intent
In spite of the low transduction efficiency observed in human gene marking studies, a wealth of clinical protocols with therapeutic intent were subsequently approved throughout the world (Table 1-First generation HSC gene therapy clinical trials). As in the murine preclinical models, SCID resulting from ADA deficiency has been the focus of the initial gene therapy attempts. T cells,70,71 BM,72,73 or cord blood (CB) CD34+ cells74 were used as targets of transduction. In one study,74 the continued presence and expression of the ADA gene for 18 months demonstrated that CB cells had been genetically modified with retroviral vectors and could engraft in neonates. However, only 0.01% of the patient total BM cells were shown to contain the transferred gene, indicating that the anticipated survival advantage of transduced T cells was not substantial and far below clinical benefit.
Modeling preclinical murine transplantation models, gene therapy was also attempted for Gaucher disease.75 Akin the previous gene therapy trials, no conditioning was used in these patients prior to infusion of G-CSF mobilized or BM CD34+ cells transduced with MLV-based retroviral vectors carrying a normal GC cDNA. Unlike results obtained in the mouse, low numbers of gene corrected cells were again detected, with little or no expression and no disease correction following transplantation.
Similar results were obtained in a clinical trial for an autosomal recessive form of CGD.76 Five patients received transduced G-CSF mobilized CD34+ cells without preconditioning. Corrected granulocytes were detected in the peripheral blood of all individuals for up to 6 months after infusion but the levels (0.004–0.05%) were below the desired 5–10% required for therapeutic effects.
Murine transplantation models: poor predictors of gene transfer efficiency in human HSCs
While these initial studies established the proof of principle of transduction of primitive human repopulating hematopoietic cells, they uniformly failed to reach and maintain therapeutically relevant levels of genetically modified cells. This striking difference in outcome observed between mouse and human studies is thought to result from intrinsic differences between mice and humans, resulting in a different susceptibility to retroviral transduction and a different ability of transduced HSCs to engraft. Mice and humans differ in size, environmental exposure and lifespan, imposing different selective proliferative pressures on HSCs from each species. As a result, the balance between self-renewal and proliferation, as well as quiescence and proliferation, is altered in human compared to mouse HSCs. For instance, the number of red blood cells (RBCs) produced by mice in a 2-year lifespan is comparable to the number of RBCs produced daily by a healthy adult individual.77 Moreover, competitive transplantation studies in mice have estimated that murine HSCs divide every 2.5 weeks in vivo,78,79 significantly faster than human HSCs estimated to divide every 40–45 weeks.80,81 This difference is further amplified by a decreased sensitivity of mouse HSCs to cytotoxic agents compared to human HSCs,82 allowing the administration of 5-fluorouracil to donor mice prior to collection of HSCs to increase cell cycling of HSCs without decreasing HSC numbers significantly. These considerations are important for gene transfer with MLV-based retroviral vectors given the inability of these vectors to transduce quiescent cells.83 The minimal expression of amphotropic surface receptors used by MLV-based gene transfer vectors on human HSCs has also been proposed for the poor susceptibility of these cells to retroviral vectors.84
While culture of early mouse hematopoietic cells under stimulatory conditions improved gene transfer efficiency, these ex vivo culture conditions were subsequently shown to result in loss of at least 75% in vivo repopulating stem cell activity, compared to non-cultured BM.85–87 A similar loss in repopulating activity has also been reported after culture of human88,89 and rhesus macaque90 HSCs. This observation has recently been proposed to result from the disruption of an organized membrane domain on repopulating cells as a result of increased cell cycling during ex vivo culture91, consistent with the finding that progenitor and stem cells in G2/S/M phase of the cell cycle engraft poorly compared to cells in G0 and G1.92 However, because mouse strains used in biomedical research are inbred and maintained in a protected pathogen-free environment, unlike genetically diverse human populations exposed to more complex environmental stimuli, murine engraftment and survival could occur even when a small number of transduced HSCs were transplanted. Therefore, detection of transduced HSCs and their progeny after transplantation was robust, circumventing the need to improve upon gene transfer protocols or ensure retention of HSC functional activity during culture and transduction.
The recognition of differences in HSC behavior between mice and humans and the disappointing results in the early human HSC gene therapy clinical trials underscored the need for alternative assays similar to those available in mice that permit direct identification and characterization of human long-term reconstituting cells and their susceptibility to gene transfer. These assays allowed investigators to refocus efforts on better understanding of target cells and vector biology, optimization of in vitro transduction conditions, and use of conditioning regimens to favor engraftment of transduced cells.
Optimization of human HSC gene transfer protocols: the role of mouse xenotransplant and large animal models
Development of preclinical animal models for investigating gene transfer into human HSCs
Large animal models
Since prolonged follow-up of in vivo hematopoiesis is the most reliable parameter to identify human HSCs, large animals are more adapted than mice for evaluation of gene transfer to long-term repopulating cells, given their lifespan and their size, allowing repetitive blood and marrow sampling. The group of Zanjani, capitalizing on the immunologically tolerant environment of the fetal sheep between 50 and 60 days of gestation, successfully showed engraftment and persistence of human cells several years after intraperitoneal administration in utero of primitive human hematopoietic cells from CB or BM.93–95 Among fetuses reaching maturity, at least 50% showed long-term persistence of human cells and demonstration that retrovirally transduced human cells could be detected,96 suggesting transduction of primitive human HSCs. However, the procedure was hampered by low-level engraftment, a high rate of fetal loss, and impracticality for most research groups.
An alternative approach based on autologous HSC transplant in dogs and non-human primates, including baboons and Old World rhesus macaques, has proven to be invaluable in gene transfer preclinical research. Cells from these species cross-react with key anti-human antibodies found on progenitor and stem cells (e.g. CD34), respond to human cytokines and have stem cell dynamics approximating those found in humans. In contrast to murine studies, results from early clinical trials were very similar to data obtained in non-human primates and dogs using similar vector systems and transduction conditions,97 validating the importance of large animal models for the optimization of gene therapy strategies. The value of these models for preclinical testing was also illustrated when aggressive T cell lymphomas developed in three of ten rhesus macaques transplanted with BM cells transduced with retroviral vector stocks contaminated with replication-competent retrovirus (RCR).98 This finding resulted in the introduction of more stringent requirements for preventing and detecting RCR in vector formulations developed for clinical applications. However, given the high purchase costs of these animals, the space requirements for husbandry, and the sophisticated training required to collect stem cells and support them through myeloablative transplantation, mouse xenotransplant models have been exploited as a more practical alternative approach for preclinical gene transfer testing in human HSCs.
Mouse xenotransplant models
The most widely used murine xenotransplant model was developed using a strategy closely modeled on human BM transplantation and conventional murine HSC assays. Initially, large numbers of human BM cells were intravenously injected into sub-lethally irradiated mice lacking a functional immune system to prevent rejection of the injected human cells, including SCID and beige/nude/xid (bnx) mice.99,100 With regular treatment of engrafted SCID mice with a combination of human hematopoietic cytokines, human BM cells that migrated to the mouse marrow and spleen gave rise to a small but sustained pool of myeloid progenitors and B cells for several months, indicating that the engraftment was long-term and multipotent, fulfilling two key criteria used to define HSCs.100
The SCID mouse used in these initial studies can spontaneously generate murine T and B cells with age and has high levels of natural killer (NK) cell activity, impeding efficient and prolonged xenograft. To improve upon the available immunodeficient mouse strains, the SCID mutation was backcrossed onto the non-obese diabetic (NOD) background.101 The resultant NOD-SCID mouse has reduced innate immunity and superior engraftment of human hematopoietic cells.102 However, this strain has a high incidence of spontaneous thymic lymphomas resulting in a short lifespan, a striking preferential development of human B cells after transplantation, and a persistence of murine NK cells resulting in a lack of human T- and NK-cell differentiation in this strain. Reducing or abolishing NK activity in NOD-SCID mice by infusion of antibodies against NK1.1 or the IL2 receptor β chain (IL2Rβ, CD122),103,104 or genetic manipulation by introduction of the IL2 receptor γ chain c (IL2Rγc) deletion (NSG mice) or truncation (NOG mice) strikingly improved permissiveness towards human T and NK lymphoid differentiation, decreased incidence of thymic lymphoma, and murine T and B cell leakiness. This strain is currently the most widely used humanized mouse model for gene therapy pre-clinical testing.105–107
The immunodeficient mouse model assays a human cell population operationally defined as SCID-repopulating cells (SRCs). In contrast to preclinical standard murine transplantation studies and human in vitro progenitor assays, the xenotransplantation model was shown to provide a more accurate prediction of the low levels of HSC gene marking reported in human clinical trials and large animal models; whereas CFC and long-term culture initiating cells (LTCIC) were efficiently transduced using gene marking approaches similar to those used in mouse transplant studies and early clinical trials, SRC were rarely transduced and therefore distinct from progenitor cells.56 Using improved transduction conditions (see below), Guenechea G. et al.108 subsequently showed that individual engrafted and marked human SRCs could produce a large clone of differentiated progeny with engraftment potential in secondary NOD-SCID recipients, demonstrating the proliferative and self-renewal capacity of SRCs. Although this study did not contain a direct analysis of individual lineages, a recent investigation by the same group109 successfully tracked self-renewal and multilineage output of single human cells purified using a complex set of markers (CD34+CD38-CD45RA-CD90+CD49f+Rholo) for at least 8 months after transplantation in NSG mice. Together, these studies fulfilled the proliferative, self-renewal and multipotential criteria previously used to define murine HSCs, providing a compelling characterization of human HSCs and relevance for preclinical testing of human HSC gene therapy.
Although, in practical terms, the above studies served to validate the murine xenotransplant model as a sound surrogate assay system for human stem cells, a number of limitations have cast doubt on its usefulness to the study of human HSCs. For instance, the various human cell lineages that differentiate and proliferate from transplanted SRCs may not be detected simultaneously, with an early (2–4 weeks) but usually non-persistent RBC contribution and a late (>12 weeks) T cell detection. Thus, a careful kinetic analysis is required when evaluating human cell engraftment in these mice. This caveat is compounded by the practical 8–12 week-post-transplant endpoint adopted by most investigators for assessment of engraftment, perhaps limiting the ability to distinguish between long-term and short-term repopulating cells.109,110 A study in non-human primates (baboon) which compared autologous reconstitution of retrovirally marked cells infused in parallel in the donor non-human primate and NOD-SCID mice have suggested that clones detected in NOD-SCID mice were only found early after transplantation in the baboon but did not contribute to long-term (>6 months) hematopoiesis.111 The authors deduced that most cells assayed in murine xenografts must represent short-term repopulating cells, but their conclusion was derived from the analysis of only two clones from one baboon.
The development of better assays to evaluate gene transfer efficiency in human repopulating cells has culminated over the past two decades in various strategies to improve gene transfer protocols, including: 1- New retroviral vector designs; 2- Optimized transduction conditions; and 3- Use of conditioning regimens to favor engraftment of transduced cells.
Use of alternative gene transfer vectors based on lentiviruses
Early preclinical murine studies established MLV-based vectors as a tool of choice for efficient transduction of mouse HSCs but the contrasting poor in vivo marking levels observed in more relevant preclinical models and in actual gene therapy trials led investigators to explore alternative gene transfer vehicles for human HSCs. In the late 1990’s, retroviral vectors based on lentiviruses (e.g. human immunodeficiency virus-1, HIV-1) were designed to overcome the shortcomings of MLV-based vectors. Unlike γ-retroviral vectors, lentiviral vectors harbor several viral elements that enhance nuclear translocation and localization, thus allowing enhanced transduction of non-dividing cells such as HSCs.112–122 However, it is also generally agreed that cells must exit G0 and enter G1 for efficient transduction by HIV-1-based vectors.123–125 Because of safety concerns regarding recombination with endogenous HIV, a “self-inactivating (SIN)” feature was incorporated in these vectors by eliminating a portion of the 3’-LTR, which on proviral integration replaces and thus inactivates the 5’-LTR.126 For these SIN vectors, the efficiency of transgene expression is highly dependent on the addition of a ubiquitous internal promoter, such as murine stem cell virus (MSCV), human elongation factor-1α (EF-1α), human phosphoglycerate kinase (PGK), spleen focus-forming virus (SFFV), gibbon-ape leukemia virus (GALV), or the hybrid chicken actin promoter containing the CMV enhancer region (CAG). Wild-type HIV-1 virions infect cells that express the CD4 receptor and an appropriate co-receptor. Because murine and large animal HSCs do not express CD4, wild-type HIV or any lentiviral vectors using the HIV envelope cannot be used in HSC gene therapy applications. Expansion of the cellular tropism of HIV-1-based vectors can be accomplished by substituting the wild-type envelope protein with an envelope protein from a different virus, a process referred to as pseudotyping.127 Superior transduction of HSCs was achieved using the alternative GALV envelope protein, the cat endogenous retroviral glycoprotein (RD114), and the vesicular stomatitis virus-G protein (VSV-G).128–131
Preclinical evaluation of lentiviral vectors using murine xenotransplantation models
Given the established lack of predictability of standard murine transplantation models for evaluation of HSC transduction efficiency in humans, investigators initially chose the murine xenotransplant assay to validate the ability of HIV-1-based vectors to efficiently transduce human hematopoietic cells, including CD34+ cells and the more primitive CD34+/CD38− subset. Several groups reported efficient gene transfer in SRCs derived from human CB132–136 and mobilized peripheral blood137,138 under conditions where MLV vectors were ineffective. High level GFP transgene expression in SRCs capable of secondary136 and tertiary139 multilineage repopulation in NOD-SCID mice suggested transduction of primitive human HSCs with pluripotential, proliferative, and self-renewing capabilities.
Murine xenograft models also provided unique experimental systems to assess disease-specific lentiviral gene transfer vectors for their ability to improve disease phenotype after gene therapy. In one study, immune-deficient mice were transplanted with BM cells from patients with thalassemia major transduced with a SIN-lentiviral vector carrying the human β-globin gene and LCR regulatory sequences.140 Effective human erythropoiesis was achieved with circulating β-globin producing human erythroid cells in the genetically corrected xenografts, at levels comparable to normal BM xenograft controls. Preclinical evaluation has also been documented for X-linked adrenoleukodystrophy (ALD), an inherited demyelinating disorder of the central nervous system caused by mutations in ABCD1, a gene encoding the ALD protein (ALDP) whose function involves peroxisomal import of fatty acids in the organelle.137,141 Xenotransplantation of lentivirally transduced human ALD CD34+ cells into NOD-SCID mice demonstrated in vivo expression of ALDP in human monocytes and macrophages derived from engrafted human HSCs. Human BM-derived cells migrated into the brain of transplanted mice where they differentiated into microglia expressing the human ALDP. As described below, the validity of these preclinical data has recently been confirmed in the context of clinical trials for ALD142 and β-thalassemia.42
Murine xenotransplantation has also been utilized to establish preclinical safety and efficacy of HIV-1-based gene transfer vectors for the treatment of WAS, CGD, SCID, and lysosomal storage diseases. As reported below, patients with WAS have been treated using an MLV-based vector methodology,143 and phase I/II gene therapy clinical protocols based on infusion of lentivirally transduced autologous CD34+ cells have also recently started.144 In a preclinical study, Aiuti and colleagues145 transduced human CD34+ cells derived from the BM or mobilized blood of WAS patients using a good manufacturing practice (GMP)-grade lentiviral vector encoding WASP. Transduced human cells infused in immunodeficient mice could repopulate and differentiate into multiple lineages with a polyclonal pattern of integration in the absence of vector shedding or germline transmission, providing a rationale for the use of this vector in patients.145 Similar conclusions were derived from xenotransplant preclinical studies for X-CGD; sustained gp91phox expression and correction of the defective oxidase function was achieved in the lentivirally transduced human X-CGD myeloid cells arising from the human xenograft in the BM of NOD-SCID mice, suggesting efficacy of lentiviral vectors for clinical applications in CGD.146,147 HIV-1-based vectors for SCID gene therapy have also been investigated in immunodeficient mice.148 Human CD34+ cells from SCID patients have been transduced with a SIN lentiviral vector containing the Artemis or RAG-1 gene, followed by transplantation into NOD-SCID mice. Transduced cells differentiated into functionally mature B cells, with human IgM present in the serum of the recipient mice, providing a useful model for evaluation of gene transfer efficiency in human SCID forms affecting B cell development. The feasibility of lentiviralbased HSC gene therapy was also investigated in NOD-SCID mice for mucopolysaccharidosis type VII (MPSVII), a lysosomal storage disease (LSD) caused by β-glucuronidase (GUSB) deficiency.149 Human GUSB-deficient mobilized peripheral blood CD34+ cells from a patient with MPSVII were transduced with HIV-1-based vectors encoding human GUSB and xenotransplanted in murine recipients that were also GUSB-deficient. Approximately 10% of cells expressed the defective protein and the corrected cells distributed throughout recipient tissues, resulting in therapeutic improvement of the disease phenotype and indication of potential clinical value.
Preclinical evaluation of lentiviral vectors using large animals models
Demonstration of the utility of HIV-1-based lentiviral vectors to transduce hematopoietic cells with long-term repopulating potential was initially met with limited success in rhesus macaques150,151 and baboons.152 These Old World monkeys were found to possess cellular antiviral factors, including tripartite motif-containing 5 isoform-α (TRIM5α) and apolipoprotein B mRNA-editing catalytic polypeptide 3G (APOBEC3G), responsible for the observed resistance to HIV-1 infection. In contrast, HIV-1-based lentiviral vectors could mediate efficient gene transfer to long-term repopulating cells in the canine153 and pigtailed macaque models.154 The TRIM5α-mediated restriction to HIV-1 infection in rhesus macaques was alleviated by the use of an alternative lentiviral vector based on the simian immunodeficiency virus (SIV).155–159 Modification of HIV-1-based vectors by introduction of mutations in the cyclophilin A (CypA) binding site160,161 or by substituting SIV capsid and SIV viral infectivity factor (Vif) in HIV-1 vectors were shown to escape TRIM5α resistance, resulting in superior in vivo marking levels (up to 30%) in all blood lineages in rhesus macaques 3 to 7 months post-infusion.162 Thus, rhesus macaques provide a unique model for preclinical testing of lentiviral vectors intended for clinical applications, as recently shown with a lentiviral vector (TNS9) based on HIV-1 encoding the human β-globin gene flanked by regulatory sequences.163
Only rare human disease phenotypes that can be targeted by gene therapy of HSCs have been recapitulated in large animal models (reviewed in Bauer et al.164). Gene therapy has been investigated in a canine model of Glanzmann thrombasthenia (GT), a platelet adhesion and aggregation disorder resulting from a defect in one of two glycoproteins (GPs), GPIIb or GPIIIa, and leading to bleeding episodes inolving mucosal membranes.165 Autologous canine GT CD34+ cells were collected, transduced using a SIN lentiviral vector carrying a human GPIIb promoter driving the canine GPIIb cDNA, and reinfused into the affected animals after non-myeloablative conditioning. Long-term follow-up revealed expression of the GPIIb/GPIIIa heterodimers in 10% of circulating platelets, buccal bleeding was reduced, and animals were less susceptible to bruising.166 Gamma-retroviral vectors were investigated for in vivo and ex vivo gene therapy of canine X-SCID167 and leukocyte adhesion deficiency (CLAD),168 respectively, and a foamy virus-based vector has been used to phenotypically correct CD18 integrin deficiency in a canine model of LAD169, but no other reports of preclinical testing of lentiviral vectors in large animal disease models are available.
Preclinical evaluation of lentiviral vectors using standard murine transplantation models
Despite the lack of predictability of standard murine transplantation studies for evaluation of gene transfer into human HSCs, mouse disease models developed by gene targeting or arising from spontaneous gene mutations may represent the only available option for preclinical testing of the impact of gene transfer vectors on disease phenotype; large animal disease models do not exist for most disorders treated by gene therapy, and for a number of orphan diseases that are common targets of gene transfer protocols, it is impractical to obtain biospecimens from a large cohort of patients to perform preclinical murine xenotransplant efficacy studies.
A large number of investigations using murine disease models as surrogates in gene therapy preclinical studies have shown partial or complete correction of the underlying abnormal phenotype and thus have encouraged in some cases instigation of new clinical trials. All disorders with active HSC gene therapy clinical trials have employed murine models of human diseases to evaluate efficacy of lentiviral vectors for correction of disease phenotype, including ALD,142 WAS,170–175 β-thalassemia,176–179 SCID,180–184, and CGD.185 Human gene therapy trials employing lentiviral vectors are currently underway for ALD, β-thalassemia and WAS. However, in several cases, these murine homologues were limited as translational models by the fact that mutations in the affected gene do not faithfully reflect the clinical manifestations of such mutations in humans. For instance, because the ALD mice do not develop cerebral demyelination,186 the neuropathological and clinical effects of lentiviral gene transfer could not be assessed in this model. Also, expression of human proteins in a murine disease model may not provide optimal correction of the human disease characteristics. This is illustrated in β-thalassemia mouse models using vectors encoding the human β-globin gene; tetramers of human β-globin must form with mouse α-globin, likely underestimating the degree of correction that would be achieved with similar vectors in human cells, where natural tetramers of human α and β globin would be formed.
Optimization of transduction conditions
Cytokine combinations
Various procedures have been refined by different laboratories to optimize retrovirus-mediated transduction in HSCs. A balance must be reached to reconcile the need for cell cycling for productive transduction without impairing the repopulating ability of HSCs. In the mid-1990’s, the addition of fms-related tyrosine kinase 3 ligand (Flt3L) to the cytokine combination IL3, IL6 and SCF used in original murine studies helped preserve human CD34+ cells to sustain long-term hematopoiesis in the murine xenotransplant model after retroviral-mediated transduction.187 Subsequently, consistent with evidence that the early acting hematopoietic growth factor thrombopoietin (TPO) or its truncated homologue, megakaryocyte growth and development factor (MGDF), could better support primitive human hematopoietic cells in vitro,188,189 studies in non-human primates showed that IL3 and IL6 could be replaced by MGDF during ex vivo transduction.190–192 While the addition of IL3 during transduction was shown to impair the engraftment of murine HSCs cultured ex vivo,193,194 this finding was not confirmed in large animal models191 and most groups have thus continued to use IL3 during transduction of human HSCs in clinical applications. The mechanism of action of these early acting cytokines is generally related to their effect on cell cycle status, but they have also been proposed to enhance transduction by down-regulating proteasome activity in HSCs.195,196 Overall, the current consensus based on large animal and murine xenograft models favors a transduction step in the presence of an early-acting cytokine cocktail composed of SCF, Flt3L, TPO/MGDF, with the optional addition of IL3.
Use of Fibronectin during transduction
The function of HSCs depends upon the signals from surrounding stromal cells (e.g. fibroblasts, osteoblasts) and extracellular matrix molecules (e.g. fibronectin, FN) found within the highly specialized BM microenvironment. In murine studies, co-culture of the hematopoietic target cells with the fibroblast-derived retroviral producer cells has been shown to be the most efficient gene transfer method. However, this approach is inappropriate for use in human clinical trials because of the risk of co-infusing the producer cells into patients along with the transduced hematopoietic cells.
To expand clinical applicability of murine gene transfer protocols, several investigators showed increased gene transfer in human hematopoietic cells adherent to autologous or allogeneic BM stromal cells using in vitro assays.197–199 Similarly, addition of autologous stromal cells during the transduction period promoted gene transfer into HSCs in the rhesus macaque model.200 However, the technical difficulties associated with extensive scale-up and the lack of clear benefit in a gene marking clinical trial have limited widespread clinical application of this approach.67 Moritz et al. took a different tactic by transducing human hematopoietic cells cultured in vessels coated with the chemotrypic 35-kd carboxy-terminal fragment of human fibronectin (FN35) and noted enhanced transduction by retroviral vectors.201 A recombinant version of this fragment (CH-296), commercially available as Retronectin (Takara Biomedical, Otsu, Japan), was shown to enhance gene transfer in human CD34+ cells by co-localization of the cells with the vector,202–204 unless an excess of retroviral particles was used during transduction.205 The colocalization of vectors and target cells can be enhanced using spinoculation, a neologism coined to describe low speed centrifugation used at the start of transduction to augment gene transfer.206 The enhancement effect achieved with FN was also attributed to biological effects on HSCs, such as preservation of repopulating potential,203,207–209 and decreased apoptosis.210 Extensive pre-clinical studies showed successful gene transfer into murine long-term repopulating HSCs as well as into human and non-human primate CD34+ cells derived from BM, CB or mobilized peripheral blood when transduction was performed in vitro210–213 or in vivo213 with retroviral vectors and Retronectin. Correspondingly, in most recent clinical gene therapy trials showing therapeutic benefits, retroviral transduction of human CD34+ cells has been conducted in the presence of human recombinant FN.
Conditioning regimens to enhance in vivo gene marking
Engraftment of HSCs is a competitive process between endogenous and infused stem cells. Conditioning of the patient, traditionally achieved by delivering chemotherapy agents with or without radiation before cell infusion, damages or destroys endogenous stem cells and thus provides a competitive advantage to the infused HSCs. This is of particular interest to enhance engraftment of low numbers of genetically marked HSCs in gene therapy applications. In preclinical models190,200 and in patients with malignancies,60,66,67 myeloablative regimens have been used routinely to enable engraftment of gene-modified primitive hematopoietic cells. However, many of the diseases that could be targeted by gene therapy are chronic, indolent disorders in which the risks of myeloablative regimens may outweigh the potential benefits.
Reduced intensity (non-myeloablative) conditioning regimens were tested in preclinical models to assess engraftment of marked HSCs while reducing transplant-related toxicities. Retrovirally modified hematopoietic cells have been detected at low levels (<1%) in hematologically normal mice even with no myeloablation but infusion of extraordinarily high cell doses obtained from multiple syngeneic donors, impossible to achieve in large animals or humans, was required.214 Moderate-dose irradiation or antimetabolite-based non-myeloablative conditioning allowed correction of the CGD phenotype in knockout mice receiving cells transduced with a retroviral vector containing a normal copy of the defective gene.48,215–218 Similarly, improved immune reconstitution was detected in a mouse model of X-SCID by applying mild conditioning prior to infusion of genetically corrected BM cells.219 Alternative conditioning strategies to promote engraftment of infused HSCs with increased safety have been investigated in mice and are of potential relevance for gene therapy applications. For instance, administration of an antibody that blocks c-kit, the receptor for SCF, transiently reduced endogenous murine HSCs, facilitating engraftment with donor HSCs in immunodeficient Rag2−/−/γc−/− mice but not in immunocompetent mice.220 Combination of this antibody with low-dose irradiation profoundly decreased endogenous repopulating activity, enabling efficient and durable engraftment of fresh or lentivirus-transduced BM cells in wild-type and CGD mice.221 In another study, HSC mobilization with AMD3100, a CXCR4 antagonist with a favorable safety profile in clinical studies, was shown to vacate niches in the mouse BM microenvironment and thus could be used in this model as a preparative regimen for HSC transplantation.222
In large animal models, no engraftment with corrected cells has been observed without conditioning, except for rare detectable neomycin-resistant (NeoR) marrow CFU-GM in dogs receiving cells transduced over a 3-week period in a long-term culture system.223 In one study, 12% gene marked leukocytes persisted in the peripheral blood of a rhesus macaque up to 33 weeks after low dose non-myeloablative total body irradiation.224 In other studies, autologous transplantation of either γ-retrovirus- or lentivirus-transduced HSCs in non-human primates conditioned with low-dose irradiation or busulfan have generally resulted in low but measurable (~1%) long-term marking,158,225–227 providing proof-of-principle that partial marrow cytoreduction allowed engraftment of gene-modified cells without significant toxicity. A possible concern is the unexpected finding that in partially ablated recipient mice, in which the BM is a more competitive environment for repopulation compared to the fully ablated marrow, HSCs carrying survival- or proliferation-activating lentiviral insertions had an advantage for engraftment, perhaps increasing the risk of leukemic progression.228
Initial gene therapy efforts using HSCs for non-malignant disorders did not administer preparative regimens to avoid potential toxicities from chemotherapy agents when benefits of the procedure were unproven (Table 1-First generation HSC gene therapy clinical trials).72,74,76 It was hypothesized that the low marking levels achieved in HSCs in these trials could only lead to clinical benefits if the cells derived from the transduced HSCs had a biological selective advantage in vivo. Modeling conditioning protocols developed in preclinical assays, subsequent gene therapy trials for ADA-SCID,229–233 X-CGD,234–236 WAS,143 and β-thalassemia42 introduced partial myeloablation with busulfan or melphalan prior to infusion of the genetically corrected cells (Table 1). As outlined below, in most cases, successes were detected after non-myeloablative conditioning when augmented by a recognized biological selection for successfully treated cells in vivo or by retroviral-mediated clonal expansion. As predicted by preclinical studies, it appears that reduced intensity regimens employed to date for disorders that lack a biological selective advantage in gene-corrected cells, such as CGD, may be insufficient to mediate substantial HSC engraftment and that more ablative approaches may be necessary. The recent clinical success achieved in X-linked ALD using a conventional myeloablative conditioning (cyclophosphamide 200mg/Kg and busulfan 16mg/Kg) is a useful example of a relatively non-toxic regimen that resulted in clinically relevant levels of HSC marking (10–15%) and long-term myeloid engraftment in the absence of a selective advantage (Table 1).142
Optimized gene therapy protocols
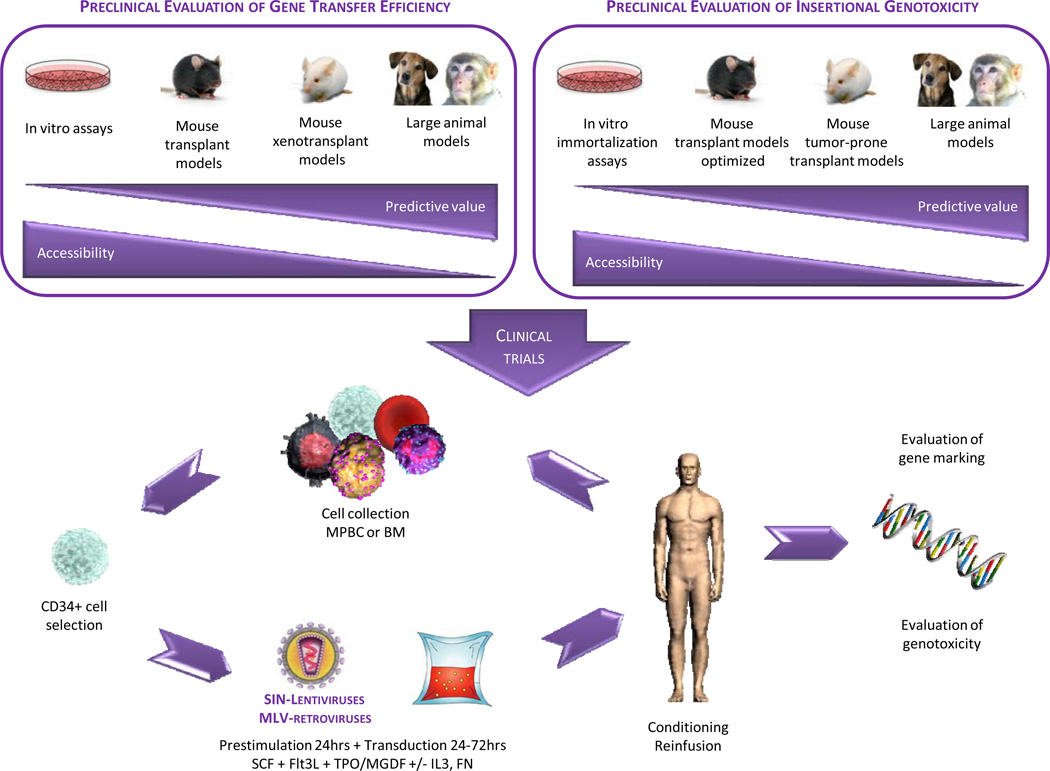
The preclinical studies above (Figure 1, upper left panel), augmented by the clinical experience of the early gene therapy efforts, have led to an optimized protocol for ex vivo manipulation of HSCs that has been employed with success in recent clinical trials (Figure 1, lower panel).237,238 Hematopoietic stem and progenitor cells expressing the CD34 cell surface marker are purified from BM harvests or granulocyte-colony stimulating factor (G-CSF)-mobilized peripheral blood cell (MPBC) collections. Following a brief (~24 hours) ex vivo culture in conditions that favor cell cycle transition from G0 to G1 (e.g. X-vivo medium supplemented with SCF, Flt3L, TPO/MGDF +/− IL3), the CD34+ cells are incubated on a surface coated with the C-terminal fragment of FN in the presence of the same cytokines for an additional 72 hours with MLV-based vectors or for an additional 24 hours with VSV-G-pseudotyped SIN HIV-1-based vectors expressing the desired therapeutic gene from an internal promoter. As discussed below, the increased incidence of genotoxicity in patients transplanted with cells transduced with standard MLV-based vectors has led investigators and regulators to abandon their use in further HSC gene therapy applications.239 For disorders in which corrected cells do not have a competitive repopulating advantage over uncorrected endogenous HSCs and their progeny, the patient is conditioned with a reduced intensity (e.g. Busulfan 2–10mg/Kg) or a fully ablative regimen (e.g. busulfan 16 mg/kg and cyclophosphamide 200 mg/kg), prior to intravenous reinfusion of the treated cells. After autologous transplantation, transduced cells home to the BM where they initiate hematopoiesis. Transduction efficiency and safety of the procedure are evaluated by periodic collection of peripheral blood samples over extended periods of time.
Figure 1. Approach for the genetic manipulation of hematopoietic stem cells (HSCs).
The upper panel compares the predictive value and accessibility of various preclinical models in the evaluation of gene transfer efficiency and insertional genotoxicity of vectors used in HSC gene therapy trials. The lower panel depicts the optimized protocol for transduction of hematopoietic stem and progenitor cells, as used in recent clinical trials.
BM (bone marrow); Flt3L (fms-related tyrosine kinase 3 ligand); FN (fibronectin); IL3 (interleukin 3); MGDF (megakaryocyte growth and development factor); MLV (murine leukemia virus); MPBC (mobilized peripheral blood cells); SCF (stem cell factor); SIN (self-inactivating); TPO (thrombopoietin)
Clinical benefits in gene therapy trials using protocols optimized in preclinical studies
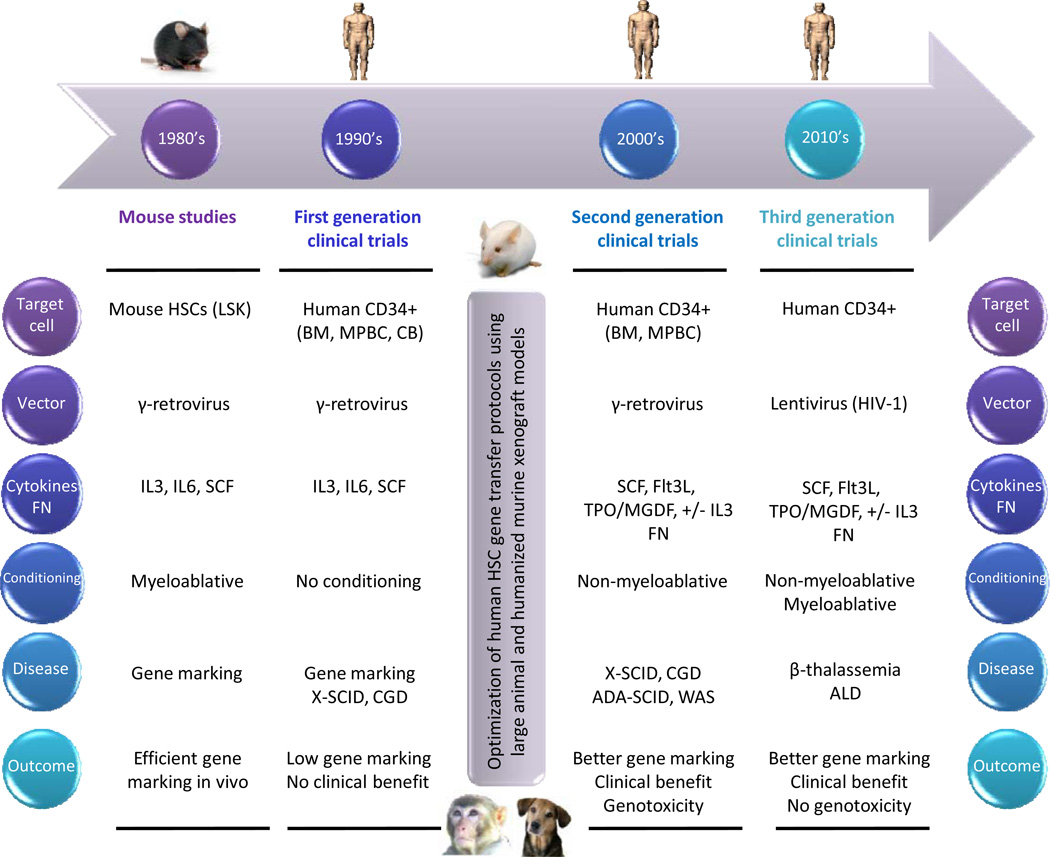
The advances derived from preclinical models were rapidly applied in a second generation of clinical trials, with actual therapeutic intent, for immunodeficiency disorders, including X-SCID,240–245 ADA-SCID,229–231,233 CGD,236,246 and WAS143,247 (Figure 2, Table 1). Third generation HSC gene therapy trials have recently emerged, pioneering the use of SIN-lentiviral vectors for correction of ALD142 and β-thalassemia42 (Figure 2, Table 1).
Figure 2. Timeline for the development of human gene therapy protocols targeting hematopoietic stem cells (HSCs).
The main phases in the evolution of HSC gene therapy clinical trials are ordered chronologically. The six principal features defining each phase are outlined.
ADA (adenosine deaminase); ALD (adrenoleukodystrophy); BM (bone marrow); CB (cord blood); CGD (chronic granulomatous disease); Flt3L (fms-related tyrosine kinase 3 ligand); FN (fibronectin); HIV (human immunodeficiency virus); IL3 (interleukin 3); IL6 (interleukin 6); LSK (Lin−, Sca1+, Kit+); MGDF (megakaryocyte growth and development factor); MPBC (mobilized peripheral blood cells); SCF (stem cell factor); SCID (severe combined immunodeficiency disease); TPO (thrombopoietin); WAS (Wiskott-Aldrich syndrome)
Second generation HSC gene therapy clinical trials
Severe combined immunodeficiency disease
The first HSC gene therapy trial demonstrating unequivocal clinical benefit was led by Fischer and Cavazzana-Calvo at the Necker Hospital in France240,243,244. Ten boys with X-SCID were treated, and each patient received autologous CD34+ BM cells, transduced with a γ-retroviral vector expressing the corrective IL2Rγ gene and reinfused without conditioning. Rapid functional B and T cell immune reconstitution was observed in all but one child. The same vector insertion site could be detected in both myeloid and lymphoid cells, suggesting that at least some true HSCs had been corrected. Unfortunately, the elation after this success was short-lived; four subjects in the trial developed acute T cell leukemia 248–250. The gene therapy vector had inserted near the locus for LMO2, CCND or BMI1 resulting in increased expression of these proto-oncogenes through the vector’s enhancer activity. Chemotherapy led to sustained remission in 3 of the 4 cases of T-lineage acute lymphoblastic leukemia (T-ALL), but failed in the fourth. Patients that were successfully treated with chemotherapy continued to benefit from therapeutic gene transfer as a result of restoration of polyclonal transduced T cell populations after chemotherapy.
A similar gene therapy trial was also conducted in London on ten children with X-SCID. All patients experienced substantial immunological recovery and in most patients this was accompanied by recovery of humoral immunity and withdrawal of immunoglobin therapy232,242,251. Similar to the first clinical study of gene therapy for X-SCID, a single insertion 35 kb upstream of the transcription start site caused substantial overexpression of LMO2 and neighboring genes in one of ten patients252. Leukemogenesis was thought to have been precipitated by the acquisition of other genetic abnormalities, including a gain-of-function mutation in NOTCH1, deletion of the tumor suppressor gene locus CDKN2A, and translocation of the TCR- β region to the STIL-TAL1 locus. These data indicated that conventional murine γ-retroviral vectors (based on MLV) present a high risk of leukemogenesis as a result of their powerful enhancer sequences in the intact LTR regions. While gene therapy has been effective in infants with X-SCID, minimal clinical improvement was detected in 2 of 3 pre-adolescents despite effective transduction and engraftment of G-CSF mobilized CD34+ cells241, and complete failure of gene therapy in two adult patients was reported after transplantation of transduced BM CD34+ cells245.
Bordignon’s and Thrasher’s group also reported sustained engraftment of engineered HSCs and good immune reconstitution in patients with SCID resulting from ADA enzyme deficiency following transplantation of BM-derived transduced CD34+ cells230,233,253. No cases of leukemia have been reported to date in these trials consistent with the long-term analysis of integration sites revealing a polyclonal pattern along with a lack of in vivo skewing for risky insertions.
Chronic granulomatous disease
In an attempt to genetically correct the defect in CGD patients, Manuel Grez and collaborators in Switzerland obtained G-CSF-mobilized CD34+ cells from two CGD patients and, using a vector similar to that used in the X-SCID trials, inserted a corrective copy of one gp91phox gene needed to make a functional NADPH oxidase236. Unlike other CGD trials,76,234,235 three weeks after the cells were reinfused into the patients, a surprisingly large fraction of circulating myeloid cells, more than 20%, carried the corrective gene. This sustained engraftment of functionally corrected cells with therapeutically relevant levels of NADPH oxidase was unexpectedly followed by further in vivo expansion of cell clones containing insertionally activated growth-promoting genes, including MDS1-EVI1, PRDM16 or SETBP1.These activating insertions facilitated expansion of the gene-corrected clones in both individuals, with up to 60% of their white blood cells containing the corrective gene by a year after transplantation. However, a progressive decline in blood counts was observed in both CGD patients at 15 and 28 months, respectively, after gene therapy.239,246 BM examination was consistent with a myelodysplastic syndrome (MDS). Recent evidence suggests that overexpression of EVI1 could have been an initiating event that then resulted in chromosomal instability, stimulating further chromosomal abnormalities, such as loss of chromosome 7, and progression to MDS239,246. Despite the persistent high frequency of vector-corrected neutrophils, expression of NADPH oxidase dropped precipitously in both subjects over time. The silencing of NADPH oxidase occurred through progressive CpG methylation of the promoter contained in the long terminal repeat (LTR) of the vector, a region important for transgene expression. These events led to a series of infections and eventual death of one patient. The second subject was referred for unrelated donor stem cell transplantation while still infection free.
Wiskott-Aldrich syndrome
A German group has recently reported long-term (up to 5 years) correction of WAS in 9 of 10 patients through γ-retroviral infection of G-CSF mobilized CD34+ cells transfused after busulfan-induced transient myelosuppression, as evidenced by a decreased frequency and severity of infections, and resolution of signs and symptoms of autoimmunity, including autoimmune hemolytic anemia, thrombocytopenia, neutropenia and eczema.143,247 However, in an initial report, 2 of 9 patients that achieved an efficient correction of the disease developed T-ALL 5 years and 16 months after gene therapy, respectively. Two additional patients also developed T-ALL more recently (unpublished data). In 3 of 4 patients with T-ALL analyzed to date, an increase of a cell clone harboring an integration site upstream of LMO2 could be observed at the onset of leukemia, the same gene locus activated by vector insertion in most of the X-SCID patients that developed T-ALL. One patient underwent an allogeneic stem cell transplant and the other individuals have responded to chemotherapy with recovery of a polyclonal integration site pattern.
Third generation gene therapy clinical trials
Adrenoleukodystrophy
A third generation of clinical trials have been designed, supported by preclinical data suggesting that HIV-derived lentiviral vectors may be safer and more efficient for transduction of HSCs than vectors based on γ-retroviral vectors113,125,154,156,254–257. The first use of a lentiviral vector for HSC gene therapy was reported for the treatment of two children with X-linked ALD142. This is also the first study using a conventional myeloablative conditioning regimen before reinfusion of the genetically manipulated G-CSF mobilized CD34+ cells. Functional myelomonocytic cells derived from corrected CD34+ cells migrated into the patient’s central nervous system to replace diseased microglia cells. At 24 and 30 months post-transplantation, both patients had highly polyclonal reconstitution of hematopoiesis and normal levels of ALD protein, which appeared to retard the progressive cerebral demyelination process. No evidence of genotoxicity has yet been reported in this trial.
β-thalassemia
Cavazzana-Calvo and colleagues42 have recently provided another example of the clinical potential of gene therapy using lentiviral vectors by treating an 18-year-old male patient suffering from transfusion-dependent βE/β0-thalassemia. CD34+ cells were isolated from the individual’s BM and transduced with an HIV-derived lentiviral vector containing a functional β-globin gene. Compared to previous trials, a conditioning regimen with higher doses of busulfan (12.8 mg/kg) was used in this study. The levels of genetically modified cells gradually rose up to 11% at 33 months post-transplant with concomitant increases in levels of the normal β-globin protein and improved production and quality of normal RBCs. Remarkably, a year after treatment, the patient no longer needed RBC transfusions. This therapeutic benefit, however, was observed in the setting of clonal expansion resulting from integration of lentiviral vectors in the high mobility group AT-hook 2 (HMGA2) gene258. Marked expansion of a single corrected clone, persisting without malignant transformation for many years, has been documented in an early ADA-SCID gene therapy trial,259 suggesting that clonal expansion does not irrevocably progress to malignancy, but the known association between HMGA2 overexpression and benign/malignant neoplasias imposes further caution.258,260,261
EVALUATION OF INSERTIONAL GENOTOXICITY USING MOUSE MODELS
MLV-based retroviral vectors pose a high risk of insertional mutagenesis
Studies performed prior to the availability of the human and murine genome sequences and without high-throughput sequencing methodologies suggested the paradigm that retroviral integration was random, and that insertional mutagenesis was thus likely to be an exceedingly rare event in the absence of replication competent vectors.262 Given the powerful tumor induction models reported by mouse geneticists and cancer biologists, who injected replication-competent MLV viruses into neonatal mice to produce hematopoietic tumors at a high rate, allowing identification of novel proto-oncogenes,263,264 it seems surprising that the issue of insertional mutagenesis was not further seriously considered until the first child enrolled in the X-SCID gene therapy trial developed leukemia in 2003. This paradigm was so pervasive that a paper reporting what in retrospect was clearly a murine myeloid leukemia resulting from a single insertion activating the EVI1 locus with a replication-incompetent MLV vector instead attempted to link the leukemia to aberrant signaling from the truncated nerve growth factor receptor (NGFR) marker gene.265 Another paper suggesting that the multidrug resistance gene 1 (MDR1) could be oncogenic, due to a high rate of leukemia in mice transplanted with cells transduced with MDR1 versus control NeoR vectors, can also be reinterpreted in retrospect as almost certainly representing insertional genotoxicity, given the very high titer and vector copy number associated with a novel Harvey sarcoma virus vector carrying the MDR1 transgene, compared to a standard MLV control NeoR vector.266
Standard murine transplantation models: poor predictors of insertional genotoxicity
Standard murine gene transfer experiments, as performed for twenty years, do not provide robust information on genotoxicity, for a number of reasons. The accepted time point of analysis to document successful HSC gene transfer is only 4–6 months, far shorter than time to induction of murine tumors via insertional mutagenesis and the clinical experience with such tumors, given the multistep nature of their pathogenesis. Mice receive ablative irradiation in most studies, and have such a high rate of radiationinduced tumors that longer term follow-up is challenging, even when a study is designed to monitor insertional mutagenesis.267 A survey of the literature and unpublished studies completed following the 2003 reports of human leukemias in the X-SCID trial confirmed lack of follow-up of longer than a few months in the vast majority of murine studies.268
Most studies utilize the equivalent of one million starting nucleated marrow cells (i.e. ~50 HSCs) to transduce and then transplant per recipient mouse. Ex vivo transduction conditions result in gene transfer efficiencies of less than 50% even in progenitors and in loss of at least 75% repopulating activity, compared to fresh marrow.85 In addition, relatively low-titer vector was used to transduce murine HSCs, resulting in vector copy number per transduced cell of only 1–2. The net result of these factors is transplantation of each mouse with very few transduced HSCs, and thus very limited numbers of insertions to follow for genotoxicity long-term. Early clonal tracking studies utilizing Southern blot documented oligoclonal patterns of contribution from vector-containing cells.10 Even more modern studies, utilizing ligation-mediated PCR (LM-PCR) to retrieve vector insertions and improved transduction conditions, generally only catalog 2–5 insertions per mouse.269,270 Therefore, even cohorts of 10 or more mice per experimental group interrogate less than 100 viral insertions, far fewer than the thousands of insertions documented in the X-SCID and other trials.251,271
Preclinical models to assess the risks of insertional mutagenesis
The high rate of leukemia induction first in the X-SCID clinical trial, followed by similar issues in trials for CGD and WAS, accelerated the paradigm shift towards the realization that all integrating vectors have a risk for insertional oncogenesis, and a focus on the development of predictive models to prevent further unexpected complications in human clinical trials of HSC gene therapy.246,249,252,257 The availability of complete sequences for human, murine and other model organism genomes, and the development of PCR and high-throughput sequencing methodologies for insertion site retrieval from highly complex and polyclonal samples have concurrently permitted a much deeper understanding of the integration patterns and biases inherent to different vector classes and target cells. Over the past decade, many investigators and regulators have thus focused on the development of predictive models of insertional mutagenesis.
In vitro immortalization assays
The quickest, simplest and most quantitative models have utilized murine cells in vitro to document behavior consistent with malignant transformation. Factor-dependent immortalized myeloid cell lines such as 32D convert to cytokine-independence as a result of insertional events.272 Primary murine BM cells transduced with vectors at a high MOI become immortalized at a low rate, and this process can be semi-quantitated, allowing comparisons between different vector backbones.273,274 Both assays monitor only myeloid transformation events, and are skewed very markedly towards a few genes in each assay, those involved in cytokine signaling pathways in the first instance, and EVI1 or genes that upregulate EVI1 in the second. The relevance to human cell immortalization and transformation is not clear, given the inability to create factor-dependent similar human myeloid cell lines, or immortalize human cells via similar approaches or assays.
Murine transplantation assays
Optimization of insertional load using improved transduction and transplantation conditions
Based on the drawbacks of the standard murine transplantation assays detailed above, a number of investigators have attempted to improve their relevance, reproducibility, and speed. Transduction of murine HSCs in the presence of improved cytokine combinations and Retronectin support and using concentrated vector supernatants has increased gene transfer efficiency and vector copy number per cell, and likely maintained a higher number of HSCs during culture and transduction, resulting in a much higher “insertional load” per recipient mouse. Animals are followed longer and secondary transplants further extend follow-up and provide additional selective pressure to help identify dominant or eventually leukemic clones.
A series of landmark papers from Baum’s group reported important insights into vector safety and hematopoiesis, using serial transplantation to enhance detection.269,275,276 Only a relatively small number of clonal insertions leading to dominance or leukemia could be studied, 29 in the initial paper, expanded to 276 via combining information from a number of laboratories. Twenty two percent overlapped with cancer genes identified using retroviral insertional mutagenesis by Copeland and coworkers (the RTCGD database277), and both EVI1 as well as LMO2, the two loci linked to leukemias most frequently in the human gene therapy clinical trials, were detected in these murine transplantation assays. The RTCGD database had already been shown to predict genes dysregulated in spontaneous human AML.278
Tumor-prone mouse models
In order to speed the detection process and have a readout directly linked to cancer, instead of simply clonal dominance, several groups have utilized murine “tumor-prone” models. The Cdkn2a gene product is a tumor suppressor and has a central role in controlling hematopoietic cell senescence and preventing transformation. It is inactivated in a large fraction of human cancers, including leukemias, and was a secondary event, preceding full leukemic transformation, in at least two of the X-SCID leukemias. All mice transplanted with Cdkn2a−/− marrow develop both lymphoid and myeloid leukemias relatively rapidly. Elegant studies from Naldini’s group have utilized this model to study the impact of transducing Cdkn2a−/− marrow with various vectors on latency of leukemia development and tumor type.279,280 The model reads out both myeloid and lymphoid tumors, and the total number of integrations administered and the vector copy number per cell are relevant. The relative risk for leukemia induction could be compared, and MLV vectors with an intact LTR were found to be most potent in shortening latency, followed by lentiviral vectors with an MLV enhancer inserted into their LTR regions. Lentiviral vectors with an internal enhancer/promoter, even a very strong one such as an MLV LTR, were not active in enhancing leukemia induction in this model, and MLV vectors with deletion of the LTR enhancer (SIN design) or lentiviral vectors with an internal non-viral promoter were also no different than mock-transduced cells in leukemia induction. These studies could be carried out in less than a year and with practical mouse cohort sizes of 15–30. That the results from these studies are predictive of genotoxicity in more relevant clinical situations is supported by the lack of clonal dominance and known “dangerous clones” such as EVI1 and LMO2 when comparing standard MLV insertions to LV insertions, even with the presence of a strong viral internal promoter, in both rhesus macaque long-term follow-up studies and human clinical trials.142,156,281–283
Large animal models
Large animal models have provided important information validating the murine models, uniquely provided very long-term follow-up of a much larger number of insertions than have been followed in mice, and uncovered risks associated with specific transgenes or integration sites that were not apparent from the murine models. Soon after the report of T-ALL linked to vector integration in the X-SCID trial, there was intense consternation and attempts by investigators to blame the adverse events on the IL2Rγ transgene or the underlying patient population, instead of integration events alone.284,285 The initial combined data regarding primate and dog HSC gene marking studies was reassuring, with all animals remaining polyclonal and healthy in our initial report, however, few animals in this cohort had high level marking, and actual insertion sites were not identified.286 Therefore it was a shock to us and an alarm for regulators and clinicians when we observed the development of an acute myeloid clonal vector-linked leukemia in a rhesus macaque 5 years following transplantation, with a vector containing only marker genes.287 The insertion site was in the Bcl2A1 gene, encoding an anti-apoptotic protein. We and others then rapidly completed surveys of large numbers of insertions in monkeys and dogs, providing the first evidence in primary cells for a number of very important principles, including a pattern of integrations favoring expressed genes with both MLV- and lentivirus-based vectors, and transcription start sites for MLV-based vectors,283 and marked over-representation of integrations in specific proto-oncogenes such as EVI1, MDS1, HDAC7 and others, in a manner suggesting in vivo selection.282,288 The identification of EVI1/MDS1 as a particularly worrisome locus was prescient, as the CGD trial adverse events demonstrated a few years later.246 Novel vectors were reported as having much less concerning integration patterns in large animal models, including human foamy virus289 and avian leucosis sarcoma virus.290
Kiem and coworkers have demonstrated the unique importance of large animals in their report of the consequences of inclusion of homeobox B4 (HOXB4) in gene transfer vectors. Years of research in the murine model suggested that HOXB4 overexpression from a retrovirus vector could facilitate a benign and still homeostatically-controlled expansion of HSCs, with many potential clinical applications in gene therapy and stem cell transplantation.291 However, Kiem first showed that the impact on large animal HSCs was much less pronounced than in rodents in terms of long-term repopulating stem cell expansion,292 and then reported that two of two dogs and one of two rhesus macaques transplanted with CD34+ cells transduced with a HOXB4 retrovirus vector developed acute leukemias within two years of transplantation, and more recently have shown that other rhesus macaques developed a myelodysplasia-like syndrome.293,294 These findings have convinced the community that any approach utilizing HOXB4 in a gene transfer vector is not safe for clinical applications.
Approach to evaluate the risks of insertional genotoxicity
We believe the most practical, prudent and informative approach to the investigation of relative genotoxicity of gene transfer vectors would involve initial screening utilizing the in vitro immortalization assays, testing of a subset of candidate vectors in optimized murine transplant models or, preferably, in the murine tumor-prone models, followed by testing of promising novel vectors in long-term primate or canine transplantation studies, allowing retrieval of a large panel of insertions and very long-term follow-up. Large animals are not practical for screening of new vectors, and in contrast to gene transfer efficiency predictions, more modern rodent models have proven useful and overall predictive for genotoxicity (Figure 1, top right panel).
CONCLUSIONS AND FUTURE DIRECTIONS
In the hierarchy of laboratory animal species, the mouse rules and has become a central tool of biomedical research. However, the efficiency of gene transfer into human HSCs is poorly predicted by gene transfer studies in mouse HSCs and conventional murine transplant models have fallen short of predicting the risks of insertional mutagenesis. Instead, more modern mouse transplantation assays, namely the xenotransplant and tumor-prone mouse models, together with large animal systems have provided the essential preclinical platform for advancing approaches to improve the efficacy and safety of gene transfer into human HSCs that have underpinned the recent successful clinical applications.
The influence of modern mouse transplant assays and large animal models on approaches to gene therapy is likely to continue growing as new prospects in HSC engineering are being developed. Current preclinical studies are evaluating gene transfer vectors containing a suicide gene, such as the human-derived dimerizable caspase-9 gene,295,296 which would allow ablation of the vector-containing cells in the event of an adverse outcome. Approaches are also being developed to restrict expression of a transgene to a given target cell population, either by modification of the vector envelope to restrict tropism (transductional targeting) or by insertion of a tissue-specific promoter upstream of the therapeutic transgene (transcriptional targeting; reviewed in Frecha C. et al.297). Another promising methodology in gene therapy is the targeting of transgene delivery to a predetermined chromosomal location or the repair of a mutated locus using homologous recombination (HR). Maximization of HR efficiency is a sine qua non goal to achieve clinical relevance, and various techniques have been explored, including the transient expression of an endonuclease, which can be directed to a specific sequence using modular zinc finger proteins,298 homing endonucleases,299 or transcription activator-like effectors (TALEs).300 Xenograft mouse models and large animal studies will be required to assess off-target genotoxicity and efficiency of long-term HSC correction using these methodologies. Finally, the advent of patient-specific induced pluripotent stem cells (iPSCs) may offer a new avenue to generate and select genetically corrected hematopoietic cells, as well as to perform extensive efficacy and biosafety testing in the selected clonal derivatives using murine xenograft and large animal models. The inability to efficiently generate hematopoietic progenitors from iPSCs has limited this approach but recent progress in directly reprogramming somatic cells to a multipotent progenitor via introduction of a single transcription factor (Oct-4), may provide an alternative cell type with therapeutic potential in gene therapy applications.301
We anticipate that the trend established over the past decade will continue as investigators hoping to use these novel technologies to treat humans will need to provide substantial safety and efficacy data in a variety of comparative murine and large animal assays before returning to the clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 2.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 3.Larochelle A, Dunbar CE. Genetic manipulation of hematopoietic stem cells. Semin. Hematol. 2004;41:257–271. doi: 10.1053/j.seminhematol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Micklem HS, Ford CE, Evans EP, Gray J. Interrelationships of myeloid and lymphoid cells: studies with chromosome-marked cells transfused into lethally irradiated mice. Proc. R. Soc. Lond B Biol. Sci. 1966;165:78–102. doi: 10.1098/rspb.1966.0059. [DOI] [PubMed] [Google Scholar]
- 5.Abramson S, Miller RG, Phillips RA. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J. Exp. Med. 1977;145:1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 7.Ford CE, Hamerton JL, Barnes DW, Loutit JF. Cytological identification of radiationchimaeras. Nature. 1956;177:452–454. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- 8.Wu AM, Till JE, Siminovitch L, McCulloch EA. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J. Exp. Med. 1968;127:455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick JE, Magli MC, Huszar D, Phillips RA, Berstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of w/wv mice. Cell. 1985;42:71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- 10.Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 11.Keller G, Paige C, Gilboa E, Wagner EF. Expression of a foriegn gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985;318:149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- 12.Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45:917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 13.Roe T-Y, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolta JA, Smogorzewska EM, Kohn DB. Analysis of optimal conditions for retroviralmediated transduction of primitive human hematopoietic cells. Blood. 1995;86:101–105. [PubMed] [Google Scholar]
- 15.Kaleko M, Garcia JV, Osborne WRA, Miller AD. Expression of human adenosine deaminase in mice after transplantation of genetically-modified bone marrow. Blood. 1990;75:1733–1739. [PubMed] [Google Scholar]
- 16.Lim B, Apperly JF, Orkin SH, Williams DA. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8892–8897. doi: 10.1073/pnas.86.22.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore KA, Fletcher FA, Villalon DK, Utter AE, Belmont JW. Human adenosine deaminase expression in mice. Blood. 1990;75:2085–2092. [PubMed] [Google Scholar]
- 18.Osborne WR, Hock RA, Kaleko M, Miller AD. Long-term expression of human adenosine deaminase in mice after transplantation of bone marrow infected with amphotropic retroviral vectors. Hum. Gene Ther. 1990;1:31–41. doi: 10.1089/hum.1990.1.1-31. [DOI] [PubMed] [Google Scholar]
- 19.Van Beusechem VW, et al. Expression of human adenosine deaminase in mice transplanted with hematopoietic stem cells infected with amphotropic retroviruses. J. Exp. Med. 1990;172 doi: 10.1084/jem.172.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson JM, Danos O, Grossman M, Raulet DH, Mulligan RC. Expression of human adenosine deaminase in mice reconstituted with retrovirus-transduced hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1990;87:439–445. doi: 10.1073/pnas.87.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson S. Treatment of genetic defects in hematopoietic cell function by gene transfer. Blood. 1991;78:2481–2492. [PubMed] [Google Scholar]
- 22.Lo M, et al. Restoration of lymphoid populations in a murine model of X-linked severe combined immunodeficiency by a gene-therapy approach. Blood. 1999;94:3027–3036. [PubMed] [Google Scholar]
- 23.Otsu M, et al. Lymphoid development and function in X-linked severe combined immunodeficiency mice after stem cell gene therapy. Mol. Ther. 2000;1:145–153. doi: 10.1006/mthe.1999.0020. [DOI] [PubMed] [Google Scholar]
- 24.Soudais C, et al. Stable and functional lymphoid reconstitution of common cytokine receptor γ chain deficient mice by retroviral-mediated gene transfer. Blood. 2000;95:3071–3077. [PubMed] [Google Scholar]
- 25.Bunting KD, Sangster MY, Ihle JN, Sorrentino BP. Restoration of lymphocyte function in Janus kinase 3-deficient mice by retroviral-mediated gene transfer. Nat. Med. 1998;4:58–64. doi: 10.1038/nm0198-058. [DOI] [PubMed] [Google Scholar]
- 26.Otsu M, et al. Reconstitution of lymphoid development and function in ZAP-70-deficient mice following gene transfer into bone marrow cells. Blood. 2002;100:1248–1256. doi: 10.1182/blood-2002-01-0247. [DOI] [PubMed] [Google Scholar]
- 27.Yates F, et al. Gene therapy of RAG-2-/- mice: sustained correction of the immunodeficiency. Blood. 2002;100:3942–3949. doi: 10.1182/blood-2002-03-0782. [DOI] [PubMed] [Google Scholar]
- 28.Tybulewicz VL, et al. Animal model of Gaucher's disease from targeted disruption of the mouse glucocerebrosidase gene. Nature. 1992;357:407–410. doi: 10.1038/357407a0. [DOI] [PubMed] [Google Scholar]
- 29.Correll PH, et al. Expression of human glucocerebrosidase in long-term reconstituted mice following retroviral-mediated gene transfer into hematopoietic stem cells. Hum. Gene Ther. 1990;1:277–283. doi: 10.1089/hum.1990.1.3-277. [DOI] [PubMed] [Google Scholar]
- 30.Correll PH, Colilla S, Dave HP, Karlsson S. High levels of human glucocerebrosidase activity in macrophages of long-term reconstituted mice after retroviral infection of hematopoietic stem cells. Blood. 1992;80:331–336. [PubMed] [Google Scholar]
- 31.Correll PH, Fink JK, Brady RO, Perry LK, Karlsson S. Production of human glucocerebrosidase in mice after retroviral gene transfer into multipotential hematopoietic progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8912–8916. doi: 10.1073/pnas.86.22.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolta JA, Sender LS, Barranger JA, Kohn DB. Expression of human glucocerebrosidase in murine long-term bone marrow cultures after retroviral vector-mediated transfer. Blood. 1990;75:787–797. [PubMed] [Google Scholar]
- 33.Ohashi T, et al. Efficient transfer and sustained high expression of the human glucocerebrosidase gene in mice and their functional macrophages following transplantation of bone marrow transduced by a retroviral vector. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinthal J, et al. Expression of human glucocerebrosidase following retroviral vector-mediated transduction of murine hematopoietic stem cells. Bone Marrow Transplant. 1991;8:403–412. [PubMed] [Google Scholar]
- 35.Bender MA, Gelinas RE, Miller AD. A majority of mice show long-term expression of a human beta-globin gene after retrovirus transfer into hematopoietic stem cells. Mol. Cell Biol. 1989;9:1426–1434. doi: 10.1128/mcb.9.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodine DM, Karlsson S, Nienhuis AW. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cone RD, Weber-Benarous A, Baorto D, Mulligan RC. Regulated expression of a complete human β-globin gene encoded by a transmissible retrovirus vector. Mol. Cell. Biol. 1987;7:887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzierzak EA, Papayannopoulou T, Mulligan RC. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988;331:35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson S, Bodine DM, Perry L, Papayannopoulou T, Nienhuis AW. Expression of the human β-globin gene following retroviral-mediated transfer into multipotential hematopoietic progenitors of mice. Proc. Natl. Acad. Sci. U. S. A. 1988;85:6062–6066. doi: 10.1073/pnas.85.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlsson S, Papayannopoulou T, Schweiger SG, Stamatoyannopoulos G, Nienhuis AW. Retroviral-mediated transfer of genomic globin genes leads to regulated production of RNA and protein. Proc. Natl. Acad. Sci. U. S. A. 1987;84:2411–2415. doi: 10.1073/pnas.84.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li CL, Dwarki VJ, Verma IM. Expression of human alpha-globin and mouse/human hybrid beta-globin genes in murine hemopoietic stem cells transduced by recombinant retroviruses. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4349–4353. doi: 10.1073/pnas.87.11.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavazzana-Calvo M, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jirapongsananuruk O, Niemela JE, Malech HL, Fleisher TA. CYBB mutation analysis in X-linked chronic granulomatous disease. Clin. Immunol. 2002;104:73–76. doi: 10.1006/clim.2002.5230. [DOI] [PubMed] [Google Scholar]
- 44.Dinauer MC, Li LL, Björgvinsdóttir H, Ding C, Pech N. Long-term correction of phagocyte NADPH oxidase activity by retroviral-mediated gene transfer in murine X-linked chronic granulomatous disease. Blood. 1999;94:914–922. [PubMed] [Google Scholar]
- 45.Bjorgvinsdottir H, et al. Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against Aspergillus fumigatus in murine X-linked chronic granulomatous disease. Blood. 1997;89:41–48. [PubMed] [Google Scholar]
- 46.Dinauer MC, Gifford MA, Pech N, Li LL, Emshwiller P. Variable correction of host defense following gene transfer and bone marrow transplantation in murine X-linked chronic granulomatous disease. Blood. 2001;97:3738–3745. doi: 10.1182/blood.v97.12.3738. [DOI] [PubMed] [Google Scholar]
- 47.Johnston RB, III, Harbeck RJ, Johnston RB., Jr Recurrent severe infections in a girl with apparently variable expression of mosaicism for chronic granulomatous disease. J. Pediatr. 1985;106:50–55. doi: 10.1016/s0022-3476(85)80463-7. [DOI] [PubMed] [Google Scholar]
- 48.Mardiney M, III, et al. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood. 1997;89:2268–2275. [PubMed] [Google Scholar]
- 49.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 50.Notarangelo LD, Miao CH, Ochs HD. Wiskott-Aldrich syndrome. Curr. Opin. Hematol. 2008;15:30–36. doi: 10.1097/MOH.0b013e3282f30448. [DOI] [PubMed] [Google Scholar]
- 51.Puck JM, Candotti F. Lessons from the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2006;355:1759–1761. doi: 10.1056/NEJMp068209. [DOI] [PubMed] [Google Scholar]
- 52.Strom TS, et al. Defects in T-cell-mediated immunity to influenza virus in murine Wiskott-Aldrich syndrome are corrected by oncoretroviral vector-mediated gene transfer into repopulating hematopoietic cells. Blood. 2003;102:3108–3116. doi: 10.1182/blood-2002-11-3489. [DOI] [PubMed] [Google Scholar]
- 53.Klein C, et al. Gene therapy for Wiskott-Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood. 2003;101:2159–2166. doi: 10.1182/blood-2002-05-1423. [DOI] [PubMed] [Google Scholar]
- 54.Cassel A, Cottler-Fox M, Doren S, Dunbar CE. Retroviral-mediated gene transfer into CD34-enriched human peripheral blood stem cells. Exp. Hematol. 1993;21:585–591. [PubMed] [Google Scholar]
- 55.Hughes PFD, Eaves CJ, Hogge DE, Humphries RK. High efficiency gene transfer to human hematopoietic cells maintained in long-term marrow culture. Blood. 1989;74:1915–1922. [PubMed] [Google Scholar]
- 56.Larochelle A, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat. Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 57.Fink JK, Correll PH, Perry LK, Brady RO, Karlsson S. Correction of glucocerebrosidase deficiency after retroviral-mediated gene transfer into hematopoietic progenitor cells from patients with Gaucher disease. Proc. Natl. Acad. Sci. U. S. A. 1990;87:2334–2338. doi: 10.1073/pnas.87.6.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bordignon C, et al. Transfer of the ADA gene into bone marrow cells and peripheral blood lymphocytes for the treatment of patients affected by ADA-deficient SCID. Hum. Gene Ther. 1993;4:513–520. doi: 10.1089/hum.1993.4.4-513. [DOI] [PubMed] [Google Scholar]
- 59.Brenner MK, et al. Gene-marking to trace origin of relapse after autologous bone marrow transplantation. Lancet. 1993;341:85–86. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- 60.Brenner MK, et al. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet. 1993;342:1134–1137. doi: 10.1016/0140-6736(93)92122-a. [DOI] [PubMed] [Google Scholar]
- 61.Rill DR, et al. An approach for the analysis of relapse and marrow reconstitution after autologous marrow transplantation using retrovirus-mediated gene transfer. Blood. 1992;79:2694–2700. [PubMed] [Google Scholar]
- 62.Rill DR, et al. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood. 1994;84:380–383. [PubMed] [Google Scholar]
- 63.Rill DR, et al. Retrovirus-mediated gene transfer as an approach to analyze neuroblastoma relapse after autologous bone marrow transplantation. Hum. Gene Ther. 1992;3:129–136. doi: 10.1089/hum.1992.3.2-129. [DOI] [PubMed] [Google Scholar]
- 64.Deisseroth AB, et al. Genetic marking shows that Ph+ cell present in autologous transplants of chronic myelogenous leukemia (CML) contribute to relapse after autologous bone marrow transplantation in CML. Blood. 1994;83:3068–3076. [PubMed] [Google Scholar]
- 65.Alici E, et al. Long-term follow-up of gene-marked CD34+ cells after autologous stem cell transplantation for multiple myeloma. Cancer Gene Ther. 2007;14:227–232. doi: 10.1038/sj.cgt.7701006. [DOI] [PubMed] [Google Scholar]
- 66.Dunbar CE, et al. Retrovirally-marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 67.Emmons RV, et al. Retroviral gene transduction of adult peripheral blood or marrow-derived CD34+ cells for six hours without growth factors or on autologous stroma does not improve marking efficiency assessed in vivo. Blood. 1997;89:4040–4046. [PubMed] [Google Scholar]
- 68.Bachier CR, et al. Hematopoietic retroviral gene marking in patients with follicular non-Hodgkin's lymphoma. Leuk. Lymphoma. 1999;32:279–288. doi: 10.3109/10428199909167388. [DOI] [PubMed] [Google Scholar]
- 69.Cornetta K, et al. Retroviral gene transfer in autologous bone marrow transplantation for adult acute leukemia. Hum. Gene Ther. 1996;7:1323–1329. doi: 10.1089/hum.1996.7.11-1323. [DOI] [PubMed] [Google Scholar]
- 70.Blaese RM, et al. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 71.Culver K, et al. Lymphocytes as cellular vehicles for gene therapy in mouse and man. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3155–3159. doi: 10.1073/pnas.88.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bordignon C, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 73.Hoogerbrugge PM, et al. Bone marrow gene transfer in three patients with adenosine deaminase deficiency. Gene Ther. 1996;3:179–183. [PubMed] [Google Scholar]
- 74.Kohn DB, et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat. Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunbar CE, et al. Retroviral transfer of the glucocerebrosidase gene into CD34+ cells from patients with Gaucher Disease: in vivo detection of transduced cells without myeloablation. Hum. Gene Ther. 1998;9:2629–2640. doi: 10.1089/hum.1998.9.17-2629. [DOI] [PubMed] [Google Scholar]
- 76.Malech HL, et al. Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12133–12138. doi: 10.1073/pnas.94.22.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abkowitz JL, et al. Behavior of hematopoietic stem cells in a large animal. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2031–2035. doi: 10.1073/pnas.92.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abkowitz JL, Catlin SN, Guttorp P. Evidence that hematopoiesis may be a stochastic process in vivo. Nat. Med. 1996;2:190–197. doi: 10.1038/nm0296-190. [DOI] [PubMed] [Google Scholar]
- 79.Abkowitz JL, Golinelli D, Harrison DE, Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96:3399–3405. [PubMed] [Google Scholar]
- 80.Catlin SN, Busque L, Gale RE, Guttorp P, Abkowitz JL. The replication rate of human hematopoietic stem cells in vivo. Blood. 2011;117:4460–4466. doi: 10.1182/blood-2010-08-303537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shepherd BE, Guttorp P, Lansdorp PM, Abkowitz JL. Estimating human hematopoietic stem cell kinetics using granulocyte telomere lengths. Exp. Hematol. 2004;32:1040–1050. doi: 10.1016/j.exphem.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 82.Mohrin M, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orlic D, et al. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bryder D, Bjorgvinsdottir H, Sasaki Y, Jacobsen SE. Deficiency of oncoretrovirally transduced hematopoietic stem cells and correction through ex vivo expansion. J. Gene Med. 2005;7:137–144. doi: 10.1002/jgm.658. [DOI] [PubMed] [Google Scholar]
- 86.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 87.Peters SO, Kittler ELW, Ramshaw HS, Quesenberry PJ. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp. Hematol. 1995;23:461–466. [PubMed] [Google Scholar]
- 88.Gan OI, Murdoch B, Larochelle A, Dick JE. Differential maintenance of primitive human SCID-repopulating cells, clonogenic progenitors, and long-term culture-initiating cells after incubation on human bone marrow stromal cells. Blood. 1997;90:641–650. [PubMed] [Google Scholar]
- 89.Gothot A, van der Loo JCM, Clapp DW, Srour EF. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34+ cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 90.Takatoku M, et al. Avoidance of stimulation improves engraftment of cultured and retrovirally transduced hematopoietic cells in primates. J. Clin. Invest. 2001;108:447–455. doi: 10.1172/JCI12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larochelle A, et al. Bone marrow homing and engraftment of human hematopoietic stem and progenitor cells is mediated by a polarized membrane domain. Blood. 2012;119:1848–1855. doi: 10.1182/blood-2011-08-371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glimm H, Oh IH, Eaves CJ. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0) [In Process Citation] Blood. 2000;96:4185–4193. [PubMed] [Google Scholar]
- 93.Zanjani ED, et al. Engraftment and long-term expression of human fetal hemopoietic stem cells in sheep following transplantation in utero. J. Clin. Invest. 1992;89:1178–1188. doi: 10.1172/JCI115701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zanjani ED, Flake AW, Rice H, Hedrick M, Tavassoli M. Long-term repopulating ability of xenogeneic transplanted human fetal liver hematopoietic stem cells in sheep.[see comment] Journal of Clinical Investigation. 1994;93:1051–1055. doi: 10.1172/JCI117054. 1994 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zanjani ED, Almeida-Porada G, Flake AW. The human/sheep xenograft model: a large animal model of human hematopoiesis. Int. J. Hematol. 1996;63:179–192. doi: 10.1016/0925-5710(96)00445-8. [DOI] [PubMed] [Google Scholar]
- 96.Porada CD, et al. Transduction of long-term-engrafting human hematopoietic stem cells by retroviral vectors. Hum. Gene Ther. 2002;13:867–879. doi: 10.1089/10430340252899037. [DOI] [PubMed] [Google Scholar]
- 97.Donahue RE, Dunbar CE. An update on the use of non-human primate models for preclinical testing of gene therapy approaches targeting hematopoietic cells. Hum. Gene Ther. 2001;12:607–617. doi: 10.1089/104303401300057289. [DOI] [PubMed] [Google Scholar]
- 98.Donahue RE, et al. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamel-Reid S, Dick JE. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988;242:1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- 100.Lapidot T, et al. Cytokine stimulation of multilineage hematopoietsis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 101.Shultz LD, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSzscid mice. J. Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 102.Kollet O, et al. β2 microglobulin-deficient (B2mnull) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood. 2000;95:3102–3105. [PubMed] [Google Scholar]
- 103.Kerre TC, et al. Adapted NOD/SCID model supports development of phenotypically and functionally mature T cells from human umbilical cord blood CD34(+) cells. Blood. 2002;99:1620–1626. doi: 10.1182/blood.v99.5.1620. [DOI] [PubMed] [Google Scholar]
- 104.Shultz LD, et al. Regulation of human short-term repopulating cell (STRC) engraftment in NOD/SCID mice by host CD122+ cells. Exp. Hematol. 2003;31:551–558. doi: 10.1016/s0301-472x(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 105.Hiramatsu H, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 106.Ito M, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 107.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 108.Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat. Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 109.Notta F, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 110.Glimm H, et al. Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-beta2 microglobulin-null mice. J. Clin. Invest. 2001;107:199–206. doi: 10.1172/JCI11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Horn PA, et al. Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice versus nonhuman primates. Blood. 2003;102:4329–4335. doi: 10.1182/blood-2003-01-0082. [DOI] [PubMed] [Google Scholar]
- 112.Bukrinsky MI, et al. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells [see comments] Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Case SS, et al. Stable transduction of quiescent CD34+CD38- human hematopoietic cells by HIV-1-based lentiviral vectors. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 115.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 116.Gallay P, Hope T, Chin D, Trono D. Hiv-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goff SP. Intracellular trafficking of retroviral genomes during the early phase of infection: viral exploitation of cellular pathways. J. Gene Med. 2001;3:517–528. doi: 10.1002/1521-2254(200111)3:6<517::AID-JGM234>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 118.Heinzinger NK, et al. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 1998;273:13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- 120.Sirven A, et al. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood. 2000;96:4103–4110. [PubMed] [Google Scholar]
- 121.Zennou V, et al. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 122.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 123.Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sutton RE, Reitsma MJ, Uchida N, Brown PO. Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent. J. Virol. 1999;73:3649–3660. doi: 10.1128/jvi.73.5.3649-3660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uchida N, et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated Go/G1 human hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 127.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kelly PF, Vandergriff J, Nathwani A, Nienhuis AW, Vanin EF. Highly efficient gene transfer into cord blood nonobese diabetic/severe combined immunodeficiency repopulating cells by oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. Blood. 2000;96:1206–1214. [PubMed] [Google Scholar]
- 129.Marandin A, et al. Retrovirus-mediated gene transfer into human CD34+38low primitive cells capable of reconstituting long-term cultures in vitro and nonobese diabetic-severe combined immunodeficiency mice in vivo. Hum. Gene Ther. 1998;9:1497–1511. doi: 10.1089/hum.1998.9.10-1497. [DOI] [PubMed] [Google Scholar]
- 130.Movassagh M, et al. High-level gene transfer to cord blood progenitors using gibbon ape leukemia virus pseudotype retroviral vectors and an improved clinically applicable protocol. Hum. Gene Ther. 1998;9:225–234. doi: 10.1089/hum.1998.9.2-225. [DOI] [PubMed] [Google Scholar]
- 131.Porter CD, et al. Comparison of efficiency of infection of human gene therapy target cells via four different retroviral receptors. Hum. Gene Ther. 1996;7:913–919. doi: 10.1089/hum.1996.7.8-913. [DOI] [PubMed] [Google Scholar]
- 132.Guenechea G, et al. Transduction of human CD34+CD38- bone marrow and cord blood-derived SCID-repopulating cells with third-generation lentiviral vectors. Mol. Ther. 2000;1:566–573. doi: 10.1006/mthe.2000.0077. [DOI] [PubMed] [Google Scholar]
- 133.Liu Y, et al. Identification of parameters required for efficient lentiviral vector transduction and engraftment of human cord blood CD34(+) NOD/SCID-repopulating cells. Exp. Hematol. 2008;36:947–956. doi: 10.1016/j.exphem.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miyoshi H, Smith KA, Mosier DE, Verma IM, Torbett BE. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 135.Piacibello W, et al. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–2653. [PubMed] [Google Scholar]
- 136.Woods NB, et al. Lentiviral gene transfer into primary and secondary NOD/SCID repopulating cells. Blood. 2000;96:3725–3733. [PubMed] [Google Scholar]
- 137.Benhamida S, et al. Transduced CD34+ cells from adrenoleukodystrophy patients with HIV-derived vector mediate long-term engraftment of NOD/SCID mice. Mol. Ther. 2003;7:317–324. doi: 10.1016/s1525-0016(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 138.Tesio M, et al. Sustained long-term engraftment and transgene expression of peripheral blood CD34+ cells transduced with third-generation lentiviral vectors. Stem Cells. 2008;26:1620–1627. doi: 10.1634/stemcells.2008-0161. [DOI] [PubMed] [Google Scholar]
- 139.Piacibello W, et al. Lentiviral gene transfer and ex vivo expansion of human primitive stem cells capable of primary, secondary, and tertiary multilineage repopulation in NOD/SCID mice. Nonobese diabetic/severe combined immunodeficient. Blood. 2002;100:4391–4400. doi: 10.1182/blood.V100.13.4391. [DOI] [PubMed] [Google Scholar]
- 140.Puthenveetil G, et al. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104:3445–3453. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- 141.Asheuer M, et al. Human CD34+ cells differentiate into microglia and express recombinant therapeutic protein. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3557–3562. doi: 10.1073/pnas.0306431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cartier N, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 143.Boztug K, et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Galy A, Thrasher AJ. Gene therapy for the Wiskott-Aldrich syndrome. Curr. Opin. Allergy Clin. Immunol. 2011;11:545–550. doi: 10.1097/ACI.0b013e32834c230c. [DOI] [PubMed] [Google Scholar]
- 145.Scaramuzza S, et al. Preclinical Safety and Efficacy of Human CD34(+) Cells Transduced With Lentiviral Vector for the Treatment of Wiskott-Aldrich Syndrome. Mol. Ther. 2012 doi: 10.1038/mt.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Naumann N, et al. Simian immunodeficiency virus lentivector corrects human X-linked chronic granulomatous disease in the NOD/SCID mouse xenograft. Gene Ther. 2007;14:1513–1524. doi: 10.1038/sj.gt.3303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roesler J, et al. Third-generation, self-inactivating gp91(phox) lentivector corrects the oxidase defect in NOD/SCID mouse-repopulating peripheral blood-mobilized CD34+ cells from patients with X-linked chronic granulomatous disease. Blood. 2002;100:4381–4390. doi: 10.1182/blood-2001-12-0165. [DOI] [PubMed] [Google Scholar]
- 148.Lagresle-Peyrou C, et al. Restoration of human B-cell differentiation into NOD-SCID mice engrafted with gene-corrected CD34+ cells isolated from Artemis or RAG1-deficient patients. Mol. Ther. 2008;16:396–403. doi: 10.1038/sj.mt.6300353. [DOI] [PubMed] [Google Scholar]
- 149.Hofling AA, Devine S, Vogler C, Sands MS. Human CD34+ hematopoietic progenitor cell-directed lentiviral-mediated gene therapy in a xenotransplantation model of lysosomal storage disease. Mol. Ther. 2004;9:856–865. doi: 10.1016/j.ymthe.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 150.An DS, et al. Lentivirus vector-mediated hematopoietic stem cell gene transfer of common gamma-chain cytokine receptor in rhesus macaques. J. Virol. 2001;75:3547–3555. doi: 10.1128/JVI.75.8.3547-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.An DS, et al. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34+ cells. J. Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Horn PA, et al. Lentivirus-mediated gene transfer into hematopoietic repopulating cells in baboons. Gene Ther. 2002;9:1464–1471. doi: 10.1038/sj.gt.3301820. [DOI] [PubMed] [Google Scholar]
- 153.Horn PA, et al. Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol. Blood. 2004;103:3710–3716. doi: 10.1182/blood-2003-07-2414. [DOI] [PubMed] [Google Scholar]
- 154.Trobridge GD, et al. Efficient transduction of pigtailed macaque hematopoietic repopulating cells with HIV-based lentiviral vectors. Blood. 2008;111:5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hanawa H, et al. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- 156.Kim YJ, et al. Sustained high level polyclonal hematopoietic marking and transgene expression four years following autologous transplantation of rhesus macaques with SIV lentiviral vector transduced CD34+ cells. Blood. 2009;113:5434–4354. doi: 10.1182/blood-2008-10-185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Larochelle A, et al. In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene. J. Clin. Invest. 2009;119:1952–1963. doi: 10.1172/JCI37506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tarantal AF, et al. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol. Ther. 2012;20:1033–1045. doi: 10.1038/mt.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Verhoeyen E, et al. Stem cell factor-displaying SIV vectors together with a low conditioning regimen allow for long-term engraftment of gene marked autologous HSCs in macaques. Hum. Gene Ther. 2012 doi: 10.1089/hum.2012.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kootstra NA, Munk C, Tonnu N, Landau NR, Verma IM. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1298–1303. doi: 10.1073/pnas.0337541100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rits MA, van Dort KA, Munk C, Meijer AB, Kootstra NA. Efficient transduction of simian cells by HIV-1-based lentiviral vectors that contain mutations in the capsid protein. Mol. Ther. 2007;15:930–937. doi: 10.1038/mt.sj.6300091. [DOI] [PubMed] [Google Scholar]
- 162.Uchida N, et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J. Virol. 2009;83:9854–9862. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hayakawa J, et al. Transient in vivo beta-globin production after lentiviral gene transfer to hematopoietic stem cells in the nonhuman primate. Hum. Gene Ther. 2009;20:563–572. doi: 10.1089/hum.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bauer TR, Jr, Adler RL, Hickstein DD. Potential large animal models for gene therapy of human genetic diseases of immune and blood cell systems. ILAR. J. 2009;50:168–186. doi: 10.1093/ilar.50.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boudreaux MK, Lipscomb DL. Clinical, biochemical, and molecular aspects of Glanzmann's thrombasthenia in humans and dogs. Vet. Pathol. 2001;38:249–260. doi: 10.1354/vp.38-3-249. [DOI] [PubMed] [Google Scholar]
- 166.Fang J, et al. Platelet gene therapy improves hemostatic function for integrin alphaIIbbeta3-deficient dogs. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9583–9588. doi: 10.1073/pnas.1016394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ting-De Ravin SS, et al. Correction of canine X-linked severe combined immunodeficiency by in vivo retroviral gene therapy. Blood. 2006;107:3091–3097. doi: 10.1182/blood-2005-10-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bauer TR, Jr, et al. Correction of the disease phenotype in canine leukocyte adhesion deficiency using ex vivo hematopoietic stem cell gene therapy. Blood. 2006;108:3313–3320. doi: 10.1182/blood-2006-03-006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bauer TR, Jr, et al. Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nat. Med. 2008;14:93–97. doi: 10.1038/nm1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Astrakhan A, et al. Ubiquitous high-level gene expression in hematopoietic lineages provides effective lentiviral gene therapy of murine Wiskott-Aldrich syndrome. Blood. 2012;119:4395–4407. doi: 10.1182/blood-2011-03-340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Blundell MP, et al. Improvement of migratory defects in a murine model of Wiskott-Aldrich syndrome gene therapy. Mol. Ther. 2008;16:836–844. doi: 10.1038/mt.2008.43. [DOI] [PubMed] [Google Scholar]
- 172.Bosticardo M, et al. Lentiviral-mediated gene therapy leads to improvement of B-cell functionality in a murine model of Wiskott-Aldrich syndrome. J. Allergy Clin. Immunol. 2011;127:1376–1384. doi: 10.1016/j.jaci.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 173.Charrier S, et al. A lentiviral vector encoding the human Wiskott-Aldrich syndrome protein corrects immune and cytoskeletal defects in WASP knockout mice. Gene Ther. 2005;12:597–606. doi: 10.1038/sj.gt.3302440. [DOI] [PubMed] [Google Scholar]
- 174.Dupre L, et al. Efficacy of gene therapy for Wiskott-Aldrich syndrome using a WAS promoter/cDNA-containing lentiviral vector and nonlethal irradiation. Hum. Gene Ther. 2006;17:303–313. doi: 10.1089/hum.2006.17.303. [DOI] [PubMed] [Google Scholar]
- 175.Marangoni F, et al. Evidence for long-term efficacy and safety of gene therapy for Wiskott-Aldrich syndrome in preclinical models. Mol. Ther. 2009;17:1073–1082. doi: 10.1038/mt.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Imren S, et al. Permanent and panerythroid correction of murine beta thalassemia by multiple lentiviral integration in hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14380–14385. doi: 10.1073/pnas.212507099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Negre O, et al. Correction of murine beta-thalassemia after minimal lentiviral gene transfer and homeostatic in vivo erythroid expansion. Blood. 2011;117:5321–5331. doi: 10.1182/blood-2010-01-263582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Persons DA, et al. Successful treatment of murine beta-thalassemia using in vivo selection of genetically modified, drug-resistant hematopoietic stem cells. Blood. 2003;102:506–513. doi: 10.1182/blood-2003-03-0677. [DOI] [PubMed] [Google Scholar]
- 179.Zhao H, et al. Amelioration of murine beta-thalassemia through drug selection of hematopoietic stem cells transduced with a lentiviral vector encoding both gamma-globin and the MGMT drug-resistance gene. Blood. 2009;113:5747–5756. doi: 10.1182/blood-2008-10-186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Almarza E, et al. Characteristics of lentiviral vectors harboring the proximal promoter of the vav proto-oncogene: a weak and efficient promoter for gene therapy. Mol. Ther. 2007;15:1487–1494. doi: 10.1038/sj.mt.6300213. [DOI] [PubMed] [Google Scholar]
- 181.Benjelloun F, et al. Stable and functional lymphoid reconstitution in artemis-deficient mice following lentiviral artemis gene transfer into hematopoietic stem cells. Mol. Ther. 2008;16:1490–1499. doi: 10.1038/mt.2008.118. [DOI] [PubMed] [Google Scholar]
- 182.Mostoslavsky G, Fabian AJ, Rooney S, Alt FW, Mulligan RC. Complete correction of murine Artemis immunodeficiency by lentiviral vector-mediated gene transfer. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16406–16411. doi: 10.1073/pnas.0608130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang F, et al. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110:1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou S, et al. A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LMO2 expression in human T cells. Blood. 2010;116:900–908. doi: 10.1182/blood-2009-10-250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barde I, et al. Lineage- and stage-restricted lentiviral vectors for the gene therapy of chronic granulomatous disease. Gene Ther. 2011;18:1087–1097. doi: 10.1038/gt.2011.65. [DOI] [PubMed] [Google Scholar]
- 186.Pujol A, et al. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. Hum. Mol. Genet. 2002;11:499–505. doi: 10.1093/hmg/11.5.499. [DOI] [PubMed] [Google Scholar]
- 187.Dao MA, Hannum CH, Kohn DB, Nolta JA. FLT3 ligand preserves the ability of human CD34+ progenitors to sustain long-term hematopoiesis in immune-deficient mice after ex vivo retroviral-mediated trasduction. Blood. 1997;89:446–456. [PubMed] [Google Scholar]
- 188.Borge OJ, Ramsfjell V, Cui L, Jacobsen SEW. Ability of early acting cytokines to directly promote survival and suppress apoptosis of human primitive CD34+CD38- bone marrow cells with multilineage potential at the single cell level: key role of thrombopoietin. Blood. 1997;90:2282–2292. [PubMed] [Google Scholar]
- 189.Borge OJ, et al. Thrombopoietin, but not erythtopoietin promotes viability and inhibits apoptosis of multipotent murine hematopoietic progenitor cells in vitro. Blood. 1996;88:2859–2870. [PubMed] [Google Scholar]
- 190.Kiem HP, et al. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 191.Kurre P, et al. Gene transfer into baboon repopulating cells: A comparison of Flt-3 Ligand and megakaryocyte growth and development factor versus IL-3 during ex vivo transduction. Mol Ther. 2001;3:920–927. doi: 10.1006/mthe.2001.0328. [DOI] [PubMed] [Google Scholar]
- 192.Wu T, et al. Prolonged high-level detection of retrovirally-marked hematopoietic cells in non-human primates after transduction of CD34+ progenitors using clinically feasible methods. Mol. Ther. 2000;1:285–293. doi: 10.1006/mthe.2000.0034. [DOI] [PubMed] [Google Scholar]
- 193.Wognum AW, Visser TP, Peters K, Bierhuizen MF, Wagemaker G. Stimulation of mouse bone marrow cells with kit ligand, FLT3 ligand, and thrombopoietin leads to efficient retrovirus-mediated gene transfer to stem cells, whereas interleukin 3 and interleukin 11 reduce transduction of short- and long-term repopulating cells [In Process Citation] Hum. Gene Ther. 2000;11:2129–2141. doi: 10.1089/104303400750001435. [DOI] [PubMed] [Google Scholar]
- 194.Yonemura Y, Ku H, Hirayama F, Souza LM, Ogawa M. Interleukin 3 or interleukin 1 abrogates the reconstituting ability of hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4040–4044. doi: 10.1073/pnas.93.9.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Leuci V, et al. Transient proteasome inhibition as a strategy to enhance lentiviral transduction of hematopoietic CD34(+) cells and T lymphocytes: implications for the use of low viral doses and large-size vectors. J. Biotechnol. 2011;156:218–226. doi: 10.1016/j.jbiotec.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 196.Santoni de Sio FR, Cascio P, Zingale A, Gasparini M, Naldini L. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood. 2006;107:4257–4265. doi: 10.1182/blood-2005-10-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Moore KA, Deisseroth AB, Reading CL, Williams DE, Belmont JW. Stromal support enhances cell-free retroviral vector transduction of human bone marrow long-term culture initiating cells. Blood. 1992;79:1393–1399. [PubMed] [Google Scholar]
- 198.Wells S, Malik P, Pensiero M, Kohn DB, Nolta JA. The presence of an autologous marrow stromal cell layer increases glucocerebrosidase gene transduction of long-term culture initiating cells (LTCICs) from the bone marrow of a patient with Gaucher disease. Gene Ther. 1995;2:512–520. [PubMed] [Google Scholar]
- 199.Xu LC, et al. Growth factors and stromal support generate very efficient retroviral transduction of peripheral blood CD34+ cells from Gaucher patients. Blood. 1995;86:141–146. [PubMed] [Google Scholar]
- 200.Tisdale JF, et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 201.Moritz T, Patel VP, Williams DA. Bone marrow extracellular matrix molecules improve gene transfer into human hematopoietic cells via retroviral vectors. J. Clin. Invest. 1994;93:1451–1457. doi: 10.1172/JCI117122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carstanjen D, Dutt P, Moritz T. Heparin inhibits retrovirus binding to fibronectin as well as retrovirus gene transfer on fibronectin fragments. J. Virol. 2001;75:6218–6222. doi: 10.1128/JVI.75.13.6218-6222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hanenberg H, et al. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat. Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 204.Lei P, Bajaj B, Andreadis ST. Retrovirus-associated heparan sulfate mediates immobilization and gene transfer on recombinant fibronectin. J. Virol. 2002;76:8722–8728. doi: 10.1128/JVI.76.17.8722-8728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Relander T, Karlsson S, Richter J. Oncoretroviral gene transfer to NOD/SCID repopulating cells using three different viral envelopes. J. Gene Med. 2002;4:122–132. doi: 10.1002/jgm.246. [DOI] [PubMed] [Google Scholar]
- 206.Kotani H, et al. Improved methods of retroviral vector transduction and production for gene therapy. Hum. Gene Ther. 1994;5:19–28. doi: 10.1089/hum.1994.5.1-19. [DOI] [PubMed] [Google Scholar]
- 207.Dao MA, Hashino K, Kato I, Nolta JA. Adhesion to fibronectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells. Blood. 1998;92:4612–4621. [PubMed] [Google Scholar]
- 208.Sagar BM, Rentala S, Gopal PN, Sharma S, Mukhopadhyay A. Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem. Biophys. Res. Commun. 2006;350:1000–1005. doi: 10.1016/j.bbrc.2006.09.140. [DOI] [PubMed] [Google Scholar]
- 209.Yokota T, et al. Growth-supporting activities of fibronectin on hematopoietic stem/progenitor cells in vitro and in vivo: structural requirements for fibronectin activities of CS1 and cell-binding domains. Blood. 1998;91:3263–3272. [PubMed] [Google Scholar]
- 210.Donahue RE, et al. Fibronectin fragment CH-296 inhibits apoptosis and enhances ex vivo gene transfer by murine retrovirus and human lentivirus vectors independent of viral tropism in nonhuman primate CD34+ cells. Mol. Ther. 2001;3:359–367. doi: 10.1006/mthe.2001.0269. [DOI] [PubMed] [Google Scholar]
- 211.Abonour R, et al. Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells. Nat. Med. 2000;6:652–658. doi: 10.1038/76225. [DOI] [PubMed] [Google Scholar]
- 212.Hanenberg H, et al. Optimization of fibronectin-assisted retroviral gene transfer into human CD34+ hematopoietic cells. Hum. Gene Ther. 1997;8:2193–2206. doi: 10.1089/hum.1997.8.18-2193. [DOI] [PubMed] [Google Scholar]
- 213.Lee HJ, et al. Retronectin enhances lentivirus-mediated gene delivery into hematopoietic progenitor cells. Biologicals. 2009;37:203–209. doi: 10.1016/j.biologicals.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 214.Schiffman R, et al. Transfer of the human glucocerebrosidase gene into hematopoietic stem cells of nonablated recipients: successful engraftment and long-term expression of the transgene. Blood. 1995;86:1218–1227. [PubMed] [Google Scholar]
- 215.Barese C, et al. Granulocyte colony-stimulating factor prior to nonmyeloablative irradiation decreases murine host hematopoietic stem cell function and increases engraftment of donor marrow cells. Stem Cells. 2007;25:1578–1585. doi: 10.1634/stemcells.2006-0808. [DOI] [PubMed] [Google Scholar]
- 216.Goebel WS, et al. A murine model of antimetabolite-based, submyeloablative conditioning for bone marrow transplantation: biologic insights and potential applications. Exp. Hematol. 2004;32:1255–1264. doi: 10.1016/j.exphem.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 217.Goebel WS, Yoder MC, Pech NK, Dinauer MC. Donor chimerism and stem cell function in a murine congenic transplantation model after low-dose radiation conditioning: effects of a retroviral-mediated gene transfer protocol and implications for gene therapy. Exp. Hematol. 2002;30:1324–1332. doi: 10.1016/s0301-472x(02)00927-x. [DOI] [PubMed] [Google Scholar]
- 218.Goebel WS, Pech NK, Dinauer MC. Stable long-term gene correction with low-dose radiation conditioning in murine X-linked chronic granulomatous disease. Blood Cells Mol. Dis. 2004;33:365–371. doi: 10.1016/j.bcmd.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 219.Huston MW, et al. Correction of murine SCID-X1 by lentiviral gene therapy using a codon-optimized IL2RG gene and minimal pretransplant conditioning. Mol. Ther. 2011;19:1867–1877. doi: 10.1038/mt.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Xue X, et al. Antibody targeting KIT as pretransplantation conditioning in immunocompetent mice. Blood. 2010;116:5419–5422. doi: 10.1182/blood-2010-07-295949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen J, et al. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood. 2006;107:3764–3771. doi: 10.1182/blood-2005-09-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Carter RF, et al. Autologous transplantation of canine long-term marrow culture cells genetically marked by retroviral vectors. Blood. 1992;79:356–364. [PubMed] [Google Scholar]
- 224.Huhn RD, et al. Retroviral marking and transplantation of rhesus hematopoietic cells by nonmyeloablative contitioning. Hum. Gene Ther. 1999;10:1783–1790. doi: 10.1089/10430349950017464. [DOI] [PubMed] [Google Scholar]
- 225.Kahl CA, et al. Effects of busulfan dose escalation on engraftment of infant rhesus monkey hematopoietic stem cells after gene marking by a lentiviral vector. Exp. Hematol. 2006;34:369–381. doi: 10.1016/j.exphem.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 226.Kang EM, et al. Busulfan pharmacokinetics, toxicity, and low-dose conditioning for autologous transplantation of genetically modified hematopoietic stem cells in the rhesus macaque model. Exp. Hematol. 2006;34:132–139. doi: 10.1016/j.exphem.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rosenzweig M, et al. Efficient and durable gene marking of hematopoietic progenitor cells in nonhuman primates after nonablative conditioning. Blood. 1999;94:2271–2286. [PubMed] [Google Scholar]
- 228.Sadat MA, et al. Retroviral vector integration in post-transplant hematopoiesis in mice conditioned with either submyeloablative or ablative irradiation. Gene Ther. 2009;16:1452–1464. doi: 10.1038/gt.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Aiuti A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 230.Aiuti A, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 231.Gaspar HB, et al. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci. Transl. Med. 2011;3:97ra80. doi: 10.1126/scitranslmed.3002716. [DOI] [PubMed] [Google Scholar]
- 232.Gaspar HB, et al. Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2011;3:97ra79. doi: 10.1126/scitranslmed.3002715. [DOI] [PubMed] [Google Scholar]
- 233.Gaspar HB, et al. Successful reconstitution of immunity in ADA-SCID by stem cell gene therapy following cessation of PEG-ADA and use of mild preconditioning. Mol. Ther. 2006;14:505–513. doi: 10.1016/j.ymthe.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 234.Kang EM, et al. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood. 2010;115:783–791. doi: 10.1182/blood-2009-05-222760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kang HJ, et al. Retroviral gene therapy for X-linked chronic granulomatous disease: results from phase I/II trial. Mol. Ther. 2011;19:2092–2101. doi: 10.1038/mt.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ott MG, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 237.Millington M, Arndt A, Boyd M, Applegate T, Shen S. Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells. PLoS. One. 2009;4:e6461. doi: 10.1371/journal.pone.0006461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Uchida N, et al. Optimal conditions for lentiviral transduction of engrafting human CD34+ cells. Gene Ther. 2011;18:1078–1086. doi: 10.1038/gt.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dunbar CE, Larochelle A. Gene therapy activates EVI1, destabilizes chromosomes. Nat. Med. 2010;16:163–165. doi: 10.1038/nm0210-163. [DOI] [PubMed] [Google Scholar]
- 240.Cavazzana-Calvo M, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 241.Chinen J, et al. Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood. 2007;110:67–73. doi: 10.1182/blood-2006-11-058933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gaspar HB, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 243.Hacein-Bey-Abina S, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hacein-Bey-Abina S, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 245.Thrasher AJ, et al. Failure of SCID-X1 gene therapy in older patients. Blood. 2005;105:4255–4257. doi: 10.1182/blood-2004-12-4837. [DOI] [PubMed] [Google Scholar]
- 246.Stein S, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 247.Paruzynski A, et al. High-Level Clustering of Vector Integrations in the German WAS Clinical Gene Therapy Trial. Mol Ther. 2012 May 1;20(Suppl.1):S212. Ref Type: Generic. [Google Scholar]
- 248.Hacein-Bey-Abina S, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 249.Hacein-Bey-Abina S, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 251.Schwarzwaelder K, et al. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J. Clin. Invest. 2007;117:2241–2249. doi: 10.1172/JCI31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Howe SJ, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Aiuti A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 254.Baum C. Insertional mutagenesis in gene therapy and stem cell biology. Curr. Opin. Hematol. 2007;14:337–342. doi: 10.1097/MOH.0b013e3281900f01. [DOI] [PubMed] [Google Scholar]
- 255.Mazurier F, Gan OI, McKenzie JL, Doedens M, Dick JE. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood. 2004;103:545–552. doi: 10.1182/blood-2003-05-1558. [DOI] [PubMed] [Google Scholar]
- 256.Miyoshi H, Smith KA, Mosier DE, Verma IM, Torbett BE. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 257.Nienhuis AW, Dunbar CE, Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 258.Ikeda K, Mason PJ, Bessler M. 3'UTR-truncated Hmga2 cDNA causes MPN-like hematopoiesis by conferring a clonal growth advantage at the level of HSC in mice. Blood. 2011 doi: 10.1182/blood-2011-02-334425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schmidt M, et al. Clonality analysis after retroviral-mediated gene transfer to CD34+ cells from the cord blood of ADA-deficient SCID neonates. Nat. Med. 2003;9:463–468. doi: 10.1038/nm844. [DOI] [PubMed] [Google Scholar]
- 260.Inoue N, et al. Molecular basis of clonal expansion of hematopoiesis in 2 patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2006;108:4232–4236. doi: 10.1182/blood-2006-05-025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Murakami Y, et al. Deregulated expression of HMGA2 is implicated in clonal expansion of PIGA deficient cells in paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2012;156:383–387. doi: 10.1111/j.1365-2141.2011.08914.x. [DOI] [PubMed] [Google Scholar]
- 262.Baum C, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–2114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 263.Mucenski ML, et al. Identification of a common ecotropic viral integration site, Evi-1, in the DNA of AKXD murine myeloid tumors. Mol. Cell Biol. 1988;8:301–308. doi: 10.1128/mcb.8.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Suzuki T, et al. New genes involved in cancer identified by retroviral tagging. Nat. Genet. 2002;32:166–174. doi: 10.1038/ng949. [DOI] [PubMed] [Google Scholar]
- 265.Li Z, et al. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 266.Bunting KD, Galipeau J, Topham D, Benaim E, Sorrentino BP. Transduction of murine bone marrow cells with an MDR1 vector enables ex vivo stem cell expansion, but these expanded grafts cause a myeloproliferative syndrome in transplanted mice. Blood. 1998;92:2269–2279. [PubMed] [Google Scholar]
- 267.Will E, et al. Importance of murine study design for testing toxicity of retroviral vectors in support of phase I trials. Mol. Ther. 2007;15:782–791. doi: 10.1038/sj.mt.6300083. [DOI] [PubMed] [Google Scholar]
- 268.Kohn DB, et al. American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral-mediated gene transfer to hematopoietic stem cells. Mol. Ther. 2003;8:180–187. doi: 10.1016/s1525-0016(03)00212-0. [DOI] [PubMed] [Google Scholar]
- 269.Kustikova O, et al. Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science. 2005;308:1171–1174. doi: 10.1126/science.1105063. [DOI] [PubMed] [Google Scholar]
- 270.Schmidt M, et al. Detection and direct genomic sequencing of multiple rare unknown flanking DNA in highly complex samples. Hum. Gene Ther. 2001;12:743–749. doi: 10.1089/104303401750148649. [DOI] [PubMed] [Google Scholar]
- 271.Wang GP, et al. Dynamics of gene-modified progenitor cells analyzed by tracking retroviral integration sites in a human SCID-X1 gene therapy trial. Blood. 2010;115:4356–4366. doi: 10.1182/blood-2009-12-257352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Li CL, Xiong D, Stamatoyannopoulos G, Emery DW. Genomic and Functional Assays Demonstrate Reduced Gammaretroviral Vector Genotoxicity Associated With Use of the cHS4 Chromatin Insulator. Mol. Ther. 2009 doi: 10.1038/mt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Du D, Copeland NG. Insertional mutagenesis identifies genes that promote the immortalization of primary murine bone marrow progenitor cells. Blood. 2005;106:3932–3939. doi: 10.1182/blood-2005-03-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Modlich U, et al. Cell culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545–2553. doi: 10.1182/blood-2005-08-024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kustikova OS, et al. Retroviral vector insertion sites associated with dominant hematopoietic clones mark "stemness" pathways. Blood. 2007;109:1897–1907. doi: 10.1182/blood-2006-08-044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Modlich U, et al. Leukemias following retroviral transfer of multidrug resistance 1 (MDR1) are driven by combinatorial insertional mutagenesis. Blood. 2005;105:4235–4246. doi: 10.1182/blood-2004-11-4535. [DOI] [PubMed] [Google Scholar]
- 277.Akagi K, Suzuki T, Stephens RM, Jenkins NA, Copeland NG. RTCGD: retroviral tagged cancer gene database. Nucleic Acids Res. 2004;32:D523–D527. doi: 10.1093/nar/gkh013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Erkeland SJ, et al. Significance of murine retroviral mutagenesis for identification of disease genes in human acute myeloid leukemia. Cancer Res. 2006;66:622–626. doi: 10.1158/0008-5472.CAN-05-2908. [DOI] [PubMed] [Google Scholar]
- 279.Montini E, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 280.Montini E, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Biffi A, et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood. 2011;117:5332–5339. doi: 10.1182/blood-2010-09-306761. [DOI] [PubMed] [Google Scholar]
- 282.Calmels B, et al. Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells. Blood. 2005;106:2530–2533. doi: 10.1182/blood-2005-03-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hematti P, et al. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS. Biol. 2004;2:e423. doi: 10.1371/journal.pbio.0020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dave UP, Jenkins NA, Copeland NG. Gene therapy insertional mutagenesis insights. Science. 2004;303:333. doi: 10.1126/science.1091667. [DOI] [PubMed] [Google Scholar]
- 285.Woods NB, Bottero V, Schmidt M, von KC, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- 286.Kiem HP, et al. Long-term clinical and molecular follow-up of large animals receiving retrovirally transduced stem and progenitor cells: no progression to clonal hematopoiesis or leukemia. Mol. Ther. 2004;9:389–395. doi: 10.1016/j.ymthe.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 287.Seggewiss R, et al. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque. Blood. 2006;107:3865–3867. doi: 10.1182/blood-2005-10-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Xie J, et al. Repetitive busulfan administration after hematopoietic stem cell gene therapy associated with a dominant HDAC7 clone in a nonhuman primate. Hum. Gene Ther. 2010;21:695–703. doi: 10.1089/hum.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Beard BC, et al. Unique integration profiles in a canine model of long-term repopulating cells transduced with gammaretrovirus, lentivirus, or foamy virus. Hum. Gene Ther. 2007;18:423–434. doi: 10.1089/hum.2007.011. [DOI] [PubMed] [Google Scholar]
- 290.Hu J, et al. Reduced genotoxicity of avian sarcoma leukosis virus vectors in rhesus long-term repopulating cells compared to standard murine retrovirus vectors. Mol. Ther. 2008;9:1617–1623. doi: 10.1038/mt.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 292.Zhang XB, et al. Differential effects of HOXB4 on nonhuman primate short- and long-term repopulating cells. PLoS. Med. 2006;3:e173. doi: 10.1371/journal.pmed.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Murnane R, et al. Myelodysplasia in 2 pig-tailed macaques (Macaca nemestrina) associated with retroviral vector-mediated insertional mutagenesis and overexpression of HOXB4. Vet. Pathol. 2011;48:999–1001. doi: 10.1177/0300985810382673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhang XB, et al. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. J. Clin. Invest. 2008;118:1502–1510. doi: 10.1172/JCI34371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Di SA, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Straathof KC, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Frecha C, Szecsi J, Cosset FL, Verhoeyen E. Strategies for targeting lentiviral vectors. Curr. Gene Ther. 2008;8:449–460. doi: 10.2174/156652308786848003. [DOI] [PubMed] [Google Scholar]
- 298.Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005;23:967–973. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- 299.Paques F, Duchateau P. Meganucleases and DNA double-strand break-induced recombination: perspectives for gene therapy. Curr. Gene Ther. 2007;7:49–66. doi: 10.2174/156652307779940216. [DOI] [PubMed] [Google Scholar]
- 300.Boch J. TALEs of genome targeting. Nat. Biotechnol. 2011;29:135–136. doi: 10.1038/nbt.1767. [DOI] [PubMed] [Google Scholar]
- 301.Szabo E, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010 doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 302.Kohn DB, et al. T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates. Nat. Med. 1998;4:775–780. doi: 10.1038/nm0798-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hesdorffer C, et al. Phase I trial of retroviral-mediated transfer of the human MDR1 gene as marrow chemoprotection in patients undergoing high-dose chemotherapy and autologous stem-cell transplantation. J. Clin. Oncol. 1998;16:165–172. doi: 10.1200/JCO.1998.16.1.165. [DOI] [PubMed] [Google Scholar]
- 304.Kohn DB, et al. A clinical trial of retroviral-mediated transfer of a rev-responsive element decoy gene into CD34(+) cells from the bone marrow of human immunodeficiency virus-1-infected children. Blood. 1999;94:368–371. [PubMed] [Google Scholar]
- 305.Cowan KH, et al. Paclitaxel chemotherapy following autologous stem cell transplantation and engraftment of hematopoietic cells transduced with a retrovirus containing the multidrug resistance cDNA (MDR1) in metastatic breast cancer patients. Clin. Cancer Res. 1999;5:1619–1628. [PubMed] [Google Scholar]
- 306.Liu JM, et al. Engraftment of hematopoietic progenitor cells transduced with the Fanconi Anemia Group C gene (FANCC) Hum. Gene Ther. 1999;10:2337–2346. doi: 10.1089/10430349950016988. [DOI] [PubMed] [Google Scholar]
- 307.Amado RG, et al. Anti-human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a phase I study: myeloid and lymphoid reconstitution in human immunodeficiency virus type-1-infected patients. Hum. Gene Ther. 2004;15:251–262. doi: 10.1089/104303404322886101. [DOI] [PubMed] [Google Scholar]
- 308.Mitsuyasu RT, Zack JA, MacPherson JL, Symonds GP. Phase I/II Clinical Trials Using Gene-Modified Adult Hematopoietic Stem Cells for HIV: Lessons Learnt. Stem Cells Int. 2011;2011:393698. doi: 10.4061/2011/393698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mitsuyasu RT, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 2009;15:285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]