Abstract
Repair of peripheral nerve defects with current synthetic, tubular nerve conduits generally shows inferior recovery when compared with using nerve autografts, the current gold standard. We tested the ability of composite collagen and hyaluronan hydrogels, with and without the nerve growth factor (NGF), to stimulate neurite extension on a promising aligned, nanofiber poly-L-lactide-co-caprolactone (PLCL) scaffold. In vitro, the hydrogels significantly increased neurite extension from dorsal root ganglia explants. Consistent with these results, the addition of hydrogels as luminal fillers within aligned, nanofiber tubular PLCL conduits led to improved sensory function compared to autograft repair in a critical-size defect in the sciatic nerve in a rat model. Sensory recovery was assessed 3 and 12 weeks after repair using a withdrawal assay from thermal stimulation. The addition of hydrogel did not enhance recovery of motor function in the rat model. The NGF led to dose-dependent improvements in neurite out-growth in vitro, but did not have a significant effect in vivo. In summary, composite collagen/hyaluronan hydrogels enhanced sensory neurite outgrowth in vitro and sensory recovery in vivo. The use of such hydrogels as luminal fillers for tubular nerve conduits may therefore be useful in assisting restoration of protective sensation following peripheral nerve injury.
Introduction
Gap injuries in peripheral nerves are currently repaired with sensory nerve autograft (the gold standard), processed nerve allograft, or tubular conduits manufactured from polyvinyl alcohol, porcine small intestinal submucosa, type I collagen, polyglycolic acid, or poly (DL-lactide-ɛ-caprolactone).1–5 Due to the limitations of these options, multiple synthetic tubular conduits have been developed for segmental peripheral nerve repair. Many of these grafts have been evaluated in rat sciatic nerve injury models, with different conduits stimulating varying levels of functional recovery.3,6,7 We previously demonstrated that a poly(L-lactide-co-caprolactone) (PLCL) conduit with a longitudinally aligned, nanofibrous luminal structure supported muscle mass and isometric force recovery that approached that of autograft in a rat model.8 This aligned graft also enhanced recovery of nerve conduction velocity and compound motor action potential (CMAP) amplitude compared to randomly aligned controls.9 Likewise, in vitro, dorsal root ganglia (DRG) demonstrate longer bidirectional neurite extension when cultured on aligned versus randomly oriented polylactide, polycaprolactone, polyglycolic acid, agarose, or silk fibroin scaffolds,10–14 suggesting that longitudinal alignment improves axonal growth irrespective of scaffold material.
When used as luminal fillers within tubular nerve conduits, native nerve extracellular matrix (ECM) components might enhance functional recovery by supporting axonal outgrowth. For example, in vitro and in vivo studies have shown that collagen and hyaluronan, two constituents of the neuronal ECM, improve neuronal sprouting after traumatic injury. Many studies have verified the potency of collagen to support neurite growth in conjunction with synthetic nerve conduits.15–20 Isolated sensory neurons showed a significantly better axonal guidance on a collagen/poly-ɛ-caprolactone (PCL) material than on a PCL scaffold alone.21 Hyaluronic acid is a ubiquitous ECM component that enhances peripheral nerve regeneration in vitro and in vivo by preventing the formation of scars at the repair site and by creating a hydrated open lattice at the injury site to facilitate the regeneration of axons.22,23 When Schwann cells are encapsulated in interpenetrating polymer network hydrogels of collagen and hyaluronic acid, they not only spread and proliferate, but they also secrete the nerve growth factor (NGF) and brain-derived neurotrophic factor.24,25 Intraluminal delivery of the NGF within synthetic conduits has previously been shown to improve axonal regeneration of gap injuries in rat sciatic nerves by improving sensory neuron fiber density, myelination, and Schwann cell migration.12,26,27 Therefore, in addition to directly supporting axonal regeneration, ECM luminal fillers might also be used as carriers to deliver bioactive molecules.
We tested the ability of four different hydrogels made of collagen with and without unmodified hyaluronan to promote in vitro neurite outgrowth from murine DRG explants cultured on an aligned PLCL nanofiber scaffold. We previously assessed this aligned scaffold in the form of a tubular conduit for repair of sciatic nerve defects in a rat model.9 Although hyaluronan is frequently crosslinked in tissue-engineering applications to tune its properties, we chose to use unmodified hyaluronan to avoid potential steric hindrance, toxicity, and decreased biodegradability resulting from chemical substitution. Since hyaluronan forms a semi-interpenetrating complex with collagen,28 we used a composite hydrogel of these two molecules. We also assessed whether this hydrogel could act as a carrier for the NGF in vitro and in vivo. For the latter case, this hydrogel, either with or without the NGF, was used as a luminal filler within tubular PLCL conduits, which were used to repair rat sciatic nerve defects. Regeneration was assessed using motor and sensory outcomes. Nerve histomorphometry was also performed.
Materials and Methods
In vitro hydrogel preparation
Four types of hydrogel were prepared from rat-tail type I collagen (BD, Bedford, MA) and sodium hyaluronate (Supartz; Smith & Nephew, Memphis, TN): 2 mg/mL collagen (2C), 4 mg/mL collagen (4C), 2 mg/mL collagen with 2 mg/mL hyaluronan (2C+2H), and 4 mg/mL collagen with 4 mg/mL hyaluronan (4C+4H). The lyophilized recombinant human NGF (PeproTech, Rocky Hill, NJ) was reconstituted in 0.1% bovine serum albumin to generate a 100 μg/mL stock solution and added to media (control) or 4C+4H hydrogel to achieve concentrations of 0, 10, 20, 50, 100, and 500 ng/mL NGF. Ice-cold components were combined in the following order: 1 M phosphate-buffered saline (PBS), 1 N NaOH, water, NGF, sodium hyaluronate, and collagen.
NGF release from the collagen-hyaluronan hydrogel
The 4C+4H hydrogel containing 100 ng/mL of the NGF was prepared as described above. Fifty microliters of hydrogel (containing 5 ng of NGF) was pipetted into each of 10 individual wells of a 48-well plate (PeproTech, Rocky Hill, NJ). After gelation at 37°C, the hydrogel was covered with 200 μL of Dulbecco's modified Eagle medium/F-12 media (Mediatech, Grand Island, NY) with 10% fetal bovine serum (Axenia BioLogix, Dixon, CA), penicillin/streptomycin, and amphotericin B (UCSF Cell Culture Facility, San Francisco, CA). The plate was kept in a 37°C/5% CO2 incubator. Media were collected and a new medium was added to each well after day 1, 2, 3, 4, 7, 9, 12, 15, and 20. The collected media were frozen at −20°C. After collection of the media at day 20, the remaining hydrogel was dispersed in 200 μL of a fresh medium. The amount of NGF in each of the collected samples was determined using a direct enzyme-linked immunosorbent assay (ELISA) kit (PeproTech, Rocky Hill, NJ).
DRG harvest and culture
All animal procedures were approved by the Institutional Animal Care and Use Committee. DRG explants were harvested from 3–4-day-old CD1 mice (Charles River Laboratories, Wilmington, MA). Each animal was euthanized by exposure to inhalational isoflurane (4.5% in air; Baxter Healthcare Corporation, Deerfield, IL) until respiration was absent for 5 min followed by decapitation. The spinal cord was exposed using an anterior approach and the vertebral bodies were removed en bloc. Individual explants were trimmed of nerve roots and connective tissue and placed on the longitudinally aligned, nanofibrous surface of a 9×9-mm square piece of the previously described PLCL scaffold (NanoNerve, Berkeley, CA). Five explants per scaffold and six scaffolds in total were evaluated per condition. Scaffolds were placed in the wells of a 24-well cell culture plate and covered with 1 mL of Gibco Neurobasal media plus B27 supplement, and l-glutamine (Invitrogen, Carlsbad, CA), penicillin/streptomycin, and amphotericin B (UCSF Cell Culture Facility, San Francisco, CA) and allowed to recover in a 37°C/5% CO2 incubator for 2.5 h. After recovery, the medium was removed, 100 μL of hydrogel was added to each scaffold, and the plate was returned to the incubator to allow gelation. After gelation, media were layered onto the specimens, and explants were cultured for 3 days in a 37°C/5% CO2 incubator. We chose to evaluate DRG outgrowth after 3 days for two reasons: (1) three days provided the maximum time for neurite growth before the different conditions became too dense to allow differentiation among experimental groups and (2) after 3 days, the neurites grew off of the scaffold, making it almost impossible to measure maximum neurite lengths.
Immunofluorescence, imaging, and neurite length
The scaffolds/explants were fixed in buffered zinc formalin (Anatech, Battle Creek, MI), permeabilized with 0.2% Triton X-100, blocked with 5% nonfat milk in PBS, and incubated with a neuronal-specific mouse monoclonal anti-β-III tubulin antibody (TUJ1; Covance, Berkeley, CA), followed by the Alexa Fluor 488 goat anti-mouse IgG antibody (Invitrogen, Carslbad, CA). Scaffolds were mounted on glass slides and fluorescent images were taken using a lens with a 2.5×magnification at 15.1 megapixel resolution (Zeiss Akioskop 2; Canon EOS Rebel T1i). The neurite length was used as the primary outcome for assessing DRG explant growth and was measured using previously validated methods.12,16,29,30 The neurite length was calculated as an average of the 10 longest neurites from a total of the 20 longest neurites from each explant. The neurite length was measured using ImageJ software (NIH, Bethesda, MD) and was defined as the straight line distance from the tip of the neurite to the DRG body.
Preparation of hydrogel-filled conduits for rat sciatic nerve injury and repair
Aligned nanofiber tubular conduits with 12-mm length×1.1-mm internal diameter and 200-μm wall thickness,9 were filled with 4C+4H hydrogel, with or without 100 ng/mL NGF and incubated at 37°C for 1 h to allow gelation. Thirty-six female Lewis rats weighing 200–225 grams (Charles River Laboratories, Wilmington, MA) were randomized to receive one of four nerve grafts: reversed sciatic nerve autograft (n=9), empty PLCL conduit (n=9), PLCL conduit filled with 4C+4H (n=9), and PLCL conduit filled with 4C+4H/NGF (n=9). Sciatic nerve resections and repair were performed as described previously.8 Briefly, rats were anesthetized, the sciatic trunk was exposed, and a 10-mm segment of nerve was resected. Given the size of these animals, this is the longest length of nerve that can be resected and repaired. The autograft and conduit grafts were sewn into place using 8–0 nylon sutures. The muscle and fascia were closed in layers. After surgery, rats were pair-caged and housed on a 12-h light/12-h dark cycle with free access to food and water. Analgesic (buprenorphine) was provided to the animals as needed.
Sensory testing
A single, blinded investigator evaluated sensory function recovery at postoperative weeks 3 and 12 by measuring responses to thermal hyperalgesia on the operated and contralateral hind paws.31,32 Each paw was immersed in a 46°C water bath until signs of discomfort were observed, with a limit of 15 s, and time was recorded. Each rat was tested twice with a 10-min interval between tests and a 5-min interval between contralateral paws.
Electrophysiology analysis and gastrocnemius maximum isometric tetanic force
Twelve weeks after surgery, rats were re-anesthetized and the sciatic nerve was re-exposed. Bilateral nerve conduction latencies and CMAP amplitudes of the gastrocnemius muscle were recorded, and gastrocnemius muscle mechanical force testing was performed as previously described.8
Tissue collection and nerve histomorphometry
After muscle mechanical testing, rats were sacrificed and gastrocnemius muscles were harvested and weighed. The nerve grafts were removed, fixed overnight, stained with 2% osmium tetroxide (Sigma-Aldrich, Milwaukee, WI), and sectioned in 7 μm as previously described.8 For each graft, the total number of myelinated axons in the midgraft was counted by at least two investigators (S.L., S.J., R.L.) and the mean coefficient of variation was found to be 2.1%. The axon diameters in a random sample of 10% of each cross section were measured using ImageJ software, which would result in a representative sample.33–35
Statistical analysis
All in vitro conditions were replicated three times independently except for the conditions with the NGF in media (one trial only). For in vivo testing, we chose muscle tetanic force as our primary outcome. Assuming a standard deviation of 0.10, we calculated that a sample size of nine rats in each group was required to detect a 12% difference (alpha=0.05, beta=0.80) in tetanic force recovery. For data analysis, we used restricted maximum likelihood methods for ANOVA with treatment as a fixed factor and trial as a random factor. All results are reported as means or least square means (for experiments with multiple independent replicates)±standard errors. To account for multiple comparisons, the Tukey's honestly significant difference test was used for all pairwise comparisons between conditions, with a statistically significant threshold of p<0.05. For analysis of the sensory tests, paired t-tests were performed to detect significant differences (p<0.05) in withdrawal time between control and operated sides at each time point. All analyses were performed with JMP 10 (SAS Institute, Inc., Cary, NC).
Results
Kinetics of NGF release from collagen-hyaluronan hydrogel
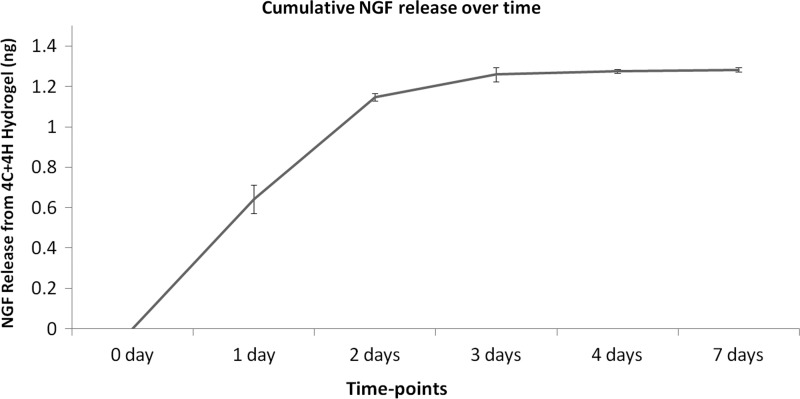
To determine the kinetics of NGF release, we loaded a hydrogel consisting of 4 mg/mL collagen and 4 mg/mL hyaluronan (4C+4H) with 100 ng/mL of the NGF. NGF release from this hydrogel was assessed over 20 days of incubation with media at 37°C. ELISA for the NGF was used to determine the amount of growth factor released into the media as well as the amount remaining in the hydrogel at the conclusion of the 20-day incubation. On average, a total of 1.3 ng of the 5 ng of NGF that was initially loaded into the hydrogel was recovered from each sample. Twenty percent (1 ng) of the initially loaded NGF was released from the hydrogel into the media over the first 3 days of incubation. An additional 6% (0.3 ng) of the loaded NGF was recovered from day 4 until day 7. After day 7, the additional NGF was not detectable either in the incubation media or in the remaining hydrogel (Fig. 1).
FIG. 1.
Cumulative release of nerve growth factor (NGF) from 4C+4H hydrogel. Amount of NGF release per time point is graphed. Means±SEM are shown. On average, a total of 1.3 ng of the 5 ng of NGF that was initially loaded into the hydrogel was recovered from each sample. 20% of the initially loaded NGF was released from the hydrogel into the media over the first 3 days of incubation. As evident from the graph, NGF showed burst release kinetics. An additional 6% (0.3 ng) of the loaded NGF was recovered from day 4 until day 7. After day 7, additional NGF was not detectable in the incubation media.
In vitro neurite outgrowth
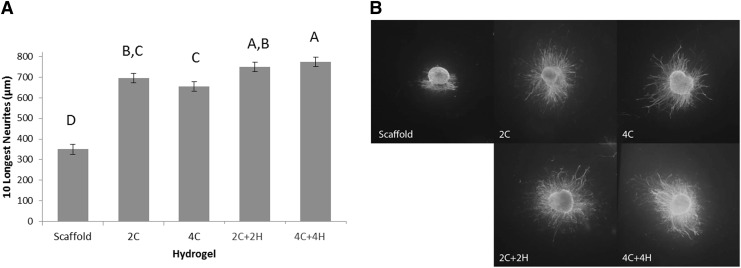
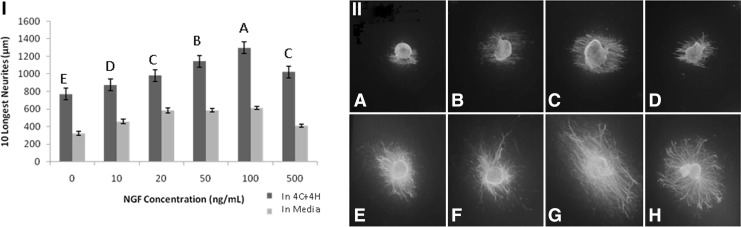
DRGs were cultured on aligned nanofibrous scaffolds at 37°C for 3 days in both media and hydrogel. Neurite outgrowth occurred in all directions in both media and hydrogel. However, the longest neurites always grew parallel to the longitudinally aligned nanofiber scaffold. Explants embedded in each of the four hydrogels showed greater neurite extension than explants cultured in media alone (p<0.0001, Fig. 2). Both of the hyaluronan-containing hydrogels (2C+2H and 4C+4H) produced longer neurite growth than the 4C hydrogel (p<0.0024), but only 4C+4H showed longer neurite growth compared to 2C (p=0.027). There was no difference in neurite growth on 2C and 4C (p=0.5). While there was not a statistically significant difference (p=0.86) between the two hyaluronan-containing hydrogels, we chose the 4C+4H hydrogel for further evaluation with the NGF because it produced the longest neurite outgrowth. The NGF stimulated dose-dependent neurite outgrowth when delivered in either the 4C+4H hydrogel or media alone (Fig. 3). From 0 to 100 ng/mL of NGF, each ascending concentration produced longer neurite growth in hydrogel (p<0.015). At 500 ng/mL of NGF, neurite outgrowth was equivalent to 20 ng/mL of the NGF and less than that with the 50 and 100 ng/mL NGF concentrations. Neurite growth was significantly lower for the NGF in media compared to hydrogel (p<0.0001 for all concentrations).
FIG. 2.
Effect of hydrogels on neurite outgrowth from dorsal root ganglia (DRG) explants. (A) Sum of the lengths of the 10 longest neurites from murine DRGs grown under the indicated conditions. DRGs were cultured on aligned nanofiber scaffolds in all cases. The scaffold group was a control grown in media alone without hydrogel. Hydrogels evaluated include 2 mg/mL type I collagen (2C), 4 mg/mL collagen (4C), 2 mg/mL collagen with 2 mg/mL hyaluronan (2C+2H), and 4 mg/mL collagen with 4 mg/mL hyaluronan (4C+4H). Least square means±SEM are shown. Different letters indicate that groups had significantly different neurite lengths (p<0.05). (B) Representative immunofluorescence images: DRG explants cultured on aligned nanofiber scaffold alone or with addition of the indicated hydrogels.
FIG. 3.
Effect of NGF on neurite outgrowth from DRG explants. (I) Sum of the lengths of the 10 longest neurites from murine DRGs grown on aligned nanofiber scaffolds in media (light bars) or 4C+4H hydrogel (dark bars) with the indicated concentrations of NGF. Least square means±SEM are shown. At all NGF concentrations, neurite outgrowth was significantly greater for DRGs grown in hydrogel compared to media (p<0.0001). Different letters over the hydrogel groups indicate significant differences in neurite lengths (p<0.05). (II) Representative immunofluorescence images: DRG explants cultured on aligned nanofiber scaffolds with NGF. A–D show NGF delivery in media (A: 0 ng/mL; B: 20 ng/mL; C: 100 ng/mL; D: 500 ng/mL). E–H show NGF delivery in 4C+4H hydrogel (E: 0 ng/mL; F: 20 ng/mL; G: 100 ng/mL; H: 500 ng/mL).
In vivo function recovery
Mean body weight in all rats was 213±2 g at the time of surgery and all rats gained weight steadily with a final mean weight of 245±2 g. There were no significant differences among groups at either time. At surgical re-exposure, all autograft and conduit repairs grossly showed intact bridging of the original nerve gap.
Gastrocnemius maximum isometric tetanic force and gastrocnemius muscle mass
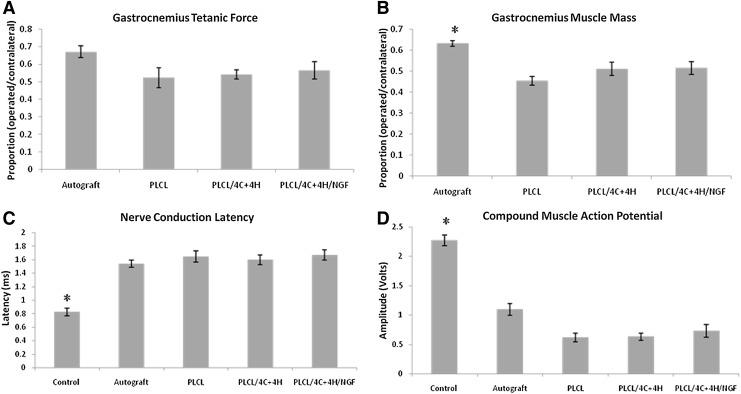
Tetanic force and muscle mass outcomes are reported as proportions between operated/contralateral sides. There was no significant difference among the groups for maximum isometric tetanic force. The percent force recovery was 67%±3% for autograft, 57%±5% for PLCL/4C+4H/NGF, 54%±3% for PLCL/4C+4H, and 52%±6% for the empty PLCL conduit (p=0.0866). There was a significant difference between autograft and the conduit groups for muscle mass (p<0.0001). The wet weight of the gastrocnemius muscles were 63%±1% for autograft, compared to 51%±3% for both PLCL/4C+4H and PLCL/4C+4H/NGF groups, and 45%±2% for the empty PLCL conduit. There were no significant differences in muscle mass among the conduit groups. (Fig. 4)
FIG. 4.
Evaluation of muscle and electrophysiologic recovery. (A) Proportion of gastrocnemius isometric tetanic force in the operated limb compared to the contralateral side. No statistically significant differences were found among the groups. (B) Proportion of gastrocnemius muscle mass in the operated limb compared to the contralateral side. Animals repaired with autograft had significantly (*p<0.0001) greater muscle mass than those repaired with any conduit. (C) Nerve conduction latency. Conduction was significantly faster in normal, control nerves compared to the four operative groups (*p<0.0001). There were no statistically significant differences among the operated groups. (D) Compound Muscle Action Potential. The CMAP amplitudes of the operative legs were significantly smaller than the control legs for all groups (*p<0.0001). There were no statistically significant differences among the operated groups. Least square means±SEM are shown for A and B. Means±SEM are shown for C and D. Poly-L-lactide-co-caprolactone (PLCL) indicates animals repaired with empty conduit.
Electrophysiologic studies
There was no statistically significant difference in the CMAP amplitude among the four groups, although values were highest in the autograft group: 1.09±0.1 V for autograft, 0.73±0.11 V for PLCL/4C+4H/NGF, 0.63±0.06 V for PLCL/4C+4H, and 0.62±0.07 V for PLCL (p<0.14). Amplitudes in the operated limb in all four groups were smaller than amplitudes in the contralateral, unoperated limbs, which served as internal controls (2.27±0.1 V, p<0.0001). Nerve conduction latency was 1.54±0.05 ms for autograft, 1.60±0.07 ms for PLCL/4C+4H, 1.65±0.08 ms for PLCL, and 1.67±0.07 ms for PLCL/4C+4H/NGF. All four were slower than normal nerve (0.82±0.05 ms, p<0.0001), but there was no statistically significant difference in nerve conduction latency among the four groups (p=0.6, Fig. 4).
Sensory results
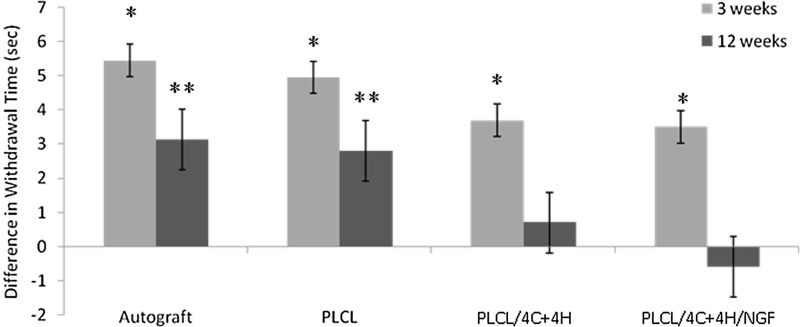
At 3 weeks, the control limbs withdrew faster from thermal stimuli than the operated limbs for all treatment groups. At 12 weeks, the withdrawal time of the operated limbs was statistically equivalent to the contralateral limbs for the PLCL/4C+4H group (p=0.47) and for the PLCL/4C+4H/NGF group (p=0.31). However, the operated limbs of the autograft (p=0.004) and PLCL (p=0.021) groups still withdrew significantly slower than the corresponding unoperated limbs (Fig. 5).
FIG. 5.
Sensory Recovery. Difference in withdrawal time from a 46°C water bath between operated and control limbs. At 3 weeks, the control limbs withdrew significantly faster than the operated limbs for all four groups (*p<0.0208). At 12 weeks, the operated limbs withdrew significantly slower than the control limbs for the autograft and PLCL (empty conduit) groups (**p<0.021). The withdrawal times for the operated limbs of the two hydrogel groups were statistically equivalent to the control limbs at 12 weeks. Means±SEM are shown.
Nerve histomorphometry
In all groups, axons regenerated through the graft and into the distal sciatic nerve. There were significantly more axons in the autograft group compared to the PLCL group (6892±316 axons for autograft and 4690±398 for PLCL; p=0.0034). There was no significant difference in the axon number between the PLCL/4C+4H (6132±447) and the PLCL/4C+4H/NGF (5997±394) groups, which also did not differ significantly from the autograft and PLCL groups (p<0.069, Fig. 6 & Table 1). There were significant differences in axon diameters among groups with autograft yielding the largest axons (3.83±0.04 μm) followed by PLCL/4C+4H/NGF (3.49±0.05 μm), PLCL/4C+4H (3.24±0.1 μm), and PLCL (3.14±0.09 μm). Autograft groups had larger axons than all other groups (p<0.0001 compared to the PLCL and PLCL/4C+4H groups; p=0.012 for the PLCL/4C+4H/NGF group). PLCL/4C+4H/NGF showed a significant difference versus PLCL (p=0.014), but not versus PLCL/4C+4H (p=0.09). The difference in the average axon diameter between PLCL/4C+4H and PLCL did not reach significance (p=0.8).
FIG. 6.
Representative light microscopy images of sciatic nerve cross sections stained with 2% osmium tetroxide. (A) Autograft, (B) PLCL, (C) PLCL/4C+4H, (D) PLCL/4C+4H/NGF. Scale bar=10 μm, 20×magnification. Color images available online at www.liebertpub.com/tea
Table 1.
Histomorphometry Results After 12 Weeks
| In vivo Histomorphometry Results | ||||
|---|---|---|---|---|
| Autograft | PLCL | PLCL/4C+4H | PLCL/4C+4H/NGF | |
| Axon count at 7 mm within graft | 6892±316 | 4690±398 | 6132±447 | 5997±394 |
| Axon diameter (μm) at 7 mm within graft | 3.83±0.04 | 3.14±0.09 | 3.24±0.1 | 3.49±0.05 |
Means±SEM are shown.
PLCL, poly-L-lactide-co-caprolactone; NGF, nerve growth factor.
Discussion
Functional recovery after segmental peripheral nerve injuries continues to be a challenging clinical problem. In this study, we tested whether composite collagen and hyaluronan hydrogels could promote axonal outgrowth on aligned nanofiber scaffolds both in vitro and in vivo.
The collagen and hyaluronan hydrogels stimulated in vitro neurite outgrowth from DRG, which are composed of sensory neurons. Consistent with this, sensory recovery was enhanced at 12 weeks when the hydrogels were used as luminal fillers within tubular, aligned nanofiber conduits used to repair segmental, rat sciatic nerve defects. While limbs repaired with conduit filled with hydrogel withdrew from thermal stimuli at the same speed as control limbs, limbs repaired with either autograft or empty conduit withdrew more slowly than controls.
In vivo autograft repairs led to the highest muscle forces, highest muscle mass, highest CMAP amplitudes, highest number of axons within the repaired segments, the highest axon diameters, and the lowest nerve conduction latencies, although statistically significant differences were only detected in muscle mass, axon numbers, and axon diameters. All groups recovered more than 50% of gastrocnemius force, which clinically could be considered an adequate result and could possibly even be greater if postoperative rehabilitation would have been administered, as it is the standard for humans. The muscle mass ratio between the operated and contralateral side ranged between 46%–63%, which is consistent with other reports in the literature, and our findings for nerve conduction latency and amplitude are in accordance with our other muscle results.34,36 The addition of hydrogel led to improvement in all motor, electrophysiologic, and histomorphometric endpoints relative to empty conduit. However, these differences did not reach statistical significance. The NGF enhanced neurite outgrowth in vitro, but did not lead to statistically significant improvements in vivo.
The ability of collagen, hyaluronan, and NGF to synergistically support neurite survival and growth is supported by other in vitro studies. Neurons from dissociated chick DRGs cultured within a 2-hydroxyethylmethacrylate hydrogel depended on the collagen, fibronectin, or NGF to grow.37 Collagen nerve guides with longitudinal alignment promote in vitro axonal extension from DRGs in concert with Schwann cell migration.38 Collagen has been combined with many different ECM components to study the effects of combinatorial hydrogels, most effective for neuronal or neuronal stem cell culture.16,39 Conduits made of hyaluronic acid have been shown to facilitate the growth of rat Schwann cells in vitro.20,40,41 The dose-dependent effect of the NGF that we observed is also consistent with previously reported studies, in which, daily NGF injections via a microinjection port produced dose-dependent effects on axonal regeneration in the rat model. Inhibitory effects were seen at high levels of NGF beyond 800 pg/μL.30
We chose the rat sciatic nerve as it is the most widely used model to study peripheral nerve injury and because of its known accessibility and reasonable size.5,42,43 In our study, a segmental gap of 10 mm was the longest possible resection, because of anatomical restrictions in the female Lewis rats that we used. We felt that this was sufficient to assess functional recovery and axonal outgrowth with our chosen conduit without introducing any tension, and the results of our study can be compared to previously reported literature.5 Our results suggest that despite limitations to our chosen 10-mm gap model, it is still successful in evaluating muscle force and sensory recovery. However, as 10-mm nerve gaps are seldom considered critical in the rat sciatic nerve model, the results from the current experiment could be reconfirmed in rat strains with longer sciatic nerves. Animals with nerves long enough to create 15-mm defects44–46 would be ideal. Such defects were impossible to create in the rats of our chosen strain, sex, and age. These small animals were chosen because they do not exhibit autotomy following sciatic nerve injury.
One limitation of our in vitro model was that we measured the axonal length rather than the total area of neurite outgrowth. Nonetheless, the mean length of the 10 longest axons is a well-established method of assessing neuronal outgrowth from DRG explants.12,16,29,30
The positive effect of hydrogel on sensory function could be particularly promising clinically, since recovery of protective sensation is critically important following peripheral nerve injury.47 One concern about the use of whole paw immersion in an isolated sciatic nerve injury model is the persistence of saphenous nerve sensory innervation of the dorsal medial paw. However, differences in withdrawal from whole paw thermal stimulation between the operated and nonoperated hindlimbs were universally found despite the presence of intact saphenous nerves. Therefore, in agreement with multiple other studies,31,48–50 we believe that this test is valid for assessing overall sensory recovery after injury of the rat sciatic nerve. The minimal sensory recovery seen following autograft repair is in line with another investigation where at 12 weeks, only 42% of the rats repaired with autograft showed a response to a thermal stimulus.36 Further studies should be conducted to explain the poor outcome of the current gold standard.
Although the collagen/hyaluronan composite supported neurite outgrowth both in vitro and in vivo, the results of the NGF release and the sciatic nerve repair experiments suggest that the hydrogel is not an effective carrier for the NGF. We observed burst release of the NGF from the collagen/hyaluronan hydrogel with most release occurring within 3 days. Such findings are similar to the kinetics of release using several other methods of NGF delivery.51–53 In addition, we were able to recover only 26% of the NGF that was initially loaded into our hydrogel, suggesting that the hydrogel does not prevent degradation of the growth factor. These factors may explain why the addition of the NGF to the hydrogel in vivo did not enhance sciatic nerve recovery. In contrast, the initial burst release of NGF would be consistent with the enhanced DRG outgrowth found in the 3-day in vitro experiments. Other studies have shown a total release of NGF ranging from 20%–45% using poly(lactide-co-glycolide) microspheres52 and 50%–91% using NGF-loaded chitosan microspheres.53 The ideal carrier would allow long-term release of NGF, while protecting the growth factor from degradation.
In summary, the addition of a novel composite collagen and hyaluronan hydrogel luminal filler to an aligned, nanofiber tubular nerve conduit allowed improved recovery of sensory function 3 months after repair compared to nerve autograft. This combination also led to motor outcomes that approached, but did not reach, that of autograft repair. Additional delivery of bioactive molecules that stimulate motor and sensory function may lead to a composite nerve conduit that rivals the performance of autograft without the morbidity of donor nerve harvest.
Acknowledgments
We thank Professor Song Li and Shyam Patel for informative discussions, Bora Shin and Judy Shigenaga for technical assistance, and Eva Grotkopp for statistical assistance. This work was supported by the Department of Defense Grant (W81XWH-05-2-0094), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California.
Disclosure Statement
No competing financial interests exist.
References
- 1.Isaacs J. Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am. 2010;35:491. doi: 10.1016/j.jhsa.2009.12.009. quiz 498. [DOI] [PubMed] [Google Scholar]
- 2.Meek M.F. Coert J.H. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann Plast Surg. 2008;60:110. doi: 10.1097/SAP.0b013e31804d441c. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain L.J. Yannas I.V. Arrizabalaga A. Hsu H.P. Norregaard T.V. Spector M. Early peripheral nerve healing in collagen and silicone tube implants: myofibroblasts and the cellular response. Biomaterials. 1998;19:1393. doi: 10.1016/s0142-9612(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 4.Kehoe S. Zhang X.F. Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Angius D. Wang H. Spinner R.J. Gutierrez-Cotto Y. Yaszemski M.J. Windebank A.J. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials. 2012;33:8034. doi: 10.1016/j.biomaterials.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung S. Moghe A.K. Montero G.A. Kim S.H. King M.W. Nanofibrous scaffolds electrospun from elastomeric biodegradable poly(L-lactide-co-epsilon-caprolactone) copolymer. Biomed Mater. 2009;4:015019. doi: 10.1088/1748-6041/4/1/015019. [DOI] [PubMed] [Google Scholar]
- 7.Moore A.M. Kasukurthi R. Magill C.K. Farhadi H.F. Borschel G.H. Mackinnon S.E. Limitations of conduits in peripheral nerve repairs. Hand (N Y) 2009;4:180. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin J. Park M. Rengarajan A. Zhang Q. Limburg S. Joshi S.K., et al. Functional motor recovery after peripheral nerve repair with an aligned nanofiber tubular conduit in a rat model. Regen Med. 2012;7:799. doi: 10.2217/rme.12.87. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y. Wang A. Patel S. Kurpinski K. Diao E. Bao X., et al. Engineering bi-layer nanofibrous conduits for peripheral nerve regeneration. Tissue Eng Part C Methods. 2011;17:705. doi: 10.1089/ten.tec.2010.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S. Kurpinski K. Quigley R. Gao H. Hsiao B.S. Poo M.M., et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 11.Johnson P.J. Skornia S.L. Stabenfeldt S.E. Willits R.K. Maintaining bioactivity of NGF for controlled release from PLGA using PEG. J Biomed Mater Res A. 2008;86:420. doi: 10.1002/jbm.a.31635. [DOI] [PubMed] [Google Scholar]
- 12.Madduri S. Papaloizos M. Gander B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials. 2010;31:2323. doi: 10.1016/j.biomaterials.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 13.Zander N.E. Orlicki J.A. Rawlett A.M. Beebe T.P., Jr. Surface-modified nanofibrous biomaterial bridge for the enhancement and control of neurite outgrowth. Biointerphases. 2010;5:149. doi: 10.1116/1.3526140. [DOI] [PubMed] [Google Scholar]
- 14.Griffin J. Delgado-Rivera R. Meiners S. Uhrich K.E. Salicylic acid-derived poly(anhydride-ester) electrospun fibers designed for regenerating the peripheral nervous system. J Biomed Mater Res A. 2011;97:230. doi: 10.1002/jbm.a.33049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbonetto S. Gruver M.M. Turner D.C. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983;3:2324. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deister C. Aljabari S. Schmidt C.E. Effects of collagen 1, fibronectin, laminin and hyaluronic acid concentration in multi-component gels on neurite extension. J Biomater Sci Polym Ed. 2007;18:983. doi: 10.1163/156856207781494377. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez Hamann M.C. Tsai E.C. Tator C.H. Shoichet M.S. Novel intrathecal delivery system for treatment of spinal cord injury. Exp Neurol. 2003;182:300. doi: 10.1016/s0014-4886(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez Hamann M.C. Tator C.H. Shoichet M.S. Injectable intrathecal delivery system for localized administration of EGF and FGF-2 to the injured rat spinal cord. Exp Neurol. 2005;194:106. doi: 10.1016/j.expneurol.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Belkas J.S. Munro C.A. Shoichet M.S. Midha R. Peripheral nerve regeneration through a synthetic hydrogel nerve tube. Restor Neurol Neurosci. 2005;23:19. [PubMed] [Google Scholar]
- 20.Lee J.H. Yu H.S. Lee G.S. Ji A. Hyun J.K. Kim H.W. Collagen gel three-dimensional matrices combined with adhesive proteins stimulate neuronal differentiation of mesenchymal stem cells. J R Soc Interface. 2011;8:998. doi: 10.1098/rsif.2010.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnell E. Klinkhammer K. Balzer S. Brook G. Klee D. Dalton P., et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang K.K. Nemeth I.R. Seckel B.R. Chakalis-Haley D.P. Swann D.A. Kuo J.W., et al. Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery. 1998;18:270. doi: 10.1002/(sici)1098-2752(1998)18:4<270::aid-micr11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Ozgenel G.Y. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery. 2003;23:575. doi: 10.1002/micr.10209. [DOI] [PubMed] [Google Scholar]
- 24.Suri S. Schmidt C.E. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009;5:2385. doi: 10.1016/j.actbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Suri S. Schmidt C.E. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16:1703. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 26.Dodla M.C. Bellamkonda R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich K.M. Alexander T.D. Pryor J.C. Hollowell J.P. Nerve growth factor enhances regeneration through silicone chambers. Exp Neurol. 1989;105:162. doi: 10.1016/0014-4886(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 28.Xin X. Borzacchiello A. Netti P.A. Ambrosio L. Nicolais L. Hyaluronic-acid-based semi-interpenetrating materials. J Biomater Sci Polym Ed. 2004;15:1223. doi: 10.1163/1568562041753025. [DOI] [PubMed] [Google Scholar]
- 29.Allodi I. Guzman-Lenis M.S. Hernandez J. Navarro X. Udina E. In vitro comparison of motor and sensory neuron outgrowth in a 3D collagen matrix. J Neurosci Methods. 2011;198:53. doi: 10.1016/j.jneumeth.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Kemp S.W. Webb A.A. Dhaliwal S. Syed S. Walsh S.K. Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol. 2011;229:460. doi: 10.1016/j.expneurol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Attal N. Jazat F. Kayser V. Guilbaud G. Further evidence for ‘pain-related’ behaviours in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- 32.Pu L.L. Syed S.A. Reid M. Patwa H. Goldstein J.M. Forman D.L., et al. Effects of nerve growth factor on nerve regeneration through a vein graft across a gap. Plast Reconstr Surg. 1999;104:1379. doi: 10.1097/00006534-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 33.de Boer R. Knight A.M. Borntraeger A. Hebert-Blouin M.N. Spinner R.J. Malessy M.J., et al. Rat sciatic nerve repair with a poly-lactic-co-glycolic acid scaffold and nerve growth factor releasing microspheres. Microsurgery. 2011;31:293. doi: 10.1002/micr.20869. [DOI] [PubMed] [Google Scholar]
- 34.Giusti G. Willems W.F. Kremer T. Friedrich P.F. Bishop A.T. Shin A.Y. Return of motor function after segmental nerve loss in a rat model: comparison of autogenous nerve graft, collagen conduit, and processed allograft (AxoGen) J Bone Joint Surg Am. 2012;94:410. doi: 10.2106/JBJS.K.00253. [DOI] [PubMed] [Google Scholar]
- 35.de Boer R. Borntraeger A. Knight A.M. Hebert-Blouin M.N. Spinner R.J. Malessy M.J., et al. Short- and long-term peripheral nerve regeneration using a poly-lactic-co-glycolic-acid scaffold containing nerve growth factor and glial cell line-derived neurotrophic factor releasing microspheres. J Biomed Mater Res A. 2012;100:2139. doi: 10.1002/jbm.a.34088. [DOI] [PubMed] [Google Scholar]
- 36.Koh H.S. Yong T. Teo W.E. Chan C.K. Puhaindran M.E. Tan T.C., et al. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J Neural Eng. 2010;7:046003. doi: 10.1088/1741-2560/7/4/046003. [DOI] [PubMed] [Google Scholar]
- 37.Carbonetto S.T. Gruver M.M. Turner D.C. Nerve fiber growth on defined hydrogel substrates. Science. 1982;216:897. doi: 10.1126/science.7079743. [DOI] [PubMed] [Google Scholar]
- 38.Bozkurt A. Brook G.A. Moellers S. Lassner F. Sellhaus B. Weis J., et al. In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng. 2007;13:2971. doi: 10.1089/ten.2007.0116. [DOI] [PubMed] [Google Scholar]
- 39.Lin P.W. Wu C.C. Chen C.H. Ho H.O. Chen Y.C. Sheu M.T. Characterization of cortical neuron outgrowth in two- and three-dimensional culture systems. J Biomed Mater Res B Appl Biomater. 2005;75:146. doi: 10.1002/jbm.b.30276. [DOI] [PubMed] [Google Scholar]
- 40.Zavan B. Abatangelo G. Mazzoleni F. Bassetto F. Cortivo R. Vindigni V. New 3D hyaluronan-based scaffold for in vitro reconstruction of the rat sciatic nerve. Neurol Res. 2008;30:190. doi: 10.1179/174313208X281082. [DOI] [PubMed] [Google Scholar]
- 41.Sakai Y. Matsuyama Y. Takahashi K. Sato T. Hattori T. Nakashima S., et al. New artificial nerve conduits made with photocrosslinked hyaluronic acid for peripheral nerve regeneration. Biomed Mater Eng. 2007;17:191. [PubMed] [Google Scholar]
- 42.Wood M.D. Kemp S.W. Weber C. Borschel G.H. Gordon T. Outcome measures of peripheral nerve regeneration. Ann Anat. 2011;193:321. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Jiang X. Lim S.H. Mao H.Q. Chew S.Y. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223:86. doi: 10.1016/j.expneurol.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Jiang X. Mi R. Hoke A. Chew S.Y. Nanofibrous nerve conduit-enhanced peripheral nerve regeneration. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1531. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Lin Y.C. Ramadan M. Van Dyke M. Kokai L.E. Philips B.J. Rubin J.P., et al. Keratin gel filler for peripheral nerve repair in a rodent sciatic nerve injury model. Plast Reconstr Surg. 2012;129:67. doi: 10.1097/PRS.0b013e3182268ae0. [DOI] [PubMed] [Google Scholar]
- 46.Huang J. Lu L. Hu X. Ye Z. Peng Y. Yan X., et al. Electrical stimulation accelerates motor functional recovery in the rat model of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally oriented microchannels. Neurorehabil Neural Repair. 2010;24:736. doi: 10.1177/1545968310368686. [DOI] [PubMed] [Google Scholar]
- 47.Lee S.K. Wolfe S.W. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Lee S.H. Kayser V. Desmeules J. Guilbaud G. Differential action of morphine and various opioid agonists on thermal allodynia and hyperalgesia in mononeuropathic rats. Pain. 1994;57:233. doi: 10.1016/0304-3959(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 49.Chaiyasate K. Schaffner A. Jackson I.T. Mittal V. Comparing FK-506 with basic fibroblast growth factor (b-FGF) on the repair of a peripheral nerve defect using an autogenous vein bridge model. J Invest Surg. 2009;22:401. doi: 10.3109/08941930903410775. [DOI] [PubMed] [Google Scholar]
- 50.Mansikka H. Pertovaara A. Submodality-selective hyperalgesia adjacent to partially injured sciatic nerve in the rat is dependent on capsaicin-sensitive afferent fibers and independent of collateral sprouting or a dorsal root reflex. Brain Res Bull. 1997;44:237. doi: 10.1016/s0361-9230(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 51.Oh S.H. Kim J.R. Kwon G.B. Namgung U. Song K.S. Lee J.H. Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng Part C Methods. 2013;19:233. doi: 10.1089/ten.tec.2012.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y. De Laporte L. Rives C.B. Jang J.H. Lin W.C. Shull K.R., et al. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104:433. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng W. Huang J. Hu X. Xiao W. Rong M. Yuan Z., et al. Ionically cross-linked chitosan microspheres for controlled release of bioactive nerve growth factor. Int J Pharm. 2011;421:283. doi: 10.1016/j.ijpharm.2011.10.005. [DOI] [PubMed] [Google Scholar]