Abstract
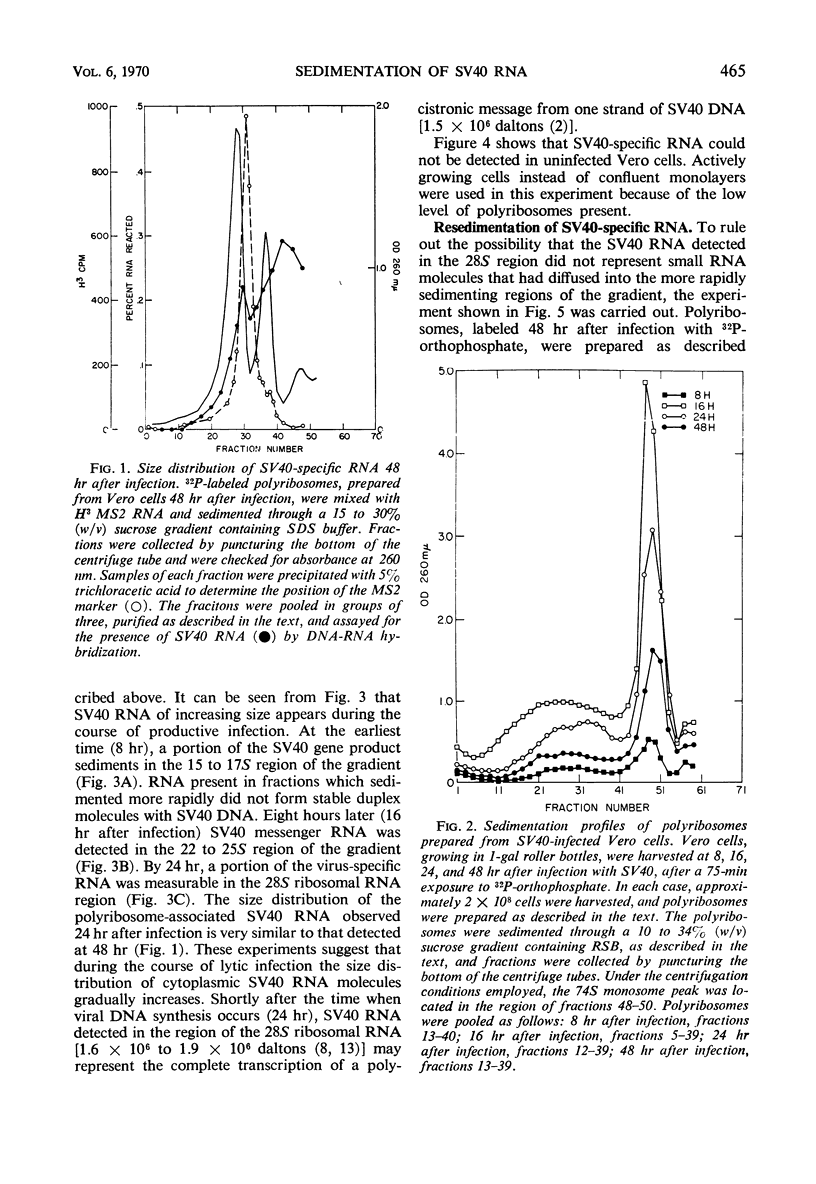
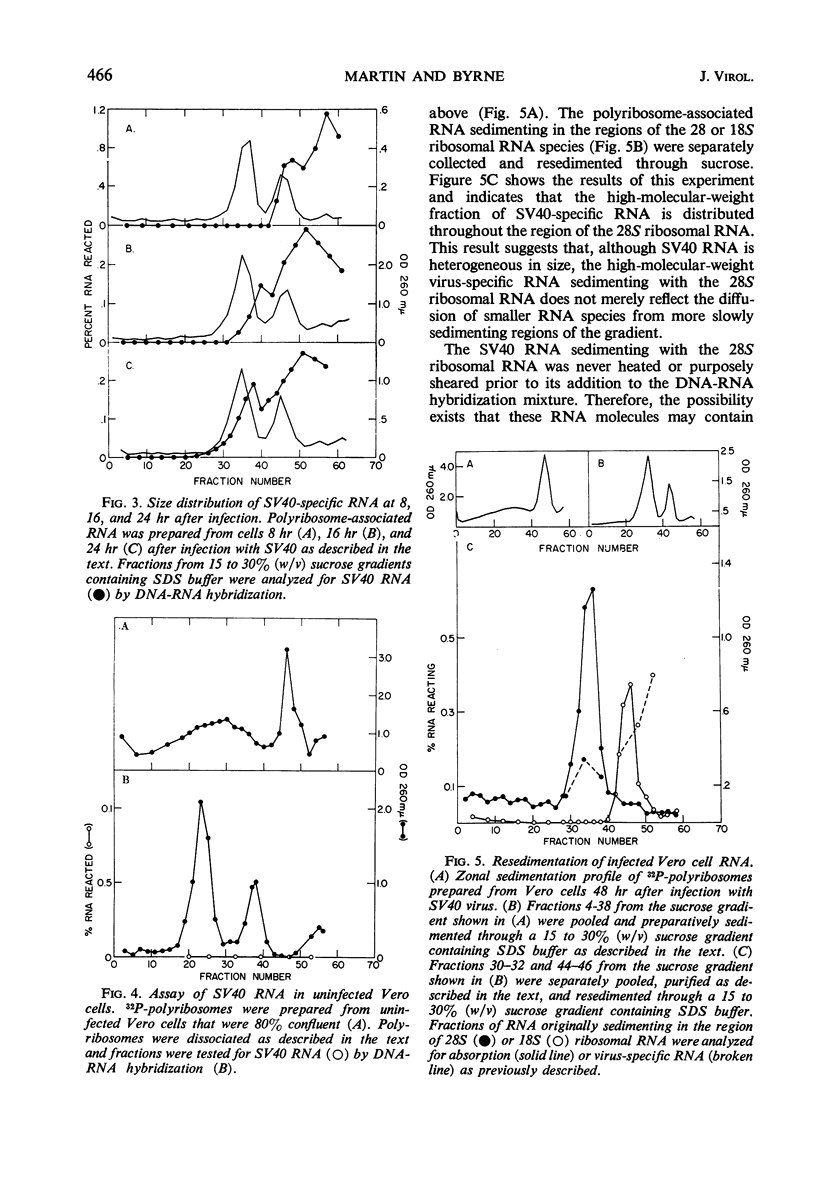
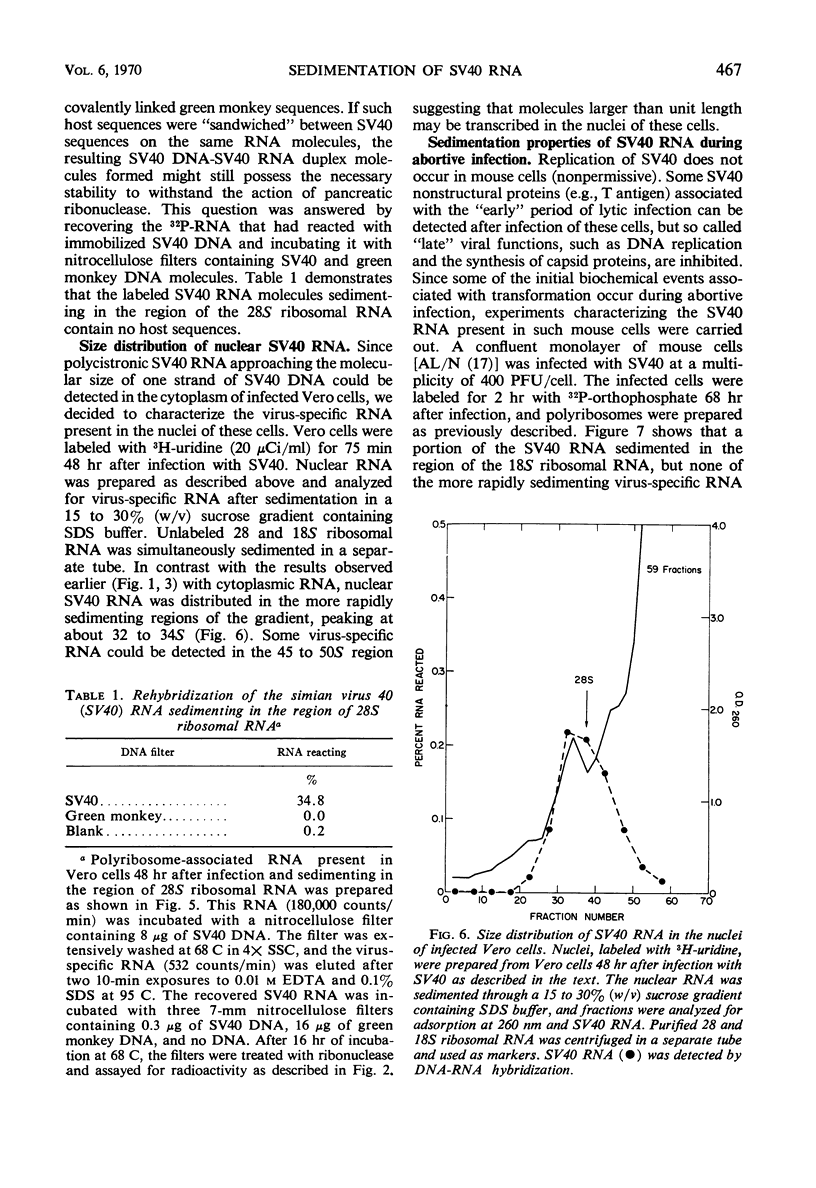
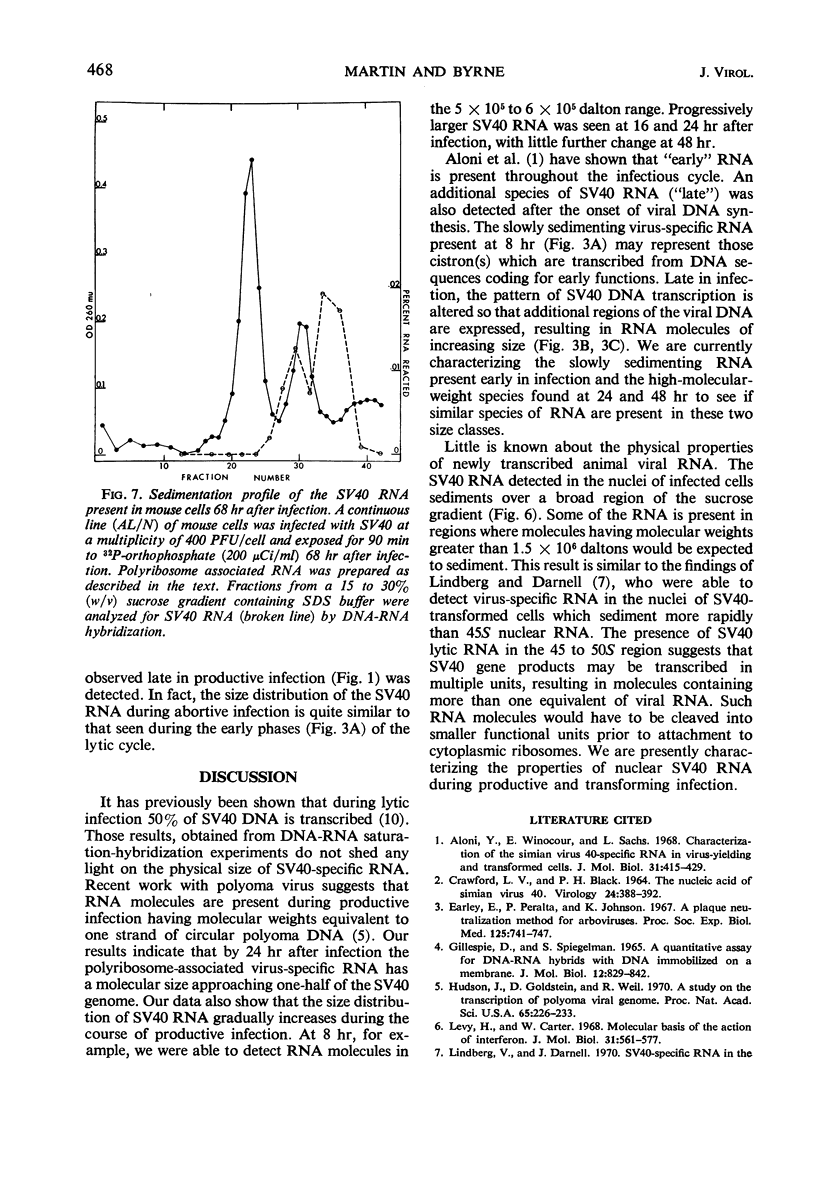
The size distribution of polyribosome-associated simian virus 40 (SV40) ribonucleic acid (RNA) was examined at various times after productive infection. Eight hours after infection, virus-specific RNA was detected in the 14 to 17S region of a sucrose gradient by deoxyribonucleic acid (DNA)-RNA hybridization; RNA present in fractions sedimenting more rapidly did not react with SV40 DNA. At successively later times, SV40 RNA was detected in more rapidly sedimenting regions. By 24 hr, a portion of the SV40 RNA was detected in the 28S region, sedimenting slightly more rapidly than a MS2 RNA marker. Nuclear SV40 RNA, prepared from cells 48 hr after infection, was distributed in more rapidly sedimenting regions of the gradient, peaking at about 32 to 34S. Some nuclear virus-specific RNA could be detected in the 45 to 50S region. During the abortive infection of mouse cells, the sedimentation profile of SV40 RNA was very similar to that observed during the early phases of the lytic cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Earley E., Peralta P. H., Johnson K. M. A plaque neutralization method for arboviruses. Proc Soc Exp Biol Med. 1967 Jul;125(3):741–747. doi: 10.3181/00379727-125-32194. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hudson J., Goldstein D., Weil R. A study on the transcription of the polyoma viral genome. Proc Natl Acad Sci U S A. 1970 Jan;65(1):226–233. doi: 10.1073/pnas.65.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. B., Carter W. A. Molecular basis of the action of interferon. J Mol Biol. 1968 Feb 14;31(3):561–577. doi: 10.1016/0022-2836(68)90428-2. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Axelrod D. Polyoma virus gene activity during lytic infection and in transformed animal cells. Science. 1969 Apr 4;164(3875):68–70. doi: 10.1126/science.164.3875.68. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Axelrod D. SV40 gene activity during lytic infection and in a series of SV40 transformed mouse cells. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1203–1210. doi: 10.1073/pnas.64.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann M. L., Pavlovec A. The subunits and structural ribonucleic acids of Jensen sarcoma ribosomes. Biochim Biophys Acta. 1966 Feb 21;114(2):264–276. doi: 10.1016/0005-2787(66)90308-x. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- Sauer G., Kidwai J. R. The transcription of the SV40 genome in productively infected and transformed cells. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1256–1263. doi: 10.1073/pnas.61.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Ting R. C., Ozer H. L., Fabisch P. Establishment of a cell line from an inbred mouse strain for viral transformation studies: simian virus 40 transformation and tumor production. J Natl Cancer Inst. 1968 Dec;41(6):1401–1409. [PubMed] [Google Scholar]
- Trilling D. M., Axelrod D. Encapsidation of free host DNA by simian virus 40: a simian virus 40 pseudovirus. Science. 1970 Apr 10;168(3928):268–271. doi: 10.1126/science.168.3928.268. [DOI] [PubMed] [Google Scholar]



