Abstract
Neo-myometrium was created by culturing isolated myocytes into decellularized rat and human myometrial scaffolds. The dual purpose of the uterus is to accommodate the growing fetus, and then expel the fetus at term by phasically contracting it. The first function requires physical robustness as well as the ability to expand and remodel. Congenital anomalies or previous surgeries can mechanically compromise the uterus and lead to major complications in pregnancy. The second function utilizes multiple interactions of complex physiological mechanisms that have yet to be fully elucidated, and this knowledge gap contributes to the continuation of serious complications of pregnancy. To address both problems, we reconstructed myometrium from isolated myocytes and scaffold. From both rat and human myometrium, myocytes were isolated using collagenase digestion, and scaffolds were isolated using ethanol/ trypsin protocols. The number of myocytes was amplified using monolayer culture, and then, the myocytes were cultured back into the scaffolds. We called this engineered tissue neo-myometrium, with allo-neo-myometrium being made from components of the same species, and xeno-neo-myometrium from across species. By artificially creating defects in rat scaffold, allo-neo-myometrium was created that demonstrated rapid scaffold remodeling. Xeno-neo myometrium (human myocytes/rat scaffold) was created and demonstrated myocytes occurring in bundles 500 μm deep in the scaffold. These experiments provide proof of principle that modest numbers of myocytes can be amplified and used to create a larger volume of engineered tissue, which may be useful for semi-autologous transplantation to repair structural defects of the human uterus. In isometric contractility experiments, coordinated contractions were observed in xeno-neo-myometrium (human myocytes, rat scaffold), but not allo-neo-myometrium (human myocytes, human scaffold).
Introduction
There are two primary functions of the pregnant uterus—to house the fetus, and then to expel the developed fetus. Myometrium is composed of two major components: myocytes (smooth muscle cells) and the connective tissue scaffold. Force production originates with the myocytes, although it does not appear that the contraction profile of the uterus or uterine tissue strips can be recapitulated by the individual myocyte.1 Coordinating the contractions of large numbers of myocytes within a syncytium seems to be necessary to mimic the phasic nature of the contractile activity that is important for labor. It is well known that cell-to-cell communication through gap junctions is necessary for tissue-level coordination,2 and it is likely that the structure imposed by the scaffold is also a key component.
In this work, tissue-engineered myometrium, or neo-myometrium, is created by culturing myocytes into a myometrial scaffold. We use pregnant myometrium as starting material to exploit the natural ability of the cells to proliferate and the scaffold to remodel.
Significant clinical problems are encountered when the uterus, through congenital or acquired structural defects, is unable to adequately remodel to accommodate the growing fetus.3 Acquired uterine defects are commonly seen after removal of fibroids or incomplete healing after a previous Cesarean delivery. Rupture of the uterus through these defects is life threatening for both mother and baby.4 In many cases, imaging techniques can visualize the defects before pregnancy.5 Therefore, one long-term goal of this work is to create a neo-myometrial tissue patch that could be placed over a uterine defect before pregnancy, with the intent of adding structural support and reducing the risk of uterine rupture. In addition, the patch should not interfere with the normal uterine expansion of advancing gestation or interfere with contractions at the time of delivery.
Attempts to create uterine patches date back to 2008, when Campbell et al.6 placed polyethylene bulbs into the peritoneal cavity and grew “tissue capsules.” These tissues were composed of myofibroblasts, which likely originated from host peritoneal fluid. After creating defects in one horn of the nonpregnant rat, the defects were repaired with the engineered tissue, and the rats were mated. That work also demonstrated that the grafted tissue remodeled during gestation and supported the development of a term fetus.
The ideal scheme to repair defects of the uterus is to create an autologous neo-myometrium that is of a greater volume than a harvested tissue specimen. While the number of myocytes can be greatly increased using monolayer culture techniques, scaffold size cannot be easily increased. Hence, it may be necessary to use a nonautologous donor scaffold. We will, therefore, culture human myometrial myocytes into rat myometrial scaffold to create xeno-neo-myometrium as proof of principle for repair of uterine defects.
Elucidating the physiology associated with dysfunctional or preterm labor is also of great clinical significance.7,8 Muscle bath experiments are often used to investigate the mechanisms of uterine contractility. This technique targets a protein with a pharmacological agent, then infers the role of the protein based on the changes of contractility. The disadvantages of this approach are that most agents have significant off-target effects, and there are many proteins in which there is no specific agent.
Here, we attempt to create neo-myometrium that retains the ability to generate force. If successful, we propose that it would then be possible to modulate gene expression of the myocytes, and test for changes of contractility. This experimental approach could provide a new method to probe the individual contributions of specific proteins to myometrial contractility using human myocytes. While here we also use rodent myocytes for proof of principle, our overarching goal is to investigate the physiology of human myocytes and myometrium. Similarly, isolating and modifying the scaffold creates a method that probes the contribution of the structure of the scaffold to myometrial contractility.
Materials and Methods
Tissues
Pregnant human myometrium
Human myometrium was procured after informed consent with procedures approved by the University of Vermont Institutional Review Board (CHRMS 07-039). Subjects were women who were undergoing term Cesarean delivery for unrelated clinical reasons, such as repeat Cesarean delivery or breech presentation. Myometrial biopsies, approximately 3×3×15 mm, were obtained after delivery of the infant and placenta at the time of term Cesarean delivery from the upper margin of the transverse incision through the lower uterine segment. Cells and scaffold were isolated as described next. Indications for Cesarean delivery included breech presentation, repeat or elective Cesarean delivery. Patients with hypertensive disorders, infection, and preterm birth were specifically excluded.
Pregnant rat myometrium
Timed pregnant rats were purchased from Charles River and used between d 20 and 21 gestations. Under a University of Vermont Institutional Animal Care and Use Committee (IACUC) approved protocol #07-055AP, rats were euthanized using pentobarbital and decapitation, and myometrial tissue was harvested.
Isolation of scaffold (decellularization)
Published protocols indicate that scaffolds from large arteries can be cleaned of cellular material by treatment with 70% ethanol for 3 days, water for 12 h, and then trypsin 0.25 g/dL for 4 days.9 When we applied this protocol to either rat or human myometrium, we found that the physical integrity of the tissue was fully degraded. To address this problem, we reduced the time of exposure to both the ethanol and trypsin steps.
Human
Myometrial specimens varied in size, but were routinely trimmed into 2×2×10 mm strips. Strips were placed on slight tension by flattening and securing at each end using pins in a Sylgard-coated Petri dish. Cells were destroyed by exposing the strips to 70% ethanol for 24 h at room temperature with mild continuous shaking. The ethanol was then removed and replaced with distilled water. After 1 h in water, cellular debris was removed by covering the scaffold with Trypsin EDTA 1×(Lonza, Walkersville, MD), 0.25% for 3 or 24 h. The trypsin was then neutralized with culture media, copiously washed, and stored in sterile phosphate-buffered saline (PBS). The culture media used was DMEM with 10% fetal bovine serum (FBS), Penicillin 10,000 U/mL, and Streptomycin 10,000 μg/mL (Lonza) added.
Rat
Full-thickness myometrium was trimmed into a rectangle shape (1.5×2.0 cm) and sutured at each corner using a braided silk 000 suture (Fig. 1, insert). The tissue was placed circular layer side up, and maintained on slight tension by securing the suture over a plastic histology cassette. The ethanol/water/trypsin decellularization process was then performed as described earlier.
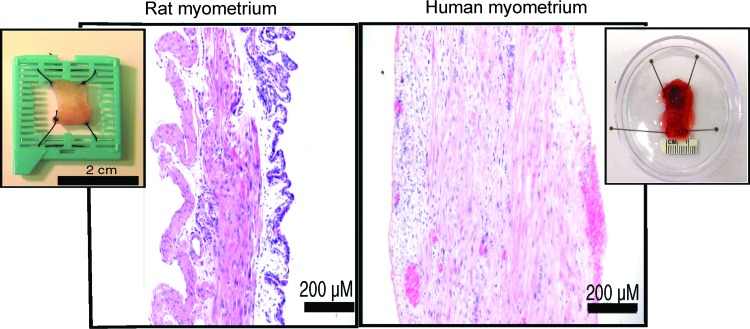
FIG. 1.
H+E staining of native rat and human myometrium. Rat myometrium displays well-defined circular and longitudinal layers. Serosal peritoneum is basophilic. Left insert: Untreated rat myometrium, hammock technique. A portion of the lid of a plastic histology cassette was removed, and a rectangular section of full-thickness pregnant rat uterus was suspended over the opening with a suture at each corner. Human myometrium is composed of fiber bundles arranged in fasciculata (bundles of bundles). Right insert: An untreated human biopsy specimen is pinned for display. H+E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tea
Isolation of human and rat myocytes (dispersion)
After procurement, myometrial tissue was washed with PBS containing 1.8 mM CaCl2 and 0.5 mM MgCl2 to remove the blood and debris. The tissue was minced into 0.5×1 mm cubes under sterile conditions, placed into a 15 mL conical centrifuge tube, and covered with calcium, magnesium-free Hank's solution. After 1 h at 37°C, the Hank's solution was then removed, and the minced tissue was exposed to Liberase™ Research Grade 0.42 U/mL (Roche Diagnostics, Indianapolis, IN) for 1 h. The tissue and cells were allowed to settle, and the Liberase solution was carefully removed by pipetting from the surface. Culture media (containing 10% FBS) was added, and cells were freed by trituration (10 times, or until the solution became cloudy) using a 5 mL serological pipette. The solution containing free cells and small tissue clumps was then centrifuged at 3500–4000 RPM for 5 min, and the supernatant was removed. 2 mL of culture media was used to re-suspend the cells, and cells were transferred to sterile culture flasks. Cells were allowed to plate in the incubator at 37°C and 5% CO2, and the next day, the flask was washed and the culture media were exchanged. Approximately 10 human myometrial cell lines and five rat myometrial cell lines were initiated. Approximately 10 experiments were performed on some cell lines.
Culture techniques
Monolayer culture
Cells were maintained under culture conditions at 37°C, 5% CO2. Media was changed every 3–4 days. As the cells achieved 80%–100% confluence, they were subcultured by lifting with Trypsin EDTA 1×, and splitting 1:4.
Three-dimensional culture
Monolayer cultured smooth muscle cells were amplified in number using standard culture techniques for approximately three passages. To accomplish three-dimensional (3D) culture, cells were lifted as if in the process of subculture, except they were resuspended in media at high density (4–5×106 cells/mL), using volumes of 250 to 500 μL per flask. The surface of the scaffold was covered with this media, using the entire volume for larger specimens. Each scaffold received cells from only a single cell line.
For rat scaffolds, the four corner sutures allowed for a hammock-like effect (Fig. 1, insert). The scaffolds were initially kept still for 1 h to allow cell attachment, and then flooded with media. The human scaffold produced less of a hammock effect, but capillary action kept the media in close proximity to the scaffold for the initial hour. 3D culture was then performed by placing the culture dishes on a plate shaker (BellCo Glass, Vineland, NJ) inside of an incubator. The samples were maintained in continuous motion using the shaker at half speed (approximately 100 cycles/minute). This two-step technique first allows the cells to attach to the scaffold, and then during long-term culture, the constant motion of the media is intended to enhance cell viability in the scaffold depths.
Histology
Standard formalin/paraffin imbedding protocols were used for creating blocks for hematoxylin and eosin (H+E), desmin, and Masson's trichrome staining.
Live/dead assays
Assays were performed following the manufacturer's recommended protocol (Invitrogen™ kit, Molecular Probes™, Eugene, Oregon). The staining solution was made by adding 4 μL Ethidium homodimer-1 (EthD-1) to phosphate-buffered saline, followed by the 1 μL Calcein AM. Samples were exposed to this solution for 45 min at 37°C and 5% CO2. Images were obtained using appropriate excitation and emission filters for live or dead assessments.
Isometric contractility studies
Strips of rat and human myometrium were trimmed to appropriate sizes and secured at both ends with a 000 silk suture before decellularization. Decellularization was then performed as described earlier. Myocytes were plated onto the scaffold, and neo-myometrium was created by 3D cultured as described earlier. The neo-myometrium was then transferred to the isometric muscle bath apparatus that has been previously described.10 The experimental bathing solution was composed of (in mM) NaCl (115), NaHCO3 (30), KCl (5), MgCl2 (0.5), CaCl2 (1.8), glucose (10), pH 7.4, bubbled with 95% O2, 5% CO2, and was continuously flowed through the recording chamber. Temperature was maintained at 37°C±1°C.
Results
Preparation of rat and human myometrial scaffolds
Native rat myometrium is composed of a well-defined circular and longitudinal layer of smooth muscle (Fig. 1). The serosal peritoneum is present in the rat (Fig. 1, basophilic cell layer at right) but not the human, because human specimens were obtained from the center of the uterine wall. Rectangular segments of rat myometrium were suspended “hammock-like” with the serosal surface facing downward before decellularization. Due to sample size limitations and the relative thickness of the tissue, native human myometrium (Fig. 1) could not be effectively stretched from the four corners, but rather was stretched linearly.
After 24 h of ethanol exposure, both rat and human tissues expressed a slightly rubbery consistency. After overnight exposure to water, the scaffolds (both human and rat) were cleaned by 24 h of trypsin exposure, leaving the scaffolds devoid of smooth muscle cells (Fig. 2). Decellularized scaffolds appeared grossly similar to native tissue (Fig. 2, inserts). In experiments in which rat scaffolds were mechanically perforated, the trypsin exposure could be reduced further (see below).
FIG. 2.

Decellularized rat and human myometrium, Masson's trichrome stain. The native structure of both rat and human myometrial scaffold is retained after decellularization. Cellular debris is largely absent after only 24 h of Trypsin exposure. Rat scaffolds retain the well-defined circular and longitudinal layers, although the circular layer can be mechanically removed before decellularization. Inserts: Gross appearances of rat and human myometrium following decellularization. Human myometrium was routinely cut into 2×2 mm strips before ethanol exposure. Color images available online at www.liebertpub.com/tea
Three-dimensional culture of myocytes into myometrial scaffolds
Human myocytes onto human scaffolds
Within the scaffold, isolated myocytes were frequently seen at depths greater than 500 μm, and rarely small clusters of 5–10 cells were observed (Fig. 3). Myocytes seemed to penetrate along natural defects, possibly old vascular pathways. Thick bundles that extended for tens of microns were not observed. After 34 days in culture, thin patches of cellular overgrowth were seen on the surface of the human scaffold (Fig. 4). Desmin staining confirmed the smooth muscle origin of the cultured cells.
FIG. 3.

Human allo-neo-myometrium created from 3rd-passage human cell lines, after 14 days in three-dimensional (3D) culture. H+E stain. Arrows demonstrate natural defects in the scaffold with early in-growth of cells. The circle demonstrates a rare small group of myocytes in the depth of the scaffold. Color images available online at www.liebertpub.com/tea
FIG. 4.
Human allo-neo-myometrium (human cells/human scaffold). First-passage human cells were 3D cultured for 34 days. Masson's trichrome stain reveals deep penetration of cells into collagen. Desmin staining reveals that the cells deep in the tissue are smooth muscle. Color images available online at www.liebertpub.com/tea
Human myocytes onto rat scaffolds
Rat scaffolds were able to support the attachment and growth of human myocytes in 3D culture (Fig. 5A). After 51 days, cells overgrew each other to depths of 20–30 cell layers on the surface of the scaffold, and also demonstrated good penetration into the scaffold. As with the human scaffold, human myocytes seemed to penetrate the rat scaffold along lines of natural defects. Live/dead assays (Fig. 5B) confirmed that living cells were close to each other, both side to side and end to end, which formed neo-bundle structures.
FIG. 5.
Xeno-neo-myometrium. (A). 3rd-passage human myocytes 3D cultured into rat scaffold for 51 days. Masson's trichrome stain demonstrated penetration and close association of myocytes into the depths of the scaffold. Desmin staining demonstrates the smooth muscle origin of the cells. (B). Live/dead assay. 3rd-passage human myocytes 3D cultured into rat scaffold for 28 days. Living cells growing in sheet-like structures within the scaffold. Color images available online at www.liebertpub.com/tea
Rat myocytes onto rat scaffolds
Interestingly, using the scaffold preparation described earlier, rat scaffold did not support culture of rat myocytes as well as human myocytes. Surface overgrowth was poor, and penetration required longer culture durations. Since cells appeared to penetrate the scaffold through defects, we created artificial defects in an attempt to enhance rat myocyte penetration into the rat scaffold. In this series, we first removed the circular layer of the rat myometrium. After the ethanol treatment, a 22-G blunt needle (inner diameter 0.41 mm) was used to manually cut an array of holes separated by 300 to 400 μm (Fig. 6A). The duration of the trypsin exposure was reduced from 24 to 3 h because of concerns that the defects in the perforated scaffold would increase fragility. Trichrome staining confirmed that cellular debris was largely removed from the perforated scaffold after 3 h of trypsin exposure (Fig. 6B).
FIG. 6.
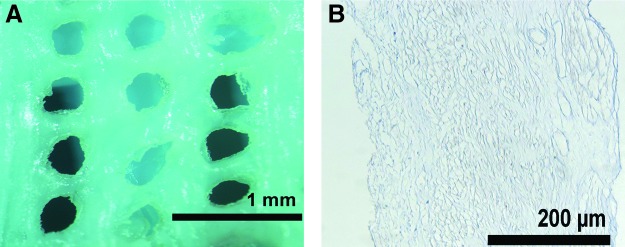
(A) Artificially created defects in ethanol-treated rat myometrium. A 22-G blunt-tipped needle was used to manually create this pattern of defects. 3 h of Trypsinization was then performed to clear much of the cellular debris from the scaffold. (B) Masson's trichrome stain, demonstrating the presence of only a small amount of cellular debris. Color images available online at www.liebertpub.com/tea
The rat myocytes quickly overgrew the scaffold defects (Fig. 7A), and then retracted. This effect has previously been reported for human myocytes on vicryl mesh.11 Most cells were alive (green), and scattered dead (red) cells were rarely seen. After 3 weeks in culture, the scaffolds structurally reorganized by losing the connections between the defects (Fig. 7B). The narrow bridge of living cells that spans large distances suggests where the scaffold was previously connected. Remodeling of the perforated rat scaffold by rat myocytes with loss of large portions of the scaffold was a consistent finding (in each of 10 experiments). Usually three or four corners of the scaffold remained attached to the supporting frame. When four corners remained attached, the remodeling effectively created two pair of 600–700 μm neo-tissue strips, which essentially traced the outer perimeter of the original rectangular-shaped tissue. One pair was oriented parallel with the original longitudinal axis, and the other pair was oriented perpendicular. Cells within the neo-tissue strips ran parallel with the long axis of the neo-tissue, even when the neo-tissue ran perpendicular to the original longitudinal axis of the tissue (Fig. 7C).
FIG. 7.
(A) Live/dead assay of rat allo-neo-myometrium after 21 days in 3D culture. Myocytes were 1st passage. Bridging/retraction of living myocytes at the corner of the defect in the scaffold. (B) Lower power view. The bridging of living cells over large distances suggests remodeling and absorption of the scaffold. (C) The suture that secured the corner of the rat myometrium is visible at the left of the image. The horizontal arm is perpendicular to the original orientation of the longitudinal layer of the scaffold. This arm contains myocytes that are oriented along the axis of the arm, and, hence, are perpendicular to the original orientation of the scaffold. Color images available online at www.liebertpub.com/tea
Artery and vein walls were cleared of smooth muscle cells during the decellularization process, but we were unable to find any evidence that they were repopulated during 3D culture. Due to this, we were unable to systematically study in-vitro neovascularization.
Contractility of neo-tissue strips
Native myometrium contracts in a coordinated, rhythmic pattern when placed on tension in a muscle bath. When human myocytes were cultured into rat scaffold, and placed on 140 mg tension, phasic contractions lasting 1 min were expressed that demonstrated peak forces as large as 15 mg. Expressed as milli-Newtons (mN) and normalized for the scaffold cross-sectional area (0.7×0.5 mm in this case), Figure 8Aa was obtained. To check that solution flow, vibration, or other artifacts were not interfering with force measurements, 70% ethanol was added to the chamber to destroy the myocytes (Fig. 8Ab). These peak forces are much less than what is typically observed in native tissues (∼2 N/cm2).
FIG. 8.
Isometric contractility experiments. (A) Xeno-neo-myometrium (human myocytes, rat scaffold). (B) Allo-neo-myometrium (human myocytes/human scaffold).
We attempted to observe similar contractions from human myocytes cultured into human scaffold (allo-neo-myometrium). These strips were able to withstand 900 mg of applied tension, but coordinated phasic contractions were not appreciated (n=5), although small irregularly appearing forces were observed (Fig. 8Ba).
Discussion
Engineering of the uterus has previously been attempted. Lu et al.12 demonstrated that it is possible to implant and support early development of embryos onto a layered co-culture of endometrium and myocytes of the rabbit. Our efforts are directed toward repairing a fraction of the uterine wall, but we remain concerned that patches would provide a suboptimal implantation site and lead to major complications of placentation later in pregnancy. Embryo implantation and placenta previa or accreta at the site of the uterine incision in women with earlier Cesarean sections is a well-established clinical issue.13
Our initial attempts to subject pregnant rat or human myometrium to published isolation protocols9 designed for vessels resulted in poor scaffold integrity. This outcome perhaps is not unanticipated, as the content of both collagen and elastin in myometrium differs from that of large anteries. We, therefore, reduced the duration of exposure to ethanol from 3 days to 24 h and to trypsin from 4 days to 24 h (and as low as 3 h for perforated rat myometrium).
One goal of this work was to provide proof of principle for engineering uterine patches in vitro. By separating myocytes from scaffold, it is possible to amplify the number of myocytes and greatly increase the volume of the neo-tissue compared with that of the original tissue specimen. Even after long-term 3D culture, the myofibroblasts retain a high degree of desmin staining (Fig. 5), suggesting that the cells retain some smooth muscle characteristics.
Creation of a variety of artificial solid organs from stem cells and synthetic scaffolds has been proposed, and techniques are currently being actively investigated. However, there are persistent long-range issues with amplification of stem cell numbers, differentiation of cells into the correct phenotype, and graft success. The uterus is relatively unique, in that myocytes readily de-differentiate into a proliferative myofibroblast phenotype,14 which allows cell numbers to be quickly amplified, then the myofibroblasts re-differentiate back into functional myocytes when placed in the correct in vivo environment.15
Modification of gene expression during the myofibroblast phase provides the opportunity for cell tracking post transplant. Neo-myometrium may also be useful to assist with repairing congenital or acquired defects in other reproductive smooth muscle structures, such as the fallopian tube or pelvic floor.
While the number and volume of host myocytes can be increased, it is not obvious how to increase the size of a host uterine scaffold for fully autologous neo-myometrium. A donor hysterectomy specimen could be used as the source of human scaffold. Due to the small size of the nonpregnant myocyte, it is likely that the scaffold would require preparation in a manner similar to the “artificial defect method” which we demonstrated with the rat (Fig. 6).
While use of other autologous scaffold material, such as fascia lata, is feasible, we elected to investigate combining human myocytes with animal scaffold to create xeno-neo-myometrium. From immunological and rejection considerations, the scaffold is likely less of a problem than the cells. Matching the scaffold to the cell type will be an ongoing topic for further research.
Rat myometrial scaffold is limited to a thickness of approximately 1 mm, and we speculate that 5 mm is the minimum thickness for a transplantable graft intended to structurally support human uterine function. Upscaling this process will require either multi-layering rat scaffolds or using thicker uterine scaffolds from larger animals. A major barrier that remains to be addressed is creation of blood supply to the graft, although angiogenesis has been shown to occur when myometrium is grafted onto the heart.16
The second long-range goal of this work is to begin to develop an additional model system for studying myometrial contractility. This model would offer the major advantage that the components can be closely controlled, and, in principle, systematically modified at the level of gene function. While selectively modifying gene function is possible with an inducible, site-specific, genetically modified mouse model, we propose to genetically modify the protein expression of the myocytes during the myofibroblast (cell number amplification) phase. This method would likely be more versatile and less expensive than creating a series of inducible conditional mouse knockout models. In addition, our method also allows for a systematic study of the influence of scaffold structure on contractility.
Human cells attached and grew in thin layers on the surface of human myometrial scaffolds. With prolonged culture, the myocytes overgrew each other, but they then detached, retracted, and never achieved a thick layer of myocytes on the scaffold surface. Even though individual myocytes penetrated the scaffold to moderate depths, they did not associate with each other or create structures that resembled native bundles. The inability to directly repopulate the human scaffold into bundle-like structures is perhaps because the physical collapse of the scaffold that occurred after the cells were removed did not allow enough space for the cells to easily penetrate or proliferate. These observations are consistent with our failure to observe coordinated contractions in allo-neo-myometrium.
When plated onto rat myometrial scaffold, the human myocytes overgrew themselves and formed multicellular layers on the surface. In contrast to the human scaffold, the surface multicellular layers were thicker, and crucially, clusters of cells were observed within the depths of the rat scaffold. This xeno-neo-myometrium demonstrated structural integrity, good cellularity, and excellent cellular viability. The observation of coordinated contractions, although not as forceful as native myometrium, provides proof of principle that neo-myometrium can potentially be used to systematically study the relationship between scaffold and myocytes in the generation of phasic contractions.
Conclusion
Tissue-engineering techniques were applied to pregnant rat and pregnant human myometrium to create allo- and xeno-neo-myometrium. Human myocytes penetrated rat scaffold, providing proof of principle for the creation of xeno-neo-myometrial grafts. Human myocytes on rat scaffold expressed coordinated contractions, and they also provided a novel model for the study of myometrial contractility.
Acknowledgments
This work was supported by NIH grant #R21 HD061875. Additional support from the Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Vermont, is gratefully acknowledged.
Disclosure Statement
No competing financial interests exist.
References
- 1.Young R.C. Myocytes, myometrium, and uterine contractions. Ann N Y Acad Sci. 2007;1101:72. doi: 10.1196/annals.1389.038. [DOI] [PubMed] [Google Scholar]
- 2.Pierce B.T. Calhoun B.C. Adolphson K.R., et al. Connexin 43 expression in normal versus dysfunctional labor. Am J Obstet Gynecol. 2002;186:504. doi: 10.1067/mob.2002.121108. [DOI] [PubMed] [Google Scholar]
- 3.Turton P. Neilson J.P. Quenby S., et al. A short review of twin pregnancy and how oxytocin receptor expression may differ in multiple pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S40. doi: 10.1016/j.ejogrb.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Turner M.J. Uterine rupture. Best Pract Res Clin Obstet Gynaecol. 2002;16:69. doi: 10.1053/beog.2001.0256. [DOI] [PubMed] [Google Scholar]
- 5.Roberge S. Boutin A. Chaillet N., et al. Systematic review of cesarean scar assessment in the nonpregnant state: imaging techniques and uterine scar defect. Am J Perinatol. 2012;29:465. doi: 10.1055/s-0032-1304829. [DOI] [PubMed] [Google Scholar]
- 6.Campbell G.R. Turnbull G. Xiang L., et al. The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and vas deferens. J Tissue Eng Regen Med. 2008;2:50. doi: 10.1002/term.66. [DOI] [PubMed] [Google Scholar]
- 7.Aguilar H.N. Mitchell B.F. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16:725. doi: 10.1093/humupd/dmq016. [DOI] [PubMed] [Google Scholar]
- 8.Kam K.Y. Lamont R.F. Developments in the pharmacotherapeutic management of spontaneous preterm labor. Expert Opin Pharmacother. 2008;9:1153. doi: 10.1517/14656566.9.7.1153. [DOI] [PubMed] [Google Scholar]
- 9.McFetridge P.S. Daniel J.W. Bodamyali T., et al. Preparation of porcine carotid arteries for vascular tissue engineering applications. J Biomed Mater Res A. 2004;70:224. doi: 10.1002/jbm.a.30060. [DOI] [PubMed] [Google Scholar]
- 10.Young R.C. Bemis A. Calcium-activated chloride currents prolongs the duration of contractions in pregnant rat myometrial tissue. Reprod Sci. 2009;16:734. doi: 10.1177/1933719109334965. [DOI] [PubMed] [Google Scholar]
- 11.Young R.C. Schumann R. Zhang P. Three-dimensional culture of human uterine smooth muscle myocytes on a resorbable scaffolding. Tissue Eng. 2003;9:451. doi: 10.1089/107632703322066633. [DOI] [PubMed] [Google Scholar]
- 12.Lu S.H. Wang H.B. Liu H., et al. Reconstruction of engineered uterine tissues containing smooth muscle layer in collagen/matrigel scaffold in vitro. Tissue Eng Part A. 2009;15:1611. doi: 10.1089/ten.tea.2008.0187. [DOI] [PubMed] [Google Scholar]
- 13.Karayalcin R. Ozcan S. Ozyer S., et al. Emergency peripartum hysterectomy. Arch Gynecol Obstet. 2011;283:723. doi: 10.1007/s00404-010-1451-z. [DOI] [PubMed] [Google Scholar]
- 14.Stegemann J.P. Hong H. Nerem R.M. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa M. Naruko T. Ikura Y., et al. A decline in platelet activation and inflammatory cell infiltration is associated with the phenotypic redifferentiation of neointimal smooth muscle cells after bare-metal stent implantation in acute coronary syndrome. J Atheroscler Thromb. 2010;17:675. doi: 10.5551/jat.3426. [DOI] [PubMed] [Google Scholar]
- 16.Taheri S.A. Yeh J. Batt R.E., et al. Uterine myometrium as a cell patch as an alternative graft for transplantation to infarcted cardiac myocardium: a preliminary study. Int J Artif Organs. 2008;31:62. doi: 10.1177/039139880803100109. [DOI] [PubMed] [Google Scholar]