Abstract
Objective
To assess the effects of zinc and iron-folic acid supplementation on motor and language milestones in Nepali children.
Methods
A total of 544 children 4–17 months old residing in Ishwarpur, Nepal were randomized to receive placebo, iron-folic acid, zinc and zinc plus iron-folic acid daily. Data were collected at baseline and at three month intervals for one year. Main effects of zinc and iron folic-acid supplementation were estimated for motor and language milestones. We modeled crude and adjusted mean cumulative changes in scores between visits 1 and 5, and adjusted rates-of-change.
Results
Adjusted differences in motor milestone scores between visits 1 and 5 and rates-of-change were not significantly different for zinc and non-zinc groups (adj. β=−0.7, 95% CI: −1.4, 0.01; adj. β=−0.1, 95% CI:−0.5, 0.3, respectively). Motor milestones in children receiving and not receiving iron supplements were not significantly different (adj. β=0.1, 95% CI:−0.7, 0.8 from visit 1 to 5; adj. β=0.1, 95% CI:−0.3, 0.5 for rate-of-change). Children receiving zinc had a 0.8 lower mean crude change in language score between visits 1 and 5 compared to children not receiving zinc (95% CI −1.3,−0.3), but significance was lost after adjustment (adj. β=−0.2, 95% CI:−0.6, 0.2, comparing visits 1 to 5; β=−0.1, 95% CI:−0.3, 0.2 for rate-of-change). We observed no significant difference in motor or language milestone scores due to iron supplementation..
Conclusion
After one year, neither zinc nor iron-folic acid supplementation in Nepali children improved attainment of motor or language milestones.
Keywords: micronutrients, iron, zinc, psychomotor performance, language development, children, Nepal
INTRODUCTION
Children in the developing world, and particularly in south central Asia, suffer from elevated levels of nutritional deficiency and malnutrition.(1) Poor diet and micronutrient deficiency can result in impaired cognitive and motor development.(2–4) Specifically, it has been proposed that effects on child development are likely causal particularly in settings where malnutrition results from extreme poverty and lack of access to food.(5) Such an environment, with very high prevalence estimates of micronutrient deficiencies, exists in our study site, the Sarlahi district of Nepal.(6, 7)
Motor and language milestone acquisition can set a child on trajectory for further developmental achievements in later childhood and adulthood (8). Because the brain develops quickly in early life (2) and nutrition provides the basis for brain functioning, nutrition during this period contributes to lasting structural changes. Adequate receipt of nutrients in early life are thought to correspond to sensitive or critical periods for several developmental outcomes.(9) Persistent adverse developmental effects can result from inadequate iron intake during the first year of life due to iron's role in brain development.(10, 11) While evidence supports improved developmental outcomes after corrective supplementation of iron-deficient children, benefits of universal preventive iron supplementation programs are unclear.(12) Moreover, it is believed that zinc is beneficial for developmental outcomes particularly for motor functioning, at least among the most vulnerable children; although more needs to be learned regarding the timing of zinc supplementation and its relation with other micronutrients (13).
In summary, while research to date points to the importance of micronutrients during early life for children’s development, it remains unknown whether supplementing all children with zinc or iron has any measureable benefit and at which ages. Therefore, we performed a randomized controlled trial to assess the effects of zinc, iron and folic-acid supplementation on motor and language milestones in Nepali children during their first year and a half of life.
METHODS
This study took place in Ishwarpur, a Village Development Committee (VDC) in the Sarlahi district of rural southern Nepal, as a sub-study of the Nepal Nutrition Intervention Project, Sarlahi (NNIPS-4), a cluster randomized 2×2 factorial micronutrient trial of the effects of iron-folic acid and zinc on mortality.(6) Within Ishwarpur, 23 geographic clusters were randomized into four arms: placebo, iron-folic acid, zinc and zinc plus iron-folic acid.
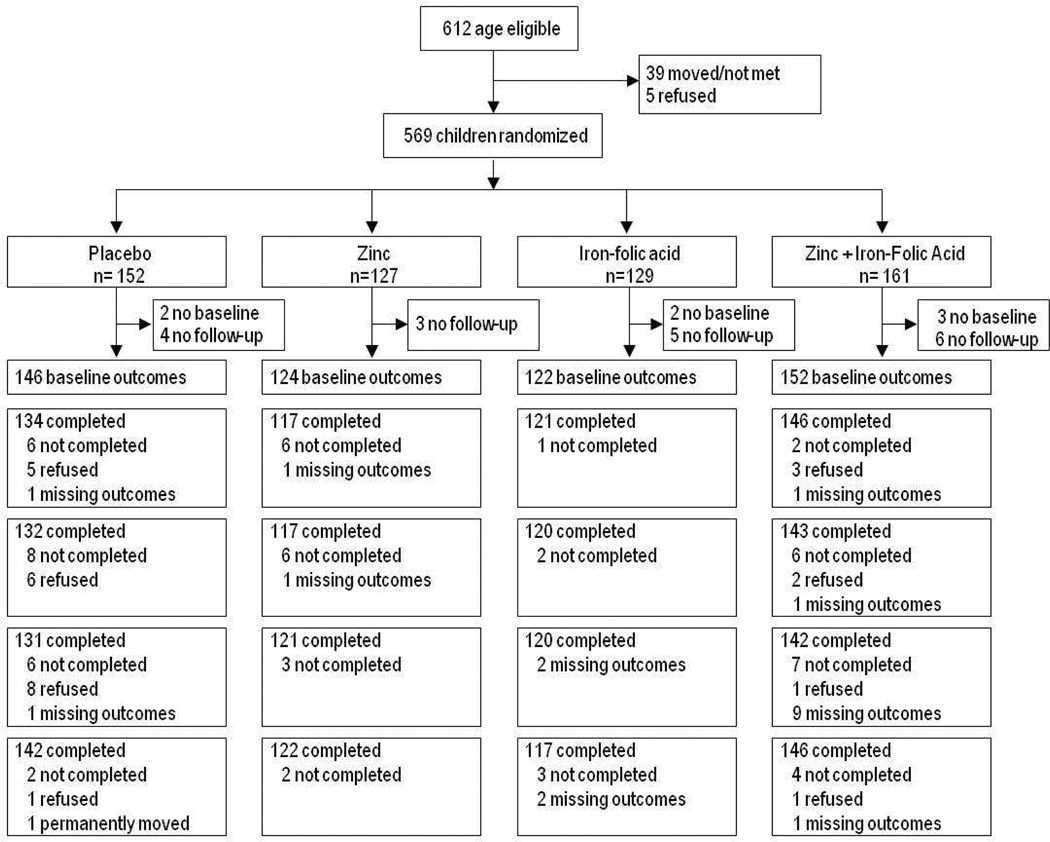
For this sub-study, 544 children 4- to 17-months old who resided in Ishwarpur between January 2002 and April 2003 are included in the analysis. Of the 613 eligible infants identified in a population-based census in this VDC conducted between December 2000 and March 2001, only 44 did not participate. These children were either not able to be located or the caregiver chose not to participate. Of the 569 children who participated, we excluded another seven children who were not assessed at baseline for the developmental outcomes of interest, and 18 additional children who had no follow-up outcome data. The study was approved by the Johns Hopkins University Committee on Human Subjects Research and the Nepal Health Research Council. Consent was obtained from all caregivers. The main trial and the developmental outcomes substudy are registered at ClinicalTrials.gov (NCT00109551).
Parents were interviewed on demographic information and child outcomes at home at baseline and four follow-up visits, occurring at approximately three month intervals for one year. At baseline, data were collected on: infant age in months (4–5, 6–8, 9–11,12–14, or 15–17), gender (male or female), caste (higher: Brahmin and Chetri; lower: Vaiysha, Shudra, or Muslim), ethnic group (Pahadi or Madeshi), maternal literacy (not literate, literate) paternal literacy (not literate, literate), paternal occupation (farmer, including also unskilled worker, laborer, or unemployed; business, including also government, or private sector worker). To ascertain the child’s age when it was not known, local festivals and lunar calendars were used to approximate the birthdate. Socio-economic status (SES) was based on data recorded by field staff including: presence of a latrine at the house, a servant, cattle, bicycle, radio, farmable land, home garden plot, second floor in the house, roof, TV, electricity at home, and bullock cart. Three categories defined SES based on ownership of these twelve items: low (0–1 items), medium (2–5 items) and high (6–12 items).
Exposure: Micronutrient Supplements
Our exposure of interest was iron-folic acid and zinc supplementation. Geographic sectors were randomized to daily supplementation groups of 1) 10 mg zinc, 2) 12.5 mg iron and 50 µg folic acid, 3) a combination of 10 mg zinc, 12.5 mg iron and 50 µg folic acid, or 4) a sugar placebo. All possible combinations of the 4 treatment groups were written on pieces of paper. The sectors were geographically ordered and senior field personnel randomly and blindly withdrew pieces of paper from a container that indicated the codes for the first 4 sectors on the list. The paper was replaced and random drawing continued until all sectors had been assigned to one of the four treatment groups. Children under age 1 year received a half-dose of supplements. Vanilla-flavored supplements were provided by Nutriset (Malaunay, France) in collaboration with the Department of Child and Adolescent Health and Development (WHO, Geneva, Switzerland). Supplements were administered by study staff twice per week, and by the child’s caregiver on days when there was no home visit. Younger children consumed tablets dissolved, either in breast milk, or purified water if milk was not available. Field staff monitored compliance by counting the number of unconsumed tablets weekly. Field staff and participating families were masked to the randomization of the intervention. All tablets looked identical.
Development outcomes: Motor and Language Milestones
The main outcomes for the analysis were parental report of motor and language milestone ascertainment at each follow-up visit. Motor and language milestone instruments were adapted for the local setting from previous work in another low-resource setting (14) that drew from the Griffiths Mental Development Scale(15) and the MacArthur Communicative Development Inventory(16) The motor milestone instrument included only those items correlated with the onset of bipedal locomotion, as identified through factor analysis of the original tool. Language milestones were additionally modified to include items from the Child Development Inventory.(17) Trained fieldworkers visited homes every three months and asked mothers to report on whether the child could perform any of 29 motor and 20 language tasks specified in the milestone instrument. Five tasks on the motor scale had to be demonstrated by the child in the presence of the fieldworker prior to receiving a yes response. Fieldworkers stopped asking questions on each scale once 5 consecutive no responses were given. Motor and language milestone tasks achieved were ordered by expected chronology of development. The milestone score was based on what parents reported as the most advanced task the child could perform by that visit (i.e., highest numerically ordered task at that visit). Thus the motor and language scores could take values from 0 to 29 and 0 to 20, respectively.
Statistical Analysis
Data were analyzed using SAS 9.2 (Cary, NC). Because randomization by ‘sector’ (i.e. a group of around 100 households) was conducted, chi-square and ANOVA tests were used to compare potential confounding characteristics across supplementation groups at baseline. Adherence was tested by comparing the mean proportion of supplements received out of those assigned.
Because of the factorial design, one group was randomized to receive a combination dose of zinc plus iron-folic acid. We tested for an interaction between the effects of zinc only and iron-folic acid only to see if when administered together the joint effect differed from what would be expected by summing each treatment under the assumption of independence. As no interaction was found, main effects of zinc and iron folic-acid were estimated separately, comparing children who received zinc to children who did not receive zinc, and children who received iron folic-acid to children who did not receive iron folic-acid.
For each milestone separately, we calculated the mean cumulative change in scores between the first and fifth visits. Then we calculated the crude and adjusted difference in mean cumulative change between the first and fifth visit in scores comparing between treatment groups. For both milestones, in multivariable models, adjustments were made for, sex, ethnicity, SES, caste, and paternal occupation, based on the fact that these were unbalanced at baseline. The analyses for language milestones were additionally adjusted for baseline language score, since this was also unbalanced. Because milestones are age dependent, all analyses adjusted for baseline age. Adjusted rates-of-change for each milestone were also estimated using Generalized Estimating Equations (GEE) (18) in order to take into account all follow-up time points. After examining correlation matrices for both milestone and language outcomes and time series graphs, we used an auto-regressive correlation structure.
RESULTS
Of 569 in the substudy, a total of 544 children with baseline and at least one follow-up milestone assessment were randomized to receive zinc (N=124), iron-folic acid (N=122), zinc plus iron-folic acid (N=152) or placebo (N=146). Until visit five, complete outcomes were collected from at least 80.2% in placebo, 86.9% in zinc plus iron-folic acid, 86.3% for zinc and 94.3% for the iron-folic acid group (Figure 1: Consort Diagram). Milestone data was missing during visits two to five for a maximum of 10.3% of children in the placebo, 2.5% in the iron-folic acid, 5.7% in the zinc and 6.6% in the zinc plus iron-folic acid groups.
Figure 1.
Participant flow
Treatment groups were comparable on age, maternal literacy, and paternal literacy, and mean motor milestone at baseline. The three treatment groups and placebo were not comparable on the distribution of child sex, caste, SES, ethnicity, paternal occupation, or mean language milestone score (Table 1). For the 544 children in this sub-study, compliance with treatment assignment by the end of follow-up for the overall study was lowest among the group taking iron-folic acid only, and highest among the placebo group. The iron group was significantly less compliant than the non-iron group, while there was no difference in the average compliance between the zinc and non-zinc group.
Table 1.
Distribution of background socio-demographic characteristics and baseline motor milestone scores by randomization group †
| Placebo n (%) |
Zinc n (%) |
Iron-Folic Acid n (%) |
Zinc + Iron-Folic Acid n (%) |
p-value* | |
|---|---|---|---|---|---|
| Total | 146 | 124 | 122 | 152 | |
| Baseline age (months) | |||||
| 4–5 | 21 (14.4) | 23 (18.5) | 16 (13.1) | 26 (17.1) | 0.42 |
| 6–8 | 22 (15.1) | 31 (25.0) | 27 (22.1) | 25 (16.4) | |
| 9–11 | 29 (19.9) | 19 (15.3) | 31 (25.4) | 33 (21.7) | |
| 12–14 | 39 (26.7) | 27 (21.8) | 29 (23.8) | 38 (25.0) | |
| 15–17 | 35 (24.0) | 24 (19.4) | 19 (15.6) | 30 (19.7) | |
| Sex | |||||
| Female | 77 (52.7) | 71 (57.3) | 55 (45.1) | 64 (42.1) | 0.05 |
| Male | 69 (47.3) | 53 (42.7) | 67 (54.9) | 88 (57.9) | |
| Caste** | |||||
| Lower | 139 (95.2) | 105 (84.7) | 116 (95.1) | 137 (90.1) | 0.01 |
| Higher | 7 (4.8) | 19 (15.3) | 6 (4.9) | 15 (9.9) | |
| Socioeconomic status*** | |||||
| 0–1 possessions | 25 (17.4) | 11 (9.2) | 39 (32.2) | 40 (26.7) | <0.01 |
| 2–5 possessions | 78 (54.2) | 72 (60.5) | 54 (44.6) | 72 (48.0) | |
| 6–12 possessions | 41 (28.5) | 36 (30.3) | 28 (23.1) | 38 (25.3) | |
| Ethnicity | |||||
| Madheshi | 131 (90.3) | 93 (75.0) | 108 (89.3) | 126 (82.9) | <0.01 |
| Pahadi | 14 (9.7) | 31 (25.0) | 13 (10.7) | 26 (17.1) | |
| Maternal literacy | |||||
| Not literate | 123 (84.2) | 103 (83.1) | 104 (85.2) | 128 (84.2) | 0.97 |
| Literate | 23 (15.8) | 21 (16.9) | 18 (14.8) | 24 (15.8) | |
| Paternal literacy | |||||
| Literate | 74 (50.7) | 71 (57.3) | 58 (47.5) | 74 (48.7) | 0.41 |
| Not literate | 72 (49.3) | 53 (42.7) | 64 (52.5) | 78 (51.3) | |
| Paternal occupation | |||||
| Farmer | 109 (74.7) | 98 (79.0) | 104 (85.2) | 100 (65.8) | <.01 |
| Businessman | 37 (25.3) | 26 (21.0) | 18 (14.8) | 52 (34.2) | |
| Baseline scores | |||||
| Mean motor milestone (sd) | 12.8 (6.3) | 12.2(7.1) | 11.8 (6.5) | 12.6 (6.5) | 0.64§ |
| Mean language milestone (sd) | 4.6(2.5) | 4.9(3.1) | 3.9 (2.5) | 4.9(2.9) | 0.01§ |
Ten children were missing baseline values for socioeconomic status, and 2 were missing ethnicity
Chi-square p-values for categorical variables
Lower caste refers to Vaiyshas, Shudras and Muslims. Higher caste refers to Brahmins and Chetris
Possessions: Presence of a latrine at the house, a servant, cattle, bicycle, radio, farmable land, home garden plot, second floor in the house, roof, TV, electricity at home, and bullock cart.
ANOVA-based p-values for continuous variables
Motor milestones
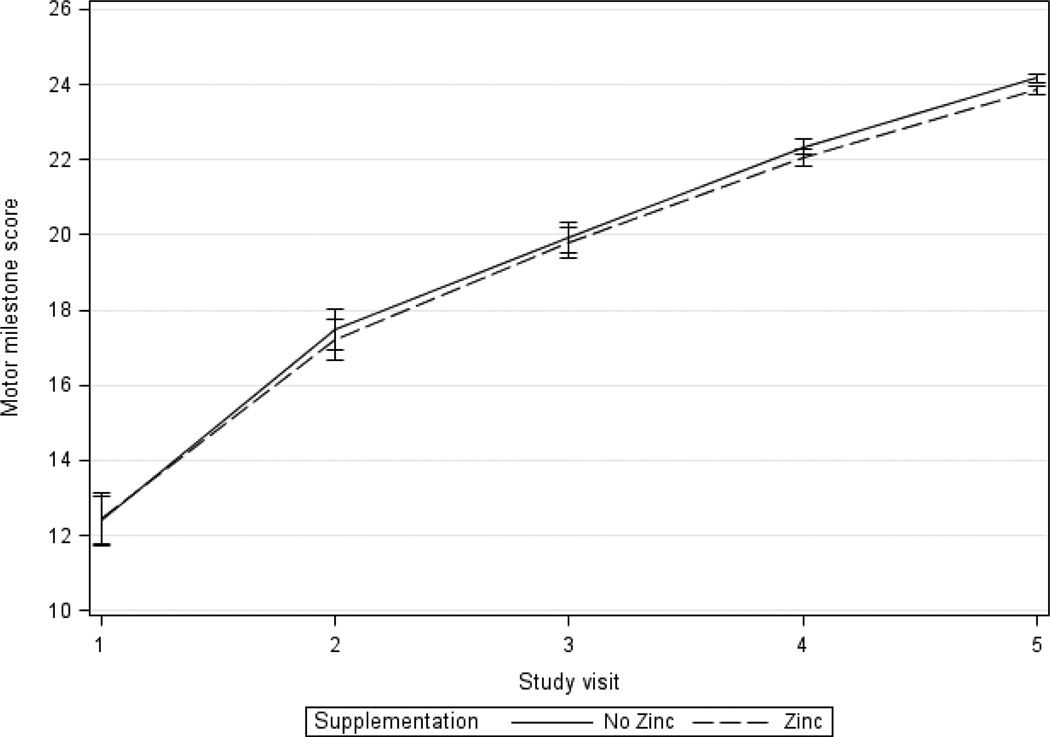
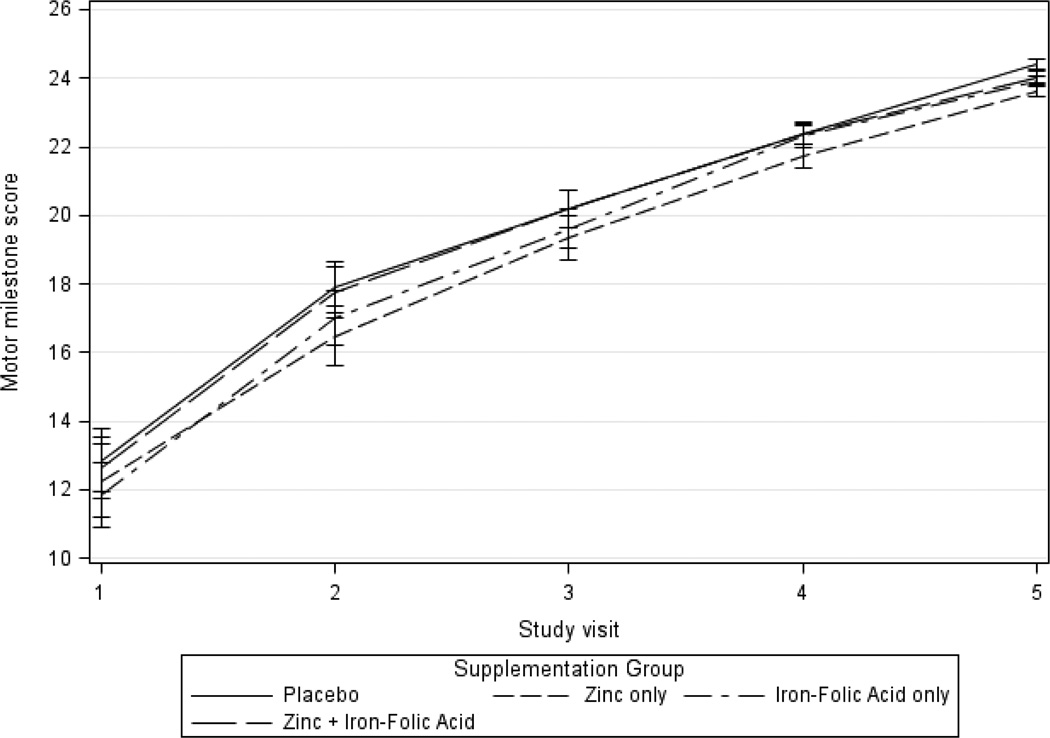
Regarding motor milestones, there was a mean increase from a score between visit 1 to visit 5 of 11.4 and 11.8 in the zinc and non-zinc groups, respectively (Table 2 and Figure 2). Analyses of unadjusted differences in motor milestones between visits 1 and 5 was not significantly different between zinc and non-zinc groups (β=−0.4, 95% CI −1.4, 0.7). Both adjusted analyses comparing visits 1 and 5 and adjusted GEE analyses using all follow-up visits showed no clear difference between the groups with and without zinc supplementation (β=−0.7, 95% CI −1.4, 0.01 visit 1 and 5 change adjusted; β=−0.1, 95% CI −0.5, 0.3 for adjusted GEE model).
Table 2.
Main effects of zinc and iron supplementation on motor milestone score
| Mean change in motor milestone score from visit 1 to visit 5 (95% CI) |
Unadjusted difference in mean motor milestone† score change β (95% CI) |
Adjusted* difference in mean motor milestone§ score change β (95% CI) |
Adjusted* difference in motor milestone Σ rate-of-change β (95% CI) (GEE) |
|
|---|---|---|---|---|
| Zinc (n=285) | 11.4 (10.7, 12.2) | −0.4 (−1.4, 0.7) | −0.7 (−1.4, 0.01) | −0.1 (−0.5, 0.3) |
| No Zinc (n=277) | 11.8 (11.1, 12.6) | ref | ref | ref |
| Iron (n=285) | 11.8 (11.0, 12.5) | 0.3 (−0.8, 1.3) | 0.1 (−0.7, 0.8) | 0.1 (−0.3, 0.5) |
| No Iron (n=277) | 11.5 (10.8, 12.3) | ref | ref | ref |
Analysis among n=527 who had outcome data at visit 1 and 5
Analysis among n=516 who had outcome data at visit 1 and 5 and complete data on relevant baseline covariates
Adjusted for age category at baseline, sex, ethnicity, SES category, caste, paternal occupation, and maternal literacy. Baseline motor scores are not controlled for because they did not differ at baseline.
Analysis among n=532 who had any outcome data for the post-intervention period (i.e. visits 2 through 5) and complete data on relevant baseline covariates
Figure 2.
Mean adjusted motor milestone score by zinc treatment over time
For iron, motor milestone score improvement from visit 1 to 5 almost was the same when comparing groups with and without iron supplementation (11.8 points change in the iron group versus a 11.5 change in score in the non-iron group)(Figure 3). Neither the unadjusted difference between the groups receiving iron supplements and not receiving iron supplements was significantly different (β=0.3, 95% CI −0.8, 1.3 unadjusted; β=0.1, 95% CI −0.7, 0.8 change from visit 1 to 5 adjusted; β=0.1, 95% CI −0.3, 0.5 for adjusted GEE model). (Table 2)
Figure 3.
Mean adjusted motor milestone score by iron treatment over time
Language Milestones
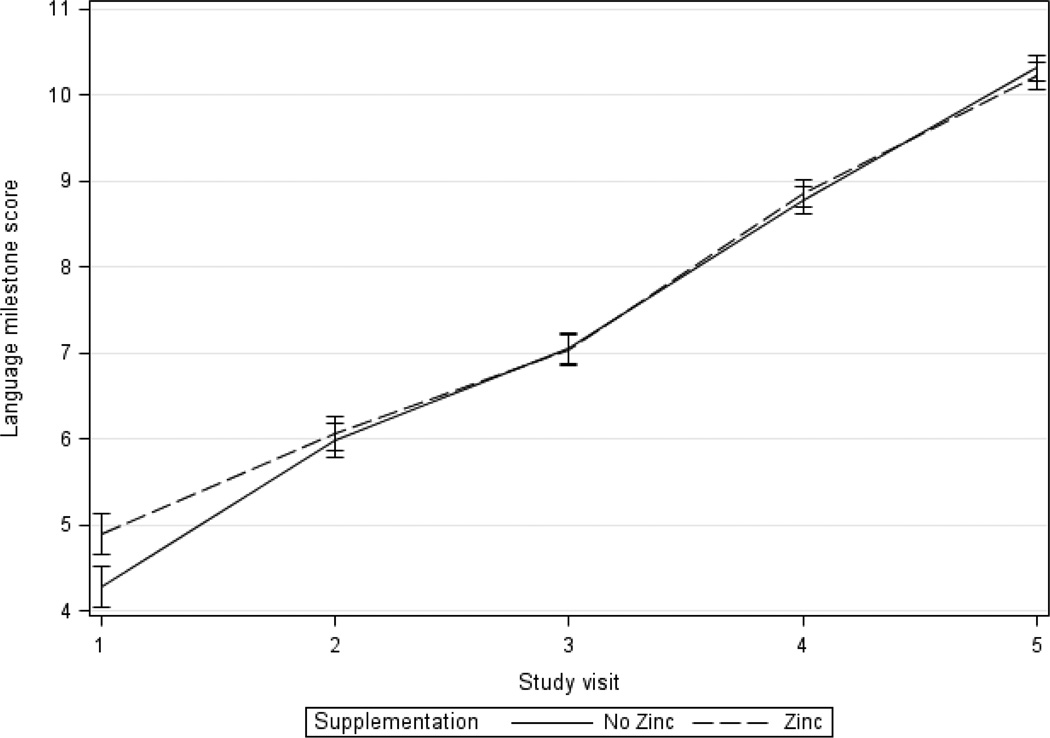
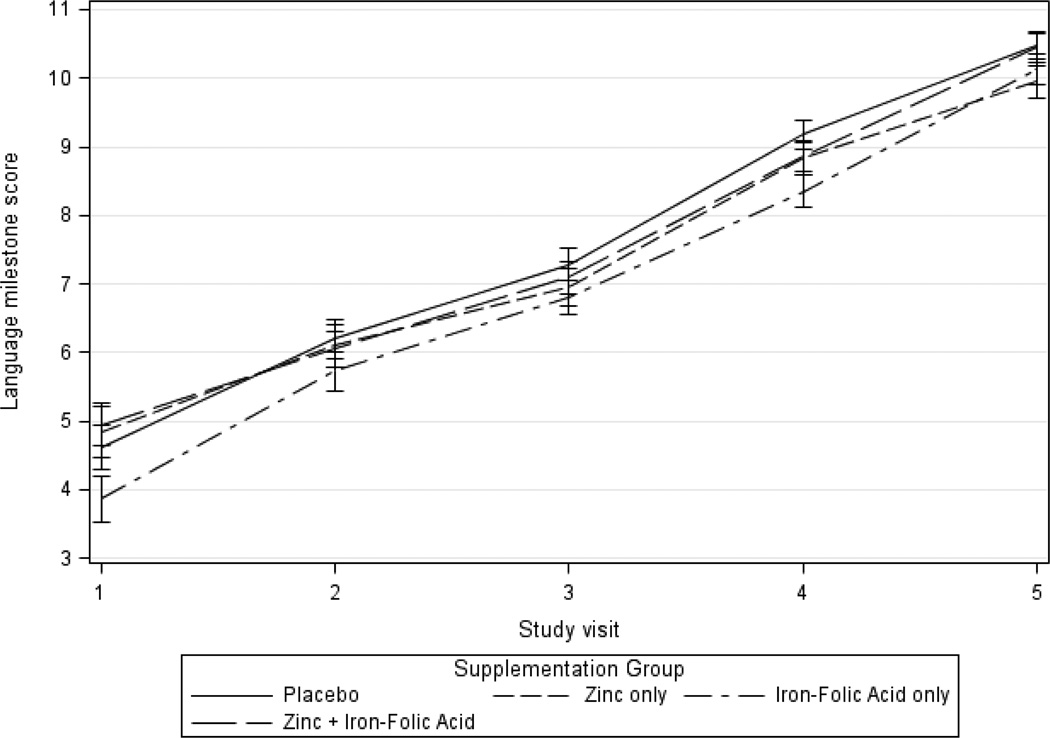
Comparing zinc and non-zinc supplement groups, language scores improved 5.3 points in the zinc supplementation group between visits 1 and 5, compared to 6.1 in the non-zinc group during this same period (Table 3 and Figure 4). Comparing groups receiving zinc or not in unadjusted analyses between visits 1 and 5, those receiving zinc had a 0.8 lower mean change in language score compared to children who did not receive zinc supplementation (β=−0.8, 95% CI −1.3, −0.3). However after adjustment for socio-demographic variables and baseline language milestone scores, this change between visits 1 and 5 lost statistical significance (β=−0.2, 95% CI −0.6, 0.2). There was also no difference in the rate of change in language milestone using data from all follow-up visits with GEE (β=−0.1, 95% CI −0.3, 0.2) (Table 3).
Table 3.
Main effects of zinc and iron supplementation on language milestone score
| Mean change in language milestone score from visit 1 to visit 5 (95% CI) |
Unadjusted difference in language milestone† score change β (95% CI) |
Adjusted* difference in language milestone§ score change β (95% CI) |
Adjusted* difference in language milestone Σ rate- of-change β (95% CI) (GEE) |
|
|---|---|---|---|---|
| Zinc | 5.3 (4.9, 5.7) | −0.8 (−1.3, −0.3) | −0.2 (−0.6, 0.2) | −0.1 (−0.3, 0.2) |
| No Zinc | 6.1 (5.7, 6.4) | ref | ref | Ref |
| Iron | 5.8 (5.4, 6.2) | 0.3 (−0.2, 0.8) | 0.2 (−0.2, 0.6) | −0.1 (−0.3, 0.1) |
| No Iron | 5.5 (5.2, 5.9) | ref | ref | Ref |
Analysis among n=527 who had outcome data at visit 1 and 5
Analysis among n=516 who had outcome data at visit 1 and 5 and complete data on relevant baseline covariates
Adjusted for age category at baseline, sex, ethnicity, SES category, caste, paternal occupation, maternal literacy, and baseline language milestone score
Analysis among n=532 who had any outcome data for the post-intervention period (i.e. visits 2 through 5) and complete data on relevant baseline covariates
Figure 4.
Mean adjusted language milestone score by zinc treatment over time
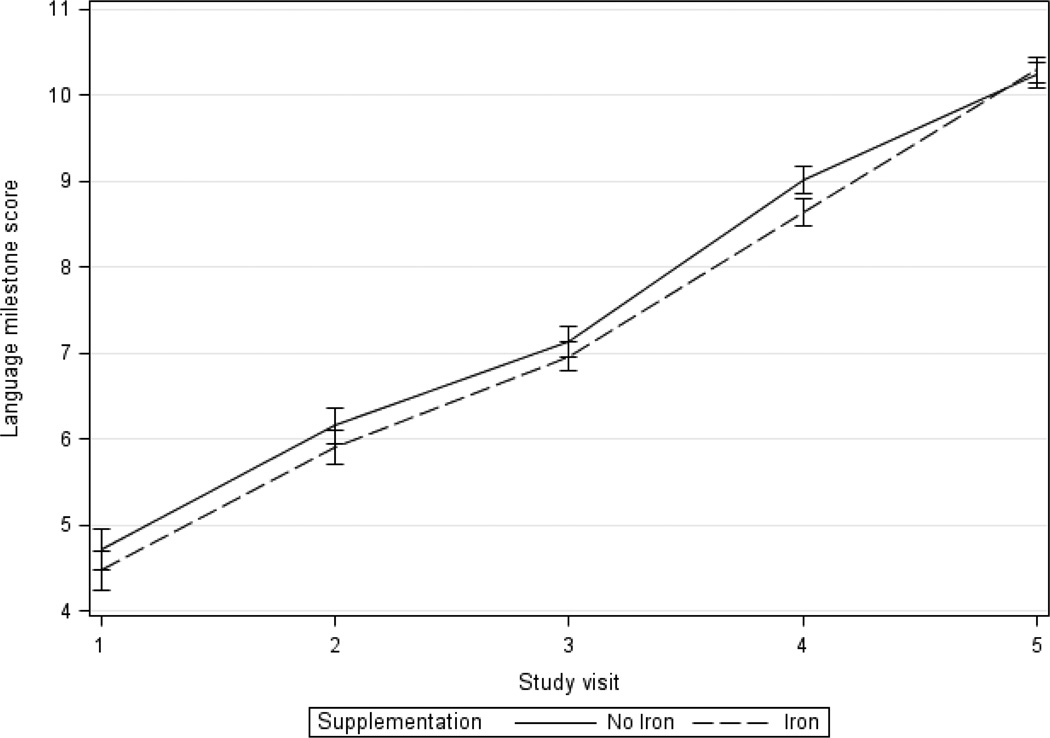
The mean increase in language milestone score between the first and last visits was 5.8 points for children in the iron compared to 5.5 in the non-iron supplementation group (Table 3 and Figure 5). This difference between these groups was non-significant in unadjusted and adjusted analyses comparing change between visits 1 and 5 (β=0.3, 95% CI −0.2, 0.8 and β=0.2, 95% CI −0.2, 0.6, respectively). Adjusted analyses looking at change over all follow-up visits using GEE also showed no significant difference in rate of change in language milestone scores (β=−0.1, 95% CI −0.3, 0.1).
Figure 5.
Mean adjusted language milestone score by iron treatment over time
DISCUSSION
In this randomized controlled trial, we found no evidence of effects of zinc or iron supplementation on motor or language milestones in a population of rural Nepali infants and children. Although basic science research has suggested pathways relating a role for zinc deficiency in child development (19, 20), previous trials on micronutrient supplementation and child motor skills and cognition have been inconclusive (13), with many of them showing null effects (21).
In terms of motor abilities, in the larger parent study of 3264 Nepali children enrolled between 1 to 35 months old, the average age of walking unassisted did not differ between children randomly assigned to receive daily iron-folic acid supplementation, except for among children < 1 year old among whom supplementation was associated with delayed walking (22). In the same subsample of Nepali children as in the present study, Siegel et al. used a 14-item pictorial scale to assess the sequential acquisition of gross motor skills (23); results showed that child hemoglobin ≥ 105 and meat consumption were related to earlier age of unassisted walking (23). In contrast, Zanzibari infants, enrolled between 5 and 11 months of age to receive daily supplementation over a year, demonstrated earlier walking when supplemented with iron (with amplified effects among iron deficient children), but observed no effects of zinc supplementation (24). Likewise, among Bangladeshi infants, positive effects of weekly combined zinc and iron supplementation were associated with a slower decline in infant motor developmental scores over time.(25) A study of Indonesian infants assessed at one year after 6 months of micronutrient supplementation found a significant effect of supplementation of iron alone on the Bayley’s Psychomotor Development Index (PDI), but an interaction was observed between iron and zinc supplementation in children receiving both, such that the benefits disappeared (26).
The beneficial effect of childhood iron supplementation on cognitive outcomes may only exist among iron-deficient children (12, 27, 28). Our findings, based on a relatively large sample, are consistent with previous studies that have found no beneficial effect of iron supplementation during early childhood on mental development. For children under 27 months, a meta-analysis by Sachdev et al (2005) of randomized controlled trials in diverse populations found no overall evidence of an effect of iron supplementation on mental development.(27) A subsequent meta-analysis by Szajewska et al (2010) of three trials found that a borderline significant improvement in mental development scores of infants who were supplemented with iron.(29) A previous investigation among a subset of our sample in infancy (at 39- and 52- weeks) found no improvement in information processing outcomes due to iron-folic acid, zinc, or their combination.(30)
In 2009 Brown et al. published a systematic review of zinc supplementation among infants and preschoolers in relation to the Bayley’s Psychomotor Development Index (PDI) and Mental Development Index (MDI). Their meta-analysis of nine studies of zinc alone compared to placebo (or zinc plus iron or another micronutrient) estimated an overall non-significant effect size of 0.025 (95% CI:−0.15 to 0.20) in relation to the Psychomotor Development Index (PDI)(21). However, studies among low birth weight (<1,500 g) infants in Canada and low-income infants in Chile found improved motor functioning (not using the PDI) among those who had been supplemented with zinc (31, 32). Similar to psychomotor findings in the same meta-analysis, Brown et al. estimated a null pooled overall effect combining nine studies that examined zinc supplementation alone (or in combination with other micronutrients) with outcomes on the Bayley’s MDI (21). One of these studies in which children received combined zinc and iron supplementation showed an adverse effect of zinc supplementation on the MDI.(33)
We expected that iron-folic acid or zinc supplementation could have an effect on motor and language outcomes, due to the biological importance of these essential elements. Zinc is thought to have a critical role in cell division and maturation and in the neurological system and metabolism, making it relevant to early motor and language development(13). Likewise, iron is believed to impact psychological functioning possibly because of reduced synthesis of hemoglobin as well as decreased activity of enzymes containing iron in the brain(3).
Research to date has been mixed and, as reviewed above, null findings consistent with most existing studies on this topic. There are several possible reasons we may not have observed an effect. First, it is possible that the Nepalese infants studied were more severely malnourished in terms of both micronutrient deficiencies and protein-energy malnutrition than most other populations studied. Thus, it is possible that other deficiencies were so severe that any true benefit of iron or zinc supplementation could not be manifest. Furthermore, given the severity of nutrition deficiencies in this population, it is possible that the window of opportunity for the benefits of supplementation was earlier than our period of supplementation. For example, Christian et al found long-term cognitive benefits to the Nepalese children at ages 7 to 9 years follow-up that were associated with prenatal iron/folic acid supplementation to the mother (34), supporting the idea that it is possible that postnatal supplementation in this trial may not have been timed optimally. Finally, it is possible that any true benefits of zinc or iron supplements to children’s brain function and cognition were too specific or subtle or to be captured by our outcomes, which reflect rather general gross milestones. Alternatively, it may be that zinc and iron do not have an effect.
Strengths of this study include that this is one of the few studies to specifically examine language abilities in relation to zinc supplementation in young children. The study used a randomized and triple masked controlled design. The rural and largely micronutrient deficient population that received supplementation over the course of a year had potential for observing effects, if they existed. There was a 93% response rate in the VDC where this sub-study was conducted, suggesting that selection bias is unlikely to have played a major role. Likewise, there were only a total of 25 children who lacked baseline or follow-up data for our outcomes of interest. Although randomization at baseline was uneven, we were able to take this into account in our analysis by adjusting for socio-demographic factors that were not equally distributed. Additional studies of effects at other ages or information on other micronutrients or micronutrient deficiencies that may be acting in conjunction with iron, zinc and folic-acid, in these children may be helpful for understanding why we may not have observed an effect.
The present study did not indicate effects of iron-folic acid or zinc supplementation on motor milestones or language abilities in Nepali children in early life. Further studies are needed to confirm these findings as well as to be sure that the domains covered by our motor milestones and the language acquisition assessment tools are the pertinent domains associated with these micronutrients. Additional research on the detailed mechanisms through which micronutrient supplementation might affect these domains, may inform future confirmatory studies.
Figure 6.
Mean adjusted motor milestone score by treatment group over time
Figure 7.
Mean adjusted language milestone score by treatment group over time
Acknowledgements
Support for this study was received from grants from the National Institutes of Health, Bethesda, MD, USA (HD 38753), the Bill and Melinda Gates Foundation, Seattle, Washington, DC, USA (810–2054), and a Cooperative Agreement between Johns Hopkins University and the Office of Health and Nutrition, US Agency for International Development, Washington, DC, USA (HRNA- 00 −97−00015−00).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
-
1.Pamela J. Surkan –led the writing of the paper, including the review and discussion of literature, and contributed to the discussions regarding the analysis plan and interpretation of results.
-
1.)Emily H. Siegel – contributed to conceptualization of the study, data analysis and analysis plan, trained data collectors, supervised data collection in the field and reviewed and edited the final version of the manuscript.
-
2.)Shivani Patel -- conducted data analysis, substantially contributed to the writing of the statistical methods and results sections, and created the consort diagram and tables.
-
3.)Joanne Katz - was instrumental in conceptualizing and designing the study, helped direct the statistical analysis, interpret the results, and gave substantive input on the writing of the manuscript.
-
4.)Subarna K. Khatry – oversaw the collection of data in the field and approved the final manuscript
-
5.)Rebecca J. Stoltzfus – was instrumental in the design of the study, gave substantial input regarding the interpretation of the results and approved the final manuscript
-
6.)Steven C. LeClerq-- oversaw the collection of data in the field and approved the final manuscript
-
7.)James M. Tielsch —was the principal investigator for the study, was instrumental in the conceptualization and design of the study, data analysis plan, interpretation of the results, and contributed to the final manuscript.
References
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008 Jan 19;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 2.Benton D. The influence of children's diet on their cognition and behavior. Eur J Nutr. 2008 Aug;47(Suppl 3):25–37. doi: 10.1007/s00394-008-3003-x. [DOI] [PubMed] [Google Scholar]
- 3.Benton D. Micronutrient status, cognition and behavioral problems in childhood. Eur J Nutr. 2008 Aug;47(Suppl 3):38–50. doi: 10.1007/s00394-008-3004-9. [DOI] [PubMed] [Google Scholar]
- 4.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007 Jan 13;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson M, Landman M. 'It's not just about food': mother-infant interaction and the wider context of nutrition. Matern Child Nutr. 2007 Oct;3:292–302. doi: 10.1111/j.1740-8709.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, Adhikari R, et al. Effect of daily zinc supplementation on child mortality in southern Nepal: a community-based, cluster randomised, placebo-controlled trial. Lancet. 2007 Oct 6;370:1230–1239. doi: 10.1016/S0140-6736(07)61539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel EH, Stoltzfus RJ, Khatry SK, Leclerq SC, Katz J, Tielsch JM. Epidemiology of anemia among 4- to 17-month-old children living in south central Nepal. Eur J Clin Nutr. 2006 Feb;60:228–235. doi: 10.1038/sj.ejcn.1602306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shonkoff JP, Phillips DA, editors. Committee On Integrating The Science of Early Childhood Development. From Neurons to Neighborhoods : The Science of Early Childhood Development. Washington, DC: National Acadamy Press; 2000. [PubMed] [Google Scholar]
- 9.Benton D. The influence of dietary status on the cognitive performance of children. Mol Nutr Food Res. 2010 Apr;54:457–470. doi: 10.1002/mnfr.200900158. [DOI] [PubMed] [Google Scholar]
- 10.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006 Sep;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008 Dec;138:2534–2536. doi: 10.1093/jn/138.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006 Dec;84:1261–1276. doi: 10.1093/ajcn/84.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black MM. The evidence linking zinc deficiency with children's cognitive and motor functioning. J Nutr. 2003 May;133:1473S–1476S. doi: 10.1093/jn/133.5.1473S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, Tielsch JM, et al. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ. 2001 Dec 15;323:1389–1393. doi: 10.1136/bmj.323.7326.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths R. The Abilities of Babies. London: University of London Press; 1954. [Google Scholar]
- 16.Fenson L, Dale P, Reznick JS, Thal D, Bates E, Hartung J. TheMacArthur Communicative Development Inventories: User’s Guide and Technical Manual. San Diego: Singular Press; 1993. [Google Scholar]
- 17.Ireton H. Child Development Inventory. Minneapolis, MN: Behavior Science Systems, Inc; 1992. [Google Scholar]
- 18.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42:121–130. [PubMed] [Google Scholar]
- 19.Salgueiro MJ, Zubillaga MB, Lysionek AE, Caro RA, Weill R, Boccio JR. The role of zinc in the growth and development of children. Nutrition. 2002 Jun;18:510–519. doi: 10.1016/s0899-9007(01)00812-7. [Review]. [DOI] [PubMed] [Google Scholar]
- 20.Sandstead HH, Frederickson CJ, Penland JG. History of zinc as related to brain function. J Nutr. 2000 Feb;130:496S–502S. doi: 10.1093/jn/130.2.496S. [Historical Article]. [DOI] [PubMed] [Google Scholar]
- 21.Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull. 2009 Mar;30:S12–S40. doi: 10.1177/15648265090301S103. [Review]. [DOI] [PubMed] [Google Scholar]
- 22.Katz J, Khatry SK, Leclerq SC, Mullany LC, Yanik EL, Stoltzfus RJ, et al. Daily supplementation with iron plus folic acid, zinc, and their combination is not associated with younger age at first walking unassisted in malnourished preschool children from a deficient population in rural Nepal. J Nutr. 2010 Jul;140:1317–1321. doi: 10.3945/jn.109.119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel EH, Stoltzfus RJ, Kariger PK, Katz J, Khatry SK, LeClerq SC, et al. Growth indices, anemia, and diet independently predict motor milestone acquisition of infants in south central Nepal. J Nutr. 2005 Dec;135:2840–2844. doi: 10.1093/jn/135.12.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, et al. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5- to 11-mo old. J Nutr. 2006 Sep;136:2427–2434. doi: 10.1093/jn/136.9.2427. [DOI] [PubMed] [Google Scholar]
- 25.Black MM, Baqui AH, Zaman K, Ake Persson L, El Arifeen S, Le K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004 Oct;80:903–910. doi: 10.1093/ajcn/80.4.903. [DOI] [PubMed] [Google Scholar]
- 26.Lind T, Lonnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, et al. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr. 2004 Sep;80:729–736. doi: 10.1093/ajcn/80.3.729. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 27.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 2005 Apr;8:117–132. doi: 10.1079/phn2004677. [DOI] [PubMed] [Google Scholar]
- 28.Hermoso M, Vucic V, Vollhardt C, Arsic A, Roman-Vinas B, Iglesia-Altaba I, et al. The effect of iron on cognitive development and function in infants, children and adolescents: a systematic review. Ann Nutr Metab. 2011;59:154–165. doi: 10.1159/000334490. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 29.Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am J Clin Nutr. 2010 Jun;91:1684–1690. doi: 10.3945/ajcn.2010.29191. [Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 30.Siegel EH, Kordas K, Stoltzfus RJ, Katz J, Khatry SK, LeClerq SC, et al. Inconsistent effects of iron-folic acid and/or zinc supplementation on the cognitive development of infants. J Health Popul Nutr. 2011 Dec;29:593–604. doi: 10.3329/jhpn.v29i6.9896. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friel JK, Andrews WL, Matthew JD, Long DR, Cornel AM, Cox M, et al. Zinc supplementation in very-low-birth-weight infants. J Pediatr Gastroenterol Nutr. 1993 Jul;17:97–104. doi: 10.1097/00005176-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Castillo-Duran C, Perales CG, Hertrampf ED, Marin VB, Rivera FA, Icaza G. Effect of zinc supplementation on development and growth of Chilean infants. J Pediatr. 2001 Feb;138:229–235. doi: 10.1067/mpd.2001.110530. [DOI] [PubMed] [Google Scholar]
- 33.Hamadani JD, Fuchs GJ, Osendarp SJ, Khatun F, Huda SN, Grantham-McGregor SM. Randomized controlled trial of the effect of zinc supplementation on the mental development of Bangladeshi infants. Am J Clin Nutr. 2001 Sep;74:381–386. doi: 10.1093/ajcn/74.3.381. [DOI] [PubMed] [Google Scholar]
- 34.Christian P, Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA. 2010 Dec 22;304:2716–2723. doi: 10.1001/jama.2010.1861. [DOI] [PubMed] [Google Scholar]