Abstract
Formation of tissue-engineered cartilage is greatly enhanced by mechanical stimulation. However, direct mechanical stimulation is not always a suitable method, and the utilization of mechanisms underlying mechanotransduction might allow for a highly effective and less aggressive alternate means of stimulation. In particular, the purinergic, adenosine 5′-triphosphate (ATP)-mediated signaling pathway is strongly implicated in mechanotransduction within the articular cartilage. We investigated the effects of transient and continuous exogenous ATP supplementation on mechanical properties of cartilaginous constructs engineered using bovine chondrocytes and human mesenchymal stem cells (hMSCs) encapsulated in an agarose hydrogel. For both cell types, we have observed significant increases in equilibrium and dynamic compressive moduli after transient ATP treatment applied in the fourth week of cultivation. Continuous ATP treatment over 4 weeks of culture only slightly improved the mechanical properties of the constructs, without major changes in the total glycosaminoglycan (GAG) and collagen content. Structure–function analyses showed that transiently ATP-treated constructs, and in particular those based on hMSCs, had the highest level of correlation between compositional and mechanical properties. Transiently treated groups showed intense staining of the territorial matrix for GAGs and collagen type II. These results indicate that transient ATP treatment can improve functional mechanical properties of cartilaginous constructs based on chondrogenic cells and agarose hydrogels, possibly by improving the structural organization of the bulk phase and territorial extracellular matrix (ECM), that is, by increasing correlation slopes between the content of the ECM components (GAG, collagen) and mechanical properties of the construct.
Introduction
Articular cartilage (AC) is a tissue highly prone to injury and pathological degeneration, that is constantly exposed to high stresses, which arise from joint motion and load bearing.1 Adult AC has limited ability for spontaneous healing, largely due to the lack of vascularization.2,3 These two factors are underlying the need for devising effective strategies for AC repair/regeneration or replacement.4,5 Various cell-based regenerative approaches have been proposed, including tissue engineering (TE) of cartilage equivalents6 using chondrocytic cells,7–9 embryonic stem cells,10–12 and mesenchymal stem cells (MSCs) from various tissues.13–16
Regardless of their origin, chondrogenic cells require an appropriate biomechanical environment to produce functional cartilage.17–20 To recreate the physiologic loading environment, a majority of TE studies have focused on various ways of applying direct compression in combination with application of growth factors.21–23 This external mechanical stimulation leads to activation of mechanotransduction cascades, which promote chemical signaling inside the cell.24 These intracellular mechanotransduction pathways are still being defined.25,26
ATP (adenosine 5′-triphosphate) has been indicated as one of the first molecules to be released in response to mechanical stimulation.27–31 Recently, efforts have been made in the direction of harnessing the ATP-mediated, purinergic signaling pathway in cartilage TE with the goal of recreating the physiologic loading environment in the absence of externally applied forces.32
The effects of extracellular ATP on mechanical properties of engineered cartilage constructs are currently not fully defined. To investigate the effects of extracellular ATP on mechanical properties of cartilaginous constructs, we evaluated the effects of low-dose supplementation of ATP (60 uM) to chondrocytes (CH) and chondrogenic human MSCs (hMSCs) encapsulated in the agarose hydrogel for 4 weeks, cultured in a chondrogenic serum-free medium supplemented with the transforming growth factor (TGF)-β3 during the first 2 weeks of culture.23,33
A recent study by Ng et al.34 reported that the tissue stiffness is not dependent on the type of the anabolic growth factor, but rather on its temporal application. We hypothesized that the same principle of temporal application can be applied to extracellular ATP when used at a dose that induces anabolic effects on engineered cartilage. To test this hypothesis, we investigated the continuous versus transient ATP application initiated at different time points during culture.
Initially, we performed a set of preliminary studies with the goal to determine if there are measurable effects of ATP supplementation in our model system, and if these effects are dependent on the timing of ATP supplementation. This is the reason why we distinguish the results of preliminary and detailed experimental studies. Based on the results of such preliminary studies on CH constructs with different starting points and durations of transient ATP treatment, we selected day 21 of culture to start the 1-week-long transient treatment with ATP. Several other intervals (1, 2, and 3 weeks in duration, initiated on day 7 or day 14 of culture) of transient ATP supplementation were tested in cartilaginous constructs with respect to the DNA content and functional mechanical properties that are the equilibrium (EY) and dynamic (G*) modulus (Supplementary Fig. 1A; Supplementary Data are available online at www.liebertpub.com/tea). The preliminary results showed that the 1-week-long ATP treatment started on day 7 led to increased proliferation (increase in total and %ww DNA content), but did not increase mechanical properties (EY and G*). When initiated on day 14, the 1-week-long ATP treatment led to increases in DNA (%ww) and EY relatively to day 21 controls, but not to the increase in G*. The 2-week-long ATP treatment initiated on day 14 did not increase the G*, DNA content, or EY.
To assess the ATP-mediated effects on CH- and hMSC-laden agarose constructs, we have evaluated the functional mechanical properties (EY and G*), biochemical content (DNA, s-glycosaminoglycan [GAG], collagen), and expression of collagen type I, II, and X as well as of P2Y2, a purinergic receptor, which has been reported as the predominant receptor in ATP binding.27,28,30,32 To investigate the relationship between the extracellular matrix (ECM) composition and mechanical moduli, we have performed the structure–function correlation analysis for each cell type in untreated, continuously ATP-treated/and transiently ATP-treated constructs.
Materials and Methods
Chondrocyte and MSC isolation and expansion
AC was harvested from fresh bovine carpometacarpal joints obtained from 4-to 6-month-old calves. Cartilage was rinsed and digested in the Dulbecco's modified essential medium (DMEM) with 0.5 mg/mL collagenase type IV (Sigma Chemicals) for 10 h at 37°C with stirring. The resulting cell suspension was filtered through a 70-μm pore size mesh to isolate individual cells.33 After rinsing the pellets, the CH were plated at high density (>1×105 cells/cm2) in the chondrocyte culture medium (high-glucose DMEM–hgDMEM supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin).
Bone-marrow derived hMSCs from passage 3 were cultured in an expansion medium (hgDMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 ng/mL of the basic fibroblast growth factor).
Cell seeding in hydrogel
To produce cell-laden agarose gels, type VII agarose (AG) (Sigma Chemicals) was dissolved in phosphate-buffered saline (PBS) at a concentration of 4% w/v, autoclaved, and cooled to 40°C. AG was combined with the cell suspension (40×106cells/mL) in a 1:1 ratio to result in a seeding density of 20×106 cells/mL in a 2% w/v AG, which was cast between two glass plates separated by 2.5-mm spacers. After cooling, cylindrical disks (4 mm in diameter×2.5 mm thick) were cored out using a biopsy punch as in our previous studies33,35 resulting in 6.2×105 cells per scaffold.
Construct cultivation and ATP supplementation
Constructs from all groups were maintained in culture for up to 28 days, with a twice weekly change of the chondrogenic serum-free growth medium known to foster cartilage tissue formation (hgDMEM supplemented with 5 mg/mL proline, 1% ITS+, 100 nM dexamethasone, 50 μg/mL ascorbate, and 10 ng/mL TGF-β3) for the first 2 weeks.6
Three days after seeding, culture media were supplemented with freshly prepared 60 uM ATP (ATP-disodium salt hydrate powder, Sigma-Aldrich Ltd.) added at each change of the medium throughout the whole 4-week culture period. Transient ATP supplementation, that is, beginning at day 21, was performed for 1 week in additional groups for each cell type.
Mechanical properties
Individual constructs were tested on a weekly basis, starting from d7, in unconfined compression using our established custom-designed testing system.23,36 Briefly, after equilibration under a tare load of 0.5 g, stress relaxation tests were conducted at a ramp rate of 1 μm/s to 10% strain. The equilibrium Young's modulus (EY) was calculated from the equilibrium stress and strain values based on the measured construct dimensions. Unconfined dynamic modulus (G*) was performed via the application of a sinusoidal deformation of 1% applied at 1.0, 0.2, and 0.1 Hz. The data for 0.2 and 0.1 Hz were included in statistical analyses only for the constructs with extremely low stiffness (very early time points for hMSC constructs), where loading at 1 Hz results in a high noise level. For all other samples, data obtained at 1 Hz were used. After mechanical testing, constructs were frozen at −20°C for biochemical evaluation or fixed in 4% paraformaldehyde (PFA) and transferred to PBS for later histology and immunohistochemistry.
Biochemical composition
Tissue constructs were blotted dry, weighed, and lyophilized overnight. Dry samples were digested with proteinase K overnight at 56°C, as described previously.22 For the GAG content, aliquots of digest were analyzed using the 1,9-dimethylmethylene blue dye binding assay.37 For the DNA content, additional aliquots were analyzed using the PicoGreen assay (Invitrogen). For the total collagen content, aliquots were acid hydrolyzed in 12N HCl at 110°C for 16 h, dried over NaOH, and resuspended in an assay buffer (24 mM citric acid monohydrate, 0.012% v/v glacial acetic acid, 85 mM sodium acetate trihydrate, 85 mM sodium hydroxide, pH 6.0). The orthohydroxyproline (OHP) content was determined via a colorimetric assay by reaction with chloramine T and dimethylaminobenzaldehyde35 that was scaled down for microplates. The OHP content was converted to total collagen content using the 1:7.64 ratio of OHP to collagen.38 For each biochemical constituent (DNA, GAG, and collagen), the total content per construct as well as concentrations after normalization to wet weight are given.
Histology and immunocytochemistry
Samples from each experimental group at each time point were fixed overnight at 4°C in 4% PFA, embedded in paraffin blocks, and cut in cross sections to 4 μm. Sections were stained for sulfated GAG (Alcian Blue), total collagen (Picrosirius Red), and overall histomorphology (hematoxylin and eosin staining H&E) using previously described protocols.39
For immunocytochemical (ICC) staining, tissue sections were first deparaffinized in Citrisolv (Fisher) and rehydrated in a series of alcohols of descending concentrations. Antigen retrieval was performed by heating in a 0.01 M citrate buffer pH=6.0 for 10 min and quenching of endogenous peroxidase through immersion in 0.3% H2O2/methanol for 10 min at room temperature. Slides were incubated for 20 min with Blocking Serum from Vectastain ABC Kit (Vector) and incubated with a primary antibody (Anti-Collagen Type II–AB2031, in 1:100 dilution, Anti-Collagen Type I–AB749P, 1:300, and Anti-Collagen Type X–234196, all from Chemicon–Millipore) overnight at 4°C. Next, the biotinylated secondary antibody (Vector) was applied to the sections, and the antibody binding was detected with a Vectastain ABC Kit (Vector). Native cartilage sections were used as a positive control, while construct samples without the applied primary antibody were used as a negative control. The samples were imaged using a color CCD camera mounted onto an inverted microscope (Olympus IX-81) and analyzed using MetaMorph (Molecular Devices).
Statistical analysis
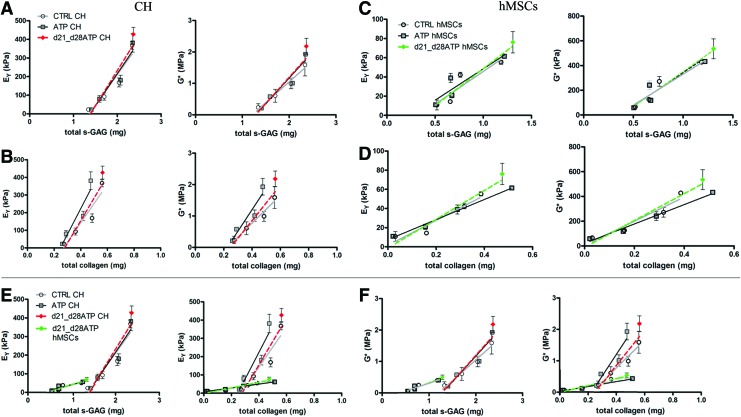
Statistics were performed with GraphPadPrism 5.01 software. For the chondrocyte groups, each data point represents the average±SD of n=4–6 samples. For the hMSC groups, n=2–5. Each group was examined for significant differences by one-way ANOVA, with EY, G*, thickness, diameter, wet weight, sGAG-, hydroxyproline-, or DNA- content as the dependent variable using the Tukey's honest significant difference test. For correlation and regression analyses, the data were fit using linear regression and t-tests were used to evaluate correlation slopes between conditions as described by Erickson et al.21 We have employed both statistical methods—correlation (testing the statistical significance of the association between variables) and linear regression (describing the relationship precisely by means of an equation that has a predictive value) to calculate the correlation slopes and coefficients for statistical comparison as well as to have appropriate graphic presentation (Fig. 3), respectively.
FIG. 3.
Structure–function correlations for cartilaginous constructs. Correlation plots relate the measured mechanical properties (Eγ and G*) and concentrations of matrix components (total s-GAG and total collagen). (A, B) Plots for CH-seeded hydrogels. (C, D) Plots for hMSC-seeded hydrogels. (E, F) Same scale comparison for both cell types. Lines show the linear fit for each group. Color images available online at www.liebertpub.com/tea
Results
Effect of continuous ATP supplementation on mechanical construct properties
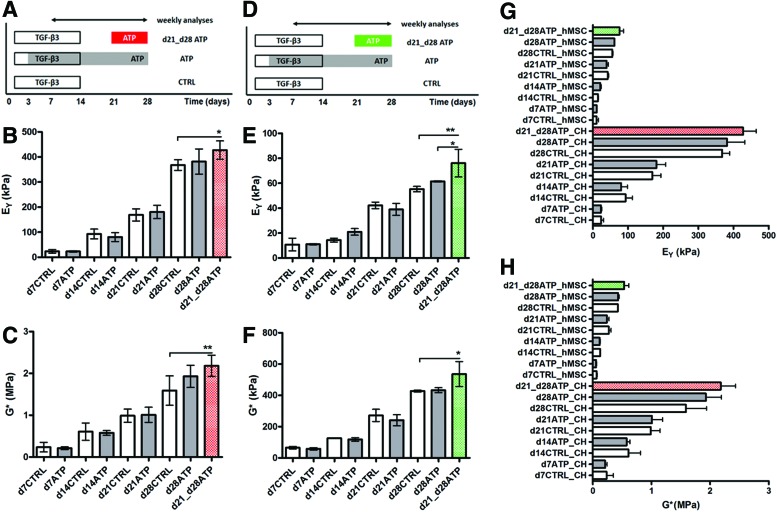
The equilibrium (EY) and dynamic (G*) compressive properties of CH and hMSC constructs were evaluated every week, starting from day 7 (d7). The EY and G* in both cell-type control constructs (CTRL, not stimulated with ATP) increased with time in culture (Fig. 1A–C). The EY and G* of hMSC constructs were significantly lower than those of CH constructs (Fig. 1G, H), consistent with previous reports.21 Continuously added ATP had no significant effect on the CH-seeded constructs versus CH-CTRL (Fig. 1B, C). A similar functional maturation process in CTRL and continuously ATP-treated groups was observed also in hMSC-seeded constructs. (Fig. 1E, F).
FIG. 1.
Experimental design. (A, D) Experimental groups: CTRL untreated, adenosine 5′-triphosphate (ATP) continuously treated, d21-d28ATP transiently ATP treated. Duration of transforming growth factor (TGF)-β3 application is also indicated. (B, E) Young's modulus and (C, F) dynamic modulus (G*) of agarose hydrogels seeded with chondrocytes (CHs) (red) or human mesenchymal stem cells (hMSCs) (green) measured weekly over a 28-day culture period. (G, H) Same scale comparison of mechanical properties for both cell types (CH and hMSCs). Data represent the mean±SD of 4–6 (CH) and 2–5 (hMSC) samples from two replicate studies. *p<0.05, **p<0.01. For clarity, the statistical differences are not labeled in G, H due to the large number of groups. Color images available online at www.liebertpub.com/tea
Effect of transient ATP supplementation on mechanical properties of cartilaginous constructs
The EY and G* of CH-seeded constructs that received transient 1-week-long ATP treatment measured on the final day of culture (d28) were significantly higher than those of CTRL constructs (Fig. 1B, C). hMSC-seeded constructs treated transiently with ATP during the last week of culture (d21–d28), also had significantly higher EY and G* values, compared to same day CTRL constructs. Furthermore, EY values for d21_d28ATP hMSC constructs were significantly higher than for continuously ATP-stimulated constructs on d28, which was not the case for CH constructs (Fig. 1E).
Effect of continuous ATP supplementation on construct compositions
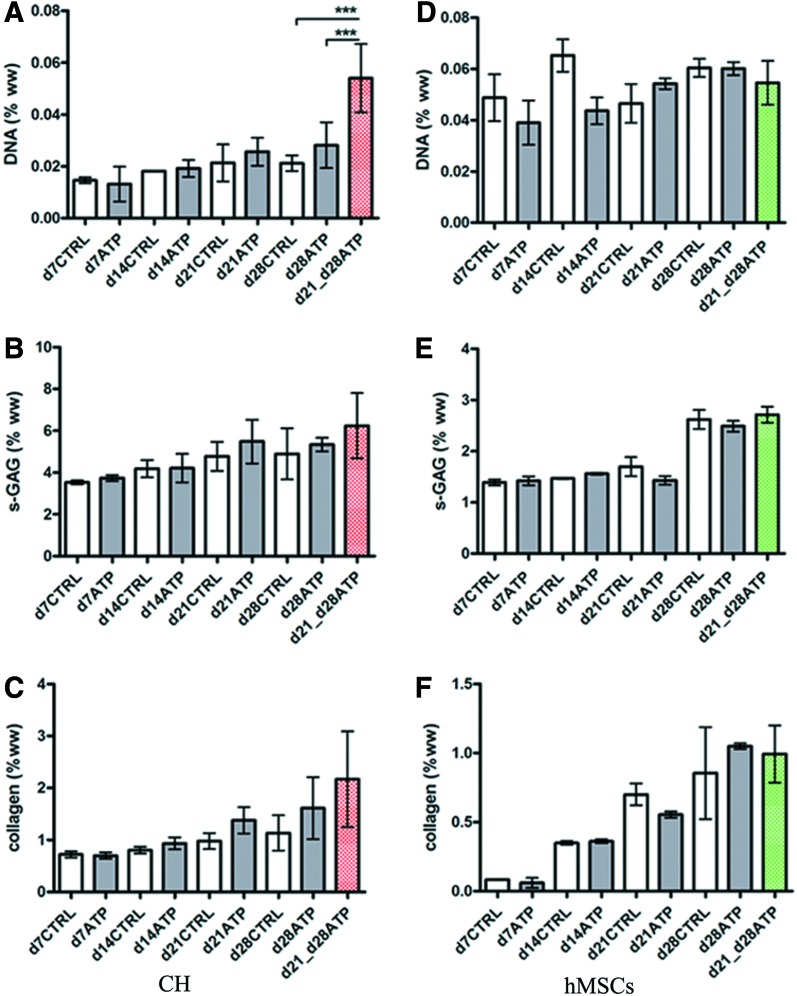
Biochemical analyses of CH and hMSC constructs were performed after each mechanical testing session. The DNA content increased over time for all CH constructs, d7CTRL versus d28CTRL, and d7ATP versus d28ATP, but there were no significant differences between CTRL and continuously ATP-supplemented constructs (Table 1). The s-GAG content (μg/construct) of CH constructs increased significantly over time, both in ATP-treated and CTRL groups: d7CTRL versus d28CTRL and d7ATP versus d28ATP. Only at d14 were the CTRL compositions significantly better, that is, the GAG and collagen content were higher than for the ATP-treated construct (Table 1). In line with this result, the d14ATP EY was lower than d14CTRL EY, but not significantly (Fig. 1B). There were no significant differences between s-GAG (% ww) of CTRL and ATP-treated CH constructs at any time point (Fig. 2B). The collagen content (μg/construct) of CH constructs increased significantly over time in both CTRL and ATP-treated constructs: d7CTRL versus d28CTRL and d7ATP versus d28ATP. In general, the collagen concentrations (% ww) were comparable among the CTRL and ATP groups (Fig. 2C).
Table 1.
Construct Dimensions and Biochemical Contents
| |
CTRL |
ATP |
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| CH | d7 | d14 | d21 | d28 | d7 | d14 | d21 | d28 | d21_d28 ATP |
| Wet weight (mg) | 37.95±0.21 | 40.85±3.32 | 43.2±6.79 | 51.3±13.29 | 37.2±0.56 | 38.3±6.22 | 38.7±9.05 | 44.65±4.59 | 36.23±8.25 |
| Thickness (mm) | 2.57±0.06 | 2.77±0.08 | 2.89±0.22 | 2.86±0.29 | 2.72±0.14 | 2.75±0.18 | 3.02±0.33 | 2.97±0.24 | 2.94±0.32 |
| Diameter (mm) | 3.99±0.06 | 4.03±0.06 | 4.06±0.07 | 4.21±0.25 | 3.92±0.12 | 4.02±0.05 | 4.03±0.03 | 4.13±0.03 | 4.14±0.05 |
| DNA (μg) | 5.71±0.71 | 7.73±0.17 | 10.27±2.51 | 13.05±1.36 | 5.06±2.03 | 8.3±0.96 | 11.44±1.83 | 13.58±3.31 | 16.12±3.54 |
| s-GAG (μg) | 1338.9±27.5 | 1702.5±29.5 | 2039. 6±23.3 | 2338.4±161.6 | 1407.1±62. 6 | 1592.3a±0.9 | 2074.9±90.5 | 2352.8±98.9 | 2367.3±22.5 |
| Collagen (μg) | 272.5±25 | 360.9±48.2 | 484.8±96.2 | 559.7±24.2 | 260.6±26.3 | 290.3b±2.3 | 418.5±43.5 | 475.3c±23 | 563.6d±26.2 |
| |
CTRL |
ATP |
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| hMSC | d7 | d14 | d21 | d28 | d7 | d14 | d21 | d28 | d21_d28 ATP |
| Wet weight (mg) | 37.4±0.14 | 39.3±7.64 | 45.4±0.85 | 46.1±1.27 | 35.8±0.28 | 39.3±7.64 | 52.5e±0.78 | 49.5±0.71 | 48.55±0.64 |
| Thickness (mm) | 2.87±0.05 | 2.99±0.13 | 3.11±0.04 | 3.19±0.04 | 2.92±0.09 | 3.07±0.07 | 3.18±0.11 | 2.95±0.15 | 3.32±0.16 |
| Diameter (mm) | 3.82±0.23 | 4.03±0.09 | 4.02±0.01 | 3.95±0.11 | 3.92±0.13 | 3.98±0.05 | 4.03±0.04 | 4.07±0.08 | 4.01±0.02 |
| DNA (μg) | 20.59±4.75 | 23.62±3.02 | 23.87±4.95 | 14.21±2.37 | 16.23±4.39 | 21.29±3.34 | 13.29±1.57 | 14.72±1.81 | 23.79±5.92 |
| s-GAG (μg) | 518.4±21.3 | 656.8±5.8 | 761±84 | 1184.9±84 | 506.2±30.8 | 674.6±8.2 | 661.8±80.2 | 1218.9±52.2 | 1306.5±19.1 |
| Collagen (μg) | 30.9±1.1 | 159.8±5.9 | 317.3±30.1 | 386±150.9 | 21.1±12.8 | 155.6±6 | 287.7±11.8 | 514.3±10 | 474.2±70.8 |
Data represent the mean±SD of 4–6 (CH) and 2–5 (hMSC).
p<0.05 versus same day control (d14CTRL); bp<0.05 versus same day control (d14CTRL); cp<0.05 versus same day control (d28CTRL); dp<0.01 versus d28ATP-CH; ep<0.01 versus same day control (d21CTRL).
CH, chondrocytes; ATP, adenosine 5′-triphosphate; GAG, glycosaminoglycan; hMSC, human mesenchymal stem cell.
FIG. 2.
Construct compositions. The biochemical content is shown as a percentage of wet weight for CH and hMSC constructs as a function of time and ATP treatment. (A, D) DNA content, (B, E) s-glycosaminoglycan (GAG) content, (C, F) total collagen content. Data represent the mean±SD of 4–6 (CH) and 2–5 (hMSC) samples from one of two replicate studies. ***p<0.001. Color images available online at www.liebertpub.com/tea
The DNA content of hMSC-CTRL constructs did not change significantly over time, and only slight decreases were observed for d7CTRL versus d28CTRL constructs. In ATP-supplemented constructs, we detected a nonsignificant decrease in DNA (Table 1). In hMSC constructs, the s-GAG content (μg/construct) increased significantly over time, both in ATP-treated and CTRL constructs: d7CTRL versus d28CTRL, and d7ATP versus d28ATP (Table 1). There were no significant differences between controls and ATP-treated constructs at any time point. However, we did observe significant increases in wet weights of ATP-treated constructs at d21, compared to d21CTRL (Table 1), along with a decrease in the GAG content (% ww, Fig. 2E). The decreases in d21ATP EY and G* were not significant compared to CTRL (Fig. 1). The bulk collagen content increased significantly with time in both CTRL and ATP-treated hMSC constructs (Table 1, Fig. 2F), but was comparable between the CTRL and ATP hMSC groups, similar to CH groups.
Effect of transient ATP supplementation on construct compositions
The total DNA content in transiently treated CH constructs (d21 vs. d28ATP) was not significantly different from CTRL constructs (Table 1). However, the DNA concentration (% ww) was significantly higher for transiently ATP-treated constructs than either d28CTRL or d28ATP constructs (Fig. 2A). Transient 1-week-long ATP treatment started on d21 did not lead to significant increases of total s-GAG on d28. In both CH constructs and hMSC constructs, there was a slight, but not significant increase of total s-GAG in comparison with respective same day controls (Table 1) (Fig. 2B, E).
The bulk collagen content in transiently ATP-treated CH constructs was similar to d28CTRL and significantly higher than in continuously ATP-treated constructs (Table 1). For hMSC-laden constructs, the DNA content in transiently treated constructs increased, but not significantly compared to CTRL and d28ATP. There was no significant difference between CTRL, d28ATP and d21-d28ATP DNA when normalized to wet weight (Fig. 2D). In both CH and hMSC d21-d28ATP constructs, the total collagen content was similar to that in d28CTRL and d28ATP constructs of the same cell type. The same was true for collagen as percentage of wet weight (Table 1) (Fig. 2C, F).
Effect of ATP supplementation on structure–function correlations for cartilaginous constructs
For better understanding of the relationship between the effects of ATP supplementation on matrix deposition and functional maturation, we performed correlation analyses between the content of each biochemical component and the specific functional mechanical properties of the constructs —EY and G* are separately correlated to the bulk contents of s-GAG and collagen in each group (CTRL, ATP, and d21_d28ATP) and for each cell type (CH and hMSC) (Fig. 3; the slopes and correlation coefficients are listed in Table 2).
Table 2.
Structure–Function Correlations
| |
EY vs. [GAG] |
EY vs. [COLL] |
||||
|---|---|---|---|---|---|---|
| CH | Slope | R2 | p | Slope | R2 | p |
| CTRL | 327.7 | 0.90 | 0.04* | 1096 | 0.89 | 0.06 ns |
| ATP | 348.2 | 0.92 | 0.03* | 1458 | 0.90 | 0.05 ns |
| d21_d28ATP | 379.0 | 0.90 | 0.01* | 1282 | 0.88 | 0.02* |
| |
G* vs. [GAG] |
G* vs. [COLL] |
||||
|---|---|---|---|---|---|---|
| Slope | R2 | p | Slope | R2 | p | |
| CTRL | 1.322 | 0.97 | 0.01* | 4.4 | 0.96 | 0.02* |
| ATP | 1.643 | 0.93 | 0.04* | 6.7 | 0.9 | 0.05 ns |
| d21_d28ATP | 1.668 | 0.89 | 0.02* | 5.6 | 0.87 | 0.02* |
| |
EY vs. [GAG] |
EY vs. [COLL] |
||||
|---|---|---|---|---|---|---|
| hMSCs | Slope | R2 | p | Slope | R2 | p |
| CTRL | 68.4 | 0.83 | 0.09 ns | 130.4 | 0.93 | 0.04* |
| ATP | 65.3 | 0.85 | 0.08 ns | 105 | 0.99 | 0.004** |
| d21_d28ATP | 76.9 | 0.91 | 0.01* | 150.6 | 0.94 | 0.006** |
| |
G* vs. [GAG] |
G* vs. [COLL] |
||||
|---|---|---|---|---|---|---|
| Slope | R2 | p | Slope | R2 | p | |
| CTRL | 544.7 | 0.94 | 0.03* | 973.6 | 0.92 | 0.04* |
| ATP | 499.2 | 0.86 | 0.06 ns | 782.2 | 0.99 | 0.007** |
| d21_d28ATP | 568.3 | 0.97 | 0.003** | 1083 | 0.95 | 0.005** |
Correlation coefficients relating measured functional mechanical properties (EY and G*) with total contents of s-GAG and collagen for CH- and MSC-seeded constructs. *p<0.05, **p<0.01; ns, no significant difference.
For CH-seeded constructs, a strong linear fit was observed for mechanical parameters (EY, G*) versus s-GAG content, with p-values ranging 0.01–0.04, and R2 values ranging 0.89–0.97, implying strong structure–function correlations in all the groups. Notably, the slopes of correlation differed among groups, with the significantly highest slope detected for the EY versus GAG content in the d21-d28ATP group relatively to CTRL and ATP groups (Table 2, Fig. 3A). The G* versus GAG content correlation in the d21-d28ATP group also had the significantly highest slope versus both CTRL and ATP groups (Table 2, Fig. 3A). For EY versus collagen content correlations, we observed the linear fit in all groups, but the only statistically significant correlation was detected for transiently treated d21-d28ATP constructs (Table 2, Fig. 3B). For G* versus total collagen content correlations, significance was detected for both the CTRL and d21-d28ATP groups, with the higher slope in the ATP-treated groups. For the ATP group, G* versus collagen correlation was not significant (Table 2, Fig. 3B).
In the hMSC-seeded constructs, we could not detect correlation in all groups for GAG. For EY versus GAG content, only the d21-d28ATP group had significant linear fit with R2=0.91; the slope for this group was also the highest (Table 2, Fig. 3C). For G* versus GAG, the group with continuous ATP treatment lacked a significant linear fit, while the slope for the d21-d28ATP group was the highest (Table 2, Fig. 3C). For both EY and G* versus total collagen in hMSC constructs, the linear fit and correlation were significant for all the groups. For EY versus collagen, transiently treated constructs exhibited the highest slope of correlation (Table 2, Fig. 3D). Similarly, for G* versus collagen correlation, the d21_d28ATP group had the highest slope, compared to CTRL and continuously ATP-treated constructs (Table 2) (Fig. 3D). The correlation slopes were significantly lower for the hMSC constructs, (p<0.001) than CH constructs, both within the same treatment groups (e.g., ATP–CH vs. ATP–hMSC) and different treatment groups (e.g., CTRL-CH vs. ATP-hMSC) (Fig. 3E, F).
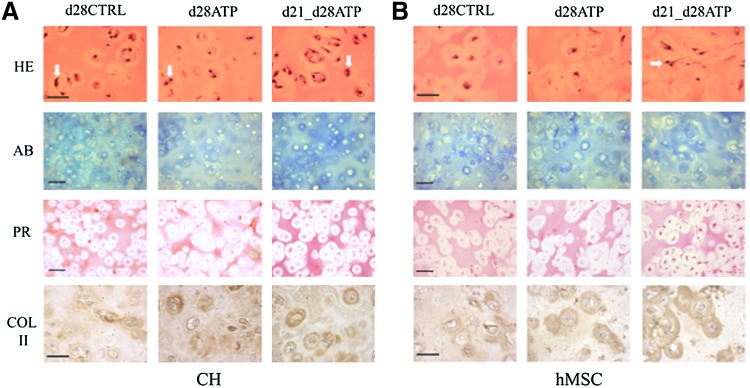
Effects of ATP supplementation on construct histomorphology
Histological and ICC evaluation were performed after mechanical testing of each construct, to obtain consistent data for structure–function analyses. Due to no detectable differences between the CTRL and ATP-treated groups at earlier time points, only day 28 results of histological/ICC evaluation are presented in Figure 4. The matrix accumulation increased with time in all groups, as evidenced by Alcian Blue staining for sulfated GAG and Picosirius Red for total collagen. For both CH and hMSC constructs, the d21-d28ATP group had the most intense GAG staining, especially in the territorial matrix, and the strongest overall collagen staining both in the inter- and territorial matrix. The collagen type II immunostaining was the strongest in the d21-d28ATP group for both CH and hMSC constructs, and prominently localized to the territorial matrix, in particular, in the hMSC constructs (Fig. 4B). Hematoxylin and eosin staining indicated that the cells were multiplying in all the groups for CH constructs (white arrows pointing to local multiplication of cell nuclei), and only in the d21-d28ATP group of hMSC constructs, in line with the measured increase of DNA content in this group, Figure 4.
FIG. 4.
Tissue morphology. Histology and immunohistochemistry data are shown for (A) CH-seeded and (B) hMSC-seeded constructs on day 28. First row: hematoxylin–eosin (H&E) staining, white arrows pointing to local multiplication of cell nuclei; second row: Alcian blue (AB) staining of deposited s-GAG; third row: Picrosirius Red (PR) staining of total collagen and immunohistochemistry for collagen type II (COL II); fourth row: scale bar=100 μm. Color images available online at www.liebertpub.com/tea
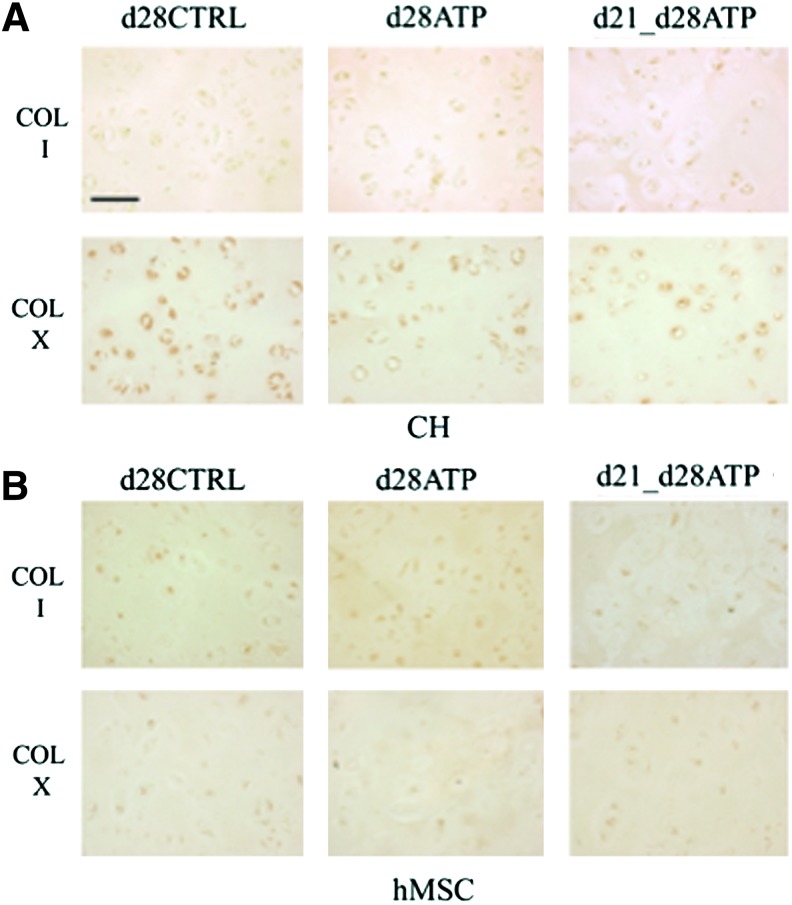
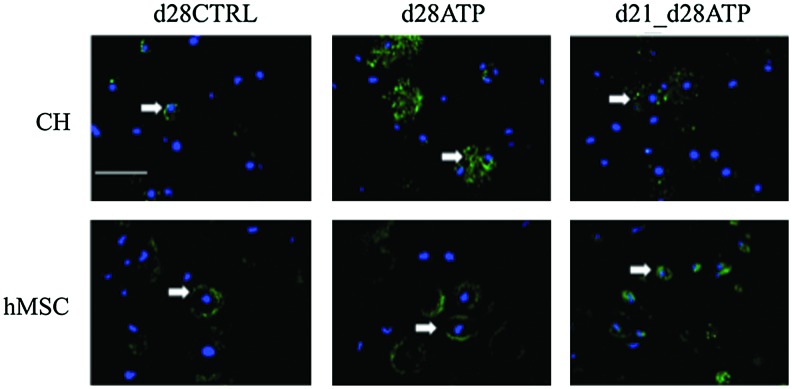
Neither CH nor hMSC constructs showed collagen type I expression, indicating that ATP treatment did not induce fibrocartilage-like collagen accumulation. Collagen type X was detectable at very low levels in all groups of CH constructs (Fig. 5A), indicating that transient ATP treatment did not induce a hypertrophic phenotype. There was no collagen type X expression detectable in hMSC-seeded constructs, Figure 5B. ICC staining for the P2Y2 receptor, which is regarded to have a predominant role in ATP binding, revealed different patterns of expression between cell types (CH or hMSC) and ATP-treatment groups (CTRL, continuous ATP, transient d21-d28ATP). For CH, P2Y2 expression on the final day of culture (d28) was increased in continuously ATP-treated (d28ATP) versus control (d28CTRL) and versus transiently ATP-treated (d21_d28ATP) constructs. The P2Y2 expression appeared to be localized over the whole cell surface in d28ATP constructs and to have a more punctuated pattern in d28CTRL and d21-d28ATP constructs. In hMSC constructs, d28CTRL and d28ATP groups had similar patterns of P2Y2 expression localized to the cell surface in the vicinity of the territorial matrix, while in the transiently ATP-treated group (d21-d28ATP), the expression was detected on the whole cell surface, similar in pattern, but in a lesser degree than for d28ATP-CH (Fig. 6).
FIG. 5.
Distributions of tissue components. Immunohistochemistry of (A) CH-seeded and (B) hMSC-seeded constructs on day 28 for collagen type I (COL I)–first rows and collagen type X (COL X)–second rows. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
FIG. 6.
Expression of P2Y2 receptors. Fluorescence immunostaining of CH-seeded (upper row) and hMSC-seeded constructs on day 28 for P2Y2 receptors (green, indicated by white arrows) and nuclear label (DAPI-blue). Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Discussion
ATP has been indicated as one of the first molecules to be released in response to mechanical stimulation. The ATP-mediated, purinergic signaling pathway may be exploited to recreate the physiologic loading environment for engineered cartilage in the absence of externally applied forces. In the current study, we hypothesized that the timing of ATP supplementation will play a role in the development of structural and mechanical properties of cartilaginous constructs. We have focused on the effects of ATP as the extracellular signaling molecule, rather than its role in the intracellular energy metabolism which, in turn, would require an entirely different set of molecular tools. We investigated the effects of transient and continuous supplementation of ATP on structural and mechanical properties of cartilaginous constructs engineered using immature bovine CH and hMSCs encapsulated in agarose hydrogel and cultured for 4 weeks in free-swelling conditions. Structure–function analyses showed that transiently ATP-treated constructs exhibited the highest correlation slopes between compositional and mechanical construct properties. This finding indicates that transient ATP supplementation leads to increased functional consequence of a given amount of ECM component (GAG and collagen), that is, to superior ECM assembly.
For both cell types (CH and hMSCs), we observed statistically significant increases in equilibrium (EY) and dynamic moduli (G*) in comparison to CTRL, after transient, but not continuous ATP treatment. In many instances, the increases in mechanical properties were not followed by corresponding increases in GAG and collagen contents, a result in line with previous reports for the effects of growth factors on engineered cartilage, which also detected disparity between the structural composition and mechanical properties and showed that this disparity is due to the changes in the type, size, structure, and/or spatial location of the matrix components affected by the growth factors.34 The fact that groups with similar biochemical contents had different mechanical properties further supports the notion that mechanical properties depend on the organization or the modulation of the size of macromolecular proteoglycan aggregates, which might explain the detected progressive Alcian blue staining in the absence of increased total GAG content and not only the amounts of the ECM components.22,39,40 In this context, several studies have shown the roles of crosslinking matrix molecules (such as cartilage oligomeric matrix protein—COMP,22 type IX collagen,41 type XI collagen, link protein33) in the development of functional mechanical properties of cartilage constructs. Ng et al.34 stressed the need to address these disparities in further studies via molecular biology techniques such as RT-PCR and calcium imaging, which would allow for better understanding of the signaling processes underlying detected macroscale phenomena. Calcium imaging would be important for better defining of purinergic signaling pathways.30,42,43 Such studies could be further supported by precise imaging of the matrix ultrastructure, extra- and pericellular distributions of matrix components,44–46 and the interactions between proteoglycans and collagen, and between collagen fibers.47,48
We are currently undertaking further studies to determine whether the effects of transient ATP stimulation are also transient, or the effects are sustained following the removal of the ATP stimulus. Other questions of interest include differential effects of ATP on the territorial and interterritorial cartilaginous matrix, and on primary CH and chondrogenic hMSCs. Notably, hMSCs were more receptive to the effects of ATP (e.g., 72% vs. 16% increase in GAG content for hMSCs and CH, respectively), suggesting a strong role of ATP during chondrogenesis. Immunostaining for the purinergic receptor P2Y2 also indicated different responses of CH and chondrogenic hMSCs to exogenous ATP stimulation, although in all the groups, the P2Y2 expression was limited to the cell surface and not the nucleus, which is in line with further reports.49 For CH, P2Y2 expression was increased in continuously ATP-treated (d28ATP) versus control (d28CTRL) and versus transiently ATP-treated (d21_d28ATP) constructs, while in hMSCs, similar patterns as in the d28ATP-CH group was detected for the transient d21_d28ATP-hMSC group, but in a lesser degree. At this point, it is difficult to directly correlate the effect of the P2Y2 overexpression/change in the expression pattern detected in different groups to the structural/mechanical properties. Xing et al. investigated the impact of P2Y2 overexpression in chondrocyte mechanosensitivity using a murine inducible chondrogenic cell line (ATDC5) and concluded that different expressions of ATP receptors (such as P2Y2 receptor) and their regulators (such as GRK2) may contribute to the enhanced mechanosensitivity in differentiated cells primarily via the ERK1/2 response.50 Knight et al. detected the difference in P2Y2 expression between superficial and deep zone cells in human cartilage and even suggest the possibility that different signaling mechanisms may occur within these two cell populations.51
Future studies will be necessary to quantify and interpret these differences in detail as well as to determine if such changes in expression also occur with other purinergic receptors sensitive to ATP. It would also be of interest to examine the age dependence of the bovine chondrocyte response to ATP stimulation. As we have detected differences in P2Y2 expression between chondrogenic hMSCs, which might be taken as analogs of fetal cartilage,52 and native juvenile CH, it could be hypothesized that P2 expression pattern changes during chondrogenesis, affecting the tissue response to ATP supplementation. Temporal expression dependency, that is, different expression at different developmental stages has been established for a number of receptors detected in developing CH.53
Previous studies have reported negative effects of extracellular nucleotides on cartilage metabolism such as promotion of proteoglycan breakdown,54 GAG release55,56 and stimulation of inflammatory mediators, nitric oxide (NO) and prostaglandin E2.32,57 Other studies reported chondroprotective effects of extracellular ATP,56 such as the upregulation of proteoglycan synthesis, collagen accumulation,58,59 increase in indentation modulus,32 suppression of inflammatory mediator (NO) production,60 a dose-dependent stimulation of formation of chondrocytic nodules,61 elevated protein expression of chondrogenic transcription factor Sox 9 and mRNA levels of collagen II and aggrecan.59
The ATP concentration and lifetime in culture are likely to play major roles in the resulting effects. The concentrations of ATP released in response to mechanical loading were at the order of 10−5M,27 and varied with the magnitude of applied loading.27 In contrast, higher concentrations of ATP, at the order of 10−4 to 10−3 M, caused the release of inflammatory mediators57,62 and matrix mineralization.63,64 We therefore applied exogenous ATP in the low-dose range (60 μM) to avoid potential catabolic effects. The half-life for ATP was measured to be 2.4 h in pellet culture58 and 5.3 h in bovine AC explants.56 Notably, significant long-term effects were observed in spite of the short ATP half-life, suggesting that extracellular ATP quickly binds to its receptors eliciting further downstream signaling cascades. Another study determined that ATP can pass through the agarose gel and pericellular matrix, bind to purinoceptors, and increase intracellular calcium concentrations.42
Purinergic signaling data for hMSCs are scarce and controversial.65 Recent studies demonstrated that P2 receptors are expressed and functional in hMSCs and that activation by their natural ligand ATP can mediate fast changes in the intracellular ion homeostasis.65,66 Another study documented the flow rate-dependent release of ATP from hMSCs and showed that ATP is unique, among nucleotides, in its ability to induce hMSC proliferation.67 In contrast to the previous studies, where ATP was applied over very short time periods,65–67 our study has focused on the effects of prolonged (continuous ∼4 week long and transient 1 week long) ATP application on CH and chondrogenic hMSCs in a 3D setting, under serum-free conditions with TGF-β3 supplementation, which is a state-of-the-art protocol for inducing and maintaining chondrogenesis.6,22,34
The highest improvements of mechanical properties (equilibrium modulus EY and dynamic modulus G* in unconfined compression) were observed for cartilaginous constructs subjected to transient ATP treatment for both the CH and hMSCs. The related increase in the DNA concentration (%ww) without a significant increase in the total DNA content, but with detected cell nuclei multiplication implies that transient ATP treatment affected also the tissue hydration and not solely the cell proliferation. These effects are in line with the results of studies reported by Waldman et al.32
The cells were multiplying in all the groups of CH constructs, but only in the d21-d28ATP group of hMSC constructs, consistent with the detected increase in the DNA content in this group. The absence of intensive proliferation in CTRL and continuously ATP-treated hMSC groups is in line with the results of Coppi et al.66 who detected that hMSCs at early stages of culture spontaneously release ATP, which decreases cell proliferation. Apparently, continuously added ATP intensified this effect in our study. In addition, the slight decrease in the DNA content observed for d7CTRL versus d28CTRL hMSC constructs can be explained by the absence of proliferation combined with the low level of apoptosis, which is inevitable in the process of chondrogenesis.68 However, transient ATP treatment at a later stage of culture (d21_d28) did not decrease proliferation, evidenced by the cell multiplication. Therefore, ATP effects on proliferation might be dependent on the maturation stage of the chondrogenic cells. Further studies are necessary to investigate in detail the multiple effects of ATP and the timing of its supplementation on the cellularity in correlation to the matrix composition and mechanical properties of cartilage constructs.
The most significant changes in the functional properties in ATP-treated constructs observed after the cessation of TGF-β3 supplementation in both CH and hMSC constructs suggest interactions between ATP and TGF-β3. In CH, TGF-β1-dependent signaling has been implicated in the elaboration of inorganic pyrophosphate (PPi).69 On the other hand, exogenous ATP can be directly involved in the generation of PPi, via purinergic receptors.63,70 In addition, the levels of exogenous ATP are modulated by TGF-β1 signaling,63 and the responses of CH to ATP were enhanced by TGF-β1.55 In light of these data, it is interesting that the ATP treatment was most effective when started after the end of TGF-β3 supplementation (i.e., d14-d21 and d14-d28 groups, Supplementary Fig. 1). The significant increase in EY and G* observed only in the d21-d28ATP group, further supports the hypothesis of TGF-β3/ATP interactions in cartilaginous constructs. ATP binding was shown, using other experimental systems, to be essential for the bioactivity of several other growth factors such as the FGF-271 and vascular endothelial growth factor.72 However, it is also possible that factors other than the TGF-β3/ATP interaction had similar or more influential effect. The efficiency of ATP stimulation might be dependent on the amount of ECM the cells had produced before ATP application, or it might be necessary to have a certain number and/or certain types of P2 receptors expressed at the time of ATP stimulation. The expression of P2 receptors may be influenced by the developmental stage73 as well as by the factors, which vary during culture time (e.g., pH level.74).
Our study and the study reported by Waldman et al.32 differ in their scope, model system, and methodology. In the Waldman et al.32 study, CH were cultured on type II collagen-coated filters to form a thin (≤100 μm) layer of cartilaginous tissue, as compared to three-dimensional tissue constructs formed in our study by cell encapsulation in agarose hydrogel. ATP diffusion through agarose gels and pericellular matrix, which was earlier described by Elfervig et al.,42 has likely changed the effective concentrations at the cell surfaces. We selected the agarose hydrogel cell encapsulation system as the only approach that resulted in the formation of physiologically stiff cartilaginous constructs,23,34,75 and because human chondrocyte-laden agarose alginate hydrogels have shown good clinical outcomes in Phase III clinical trials (http://clinicaltrials.gov/ct2/show/NCT00945399).76,77 Another difference is that Waldman et al.32 used a medium supplemented with serum, while we opted for the serum-free medium. Substantial ATP degradation activity of serum55 makes the estimation of the actual ATP dose that the cells receive rather difficult. Finally, serum variability78 and its negative effects on the chondrocyte phenotype79 may complicate potential clinical applications. Some of the differences in measured mechanical data (in particular the magnitude of detected increase in mechanical moduli) also come from the methodological differences between the two studies. Waldman et al. used unconfined indentation testing of the tissue filter unit,32 a method that determines the elastic modulus based on scaling factors (cartilage thickness, indenter radius, and Poisson's ratio) and yields Young moduli that are significantly higher (30%–107%) compared to unconfined compression testing.80
In summary, our aim was to use a model system with biological fidelity (three-dimensional hydrogel), under conditions relevant to potential clinical application (serum-free medium), and with conservative evaluation of the effects of ATP on the compositions and mechanical properties of cartilaginous constructs. Under these conditions, we evaluated two types of cells of interest (CH and hMSCs) and several regimes of ATP supplementation. We propose that purinergic stimulation is a promising mechanism for improving results of the functional cartilage engineering, both via exogenous supplementation of culture media with purinergic nucleotides that could improve biochemical and functional mechanical properties of the in vitro engineered cartilaginous tissues and via pharmacological manipulation of P2 receptors. The ultimate goal of such a strategy is the improvement of tissue function, which in turn implies both the compositional and organizational aspects of tissue development.
Further studies are necessary to define the most efficient protocol for cartilage TE using purinergic stimulation. Possible avenues for optimization include the use of ATP analogues, such as adenosine-50-O-3-thiotriphosphate (ATPγS), and nonhydrolyzable ATP analogues (a,b-methylene ATP or b,c-methylene ATP), which are described as more stable than ATP itself73,81 and adenosine-diphosphate or adenosine itself to dissect more subtle effects of these products of ATP hydrolysis. Another possibility includes pharmacological manipulation of P2 receptors via application of agonists/antagonists. In this context, we are undertaking studies on P2 receptor expression in the process of chondrogenesis.
Finally, there are still many insufficiently defined aspects of the purinergic signaling cascades, which hinder safe clinical application, for example, as intra-articular injection of purinergic nucleotides. While anabolic effects of ATP on chondrogenic cell constructs were reported,28,32,58 there are a number of ATP-induced tissue reactions that could interfere with the normal function of cartilage cells and other cell types in the joints such as synoviocytes and bone cells, which also express P2 receptors.82 For example, exogenous ATP could activate the P2X7 receptor in synoviocytes, which further may induce the release of the proinflammatory factors into the synovial fluid (e.g., interleukin-1β, prostaglandins, proteases that are associated with clinical symptoms of rheumatoid arthritis).83,84 Besides the release of inflammatory molecules, human therapy-challenging effects of exogenous ATP include the induction of catabolic processes, disturbed regulation of PPi, and mineralization.55,63,85–87 The therapeutic modalities that could utilize ATP signaling, as a substitute for mechanical stimulation, are numerous, even though the effects of ATP are not necessarily identical to those of mechanical stimulation. We anticipate that it will be possible to optimize the cell therapy protocols, such as autologous chondrocyte implantation and matrix-induced autologous chondrocyte implantation, where usually cells are grown in vitro for 4 to 6 weeks,88 to incorporate 1 week of exogenous ATP stimulation during the in vitro expansion protocol. This could motivate further studies of the effects and mechanisms of ATP stimulation in the context of cartilage regeneration.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge research funding (NIH grants DE016525, EB002520, and EB011869 to GVN; Fulbright Visiting Scholar grant and grants ON174028 and III41007 by the Ministry of education and science of Republic of Serbia to IG, and Royal Thai Graduate Fellowship to SY).
Disclosure Statement
No competing financial interests exist.
References
- 1.Adams M.A. The mechanical environment of chondrocytes in articular cartilage. Biorheology. 2006;43:537. [PubMed] [Google Scholar]
- 2.Hildner F., et al. State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med. 2011;5:e36. doi: 10.1002/term.386. [DOI] [PubMed] [Google Scholar]
- 3.Chiang H., et al. Differences between chondrocytes and bone marrow-derived chondrogenic cells. Tissue Eng Part A. 2011;17:2919. doi: 10.1089/ten.tea.2010.0732. [DOI] [PubMed] [Google Scholar]
- 4.Huang A.H., et al. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed T.A. Hincke M.T. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 6.Byers B.A., et al. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo C.K., et al. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 8.Tew S.R., et al. Cellular methods in cartilage research: primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods. 2008;45:2. doi: 10.1016/j.ymeth.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Cheng N.C., et al. Engineered cartilage using primary chondrocytes cultured in a porous cartilage-derived matrix. Regen Med. 2011;6:81. doi: 10.2217/rme.10.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jukes J.M. van Blitterswijk C.A. de Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med. 2010;4:165. doi: 10.1002/term.234. [DOI] [PubMed] [Google Scholar]
- 11.Varghese S., et al. Engineering musculoskeletal tissues with human embryonic germ cell derivatives. Stem Cells. 2010;28:765. doi: 10.1002/stem.325. [DOI] [PubMed] [Google Scholar]
- 12.Toh W.S. Lee E.H. Cao T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev. 2011;7:544. doi: 10.1007/s12015-010-9222-6. [DOI] [PubMed] [Google Scholar]
- 13.Pelttari K. Steck E. Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Hwang N.S. Elisseeff J. Application of stem cells for articular cartilage regeneration. J Knee Surg. 2009;22:60. doi: 10.1055/s-0030-1247728. [DOI] [PubMed] [Google Scholar]
- 15.Puetzer J.L. Petitte J.N. Loboa E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev. 2010;16:435. doi: 10.1089/ten.TEB.2009.0705. [DOI] [PubMed] [Google Scholar]
- 16.Aicher W.K., et al. Regeneration of cartilage and bone by defined subsets of mesenchymal stromal cells—potential and pitfalls. Adv Drug Deliv Rev. 2011;63:342. doi: 10.1016/j.addr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins R.J. Browning J.A. Urban J.P. Chondrocyte regulation by mechanical load. Biorheology. 2000;37:67. [PubMed] [Google Scholar]
- 18.Bader D.L. Salter D.M. Chowdhury T.T. Biomechanical influence of cartilage homeostasis in health and disease. Arthritis. 2011;2011:979032. doi: 10.1155/2011/979032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota H. Leong D.J. Sun H.B. Mechanical loading: bone remodeling and cartilage maintenance. Curr Osteoporos Rep. 2011;9:237. doi: 10.1007/s11914-011-0067-y. [DOI] [PubMed] [Google Scholar]
- 20.Sun H.B. Cardoso L. Yokota H. Mechanical intervention for maintenance of cartilage and bone. Clin Med Insights Arthritis Musculoskelet Disord. 2011;4:65. doi: 10.4137/CMAMD.S6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson I.E., et al. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima E.G., et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauck R.L., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 24.Ramage L. Nuki G. Salter D.M. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports. 2009;19:457. doi: 10.1111/j.1600-0838.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 25.Bougault C., et al. Molecular analysis of chondrocytes cultured in agarose in response to dynamic compression. BMC Biotechnol. 2008;8:71. doi: 10.1186/1472-6750-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bougault C., et al. Investigating conversion of mechanical force into biochemical signaling in three-dimensional chondrocyte cultures. Nat Protoc. 2009;4:928. doi: 10.1038/nprot.2009.63. [DOI] [PubMed] [Google Scholar]
- 27.Graff R.D., et al. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 2000;43:1571. doi: 10.1002/1529-0131(200007)43:7<1571::AID-ANR22>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Graff R.D. Picher M. Lee G.M. Extracellular nucleotides, cartilage stress, and calcium crystal formation. Curr Opin Rheumatol. 2003;15:315. doi: 10.1097/00002281-200305000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Picher M. Graff R.D. Lee G.M. Extracellular nucleotide metabolism and signaling in the pathophysiology of articular cartilage. Arthritis Rheum. 2003;48:2722. doi: 10.1002/art.11289. [DOI] [PubMed] [Google Scholar]
- 30.Pingguan-Murphy B., et al. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol. 2006;209:389. doi: 10.1002/jcp.20747. [DOI] [PubMed] [Google Scholar]
- 31.Garcia M. Knight M.M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res. 2010;28:510. doi: 10.1002/jor.21025. [DOI] [PubMed] [Google Scholar]
- 32.Waldman S.D., et al. Harnessing the purinergic receptor pathway to develop functional engineered cartilage constructs. Osteoarthritis Cartilage. 2010;18:864. doi: 10.1016/j.joca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Mauck R.L., et al. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 34.Ng K.W., et al. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann Biomed Eng. 2011;39:2491. doi: 10.1007/s10439-011-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao P.H., et al. Silk hydrogel for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;95:84. doi: 10.1002/jbm.b.31686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hung C.T., et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 37.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 38.Vunjak-Novakovic G., et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 39.Mauck R.L., et al. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Kelly T.A., et al. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 41.Kelly T.A., et al. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41:223. [PubMed] [Google Scholar]
- 42.Elfervig M.K., et al. ATP induces Ca(2+) signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis Cartilage. 2001;9:518. doi: 10.1053/joca.2000.0435. [DOI] [PubMed] [Google Scholar]
- 43.Pingguan-Murphy B., et al. Activation of chondrocytes calcium signalling by dynamic compression is independent of number of cycles. Arch Biochem Biophys. 2005;444:45. doi: 10.1016/j.abb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Poole C.A. Flint M.H. Beaumont B.W. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res. 1987;5:509. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- 45.Alexopoulos L.G. Setton L.A. Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1:317. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Choi J.B., et al. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poole C.A. Ayad S. Gilbert R.T. Chondrons from articular cartilage. V. Immunohistochemical evaluation of type VI collagen organisation in isolated chondrons by light, confocal and electron microscopy. J Cell Sci. 1992;103(Pt 4):1101. doi: 10.1242/jcs.103.4.1101. [DOI] [PubMed] [Google Scholar]
- 48.van Turnhout M.C. Kranenbarg S. van Leeuwen J.L. Contribution of postnatal collagen reorientation to depth-dependent mechanical properties of articular cartilage. Biomech Model Mechanobiol. 2011;10:269. doi: 10.1007/s10237-010-0233-7. [DOI] [PubMed] [Google Scholar]
- 49.Namba K. Suzuki T. Nakata H. Immunogold electron microscopic evidence of in situ formation of homo- and heteromeric purinergic adenosine A1 and P2Y2 receptors in rat brain. BMC Res Notes. 2010;3:323. doi: 10.1186/1756-0500-3-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xing Y., et al. P2Y(2) receptors and GRK2 are involved in oscillatory fluid flow induced ERK1/2 responses in chondrocytes. J Orthop Res. 2011;29:828. doi: 10.1002/jor.21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knight M.M., et al. Articular chondrocytes express connexin 43 hemichannels and P2 receptors - a putative mechanoreceptor complex involving the primary cilium? J Anat. 2009;214:275. doi: 10.1111/j.1469-7580.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hillel A.T., et al. Characterization of human mesenchymal stem cell-engineered cartilage: analysis of its ultrastructure, cell density and chondrocyte phenotype compared to native adult and fetal cartilage. Cells Tissues Organs. 2010;191:12. doi: 10.1159/000225985. [DOI] [PubMed] [Google Scholar]
- 53.Gadjanski I. Spiller K. Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Rev. 2012;8:863. doi: 10.1007/s12015-011-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer M.P., et al. The extracellular ATP receptor, cP2Y(1), inhibits cartilage formation in micromass cultures of chick limb mesenchyme. Dev Dyn. 2001;222:494. doi: 10.1002/dvdy.1196. [DOI] [PubMed] [Google Scholar]
- 55.Leong W.S. Russell R.G. Caswell A.M. Stimulation of cartilage resorption by extracellular ATP acting at P2-purinoceptors. Biochim Biophys Acta. 1994;1201:298. doi: 10.1016/0304-4165(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 56.Brown C.J., et al. Proteoglycan breakdown from bovine nasal cartilage is increased, and from articular cartilage is decreased, by extracellular ATP. Biochim Biophys Acta. 1997;1362:208. doi: 10.1016/s0925-4439(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 57.Varani K., et al. Pharmacological characterization of P2X1 and P2X3 purinergic receptors in bovine chondrocytes. Osteoarthritis Cartilage. 2008;16:1421. doi: 10.1016/j.joca.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Croucher L.J., et al. Extracellular ATP and UTP stimulate cartilage proteoglycan and collagen accumulation in bovine articular chondrocyte pellet cultures. Biochim Biophys Acta. 2000;1502:297. doi: 10.1016/s0925-4439(00)00055-7. [DOI] [PubMed] [Google Scholar]
- 59.Fodor J., et al. Ionotropic purinergic receptor P2X4 is involved in the regulation of chondrogenesis in chicken micromass cell cultures. Cell Calcium. 2009;45:421. doi: 10.1016/j.ceca.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Chowdhury T.T. Knight M.M. Purinergic pathway suppresses the release of .NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol. 2006;209:845. doi: 10.1002/jcp.20768. [DOI] [PubMed] [Google Scholar]
- 61.Orriss I.R. Burnstock G. Arnett T.R. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10:322. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Caswell A.M. Leong W.S. Russell R.G. Evidence for the presence of P2-purinoceptors at the surface of human articular chondrocytes in monolayer culture. Biochim Biophys Acta. 1991;1074:151. doi: 10.1016/0304-4165(91)90054-k. [DOI] [PubMed] [Google Scholar]
- 63.Costello J.C., et al. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res. 2011;52:139. doi: 10.3109/03008207.2010.491928. [DOI] [PubMed] [Google Scholar]
- 64.Rosenthal A.K. Crystals, inflammation, and osteoarthritis. Curr Opin Rheumatol. 2011;23:170. doi: 10.1097/BOR.0b013e3283432d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrari D., et al. Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12, increases the homing capacity, production of proinflammatory cytokines. Exp Hematol. 2011;39:360. doi: 10.1016/j.exphem.2010.12.001. 374.e1. [DOI] [PubMed] [Google Scholar]
- 66.Coppi E., et al. ATP modulates cell proliferation and elicits two different electrophysiological responses in human mesenchymal stem cells. Stem Cells. 2007;25:1840. doi: 10.1634/stemcells.2006-0669. [DOI] [PubMed] [Google Scholar]
- 67.Riddle R.C., et al. ATP release mediates fluid flow-induced proliferation of human bone marrow stromal cells. J Bone Miner Res. 2007;22:589. doi: 10.1359/jbmr.070113. [DOI] [PubMed] [Google Scholar]
- 68.Wang C.Y., et al. Apoptosis in chondrogenesis of human mesenchymal stem cells: effect of serum and medium supplements. Apoptosis. 2010;15:439. doi: 10.1007/s10495-009-0431-x. [DOI] [PubMed] [Google Scholar]
- 69.Cailotto F., et al. Calcium input potentiates the transforming growth factor (TGF)-beta1-dependent signaling to promote the export of inorganic pyrophosphate by articular chondrocyte. J Biol Chem. 2011;286:19215. doi: 10.1074/jbc.M110.175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenthal A.K., et al. Purine receptors modulate chondrocyte extracellular inorganic pyrophosphate production. Osteoarthritis Cartilage. 2010;18:1496. doi: 10.1016/j.joca.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowler W.B., et al. Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001;28:507. doi: 10.1016/s8756-3282(01)00430-6. [DOI] [PubMed] [Google Scholar]
- 72.Gast R.E., et al. Binding of ATP to vascular endothelial growth factor isoform VEGF-A165 is essential for inducing proliferation of human umbilical vein endothelial cells. BMC Biochem. 2011;12:28. doi: 10.1186/1471-2091-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burnstock G. Ulrich H. Purinergic signaling in embryonic and stem cell development. Cell Mol Life Sci. 2011;68:1369. doi: 10.1007/s00018-010-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoebertz A., et al. Expression of P2 receptors in bone and cultured bone cells. Bone. 2000;27:503. doi: 10.1016/s8756-3282(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 75.Buschmann M.D., et al. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 76.Selmi T.A., et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 77.Jacobi M., et al. MACI - a new era? Sports Med Arthrosc Rehabil Ther Technol. 2011;3:10. doi: 10.1186/1758-2555-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Honn K.V. Singley J.A. Chavin W. Fetal bovine serum: a multivariate standard. Proc Soc Exp Biol Med. 1975;149:344. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- 79.Ng K.W., et al. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 2008;35:433. doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Julkunen P., et al. Uncertainties in indentation testing of articular cartilage: a fibril-reinforced poroviscoelastic study. Med Eng Phys. 2008;30:506. doi: 10.1016/j.medengphy.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baroja-Mazo A. Pelegrin P. Modulating P2X7 receptor signaling during rheumatoid arthritis: new therapeutic approaches for bisphosphonates. J Osteoporos. 2012;2012:408242. doi: 10.1155/2012/408242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lister M.F., et al. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varani K., et al. P2X(1) and P2X(3) purinergic receptors differentially modulate the inflammatory response in human osteoarthritic synovial fibroblasts. Cell Physiol Biochem. 2010;25:325. doi: 10.1159/000276565. [DOI] [PubMed] [Google Scholar]
- 86.Terkeltaub R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281:C1. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 87.Johnson K. Terkeltaub R. Inorganic pyrophosphate (PPI) in pathologic calcification of articular cartilage. Front Biosci. 2005;10:988. doi: 10.2741/1593. [DOI] [PubMed] [Google Scholar]
- 88.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.