Abstract
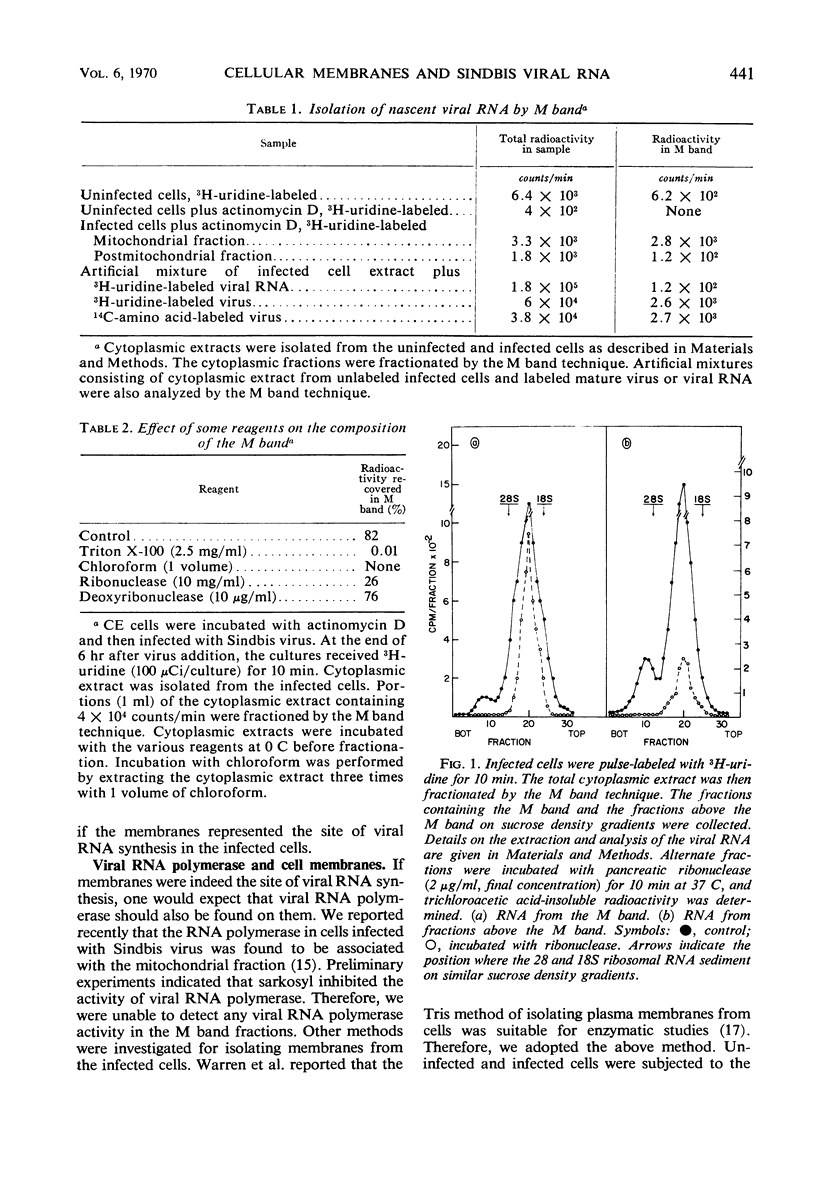
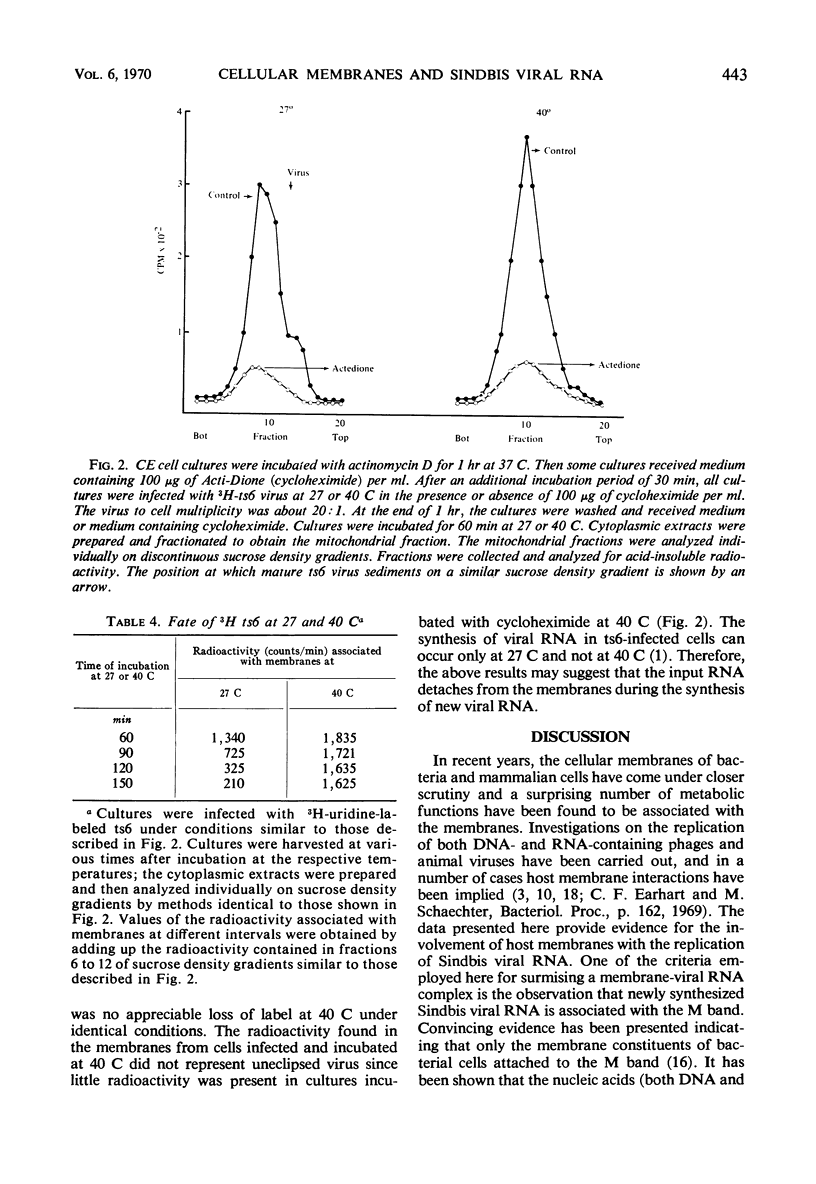
Membranes from cells infected with Sindbis virus had associated with them viral ribonucleic acid (RNA) polymerase and about 60 to 70% of the viral RNA labeled when short pulses were used. This RNA contained most of the replicative intermediate and replicative form of viral RNA found in the infected cells. The use of “Mg2+ sarkosyl crystals” permitted the isolation of membrane-bound nucleic acids and allowed the demonstration that Sindbis virus RNA was synthesized on a membrane-viral RNA complex. Viral RNA from the infecting virions first became associated with the membranes during the latent period and, subsequently, slowly detached. The attachment of the viral RNA to the membranes did not require active viral RNA polymerase, since RNA from ts6, an RNA− temperature-sensitive mutant of Sindbis virus, associated with cellular membranes at a nonpermissive temperature. However, the subsequent detachment of the RNA from the membranes was restricted in the absence of viral RNA synthesis. The results indicate that association of viral RNA with cellular membranes may represent an early step occurring during the replication of Sindbis virus RNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969 Nov 14;166(3907):885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Majumdar C., Weintraub S., Frankel D. M. DNA polymerase and the cell membrane after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:495–500. doi: 10.1101/sqb.1968.033.01.057. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Replicative intermediate of an arbovirus. J Virol. 1968 Jun;2(6):547–552. doi: 10.1128/jvi.2.6.547-552.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Sreevalsan T. Membrane binding of input arbovirus ribonucleic acid: effect of interferon or cycloheximide. J Virol. 1970 Aug;6(2):169–175. doi: 10.1128/jvi.6.2.169-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. S. The use of tricine buffer in animal tissue cultures. J Cell Biol. 1969 Jul;42(1):320–321. doi: 10.1083/jcb.42.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick L., Boyce R. P., Echols H. Membrane association by bacteriophage lambda-DNA: possible direct role of regulator gene N. Nature. 1969 Sep 20;223(5212):1239–1242. doi: 10.1038/2231239a0. [DOI] [PubMed] [Google Scholar]
- Haywood A. M., Cramer J. H., Shoemaker N. L. Host-virus interaction in ribonucleic acid bacteriophage-infected Escherichia coli. I. Location of "late" MS2-specific ribonucleic acid synthesis. J Virol. 1969 Oct;4(4):364–371. doi: 10.1128/jvi.4.4.364-371.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- Polatnick J., Arlinghaus R. B. Foot-and-mouth disease virus-induced ribonucleic acid polymerase in baby hamster kidney cells. Virology. 1967 Apr;31(4):601–608. doi: 10.1016/0042-6822(67)90188-2. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Allen P. T. Replication of Western equine encephalomyelitis virus. II. Cytoplasmic structure involved in the synthesis and development of the virions. J Virol. 1968 Oct;2(10):1038–1046. doi: 10.1128/jvi.2.10.1038-1046.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Yin F. H. Sindbis virus-induced viral ribonucleic acid polymerase. J Virol. 1969 Jun;3(6):599–604. doi: 10.1128/jvi.3.6.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Yarus M. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. 8. Evidence for an essential bacterial "site". J Virol. 1967 Feb;1(1):135–144. doi: 10.1128/jvi.1.1.135-144.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



