Abstract
This study aimed to investigate the ability of osteoclasts during bone resorption activities to regulate the differentiation and calcification of osteoblast precursor cells. The bone resorption model was established using in vitro cortical bone slices and mouse RAW264.7 cells, which were differentiated into osteoclasts by stimulation with the receptor activator of nuclear factor-κB ligand and macrophage colony-stimulating factor. Tartrate-resistant acid phosphatase (TRAP) staining, reverse transcriptase–polymerase chain reaction (RT-PCR), and scanning electron microscopy (SEM) were used to detect osteoclast differentiation. The osteoblast precursor cell line MC3T3-E1 was cultured with the bone resorption supernatant (BRS). Involvement of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway in osteogenesis was evaluated by Western blotting, RT-PCR, and ELISA analysis of markers of the early (runt-related transcription factor-2 and alkaline phosphatase) and late (osteocalcin [OCN]) stages of osteogenesis, and Alizarin Red S staining of matrix mineralization. TRAP staining, RT-PCR, and SEM analysis demonstrated the successful establishment of the bone resorption model. Osteoclast BRS effectively increased the differentiation and calcification of MC3T3-E1 cells. Western blot analysis indicated that the BRS enhanced AKT and p-AKT expression levels in MC3T3-E1 cells. Following AKT2 knockdown and treatment with the PI3K/AKT pathway inhibitor LY294002, the expression of OCN in MC3T3-E1 cells was decreased (p<0.05), as was the calcification area (p<0.05). The data obtained in this study indicated that the osteoclast bone resorption medium promoted the differentiation and calcification of MC3T3-E1 cells and that the PI3K/AKT pathway played a role in this process.
Introduction
Periodontitis is a chronic inflammatory disease characterized by alveolar bone loss leading to tooth movement, and ultimately, to tooth loss.1 Reconstruction of the lost bone tissue is the main goal of periodontal treatment. Bone tissue-engineering techniques, including guided tissue regeneration, autografting, and allografting are novel methods currently being evaluated in the clinic that may yield beneficial clinical outcomes. However, these techniques are in their infancy and currently have poor clinical predictability.
Bone tissue-engineering techniques require three key factors: cells, scaffolds, and growth factors (GFs),2 delivery of the latter being the most feasible for use in clinical applications. Several GFs such as the bone morphogenetic protein,2 transforming growth factor (TGF)-β,3 insulin-like growth factor (IGF)-1,3 and platelet-derived growth factor (PDGF),4 have been shown to promote bone regeneration through induction of osteoblast precursor cell differentiation and mineralization. However, since GFs are normally produced by healthy cells, the mechanism by which normal bone tissue formation occurs and the molecular signaling pathways involved remain to be elucidated.
The homeostatic balance of the bone system is based on the communication between osteoblasts and osteoclasts. Bone resorbing osteoclasts are multinucleated cells derived from hematopoietic cells of monocyte/macrophage lineage,5 whereas bone-forming osteoblasts are derived from the mesenchymal lineage.6 Osteoblasts regulate the differentiation of osteoclasts via the plasma membrane-bound receptor activator of nuclear factor-κB ligand (RANKL) and secreted osteoprotegerin.7 However, the influence of osteoclasts on osteoblasts remains controversial. In the transition phase of the bone remodeling cycle, osteoblast precursors are recruited to the resorbed surface followed by differentiation, mineralization, and new bone formation.8 The reason why the resorption of bone by osteoclasts is followed by the differentiation and activity of osteoblasts9 and how osteoblasts are recruited to the resorbed bone surface are still unknown. In 2005, Martin et al. reported the involvement of some GFs (IGF-I and II and TGF-β,3 in particular) released from the bone matrix as a result of osteoclastic bone resorption in this phenomenon, although this theory is still not clear enough to resolve some key issues in the bone remodeling stage. Since the transition phase occurs in a bone resorption microenvironment, we speculate that osteoclasts have the ability to recruit and regulate the osteogenic activity of osteoblasts in this microenvironment. Previous studies on the osteogenic effects of GFs on osteoblasts are far from being comprehensive. Therefore, we established a bone resorption model in vitro for the analysis of the osteogenic effects of the bone resorption supernatant (BRS), which may contain GFs released from the bone matrix and by osteoclasts.
The phosphatidylinositol 3-kinase (PI3K) pathway is an important promoter of cellular growth, metabolism, differentiation, proliferation, survival, and angiogenesis.10 Recently, it has been demonstrated that PI3K and its downstream target AKT serine/threonine kinases play a significant role in the osteoblast differentiation of progenitor cells into mature osteoblasts.11 The AKT family consists of three separate genes (AKT1-3) encoding serine/theronine protein kinases. AKT1 and AKT2 are believed to have specific roles in bone. Furthermore, AKT2 has been shown to be required for osteoblast differentiation.12
In this study, we investigated osteogenic effects of BRS-containing complex GFs produced in the bone resorption microenvironment and the possible involvement of the PI3K/AKT pathway in the osteogenic process by using RNA interference to silence the AKT2 gene in the mouse osteoblastic cell line MC3T3-E1, which has been well studied and has the capacity to form calcified bone tissue in vitro.13
Materials and Methods
Experimental design
We chose the RAW264.7 cell line here as osteoclast precursor cells, which were induced with RANKL and macrophage colony-stimulating factor (M-CSF) and plated on cortical bone slices to establish a bone resorption model in vitro. The culture medium of RAW264.7 cells was then collected to culture MC3T3-E1 cells with or without the PI3K/AKT pathway inhibitor LY294002 (5, 10, and 20 μM) and AKT2 gene knockdown cells. We examined the following parameters: osteoblastic gene expression, osteocalcin (OCN) expression, and cell calcification.
Establishment of in vitro bone resorption model
Details of the in vitro bone resorption model establishment and collection of the BRS are provided in the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/tea).
Cell culture
MC3T3-E1 cells (purchased from Chinese Academy of Sciences cell library) were cultured in α-MEM (Gibco) as described for the culture of RAW264.7 cells. Exponentially growing cells were plated in six-well plates (6×103 cells/well) for reverse transcriptase–polymerase chain reaction (RT-PCR) analysis, in 24-well plates (1×104 cells/well) for OCN detection and in six-well plates (1×104 cells/well) for Alizarin Red S staining and Western blot analysis. After incubation in the regular medium for 24 h, cells were transferred to a medium containing 25% (V/V) BRS. Groups N0, N1, N2, and N3 (negative controls) were incubated in a normal culture medium plus the PI3K/AKT pathway inhibitor LY294002 (5, 10, and 20 μM). Groups S0, S1, S2, and S3 were treated with a medium containing 25% BRS plus LY294002 (5, 10, and 20 μM). Groups SH1, 2, 3, and NS were MC3T3-E1 cells transfected with AKT2 RNAi plasmids AKT2-1, AKT2-2, AKT2-3 and scrambled plasmids, respectively. Group WT referred to untransfected cells.
BRS selection test
The biological activity of the BRS collected on different days (day 5, 7, 10, 12, 14) was evaluated using the OCN ELISA kit (R&D).
RT-PCR evaluation of early stage of cell differentiation
Early stage differentiation of MC3T3-E1 cells collected on days 7 and 14 was evaluated by analysis of alkaline phosphatase (ALP), runt-related transcription factor-2 (RUNX-2) mRNA expression levels using the RT-PCR conditions described previously with the exception of annealing at 51°C for 30 s and extension at 72°C for 30 s for 35 cycles. The primer sets used were ALP (forward: TCAGGGCAATG AGGTCACATC; reverse: CACAATGCCCACGGACTTC), RUNX-2 (forward: TTTAGGGCGCATTCCTCATC; reverse: TGTCCTTGTGGAT TAAAAGGACTTG), β-actin (forward: TGGGTATGGAATC CTGTGGC; reverse: CCAGACAGCACTGTGTTGGC).
AKT2 knockdown
Knockdown of AKT2 (NCBI reference sequence: NM_001110208.1) was achieved by RNA interference. Three pairs of AKT2-specific RNAi oligos were designed and synthesized (Table 1) and used to generate three different AKT2-specific RNAi plasmids, which were transfected into MC3T3-E1 cells. AKT2 mRNA expression was detected by real-time PCR (using a previously described method14) to validate the knockdown effect and to select the most effective AKT2-specific RNAi plasmid. The primer sets used were AKT2 (forward: GCCG CCTGCCATTCTACAACC; reverse: GCCGAGCCTCTGCTTT GGGT), and mouse 18s rRNA (forward: CGGACACGGAC AGGATTGACA; reverse: CCAGACAAATCGCTCCACCAA CTA) as the house keeping gene. MC3T3-E1 cells were then transfected with the most effective AKT2-specific RNAi plasmid. Negative control group cells were transfected with a scrambled plasmid. Relative AKT2 mRNA expression was analyzed by 2ΔCt×106.
Table 1.
Sequences of Three AKT2-Specific RNAi Oligos and Scramble Oligos
| Plasmid | Oligo sequence (5′–3′) |
|---|---|
| AKT2-1 | CCGGGCTCATTCTTATGGAGGAGATCTCGAGATCTCCTCCATAAGAATGAGCTTTTTG |
| AKT2-2 | CCGGGATGGATCTTTCATTGGGTATCTCGAGATACCCAATGAAAGATCCATCTTTTTG |
| AKT2-3 | CCGGTGACCATGAATGACTTCGATTCTCGAGAATCGAAGTCATTCATGGTCATTTTTG |
| Scramble | CCGGTCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCCTTAACCTTAGGTTTTTG |
Underline indicates the AKT2-specific sequence.
Analysis of OCN in culture medium
To assess the terminal differentiation of MC3T3-E1 cells stimulated by the BRS and the link with the PI3K/AKT pathway, OCN secreted into the cell culture medium was determined. Mediums from different groups were collected on day 14. Then, measure the OCN value in the culture medium following the instructions of the osteocalcin ELISA kit (R&D). The cells were lysed in the radio-immunoprecipitation assay buffer (RIPA; Beyotime) and centrifugated at 14,000 rpm for 15 min at 4°C. The concentration of the protein was determined by the BCA protein assay kit (Pierce). The OCN level was normalized against the total protein content.
Analysis of cell calcification
Calcification of MC3T3-E1 cells was assessed by Alizarin Red S staining of matrix mineralization as described previously.15 MC3T3-E1 cells were prepared as previously described, and then cultured with a mineralizing medium (10% fetal bovine serum, 5 mM β-glycerophosphate, and 50 μg/mL ascorbic acid; Sigma-Aldrich) containing 25% BRS. The medium was replaced every 2 days. After 2 weeks, the medium was removed and the cells were washed with phosphate-buffered saline (×3) and fixed in 10% paraformaldehyde (10–30 min, 4°C). Cells were stained in a solution of 0.1% Alizarin Red S/Tris-HCl (pH 8.3) (30 min, 37°C), washed three times with distilled water, and air-dried before being photographed (5 images per well) using a NIS-Elements F2.20 (NikonEclipse 80i). Images were analyzed using Photoshop CS3. The mean percentage of the calcified area was normalized against the total protein content determined as previously described. This experiment was repeated three times.
Western blot analysis
The extraction of protein and determination of the concentration of total protein was the same as described above in the Analysis of OCN in culture medium section. Protein samples (60 μg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membranes (Millipore). Membranes were blocked with 5% skimmed milk (1 h, room temperature) and incubated with primary antibodies (overnight, 4°C). Membranes were then washed (×3, 10 min) and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:5000; Sigma) (room temperature, 1 h). Immunoreactive bands were visualized using the ECL kit (Pierce). Membranes were washed and reprobed with the anti-GAPDH antibody (Santa Cruz) to ensure equal protein loading.
Statistical analysis
Differences between the means of OCN and RT-PCR were calculated using one-way ANOVA plus the Dunnett's T3 test. Analysis of calcified areas was conducted using one-way ANOVA plus the LSD-t multiple comparison test. The significance level was set at p<0.05. Data are expressed as the mean±SD.
Results
Osteogenic effect of osteoclast excreted factors on MC3T3-E1 cells
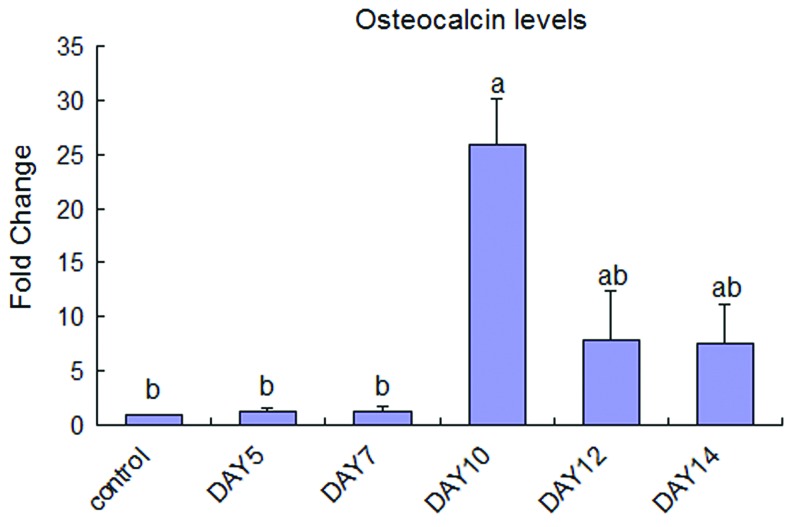
MC3T3-E1 cells were cultured with the BRS collected on days 5, 7, 10, 12, and 14. Increased OCN production was detected in the supernatants collected at day 10, 12, and 14 time-points, with the most significant increase detected on day 10 (p<0.05) (Fig. 1). Therefore, supernatants collected on day 10 were used in all experiments to detect the osteogenic effects of osteoclast secreted factors on MC3T3-E1 cells.
FIG. 1.
Osteocalcin (OCN) levels in MC3T3-E1 cells treated without (control) or with the bone resorption supernatant (BRS) were determined by ELISA. BRS were collected days 5, 7, 10, 12, and 14 (n=6). The OCN level of each group was normalized to the total protein level, and then compared to that of the control (ap<0.05 vs. control; bp<0.05 vs. day 10). Color images available online at www.liebertpub.com/tea
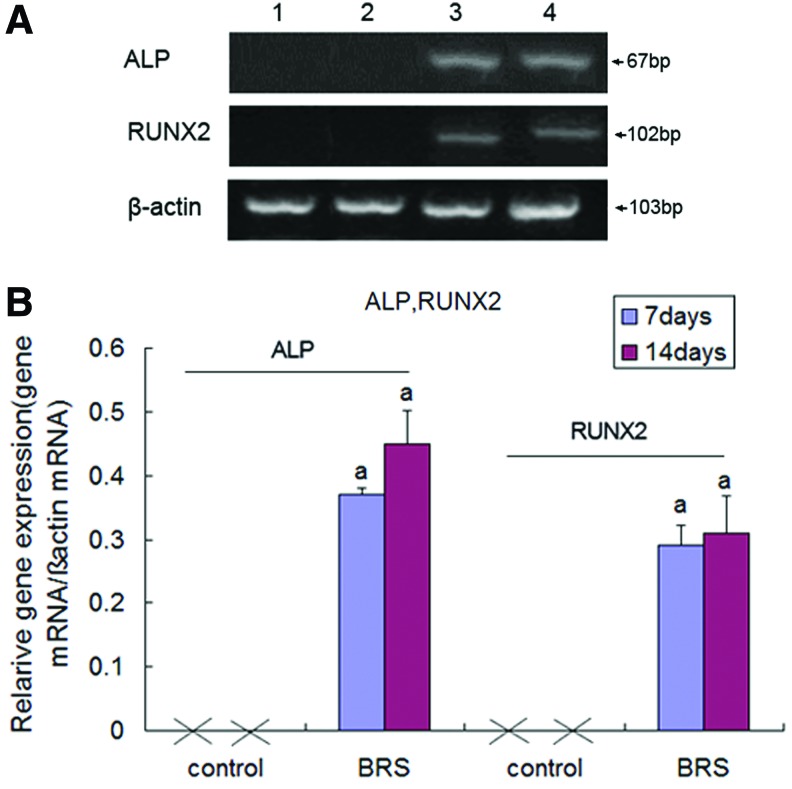
ALP and RUNX-2 mRNA expression
ALP and RUNX-2 are the key genes required for the early differentiation of osteoblasts.16,17 Expression of RUNX-2 and ALP mRNA was not detected in negative controls (MC3T3-E1 cells cultured in α-MEM alone) (Fig. 2A). However, expression of RUNX-2 and ALP mRNA was detected in MC3T3-E1 cells treated with the BRS at days 7 and 14, although there was no difference between the levels detected at these time points (p>0.05) (Fig. 2B).
FIG. 2.
Reverse transcriptase–polymerase chain reaction (RT-PCR) detection of alkaline phosphatase (ALP) and runt-related transcription factor-2 (RUNX-2) expression. (A) Representative images of three repeated experiments. Lane 1: negative control, MC3T3-E1 cells treated without BRS for 7 days; lane 2: negative control, 14 days; lane 3: MC3T3-E1 cells treated with BRS for 7 days; lane 4: 14 days. (B) Densitometric quantification of RUNX-2 and ALP mRNAs expressed as the ratio of β-actin expression in the same sample (ap<0.05 vs. control). Color images available online at www.liebertpub.com/tea
Confirmation of the reduced p-AKT expression in MC3T3-E1 cells mediated by LY294002 and RNAi knockdown
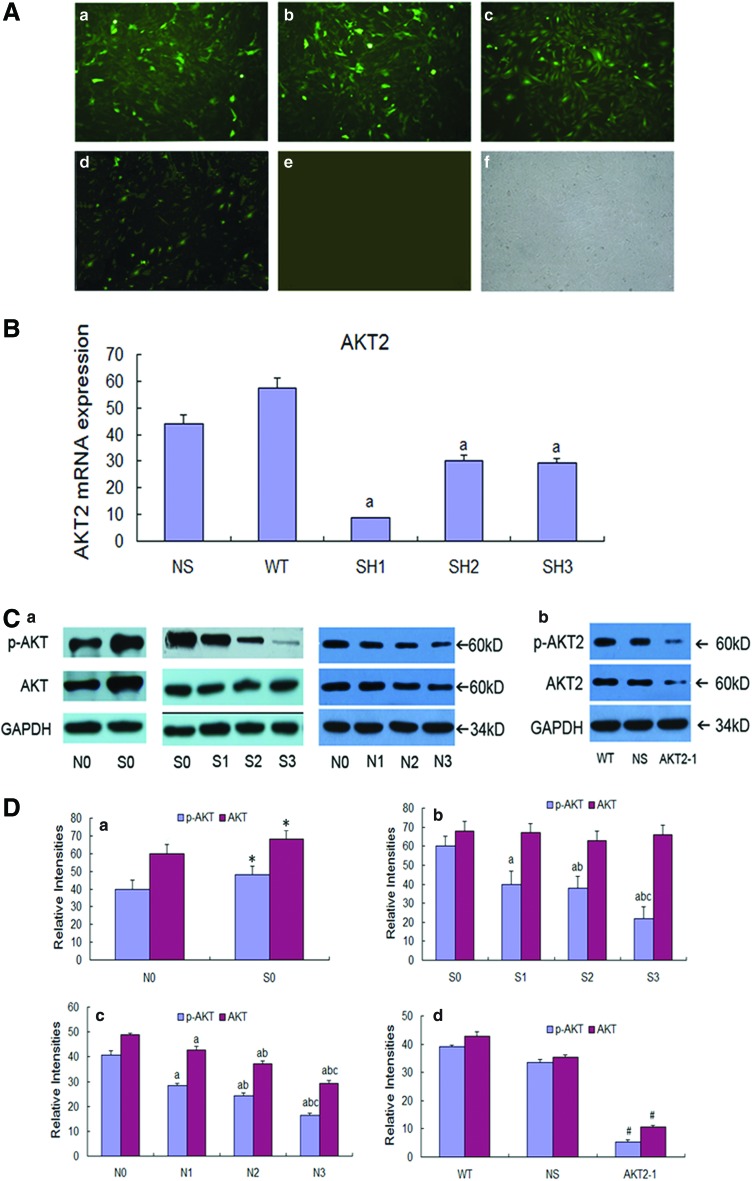
MC3T3-E1 cells were transfected with three different AKT2-specific RNAi plasmids and negative plasmids (Fig. 3A) to analyze the effects of AKT2 knockdown on the osteogenic effects of osteoclasts on osteoblast differentiation. AKT2 mRNA expression in MC3T3-E1 cells transfected with AKT2-1, AKT2-2, AKT2-3, a scrambled plasmid, and in untransfected cells was analyzed by RT-PCR (Fig. 3B). AKT2 mRNA expression was 8.70±0.12, 30.16±2.29, 29.50±1.43, 44.16±3.26, and 57.42±3.58 in each of these groups, respectively. The greatest inhibition was detected following transfection with AKT2-1 (p<0.05) (Fig. 3B). Therefore, AKT2-1 was used in all subsequent experiments.
FIG. 3.
Results of AKT2 gene knockdown in MC3T3-E1 cells. (A) Fluorescence photograph of MC3T3-E1 cells transfected with AKT2 RNAi plasmids. (a) AKT2-1; (b) AKT2-2; (c) AKT2-3; (d) cells transfected with scrambled RNAi; (e) normal MC3T3-E1 cells; (f) normal MC3T3-E1 cells detected under optical microscope. (B) RT-PCR analysis of AKT2 mRNA expression in MC3T3-E1 cells transfected with NS, scrambled plasmids; WT, untransfected cells; AKT2 RNAi plasmids AKT2-1, AKT2-2, and AKT2-3 (ap<0.05 vs. NS). (C) Western blot analysis of p-AKT, AKT, p-AKT2, and AKT2 protein expression in cells. (a) AKT and p-AKT protein expression in MC3T3-E1 cells treated with different concentrations of LY294002 with or without BRS; (b) AKT2 and p-AKT2 expression in AKT2 knockdown MC3T3-E1 cells; NS: scrambled plasmids; WT: untransfected cells. (D) The relative intensity of p-AKT, AKT, p-AKT2, and AKT2 protein in cells. (a) The relative intensity of p-AKT and AKT protein in the N0 and S0 group (*p<0.05 vs. N0). (b) The relative intensity of the p-AKT and AKT protein in the S0, S1, S2, and S3 group (ap<0.05 vs. S0, bp<0.05 vs. S1, cp<0.05 vs. S2). (c) The relative intensity of the p-AKT and AKT protein in the N0, N1, N2, and N3 group (ap<0.05 vs. N0, bp<0.05 vs. N1, c p<0.05 vs. N2). (d) The relative intensity of the p-AKT2 and AKT2 protein in the WT, NS, and AKT2-1 group (#p<0.05 vs. WT). Color images available online at www.liebertpub.com/tea
Western blot analysis showed that the expression of p-AKT in MC3T3-E1 cells was upregulated by treatment with the BRS for 14 days. This effect was inhibited in a dose-dependent manner by LY294002 treatment. BRS and LY294002 treatments used in isolation had no effect on the expression of AKT. Low-level p-AKT2 and AKT2 were detected in AKT2 knockdown cells (Fig. 3C, D)
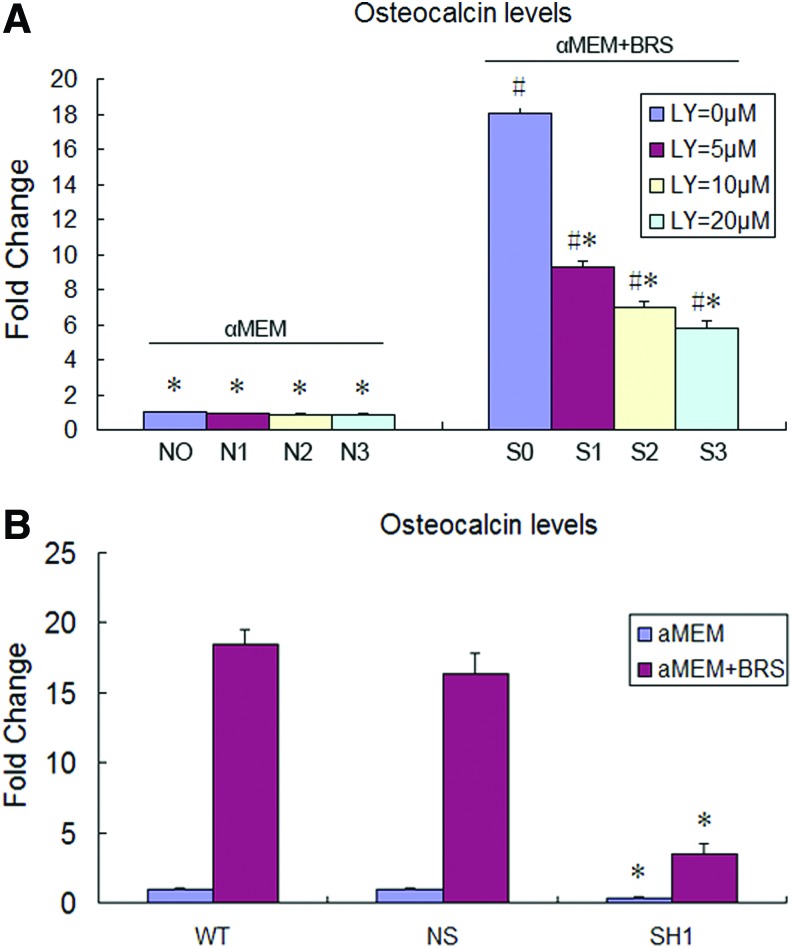
OCN expression
OCN has recently been identified as an osteoblast-secreted hormone suitable for use as an indicator of terminal differentiation of osteoblasts.18 A dose-dependent decrease in OCN was detected following treatment of MC3T3-E1 with the PI3K/AKT-specific inhibitor LY294002 (p<0.05). Furthermore, the lowest OCN expression was detected in AKT2 knockdown cells treated with the BRS (Fig. 4).
FIG. 4.
ELISA analysis of OCN expression. (A) MC3T3-E1 cells treated with the BRS (S) and without BRS (N) in the presence of LY294002 (0, 0 μM; 1, 5 μM; 2, 10 μM; and 3, 20 μM). Values represent the mean±SD (*p<0.05 vs. S0; #p<0.05 vs. N0). (B) MC3T3-E1 cells transfected with AKT2 RNAi plasmids. WT: normal MC3T3-E1 cells; NS: cells transfected with scrambled RNAi; SH1: AKT2-1. Values represent the mean±SD (*p<0.05 vs. NS). Color images available online at www.liebertpub.com/tea
Cell matrix mineralization
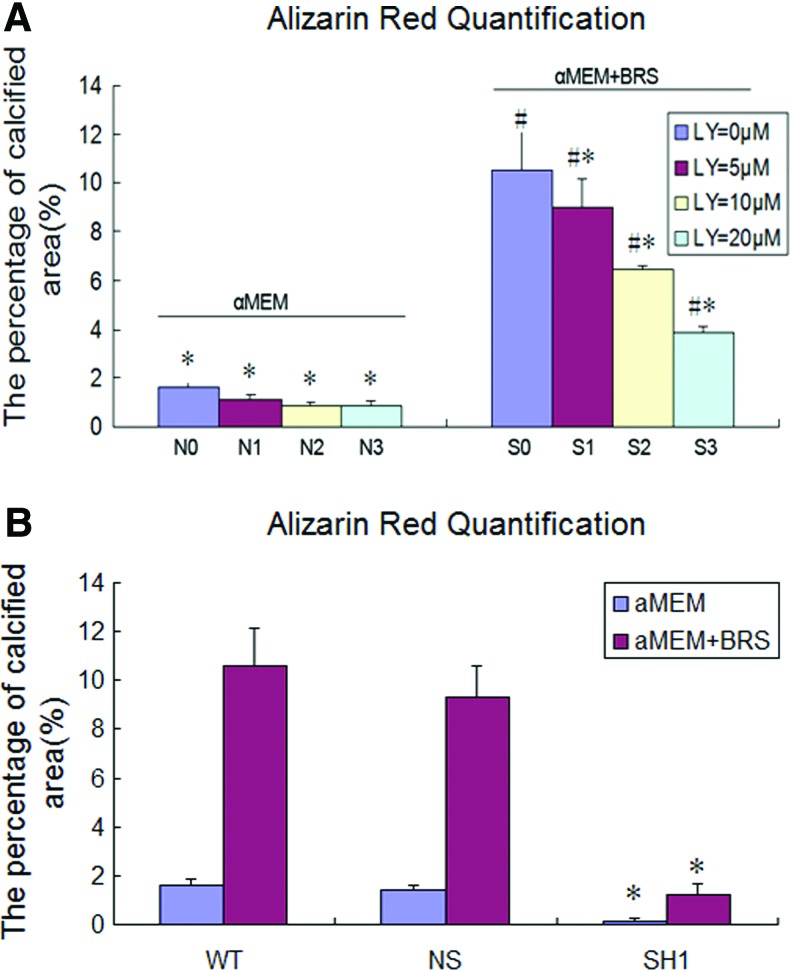
After 14 days in culture, the matrix mineralization level of MC3T3-E1 cells was detected by Alizarin Red S staining. Compared with MC3T3-E1 cells cultured with the BRS (S0), very low levels of calcified deposits were detected in MC3T3-E1 cells cultured in α-MEM alone plus LY294002 (N0-N3). In contrast, a significant and dose-dependent decrease in the calcification area of MC3T3-E1 cells incubated with the BRS was detected following treatment with different concentrations of LY294002 (S0–S3) (p<0.05). Furthermore, the AKT2 knockdown cells almost abolished the ability of MC3T3-E1 cells to generate calcified deposits (Fig. 5).
FIG. 5.
Analysis of matrix mineralization of MC3T3-E1 cells by Alizarin Red S staining. (A) MC3T3-E1 cells treated with the BRS (S) and without BRS (N) in the presence of LY294002 (0, 0 μM; 1, 5 μM; 2, 10 μM; and 3, 20 μM). Values represent the mean±SD (*p<0.05 vs. S0; #p<0.05 vs. N0). (B) MC3T3-E1 cells transfected with AKT2 RNAi plasmids. WT: normal MC3T3-E1 cells; NS: cells transfected with scrambled RNAi; SH1: AKT2-1. Values represent the mean±SD (*p<0.05 vs. NS). Color images available online at www.liebertpub.com/tea
Discussion
Osseous repair in periodontitis and similar bone defects remains a challenge. The repair methods, such as autografting and allografting currently used in the clinic are associated with various limitations. Investigation of the potential of GFs to induce bone repair for the purposes of reconstruction has become a focus of research in the field of bone tissue engineering. For example, IGF-1 and TGF-β, which are released from the bone matrix as a consequence of resorption, are responsible for the events involved in bone formation.3,19,20 Furthermore, it has been observed that osteoclasts release factors that mediate subsequent cellular events when bone resorption ceases.21 For instance, sphingosine 1-phosphate (S1P) is a bioactive phospholipid that can stimulate osteoblast migration and survival22 and the B polypeptide chain PDGF homodimer (PDGF BB) is a potent mitogenic factor that may increase osteoblast proliferation23 as well as suppress the genetic program of osteoblast differentiation and even completely abrogate mineralization in vitro.4
The aim of this study was to investigate the hypothesis that complex GFs produced during bone resorption have the potential to regulate the differentiation and calcification of osteoblast precursor cells in bone resorption activities. In this study, an in vitro bone resorption model analogous to the in vivo process was established based on the culture of mouse RAW264.7 cells with cortical bone slices prepared from fresh bovine femur in the presence of RANKL and M-CSF, which are essential for osteoclastogenesis.24 RAW264.7 cells were induced to differentiate into tartrate-resistant acid phosphatase (TRAP)-positive, multinucleate cells from day 3 following stimulation with RANKL and M-CSF. After 7 days, bone resorption lacunae were detected on the bone cortical slices. Furthermore, positive TRAP staining, detection of the TRAP activity, and the expression of TRAP and CtsK gene expression indicated the successful establishment of a bone resorption model in vitro.
In this study, the expression of ALP, RUNX-2, and OCN were detected as the markers of osteoblast precursor cell differentiation.16–18 RUNX-2, which potently initiates the expression of major bone matrix protein genes, and ALP are markers of the early stage of osteoblast differentiation.16,25 OCN, which was recently identified as an osteoblast-secreted hormone regulating insulin secretion and sensitivity, is a terminal marker of osteoblast differentiation.26 Osteoclast supernatants (BRS) collected on day 10 had the highest OCN biological activity and were used in all subsequent experiments. Expression of ALP and RUNX-2 mRNA was detected in MC3T3-E1 cells cultured in the presence of BRS for 7 days, and this level remained unchanged by 14 days, which is probably because ALP and RUNX-2 are early osteoblast differentiation markers.16,17 These data suggested that the BRS induced MC3T3-E1 cell differentiation at both the early and late stages.
β-glycerophosphate and ascorbic acid are widely used osteodifferentiation factors in vitro.27,28 The former promotes the calcium deposition, while the latter boosts the synthesis of collagen and matrix mineralization of osteoblasts. In this study, Alizarin Red S staining of MC3T3-E1 cells showed the presence of calcified deposits following culture for 7 days, which was more apparent after 14 days, thus indicating that the BRS induced matrix mineralization of MC3T3-E1 cells.
Subsequently, the capacity of the BRS to regulate the osteogenic activity of MC3T3-E1 cells via the PI3K/AKT pathway was investigated. In 2006, Zhao and colleagues demonstrated the effect of bidirectional ephrinB2-EphB4 signaling in osteoclasts and osteoblasts in mouse models.29 They observed that forward signaling through EphB4 enhances osteoblast differentiation.29 We have confirmed upregulation of the EphB4 protein and gene expression in MC3T3-E1 cells treated with the BRS (unpublished data). The ephrin family, of which EphB4 is a member, represents the largest family of receptor tyrosine kinases (RTKs).30 Activation of RTKs by phosphorylation, in turn, activates the PI3K pathway.31 To investigate the role of the PI3K pathway in the effects of BRS on the osteogenic activity of MC3T3-E1 cells, we used the PI3K/AKT inhibitor LY294002 and an RNA interference approach to mediate AKT2-specific knockdown in MC3T3-E1 cells. Western blot confirmed decreased expression of p-AKT in MC3T3-E1 cells after treatment with LY294002 and very low expression of AKT2 and p-AKT2 in AKT2 knockdown cells. The level of the AKT level was not changed, while the BRS was shown to activate p-AKT. These data indicate that the BRS, which increased EphB4 expression in MC3T3-E1 cells, activated the PI3K/AKT pathway, and that this activity was inhibited in the presence of LY294002 or following AKT2 gene knockdown. Similarly, analysis of OCN expression and cell matrix mineralization were also shown to decline. These observations indicate that the PI3K/AKT pathway plays a fundamental role in the differentiation and calcification of MC3T3-E1 cells induced by the BRS. However, other signaling pathways may be associated with the induction of osteoblastic activity by the BRS. RTKs are key regulators of cell-signaling pathways involved in proliferation, differentiation, migration, invasion, and cell death.32 However, RTKs can also activate other signaling pathways such as the MAPK pathway.33 These preliminary data obtained in our study do not define signaling pathways and the specific GFs responsible for the osteogenic activity of the BRS. Thus, future investigations are required to analyze the effective component through mass spectrometry and its relative proportion in the BRS, to advance our knowledge of the potential of GFs for tissue engineering.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 30872884, 81271142, and 81000447), the Science and Technology Department of Zhejiang Province (grant number 2012C33009), and Natural Science Foundation of Zhejiang province (grant number Y2090060).
Disclosure Statement
No competing financial interests exist.
References
- 1.Galli C. Passeri G. Macaluso G.M. FoxOs, Wnts and oxidative stress-induced bone loss: new players in the periodontitis arena? J Periodont Res. 2011;46:397. doi: 10.1111/j.1600-0765.2011.01354.x. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y. Wu G. Zhao J. Wang L. Sun P. Gu Z. rhBMP2/7 heterodimer: anosteoblastogenesis inducer of not higher potency but lower effective concentration compared with rhBMP2 and rhBMP7 homodimers. Tissue Eng PartA. 2010;16:879. doi: 10.1089/ten.TEA.2009.0312. [DOI] [PubMed] [Google Scholar]
- 3.Martin T.J. Sims N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Kubota K. Sakikawa C. Katsumata M. Nakamura T. Wakabayashi K. Platelet-derived growth factor BB secreted from osteoclasts acts as anosteoblastogenesis inhibitory factor. J Bone Miner Res. 2002;17:257. doi: 10.1359/jbmr.2002.17.2.257. [DOI] [PubMed] [Google Scholar]
- 5.Ash P. Loutit J.F. Townsend K.M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980;283:669. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- 6.Manolagas S.C. Jilka R.L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 7.Silva I. Branco J.C. Rank/Rankl/opg: literature review. Acta Reumatol Port. 2011;36:209. [PubMed] [Google Scholar]
- 8.Matsuo K. Irie N. Osteoclasts–osteoblasts communication. Arch Biochem Biophys. 2008;473:201. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Hattner R. Frost H.M. Fluorescence of tetracyclines in bone. Absorption maximum, hydration shell and polarization effects. J Surg Res. 1962;2:262. doi: 10.1016/s0022-4804(62)80019-5. [DOI] [PubMed] [Google Scholar]
- 10.Naumann R.W. The role of the phosphatidylinositol 3-kinkinase (PI3K) pathway in the development and treatment of uterine cancer. Gynecol Oncol. 2011;123:411. doi: 10.1016/j.ygyno.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh-Choudhury N. Abboud S.L. Nishimura R. Celeste A. Mahimainathan L. Choudhury G.G. Requirement of BMP-2-induced phosphatidylinositol 3-kinase andAkt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee A. Wilson E.M. Rotwein P. Selective signaling by Akt2 promotes bone morphogenetic protein2-mediated osteoblast differentiation. Mol Cell Biol. 2010;30:1018. doi: 10.1128/MCB.01401-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudo H. Kodama H.A. Amagai Y. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moffat J. Grueneberg D.A. Yang X. Kim S.Y. Kloepfer A.M. Hinkle G. Piqani B. Eisenhaure T.M. Luo B. Grenier J.K. Carpenter A.E. Foo S.Y. Stewart S.A. Stockwell B.R. Hacohen N. Hahn W.C. Lander E.S. Sabatini D.M. Root D.E. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Stanford C.M. Jacobson P.A. Eanes E.D. Lembke L.A. Midura R.J. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP) J Biol Chem. 1995;270:9420. doi: 10.1074/jbc.270.16.9420. [DOI] [PubMed] [Google Scholar]
- 16.Hoemann C.D. El-Gabalawy H. McKee M.D. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol. 2009;57:318. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Nakano-Kurimoto R. Ikeda K. Uraoka M. Nakagawa Y. Yutaka K. Koide M. Takahashi T. Matoba S. Yamada H. Okigaki M. Matsubara H. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiol Heart Circ Physiol. 2009;297:H1673. doi: 10.1152/ajpheart.00455.2009. [DOI] [PubMed] [Google Scholar]
- 18.Ferron M. Wei J. Yoshizawa T. Ducy P. Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem Biophys Res. 2010;397:691. doi: 10.1016/j.bbrc.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y. Nishida S. Elalieh H.Z. Long R.K. Halloran B.P. Bikle D.D. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21:1350. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rici R.E. Alcântara D. Fratini P. Wenceslau C.V. Ambrósio C.E. Miglino M.A. Maria D.A. Mesenchymal stem cells with rhBMP-2 inhibits the growth of canine osteosarcoma cells. BMC Vet Res. 2012;22:8. doi: 10.1186/1746-6148-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu J. Kim H.J. Chang E.J. Huang H. Banno Y. Kim H.H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sefcik L.S. Petrie Aronin C.E. Wieghaus K.A. Botchwey E.A. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29:2869. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrotra M. Krane S.M. Walters K. Pilbeam C. Differential regulation of platelet-derived growth factor stimulated migration and proliferation in osteoblastic cells. J Cell Biochem. 2004;93:741. doi: 10.1002/jcb.20138. [DOI] [PubMed] [Google Scholar]
- 24.Yao G.Q. Wu J.J. Sun B.H. Troiano N. Mitnick M.A. Insogna K. The cell surface form of colony-stimulating factor-1 is biologically active in bone in vivo. Endocrinology1. 2003;44:3677. doi: 10.1210/en.2002-221071. [DOI] [PubMed] [Google Scholar]
- 25.Burton D.G. Matsubara H. Ikeda K. Pathophysiology of vascular calcification: pivotal role of cellular senescence in vascular smooth muscle cells. Exp Geronto. 2010;l45:819. doi: 10.1016/j.exger.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Mundy G.R. Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126:441. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Anderson R.E. Kemp J.W. Jee W.S. Woodbury D.M. Ion transporting ATP-ase and matrix mineralization in cultured osteoblast-precursor cells. In Vitro. 1984;20:837. doi: 10.1007/BF02619629. [DOI] [PubMed] [Google Scholar]
- 28.Maniatopoulos C. Sodek J. Melcher A.H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 29.Zhao C. Irie N. Takada Y. Shimoda K. Miyamoto T. Nishiwaki T. Suda T. Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell S.A. Danca M.D. Blomgren P.A. Darrow J.W. Currie K.S. Kropf J.E. Lee S.H. Gallion S.L. Xiong J.M. Pippin D.A. DeSimone R.W. Brittelli D.R. Eustice D.C. Bourret A. Hill-Drzewi M. Maciejewski P.M. Elkin L.L. Imidazo[1,2-a]pyrazine diaryl ureas: inhibitors of the receptor tyrosine kinase EphB4. Bioorg Med Chem Lett. 2009;19:6991. doi: 10.1016/j.bmcl.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Blume-Jensen P. Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard S.R. Autoinhibitory mechanisms in receptor tyrosine kinases. Front Biosci. 2002;7:330. doi: 10.2741/A778. [DOI] [PubMed] [Google Scholar]
- 33.Moritz A. Li Y. Guo A. Villén J. Wang Y. MacNeill J. Kornhauser J. Sprott K. Zhou J. Possemato A. Ren J.M. Hornbeck P. Cantley L.C. Gygi S.P. Rush J. Comb M.J. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;24:3. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.