Abstract
Objective: Matrix metalloproteinase-9 (MMP-9) plays an important role in inflammation and matrix degradation involved in atherosclerosis and plaque rupture. The T allele of rs3918242 has been reported to lead to a high promoter activity and associate with the extent of coronary artery disease (CAD). And some studies have reported that the G allele of rs17576 might be associated with CAD. The aim of this study was to assess the association between the polymorphisms of the MMP-9 gene and CAD in the Chinese Han population. Methods: This case–control study comprised 258 CAD cases and 153 controls from the Chinese Han Population. The genomic DNA of MMP-9 was isolated from whole blood. Polymerase chain reaction-based restriction fragment length polymorphism was used to determine the rs3918242 and rs17576 genotypes in the MMP-9 gene and the total serum levels of MMP-9 were measured using enzyme-linked immunosorbent assay in both case and control groups. Results: Analysis of MMP-9 gene polymorphisms showed that the frequencies of the T allele and CT+TT genotypes of rs3918242 were significantly higher in the case group than in the control group (p<0.05). However, the distribution of variant genotypes of rs17576 did not differ between the case and control groups (p>0.05). The total serum level of MMP-9 was significantly higher in the case group than in the control group (p<0.05). The subjects carrying T alleles in the CAD group had higher average serum MMP-9 levels compared with CC genotypes (p<0.05). Conclusions: Our results suggest that the single-nucleotide polymorphism of rs3918242 in the MMP-9 gene is associated with CAD and high serum levels of MMP-9 are also associated with CAD in the Chinese Han population. Therefore, genetic variation of rs3918242 may participate in the development of CAD through influencing MMP-9 expression.
Introduction
Atherosclerosis is the major cause of coronary artery disease (CAD), which is a multifactorial disorder with both genetic and environmental components. However, the extent of the genetic basis in the occurrence of CAD remains incompletely understood. Atherosclerosis is characterized by artery intima damage in the blood vessel wall. Extracellular matrix (ECM) synthesis and degradation is in balance with the normal vascular intima place, but the degradation of the matrix dominates in the vascular intima of atherosclerosis, especially the vulnerable plaque areas (Heeneman et al., 2003). The accumulation of lipids and fibrous elements in the large arteries promotes smooth muscle cell (SMC) migration and proliferation. Because vascular SMC is surrounded by a basement membrane, SMC migration and proliferation necessitate breaking down the ECM (Newby and Zaltsman, 1999). Matrix metalloproteinases (MMPs) are a family of Zn2+ - dependent endopeptidases, which are considered to be the most potent proteases in the metabolism of the ECM and are present in atherosclerotic plaques and appear to be more active in unstable lesions (Galis and Khatri, 2002; Visse and Nagase, 2003). Changed expression of MMPs has been implicated in the pathogenesis of several cardiovascular disorders, including atherosclerosis (Newby, 2005). Recent observations suggest that genetic variation affects the expression of MMPs, which may contribute to the occurrence of cardiovascular disease (Henney et al., 2000).
MMP-9, also known as gelatinase B or 92-kDa type IV collagenase, is one of the important members of MMPs that may contribute to the breakdown of the ECM. MMP-9 is characterized by broad substrates, such as gelatin, type IV collagen, and elastin, but it possesses specialized proteolytic activity against type IV collagen, a major component of the coronary artery basement membrane underlying the endothelium and surrounding each vascular SMC (Birkedal-Hansen et al., 1993). MMP-9 is secreted from macrophages in the fibrous cap and has been suggested to be involved in the remodeling processes associated with atherosclerosis and plaque rupture (Loftus et al., 2000; Nanni et al., 2007). The expression of MMP-9 is regulated primarily at the transcriptional level. MMP-9 expression is increased after vascular injury (Bendeck et al., 1994) and is particularly evident in inflammatory atherosclerotic lesions. High expression of MMP-9 has been associated with coronary plaque destabilization (Galis and Khatri, 2002).
The human MMP-9 gene, which is located on chromosome 20q12.2–13.1, contains 13 exons and 12 introns. Common variants in the promoter and exon polymorphisms have been reported to be associated with CAD in recent years. Zhang et al. (1999b) reported that there was a functional single-nucleotide polymorphism (SNP) of rs3918242, the nucleotide variation from C to T gave rise to a twofold increase in the promoter activity and the T allele tended to have a high risk of severe CAD. And the other common SNP A/G polymorphism of rs17576 in the MMP-9 gene leading to an amino acid substitution in the catalytic domain of the MMP-9 enzyme has also been described (Zhang et al., 1999a), although few functional data are available. The SNP may presumably be involved in the binding of the enzyme to its substrate elastin (Shipley et al., 1996).
It has been observed that the levels of serum MMP-9 were elevated in patients with coronary atherosclerosis (Spurthi et al., 2012) and that there was an association between the levels of circulating MMP-9 and cardiovascular events and mortality (Blankenberg et al., 2003; Weiss et al., 2010). Opstad et al. (2012) reported that in T-allele carriers, mRNA levels were 1.7-fold lower compared to wild-type CC. The carriers with the minor T-allele of rs3918242 had higher MMP-9 serum levels compared to those with the major C-allele (Blankenberg et al., 2003).
The question whether SNPs in the MMP-9 gene are associated with CAD in the Chinese Han population has no consistent answers. Our objectives in the present report, therefore, were (1) to investigate a genetic association between SNPs in the MMP-9 gene and risk of CAD, (2) to evaluate the relationship between the serum level of MMP-9 and CAD, (3) to evaluate the relationship of SNP and serum MMP-9 in the Chinese Han population through a case–control design.
Subjects and Methods
Study subjects
This hospital-based case–control study included 258 unrelated patients with CAD and 153 unrelated controls to undertake a genetic analysis for association between the MMP-9 gene polymorphisms and CAD. All the subjects used for this study were the Chinese of Han descent. Patients with CAD (146 males and 112 females) were admitted to the First Hospital of Jilin University, Changchun, China from 2009 to 2011. Control subjects (83 males and 70 females) were randomly selected from the same geographical area during routine arrival checkup. The study was approved by the ethics committee of the First Hospital of Jilin University, Changchun China, and written informed consent was given by every subject.
Diagnosis was made independently by at least two well trained cardiologists based on the following criteria. All patients recruited had evidence of CAD documented by unstable angina or myocardial infarction. Unstable angina and myocardial infarction were confirmed per Chinese guidelines (Chinese Medical Cardiology Subcommittee, Chinese Editorial Committee of Cardiology Journal, 2007, 2010). Patients with nonatherosclerotic vascular diseases, congenital heart disease, cardiomyopathy, valvular disease, renal or hepatic disease, and cancer were excluded. All control subjects had ECG, chest X-ray, and serum analysis. They were classified as healthy subjects based on their physical examination coupled with the absence of personal or family history and reasons to suspect CAD.
We recorded clinical data, including age, gender, weight, height, smoking habit, and the number of cigarettes consumed per day by each smoker, the presence or absence of hypertension (defined as diastolic blood pressure >90 mmHg and/or systolic blood pressure >140 mmHg and/or if subjects were receiving antihypertensive medication), the presence or absence of type I or type II diabetes (fasting blood glucose ≥7.0 mM, and/or using glucose-lowering medication, including insulin), and the presence or absence of hyperlipidemia (defined as cholesterol >5.2 mM and/or triglyceride >1.7 mM, current medications particularly the use of lipid-lowering drugs). Overnight fasting venous blood samples were collected from each subject for total cholesterol (TC), triglyceride (TG) levels, glucose, genomic DNA extraction, and serum extraction.
Genotyping
To perform genetic analysis, we selected rs3918242 (SphI site)—the functional C1562T polymorphism located in the promoter region and rs17576 (MspI site)—the functional R279Q polymorphism located in exon 6. SNP information was taken from NCBI dbSNP Build 132 (www.ncbi.nlm.nih.gov/SNP/). The selected SNPs were restricted to a minor allele frequency of >15% in the HapMap-CHB database (www.hapmap.org). Genomic DNA used for PCR amplification was extracted from whole blood samples using a DNA extraction kit (Promega, Beijing, China). SNPs were genotyped using standard polymerase chain reaction and restriction fragment length polymorphism analysis. Primers and conditions for amplification follow. The MMP-9 rs3918242 polymorphism was amplified from 100 ng of genomic DNA in a 15-μL PCR reaction using the following primers: forward GCCTGGCACATAGTAGGCCC; reverse CTTCCTAGCCAGCCGGCATC. PCR conditions included denaturation at 95°C for 5 min followed by 35 cycles of 94°C for 35 s, 56°C for 30 s, and 72°C for 45 s, and a final extension at 72°C for 5 min. The MMP-9 rs17576 polymorphism was amplified from 100 ng of genomic DNA in a 15-μL PCR reaction using the following primers: forward TCACCCTCCCGCACTCTGG; reverse CGGTCGTAGTTGGCGGTGG. PCR conditions included denaturation at 95°C for 5 min followed by 30 cycles of 94°C for 30 s, 58°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 10 min.
Measurement of serum MMP-9
The extracted sera were stored at −80°C for MMP-9 concentration detection. The total serum MMP-9 levels were measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (MMP-9 Human ELISA Kit; Sangon Biotech, Shanghai, China) in both groups.
Statistical analysis
All statistical analyses were conducted using SPSS (version 17.0). Data are expressed as percentages of total for categorical variables, and mean±SD for continuous variables. The statistical analyses on the characteristics of the subjects were performed with the Pearson χ2 test for categorical variables such as sex, smokers, diabetes, and hypertension and with the Student's t test for the continuous variable of age, BMI, TC, and TG with normal distribution. The Hardy–Weinberg equilibrium for the genotypic distributions of SNPs was tested using the chi-square (χ2) goodness-of-fit test. p Values of <0.05 were set as a significant level. Logistic regression analysis was performed in genotypic analysis with the adjustment for confounding factors.
Results
Characteristics of study subjects
The demographic and clinical characteristics of the 258 CAD patients and 153 control subjects are listed in Table 1. Compared with the control group, the CAD group had bigger BMI, higher TC levels, higher MMP-9 concentrations, more smokers, and more individuals with hypertension although there was no significant difference in the mean age, sex, TG levels, and prevalence of diabetes mellitus between the CAD and control groups.
Table 1.
General Characteristics of Patients and Controls Included in Our Study
| Subject characteristics | Case (n=258) | Control (n=153) | p |
|---|---|---|---|
| Age (year) | 63.97±12.32 | 63.61±11.8 | >0.05 |
| Male (n/%) | 146/56.59% | 83/54.25% | >0.05 |
| BMI (kg/m2) | 24.390±5.86 | 21.07±4.17 | <0.05 |
| TC (mM) | 5.03±1.12 | 4.42±1.07 | <0.05 |
| TG (mM) | 1.71±1.18 | 1.69±1.01 | >0.05 |
| MMP-9 level (ng/mL) | 174.36±35.09 | 123.73±28.53 | <0.05 |
| Smokers (n/%) | 69/26.74% | 12/7.80% | <0.05 |
| DM (n/%) | 65/25.19% | 38/24.84% | >0.05 |
| Hypertension (n/%) | 146/56.59% | 26/16.99% | <0.05 |
BMI, body mass index; TC, total cholesterol; TG, triglyceride; DM, diabetes mellitus.
Hardy–Weinberg equilibrium
The χ2 goodness-of-fit test showed that the genotypic distributions of rs3918242 and rs17576 did not deviate from the Hardy–Weinberg equilibrium in both the CAD and control groups (p>0.05). The subjects in both groups were representative.
MMP-9 gene polymorphism analysis
For the polymorphism analysis of rs3918242, the size of the amplicon was 662 bp, we saw three genotypes after digested by SphI, cut homozygosity: TT (469 bp, 193 bp), cut heterzygosity: CT (662 bp, 469 bp, 193 bp), and uncut homozygosity: CC (662 bp) (Fig. 1).
FIG. 1.

Enzymolysis electrophoretic of Matrix metalloproteinase-9 (MMP-9) gene rs3918242 polymorphism.
For the polymorphism analysis of rs17576, the size of the amplicon was 300bp, we got three genotypes after digested by MspI, cut homozygosity: GG (170 bp, 130 bp), cut heterzygosity: AG (300 bp, 170 bp, 130 bp), and uncut homozygosity: AA (300 bp) (Fig. 2).
FIG. 2.
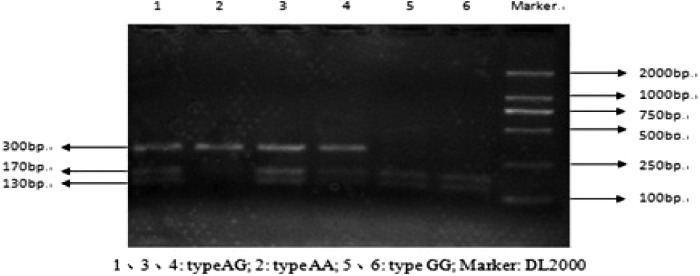
Enzymolysis electrophoretic of MMP-9 gene rs17576 polymorphism.
Allele and genotype analysis
The frequence of the T allele in the MMP-9 promoter was 14.3% in the CAD group compared with 7.2% in the control group. The rs3918242 T allele was associated with CAD (χ2=9.524, p<0.05) and survived 1000 permutations (global p<0.05) (Table 2). Furthermore, while there were no TT homozygotes in the control group, we calculated the CT plus TT genotype, and the frequencies were 25.2% in the CAD group compared with 14.4% in the control group (p<0.05). After being adjusted for confounding factors, the logistic regression test revealed genotypic associations between rs 3918242 and CAD (χ2=6.732, p<0.05) (Table 3). However, the frequencies of the G allele, AG plus GG genotype of rs17576 were similar (p>0.05) (Table 3).
Table 2.
Distribution of Allele Frequencies of Single-Nucleotide Polymorphisms in Case and Control Groups
| SNPs | Group | HWEa | Allele numbers (%) | χ2 | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| C | T | |||||||
| rs3918242 | Case | 0.061 | 442/85.7 | 74/14.3 | 9.524 | 0.002a | 0.463 | 0.281–0.762 |
| Control | 0.875 | 284/92.8 | 22/7.2 | |||||
| A | G | |||||||
| rs17576 | Case | 0.267 | 73/14.1 | 443/85.9 | 3.313 | 0.069 | 1.419 | 0.972–2.072 |
| Control | 0.431 | 58/19.0 | 248/81.0 | |||||
The adjusted p-value was 0.001 from 1,000 permutations.
HWE, Hardy–Weinberg equilibrium; SNP, single-nucleotide polymorphism; OR, odds ratio; CI, confidence interval.
Table 3.
Distribution of Genotype Frequencies of SNPs in Case and Control Groups
| SNPs | Genotype | Case (%) | Control (%) | χ2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| rs3918242 | CC | 193/74.8 | 131/85.6 | ||||
| CT+TT | 65/25.2 | 22/14.4 | 6.732 | 0.009 | 2.005 | 1.178–3.414 | |
| rs17576 | AA | 3/1.2 | 4/2.6 | ||||
| AG+GG | 255/98.8 | 145/97.4 | 0.497 | 0.481 | 2.282 | 0.504–10.335 |
The serum MMP-9 analysis
The total serum level of MMP-9 was higher in the CAD group compared with the control group (174.36±35.09 ng/mL vs. 106.73±28.53 ng/mL, p<0.05) (Fig. 3). The MMP-9 level was still an independent risk for CAD after being adjusted for covariates (χ2=4.183, p<0.05). There were also significant differences in the serum MMP-9 levels between the two groups with and without the SNP in CAD subjects. The subjects carrying T alleles (TT homozygotes and CT heterozygotes) had higher average serum MMP-9 levels compared with CC genotypes (207.10±39.43 ng/mL vs. 158.07±26.96 ng/mL, p<0.05) (Fig. 4). A multiple logistic regression analysis with a forward stepwise selection was also performed. The results revealed that the TC level, history of smoking, hypertension, and the rs3918242 C allele and MMP-9 concentration were independently associated with CAD. The most predictive risk factor for CAD was hypertension (odds ratio [OR]=12.107, 95% confidence interval [CI] 4.180–20.202, p<0.05), followed by the T allele in the MMP-9 promoter (OR=5.036, 95% CI 2.554–8.205, p<0.05).
FIG. 3.
The serum MMP-9 level between the coronary artery disease group and control group.
FIG. 4.
The serum MMP-9 level between the two groups with and without single-nucleotide polymorphisms.
Discussions
CAD is one of the main causes of death in both developed and developing countries. Atherosclerosis is apparently initiated in response to arterial endothelial injury before CAD happens, which allows increased permeability to lipids, monocytes, and lipid-laden macrophages. The MMP-9 secreted from macrophages apparently helps medial smooth muscles to migrate into the intima by degrading the ECM (Robertson et al., 2007). The MMP-9 gene is located on chromosome 20. It encodes the gelatinase enzyme for ECM metabolism. The aim of this study was to investigate the association between the common variants of the MMP-9 gene and CAD, the relationship between the serum level of MMP-9 and CAD and the relationship of SNP and serum level of MMP-9. The selected SNPs are a promoter and an exon, they are useful to find the disease-related variants because they are highly informative based on the CHB dbSNP database.
The present study showed that the promoter genetic variation in the MMP-9 gene was associated with CAD in the Chinese Han population, the frequencies of minor allele T and CT+TT genotype of rs3918242 were significantly higher in case than in control subjects (χ2=9.524, p<0.05; χ2=6.732, p<0.05). The allele frequency of rs3918242 in controls, observed in our study, was similar to that in the HapMap-CHB reference population. Our results also suggest that individuals carrying the minor allele T of rs3918242 may have a higher risk for CAD. This study is in agreement with several prior investigations indicating that genetic variation in the MMP-9 gene may affect risk of CAD. It has been reported that the MMP-9 T allele was associated with increased risk of developing CAD in patients younger than 55 years in the Tehran population of Iran (Saedi et al., 2012). Koh et al. suggested that the rs3918242 polymorphism in the MMP-9 promoter had a significant and independent role in the development of AMI due to plaque rupture in the Korean population (Koh et al., 2008). Zhang et al. performed a study in 374 patients with available angiographic data, the result revealed that the rs3918242 T allele was associated with complicated coronary lesions, 26% of those carrying 1 or 2 copies of the T allele had >50% stenosis in three coronary arteries, whereas only 15% of C/C homozygotes had triple-vessel disease (Zhang et al., 1999a). These results suggested that the MMP-9 gene rs3918242 polymorphism might play an important role in the development of CAD due to ECM degradation. The mechanism whereby the SNP of rs3918242 in the MMP-9 gene contributes to CAD is probably because the SNP exerts an allelic effect on MMP-9 promoter strength, such that the T allelic promoter has a higher transcriptional activity than the C allelic promoter, which is likely to be attributed to binding a transcriptional repressor to the C allele (Zhang et al., 1999a; Morgan et al., 2003).
However, our study did not show any significant association between the genetic variation of rs17576 and CAD. There was no significant difference in the rs17576 genotype frequency between the CAD and control groups (Zhi et al., 2010). Opstad et al. (2012) showed that the reduced endogenous MMP-9 activity was obtained in the patients with stable CAD measured by the fluorometric quantitative assay. Our result was similar to these studies. There was neither an allelic nor genotypic association between rs17576 and CAD (p<0.05) in the Chinese Han population. rs17576 did not show any association with CAD mainly because the replacement from wild type (A) to mutant type (G) and the subsequent amino acid switch from arginine to glutamine induced lower gelatinolytic activity of the zymogen.
Associations between the rs3918242 polymorphism and higher circulating concentrations of the protein have previously been reported (Blankenberg et al., 2003; Yasmin et al., 2006; Gai et al., 2009; Wu et al., 2010). Overexpression of MMP-9 was found in human atherosclerotic plaques and involved in the rupture of the plaques (Brown et al., 1995). In this study, we also study the MMP-9 activity association with CAD and gene polymorphism. We found that the patients in the CAD group had higher levels of MMP-9, and CAD patients who carried the T allele also had a significantly higher MMP-9 activity in the serum than those who carry the C allele. These findings support the hypothesis that connective tissue remodeling after artery imtima damage is mediated by MMP-9, which plays an important role in atherogenesis. MMP-9 is expressed in the shoulder region of the atherosclerotic lesion and is regulated at three levels: transcription, activation of proenzymes, and specific inhibition by endogenous tissue inhibitor of MMPs. The key step is transcriptional regulation because most MMP genes are expressed only when active physiological or pathological tissue remodeling takes place (Matrisian, 1990). This association may be explained by the enhanced ability of vascular SMCs to migrate and proliferate during atherogenesis in individuals carrying the T allele with higher transcriptional activity (Ye, 2000).
Although we got positive results in our study, the association between the MMP-9 gene polymorphism and CAD are still inconsistent in different studies. Therefore, further studies on a larger population in cohort studies will be required to confirm this polymorphism as a potential novel genetic marker for CAD or plaque rupture, and prospective studies are needed to determine whether patients who experience fatal coronary events differ from those who experience nonfatal events.
Conclusions
This randomized clinical trial showed that the SNP rs3918242 in the MMP-9 gene is very likely to be associated with CAD and that high serum levels of MMP-9 are strongly associated with CAD in the Chinese Han population. Genetic variation of rs3918242 may influence MMP-9 expression in T allele carriers. MMP-9 could be a significant risk factor for CAD and might play an important role in CAD involving the development of arteriosclerosis.
Acknowledgments
We thank the participants for their support and participation. This work was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China, and the Department of Science and Technology, Jilin Province, China (grant number 200905184).
Author Disclosure Statement
All authors have no conflict of interests.
References
- Bendeck MP. Zempo N. Clowes AW, et al. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Moore WG. Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Blankenberg S. Rupprecht HJ. Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- Brown DL. Hibbs MS. Kearney M, et al. Identification of 92- kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- Chinese Medical Cardiology Subcommittee, Chinese Editorial Committee of Cardiology Journal. Chinese guidelines for the diagnosis and treatment of patients with unstable angina and non ST-elevation myocardial infarction. Chin J Cardiol. 2007;35:295–304. [PubMed] [Google Scholar]
- Chinese Medical Cardiology Subcommittee, Chinese Editorial Committee of Cardiology Journal. Chinese guidelines for the diagnosis and treatment of patients with ST-elevation myocardial infarction. Chin J Cardiol. 2010;38:675–690. [Google Scholar]
- Gai X. Lan X. Luo Z, et al. Association of MMP-9 gene polymorphisms with atrial fibrillation in hypertensive heart disease patients. Clin Chim Acta. 2009;408:105–109. doi: 10.1016/j.cca.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Galis ZS. Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- Heeneman S. Cleutjens JP. Faber BC, et al. The dynamic extracellular matrix: intervention strategies during heart failure and atherosclerosis. J Pathol. 2003;200:516–525. doi: 10.1002/path.1395. [DOI] [PubMed] [Google Scholar]
- Henney AM. Ye S. Zhang B, et al. Genetic diversity in the matrix metalloproteinase family. Effects on function and disease progression. Ann N Y Acad Sci. 2000;902:27–38. doi: 10.1111/j.1749-6632.2000.tb06298.x. [DOI] [PubMed] [Google Scholar]
- Koh YS. Chang K. Kim PJ, et al. A close relationship between functional polymorphism in the promoter region of matrix metalloproteinase-9 and acute myocardial infarction. Int J Cardiol. 2008;127:430–432. doi: 10.1016/j.ijcard.2007.04.107. [DOI] [PubMed] [Google Scholar]
- Loftus IM. Naylor AR. Goodall S, et al. Increased matrix metalloproteinase-9 activity in unstable carotid plaques: a potential role in acute plaque disruption. Stroke. 2000;31:40–47. doi: 10.1161/01.str.31.1.40. [DOI] [PubMed] [Google Scholar]
- Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Morgan AR. Zhang B. Tapper W, et al. Haplotypic analysis of the MMP-9 gene in relation to coronary artery disease. J Mol Med (Berl) 2003;81:321–326. doi: 10.1007/s00109-003-0441-z. [DOI] [PubMed] [Google Scholar]
- Nanni S. Melandri G. Hanemaaijer R, et al. Matrix metalloproteinases in premature coronary atherosclerosis: influence of inhibitors, inflammation, and genetic polymorphisms. Transl Res. 2007;149:137–144. doi: 10.1016/j.trsl.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- Newby AC. Zaltsman AB. Fibrous cap formation or destruction-the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41:345–360. [PubMed] [Google Scholar]
- Opstad TB. Pettersen AA. Weiss TW, et al. Genetic variation, gene- expression and circulation levels of matrix metalloproteinase-9 in patients with stable coronary artery disease. Clin Chim Acta. 2012;413:113–120. doi: 10.1016/j.cca.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Robertson L. Grip L. Mattsson Hulte L, et al. Release of protein as well as activity of MMP-9 from unstable atherosclerotic plaques during percutaneous coronary intervention. J Intern Med. 2007;262:659–667. doi: 10.1111/j.1365-2796.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- Saedi M. Vaisi-Raygani A. Khaghani S, et al. Matrix metalloproteinase-9 functional promoter polymorphism 1562 C/T increased risk of early-onset coronary artery disease. Mol Biol Rep. 2012;39:555–562. doi: 10.1007/s11033-011-0770-x. [DOI] [PubMed] [Google Scholar]
- Shipley JM. Doyle GA. Fliszar CJ, et al. The structural basis for the elastolytic activity of the 92 and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. J Biol Chem. 1996;271:4335–4341. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- Spurthi KM. Galimudi RK. Srilatha G, et al. Influence of gelatinase B polymorphic variants and its serum levels in atherosclerosis. Genet Test Mol Biomarkers. 2012;16:850–854. doi: 10.1089/gtmb.2011.0299. [DOI] [PubMed] [Google Scholar]
- Visse R. Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Weiss TW. Furenes EB. Trøseid M, et al. Prediction of cardiovascular events by matrix metalloproteinase (MMP)-9 in elderly men. Thromb Haemost. 2010;103:679–681. doi: 10.1160/TH09-05-0317. [DOI] [PubMed] [Google Scholar]
- Wu S. Hsu LA. Teng MS, et al. Association of matrix metalloproteinase 9 genotypes and cardiovascular disease risk factors with serum matrix metalloproteinase 9 concentration in Taiwanese individuals. Clin Chem Lab Med. 2010;48:543–549. doi: 10.1515/CCLM.2010.099. [DOI] [PubMed] [Google Scholar]
- Yasmin McEniery CM. O'Shaughnessy KM, et al. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler Thromb Vasc Biol. 2006;26:1799–1805. doi: 10.1161/01.ATV.0000227717.46157.32. [DOI] [PubMed] [Google Scholar]
- Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000;19:623–629. doi: 10.1016/s0945-053x(00)00102-5. [DOI] [PubMed] [Google Scholar]
- Zhang B. Henney A. Eriksson P, et al. Genetic variation at the matrix metalloproteinase-9 locus on chromosome 20q12.2–13.1. Hum Genet. 1999a;105:418–423. doi: 10.1007/s004390051124. [DOI] [PubMed] [Google Scholar]
- Zhang B. Ye S. Herrmann SM, et al. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation. 1999b;99:1788–1794. doi: 10.1161/01.cir.99.14.1788. [DOI] [PubMed] [Google Scholar]
- Zhi H. Wang H. Ren L, et al. Functional polymorphisms of matrix metalloproteinase-9 and risk of coronary artery disease in a Chinese population. Mol Biol Rep. 2010;37:13–20. doi: 10.1007/s11033-009-9482-x. [DOI] [PubMed] [Google Scholar]




