Abstract
Cellular heterogeneity of mesenchymal stem cells (MSCs) impedes their use in regenerative medicine. The objective of this research is to identify potential biomarkers for the enrichment of progenitors from heterogeneous MSC cultures. To this end, the present study examines variation in expression of neuron-glial antigen 2 (NG2) and melanoma cell adhesion molecule (CD146) on the surface of MSCs derived from human bone marrow in response to culture conditions and among cell populations. Multipotent cells isolated from heterogeneous MSC cultures exhibit a greater than three-fold increase in surface expression for NG2 and greater than two-fold increase for CD146 as compared with parental and lineage-committed MSCs. For both antigens, surface expression is downregulated by greater than or equal to six-fold when MSCs become confluent. During serial passage, maximum surface expression of NG2 and CD146 is associated with minimum doubling time. Upregulation of NG2 and CD146 during loss of adipogenic potential at early passage suggests some limits to their utility as potency markers. A potential relationship between proliferation and antigen expression was explored by sorting heterogeneous MSCs into rapidly and slowly dividing groups. Fluorescence-activated cell sorting revealed that rapidly dividing MSCs display lower scatter and 50% higher NG2 surface expression than slowly dividing cells, but CD146 expression is comparable in both groups. Heterogeneous MSCs were sorted based on scatter properties and surface expression of NG2 and CD146 into high (HI) and low (LO) groups. ScLONG2HI and ScLONG2HICD146HI MSCs have the highest proliferative potential of the sorted groups, with colony-forming efficiencies that are 1.5–2.2 times the value for the parental controls. The ScLO gate enriches for rapidly dividing cells. Addition of the NG2HI gate increases cell survival to 1.5 times the parental control. Further addition of the CD146HI gate does not significantly improve cell division or survival. The combination of low scatter and high NG2 surface expression is a promising selection criterion to enrich a proliferative phenotype from heterogeneous MSCs during ex vivo expansion, with potentially numerous applications.
Introduction
Mesenchymal stem cells (MSCs) are being harnessed to develop a broad range of cellular therapies to regenerate damaged tissue.1,2 A major challenge to realizing the therapeutic potential of these adult stem cells is variation in the progenitor content and regenerative capacity of MSC cultures.3,4 This variability reflects not only different methods to isolate MSCs but also intrinsic heterogeneity among cells within an MSC culture. The latter may arise from distinct phenotypes in vivo, adaptation to ex vivo cultivation, and/or senescence upon expansion.5 The effect of MSC heterogeneity on therapeutic efficacy is evident in the preferential tissue engraftment of rapidly versus slowly proliferating MSCs6 and improved cardiac function after treatment of myocardial infarction with multipotent versus parental MSCs.7 Consequently, identification and isolation of progenitor populations in heterogeneous MSC cultures are essential to the development of highly efficacious stem cell therapies.
Characterization of MSC populations has been largely based on morphology, potency, and proliferation. MSC cultures contain small, spindle-shaped cells that rapidly proliferate and large, flat, and cuboid cells that grow more slowly.8 Clonal analysis by our laboratory and others revealed differences in trilineage potential of MSCs to exhibit osteo-, adipo-, and chondrogenesis as a measure of potency.9,10 Multipotent MSC colonies derived from single cells have a higher rate of proliferation and smaller size than more lineage-committed MSCs.11 While clonal isolation of single cells has been instrumental in resolving MSC heterogeneity, a more rapid selection method is warranted for production of MSC therapies.
An immunophenotypic characterization of MSC populations is urgently needed for high-throughput enrichment of MSC progenitors. There is limited information on cell-surface markers to identify different MSC populations. The International Society for Cellular Therapy defines human MSCs by their expression of 5′-nucleotidase (CD73), thymocyte differentiation antigen 1 (CD90) and endoglin (CD105), lack of expression of lymphocyte common antigen (CD45) and other hematopoietic markers, adherence to a plastic substrate, and trilineage potential.12 Attempts to further resolve heterogeneous MSCs into specific subsets have had only partial success. For example, Hachisuka et al.13 isolated multipotent murine MSCs based, in part, on expression of stem cell antigen 1, but they did not detect this marker on human MSCs. Nerve growth factor receptor (CD271) selects highly clonogenic, small cells in unfractioned bone marrow;14 however, the receptor is rapidly downregulated upon ex vivo expansion of MSCs.15 The objective of this research is to identify potential cell-surface markers for the enrichment of progenitors from heterogeneous MSC cultures.
To this end, we investigated the variation in cell-surface expression of neuron-glial antigen 2 (NG2) and melanoma cell adhesion molecule (CD146) in MSCs derived from human bone marrow. CD146 (also known as MCAM, Mel-CAM, S-Endo-1, A32 antigen, and MUC18) participates in heterotypic intercellular adhesion16 and promotes tumor progression in many cancers.17 Also. this surface antigen is known as a pericyte marker and is expressed by MSCs,18 reflecting the perivascular origin of the postnatal MSC niche.19,20 Positive selection for CD146 and negative selection for CD45 recovers ∼80% of the total colony-forming fibroblasts in unfractioned bone marrow.21 CD146+ cells isolated from bone marrow are capable of extensive proliferation and trilineage differentiation.22 Far less is known about NG2 expression in MSCs. NG2 (also known as MPG, CSPG4, and AN2) is a chondroitin sulfate proteoglycan, which was first observed on the surface of neural progenitors.23 Along with CD146, NG2 is a pericyte marker expressed on the surface of MSCs.18,19 While cultured MSCs are positive for NG2, uncultured bone marrow cells are negative for this antigen.24 To date, there are no reports of bone marrow cells enriched based on NG2 expression.
This study expands upon preliminary data from our laboratory, which revealed differential expression of CD146 among MSC subsets.10 As described herein, broadening the analysis to other perivascular markers revealed a significant upregulation in surface expression for only NG2 and CD146 in multipotent versus parental MSCs. We conducted an extensive investigation of the surface expression of these two markers in response to changes in culture conditions and among various MSC populations. Our research provides unique insights into the relationships of NG2 and CD146 surface expression to proliferation and potency. This enabled development of a novel enrichment strategy that selects a proliferative phenotype from heterogeneous MSCs during ex vivo expansion.
Materials and Methods
MSC cultivation
Primary MSCs were harvested from 2 mL of iliac crest bone marrow aspirate from healthy adult volunteers, ages 24–37, under a protocol approved by the Tulane Institutional Review Board as previously described.25 Plastic-adherent MSCs prior to expansion are designated as passage P0. All cell culture supplies herein were obtained from Invitrogen (Carlsbad, CA) except where noted, and 100 U/mL penicillin and 100 μg/mL streptomycin were added to all media. For routine cultivation, MSCs were inoculated at 100 cells/cm2 in growth medium of α-MEM with 2 mM L-glutamine, supplemented with 17% FBS (Hyclone, Logan, UT) and an additional 2 mM L-glutamine.26 Cultures were maintained in a 37°C humidified incubator at 5% CO2 with complete medium exchange every 3–4 days and were subcultured at 50% confluence with 0.25% trypsin/1 mM EDTA. Viable cell concentration was measured by trypan blue staining and hemocytometer counting, unless otherwise stated.
High-capacity assay of MSC potency
A high-capacity assay to quantify the potency of single cell-derived MSC colonies was developed in our laboratory. A 96-well format was employed to (1) isolate P0 MSCs labeled with CellTracker Green and detect a single cell/well with fluorescence microscopy, (2) amplify single cell-derived colonies for a week and subculture at a 1:4 ratio to generate matched P1 cultures, (3) differentiate three matched cultures per colony to quantify trilineage potential to exhibit osteo-, adipo-, and chondrogenesis as a measure of potency, and (4) cryopreserve in situ the fourth matched P1 culture per colony for future use. Russell et al.10 gives a detailed methodology.
Adipogenic, confluent, and serial passaged cultures
Single cell-derived P2 cultures of multipotent MSCs were amplified to 3×104 cells, potency of the expanded cultures was verified, and five cultures were pooled to form a sample of 1−2×105 cells. To monitor marker expression during adipogenesis, pooled MSC cultures were inoculated at 2×103 cells/cm2 into six-well plates containing 2 mL growth medium/well. Differentiation was induced in confluent cultures exposed to growth medium supplemented with 0.5 μM dexamethasone, 0.5 mM isobutylmethylxanthine (Sigma-Aldrich, St. Louis, MO), and 50 μM indomethacin (Sigma-Aldrich). Control samples were maintained in growth medium. For serial passage, the pooled cultures were inoculated at 100 cells/cm2 into T-150 flasks containing 20 mL growth medium and serial passaged at 50% confluence until most cells reached senescence. Doubling time during serial passage was calculated as in Blanch and Clark.27 Alternatively, the T-flask cultures were maintained in growth medium until they were confluent for 7 day and then passaged once to generate postconfluent MSC samples. Temporal changes in the expression of cell-surface markers were evaluated by flow cytometry.
Flow cytometry
Cell labeling. To rapidly sort from slowly dividing MSCs, heterogeneous P1 MSCs were inoculated at 500 cells/cm2 into T-150 flasks containing 20 mL growth medium, and adherent cells were stained in situ after 24 h with 6 mL of 5 μM CellTrace Violet in phosphate-buffered saline (PBS) for 20 min at 37°C. Stained MSCs were washed 2× with prewarmed growth medium and amplified for 4 days in fresh growth medium to generate ∼50% confluent cultures. Cell samples were gently trypsinized with 0.25% trypsin/1 mM EDTA for 2 min. Trypsin was deactivated with growth medium before the cells were resuspended in PBS for immunolabeling.
Cell samples for immunolabeling were obtained from subconfluent cultures unless otherwise noted. Single-cell suspensions of 2×106 MSCs/mL PBS were immunolabeled in 100–500 μL aliquots for 30 min on ice in the dark with the fluorochrome-conjugated, anti-human monoclonal antibodies listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea) at saturating concentrations recommended by the manufacturer and confirmed by titration. Labeled samples were washed 3×with PBS, resuspended at 5−6×105 cells/ml PBS for flow cytometric analysis or 1−2×106 cells/mL PBS for cell sorting, and kept on ice until analysis. Matched isotype controls were prepared in parallel at the same concentration as each antibody.
Analysis and sorting. Labeled MSCs were analyzed on either an Epics FC500 flow cytometer with CXP software version 2.2 (Beckman-Coulter, Fullerton, CA) or a Gallios flow cytometer with Kaluza software version 1.1 (Beckman-Coulter). Fluorochromes were excited with standard 488- and 633-nm lasers and detected using the following filters (nm): APC (670/20), ECD (630/20), FITC (530/20), PC5 (670/20), PC7 (720/20), and PE (560/20). Cells were sorted on a FACSVantage SE/DiVa cell sorter with FACSDiVa software version 5.02 (BD Biosciences, San Jose, CA). Fluorochromes were excited with standard 360-, 488-, and 633-nm lasers to detect APC, CellTrace Violet, and FITC using 660/20-, 450/50-, and 530/30-nm filters, respectively. Prior to fluorescence-activated cell sorting (FACS), the sample lines, sheath, sample introduction port, and nozzle were sterilized for 30 min with 70% ethanol followed by 30 min with a 10% bleach solution and finally rinsed with deionized water for an additional 30 min to remove residual decontaminant. Spectral overlap was corrected with multicolor compensation as previously described.28
Samples were analyzed and sorted by gating on live cells by forward and side scatter. Viability was confirmed in parallel by Annexin V/PI staining and was routinely greater than 90%. Fluorescence of test samples was standardized to that of the isotype control. Gates for high (HI) and low (LO) fluorescent populations were set to exclude the isotype control and adjusted to include 10% of the appropriate positive parental population. Cells were sorted in purity mode at an acquisition rate of 1,500–2,000 events/sec using a 100 micron tip, sheath pressure of 11 psi, and vibration frequency of 15.8 kHz. All analyses and sorts were repeated at least thrice, and undiluted aliquots of sorted cells were reanalyzed for purity. Cells needed for subsequent experiments were sorted into chilled growth medium, centrifuged, and resuspended into fresh growth medium for analysis.
Other assays
The efficiency of sorted MSCs to form colonies when inoculated at 100±10 viable cells in a 10 cm tissue-culture dish was evaluated as in Barrilleaux et al.26 by staining with 3% crystal violet in methanol for 5 min to detect cell colonies. Cell survival was determined by labeling sorted MSCs with 5 μM CellTracker Green for 10–15 min at 37°C, isolating the cells by limiting dilution into 96-well plates containing 50 μL/well fresh growth medium and 75 μL/well sterile-filtered growth medium, identifying wells containing single cells by fluorescence microscopy, and detecting adherent cells after 24 h by crystal violet staining. Potency of sorted MSCs to exhibit adipo-, chondro-, and osteogenesis was evaluated as in Russell et al.11 Senescence-associated β-galactosidase activity at pH 6.0 was histochemically detected in subconfluent cultures, 4 days after inoculation with the Senescence β-galactosidase Staining kit (Cell Signaling Technology, Danvers, MA).
Image analysis
Cell images were captured with an Optronics DEI-750 digital camera (Goleta, CA) mounted onto an Olympus IX50 microscope (Center Valley, PA). Projected 2D area of MSCs in culture was traced using a Graphire 4 CTE-640 tablet (Wacom Technology, Vancouver, WA) and analyzed with Image-Pro Plus software (version 6.1; Media Cybernetics, Silver Spring, MD) using the Area option in the Count/Size function to evaluate cell size.29 Results are reported as a mean value from 30 randomly selected culture images per sample.
Statistical analysis
Differentiation measurements are reported as standardized scores relative to negative control values by centering and scaling each score using the mean and standard deviation for undifferentiated MSCs. To determine appropriate cutoff values for positive differentiation, the R open-source statistical software package (version 2.15, r-project.org) was employed to generate Gaussian kernel density estimates for the distribution of measurements observed for the negative controls. Values corresponding to the 95th percentile from the fitted control densities were selected as the lower limit of detection for differentiation. Differences between two cell groups were analyzed with a two-tailed Student's t-test. A Pearson correlation quantified associations between expression of cell-surface markers and doubling time. The threshold of significance was 0.05.
Results
Immunophenotype of multipotent MSC colonies (OACs) and parental MSCs
The marrow-derived MSCs employed in this study satisfied accepted criteria that define human MSCs:12 adherence to plastic, trilineage potential, and an immunophenotype that was positive for CD73, CD90, and CD105 and negative for CD11b, CD19, CD34, CD45, and HLA-DR. Initially, we compared cell-surface expression of select antigens in multipotent MSC subsets relative to the heterogeneous parental MSCs from which they originated. The objective was to identify potential biomarkers for the prospective enrichment of progenitors from heterogeneous MSC cultures by FACS.
Multipotent MSC (OACs) subsets with osteo-, adipo-, and chondrogenic potential are designated here as OACs and were generated with the high-capacity assay described in Russell et al.10 The assay produces a large number of single cell-derived colonies of known potency that are frozen in an undifferentiated state for future use. Potency of amplified OACs was confirmed upon inoculating experimental cultures (Supplementary Fig. S1). For flow cytometry, five single cell-derived P2 OAC cultures were pooled as in our earlier studies10,11 to analyze early passage MSCs and minimize loss of antigen expression with ex vivo expansion.
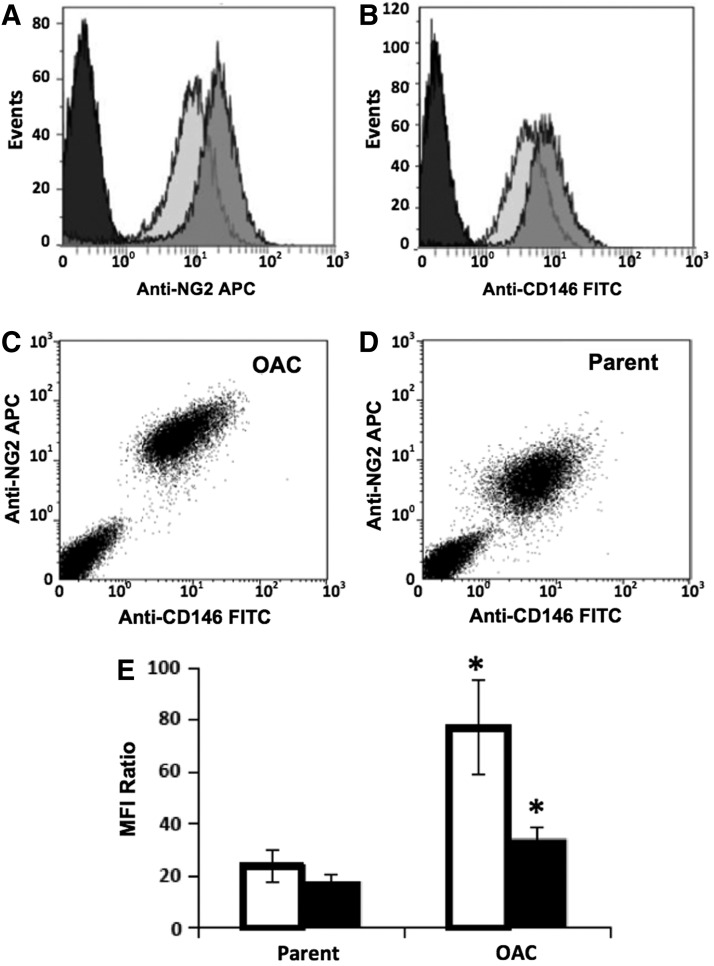
Flow cytometric analysis revealed that cell-surface expression of established MSC markers (CD73, CD90, and CD105)12 is comparable for OACs and parental MSCs (Supplementary Fig. S2). Next, we examined surface expression of perivascular markers (CD13,30 CD140a,31 CD140b,31 CD146,18 NG219, and STRO-132) given the perivascular origin of the postnatal MSC niche.19 Of these, there is a significant upregulation in surface expression for only NG2 and CD146 in OACs versus parental MSCs (Fig. 1). Cell samples labeled with anti-NG2 APC and anti-CD146 FITC monoclonal antibodies are >95% positive for each antigen (Fig. 1A–D). The distribution of fluorescence intensities for NG2 and CD146 is shifted to higher values for OACs than for parental MSCs (Fig. 1A–D). The mean fluorescence intensity (MFI) is presented as a ratio relative to that of the isotype control. OACs exhibit a greater than three-fold increase in MFI ratio for NG2 and greater than or equal to two-fold increase for CD146 (n=4) compared with parental MSCs (Fig. 1E; p<0.05). This pattern is consistent across MSCs from three different donors (Supplementary Fig. S3). Similarly, we observed higher NG2 and CD146 surface expression in OACs than in lineage-committed MSC colonies (Supplementary Fig. S4). Results for CD146 agree with preliminary data from our laboratory for this antigen from a different MSC donor.10 Based on these findings, NG2 and CD146 were selected for further analysis of surface expression in MSCs.
FIG. 1.
Differential expression of NG2 and CD146 in multipotent colonies (OACs) and parental mesenchymal stem cells (MSCs). Representative histograms (A–D) were generated from flow cytometric analysis of 10,000 cells labeled with a cocktail of anti-NG2 APC and anti-CD146 FITC or isotype controls. Surface expression of NG2 (A) and CD146 (B) was analyzed in pooled samples of five single cell-derived cultures of P2 OACs (dark grey) relative to parental MSCs (light gray) and isotypes (black). Corresponding bivariate histograms for OACs (C) and parental MSCs (D). Mean fluorescence intensity (MFI, E) for NG2 (white) and CD146 (black) surface expression is presented as a ratio relative to the MFI of the isotype. Data are reported as a mean±standard error of the mean (n=4). *p<0.05 versus parent.
Differentiation, confluence, and serial passage of OACs
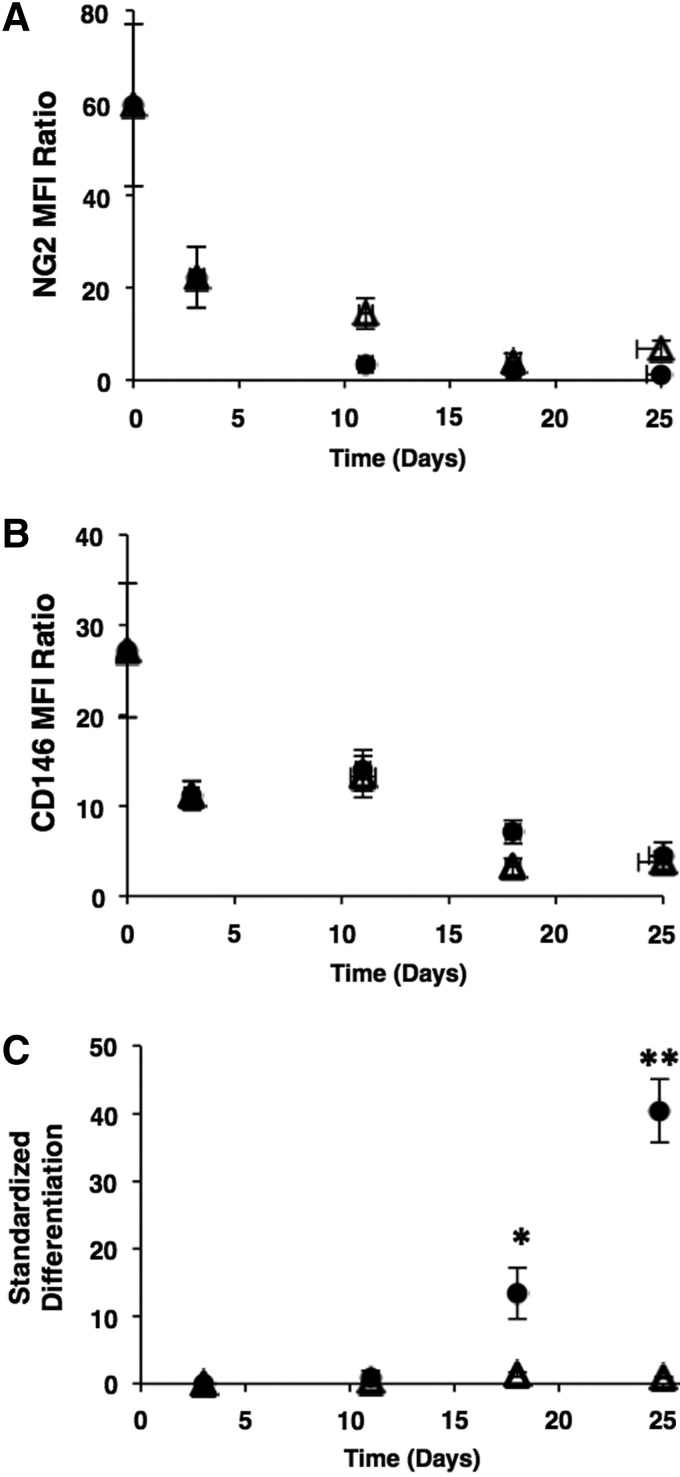
Cultures inoculated with P2 OACs were exposed to different culture conditions as a means to investigate possible relationships of NG2 and CD146 surface expression to proliferation and potency (Figs. 2–4). We employed well-defined OACs colonies for these experiments instead of heterogeneous MSCs because the complex composition in the latter may convolute results. Adipogenesis was selected as a representative mode of differentiation because adipogenic MSCs can be readily lifted from the culture surface to form a single-cell suspension for flow cytometric analysis. There is a substantial loss of NG2 and CD146 surface expression upon confluence and during adipogenesis (Fig. 2). Confluent cultures were induced to differentiate with adipogenic medium or maintained as a control in growth medium for 25 days. Temporal changes in surface expression are similar between the differentiated and control cultures (Fig. 2A, B) with a greater than or equal to six-fold decrease in MFI ratio by day 25, suggesting confluence is sufficient to downregulate both NG2 and CD146 surface expression. This trend is evident for multiple MSC donors (Supplementary Fig. S5). Downregulation is rapid, with a ≥2.5-fold decrease in MFI ratio by day 3. Lipids accumulate in adipogenic cultures, but not in confluent control cultures maintained in growth medium (Fig. 2C; p<0.05). Downregulation of NG2 and CD146 upon contact-induced growth arrest suggests a potential relationship between antigen expression and proliferation.
FIG. 2.
Loss of NG2 and CD146 expression during differentiation. Pooled P2 OAC cultures from five colonies were inoculated at 2×103 cells/cm2 into six-well plates containing 2 mL growth medium/well. Confluent cultures from a single donor were induced to differentiate on day 3 with adipogenic medium (circles) or maintained in growth medium for 25 days as control (triangles). Surface expression of NG2 (A) and CD146 (B) was monitored by flow cytometry and is reported as an MFI ratio as in Figure 1. Lipid accumulation (C) presented as a standardized score of AdipoRed staining as in Supplementary Figure S1. Data are expressed as a mean±standard error of the mean (n=3). *p<0.05 and **p<0.01 versus confluent control on the same day.
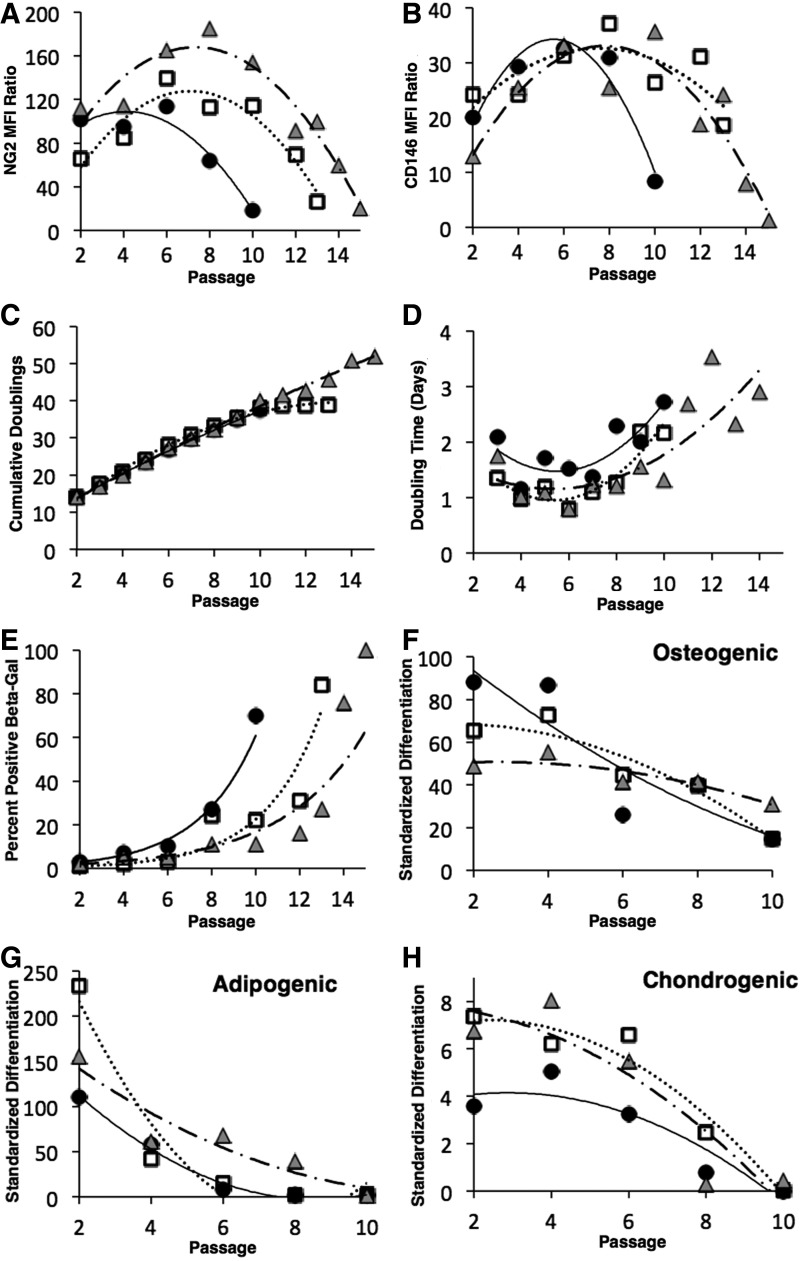
FIG. 4.
Passaging effects on OACs. Pooled P2 OAC cultures from five single cell-derived colonies were inoculated at 100 cells/cm2 into T-150 flasks containing 20 mL growth medium and serial passaged at 50% confluence. MFI ratio of NG2 (A) and CD146 (B) surface expression was determined by flow cytometry as in Figure 1. Growth properties were evaluated by monitoring cumulative doublings (C) and doubling time (D). Senescence-associated β-galactosidase activity (E) in 30 randomly selected images of subconfluent cultures. Cell potency to exhibit osteo- (F), adipo-, (G) and chondrogenesis (H) was quantified after 21 days of differentiation as in Supplementary Figure S1. Threshold values of positive differentiation are given in Supplementary Figure S1. Data are presented for n=3 independent cultures. Each symbol represents a different pooled group of five single cell-derived colonies from the same donor.
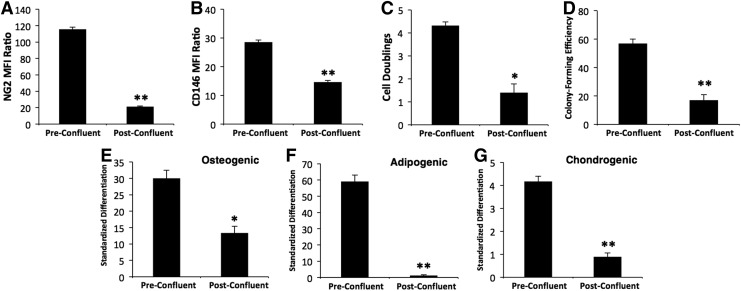
This line of investigation was continued with a comparison of pre- and post-confluent MSC cultures during ex vivo expansion (Fig. 3). The preconfluent control was inoculated with P2 OACs, and the postconfluent culture was passaged once and expanded after being confluent for a week. Both cultures were subconfluent when analyzed. There is a six- and two-fold decrease on average in the MFI ratios for NG2 and CD146 (n=3), respectively, for the postconfluent culture relative to the control (Fig. 3A, B; p<0.01). Downregulation in marker expression is accompanied by a loss in proliferation potential; cell doublings and colony-forming efficiency for the postconfluent culture are no more than half that of the control (Fig. 3C, D; p<0.05). The postconfluent culture is more lineage-committed as well, with loss of adipogenic potential and diminished osteo- and chondrogenesis (Fig. 3E–G; p<0.05). These findings support the potential relationship of marker expression to proliferation and potency depicted in Figure 2 and Supplementary Figure S4, respectively.
FIG. 3.
Effect of confluence on surface expression of NG2 and CD146, proliferation potential and potency. Pooled P2 OAC cultures from five single cell-derived colonies were inoculated at 100 cells/cm2 into T-150 flasks containing 20 mL medium. Preconfluent cultures were analyzed 5 days after inoculation. T-flask cultures that were confluent for 7 days were passaged once to generate postconfluent samples, which were analyzed 5 days after passaging when the cells were <50% confluent. Surface expression of NG2 (A) and CD146 (B) reported as an MFI ratio as in Figure 1. Proliferation potential was evaluated by monitoring (C) cell doublings during the first 5 days after inoculation and (D) colony-forming efficiency when cell samples were plated at clonogenic levels. Cell potency to exhibit osteo- (E), adipo-, (F) and chondrogenesis (G) was quantified as in Supplementary Figure S1. Data are expressed as a mean±standard error of the mean (n=3). *p<0.05 and **p<0.01 versus preconfluent culture.
Figure 4 depicts the effects of serial passaging on NG2 and CD146 surface expression in subconfluent cultures initially inoculated with P2 OACs. Cultures were passaged when the cells reached 50% confluence. Flow cytometric analysis revealed that NG2 (Fig. 4A) and CD146 (Fig. 4B) have concave expression profiles as a function of passage number with maximum expression occurring between passage 4–8. The loss of antigen expression with passaging was confirmed with three MSC donors (Supplementary Fig. S6). The cultures are capable of serial passage upward of 10–15 passages. This is equivalent to a maximum cumulative doubling of 35–50 (Fig. 4C), which is consistent with the Hayflick limit for diploid cells. The time for cultures to become 50% confluent during each passage is longer at late passage, with doubling times increasing greater than two-fold (Fig. 4D). The minimum doubling time (∼1 day; Fig. 4D) corresponds to maximum NG2 and CD146 surface expression (Fig. 4A, B). The inverse relationship between marker expression and doubling time has a Pearson correlation coefficient between −0.6 to −0.7 (Supplementary Fig. S7). As proliferation diminishes in later passages, senescent cells accumulate, accounting for 70%–100% of the cells in culture (Fig. 4E). By passage 10, the cultures retain some osteogenic potential (Fig. 4F) but lose adipo- (Fig. 4G) and chondrogenic potential (Fig. 4H). Loss of adipogenic potential (Fig. 4G) is associated with an increase in NG2 and CD146 surface expression at early passage and a decrease in marker expression at late passage. In summary, maximum surface expression of NG2 and CD146 is associated with minimum doubling time, supporting the potential relationship between marker expression and proliferation depicted in Figures 2 and 3. Serial passage revealed a complex relationship between NG2 and CD146 surface expression and adipogenic potential, suggesting some limits to their utility as potency markers.
Comparison of heterogeneous MSCs sorted into rapidly and slowly dividing cells
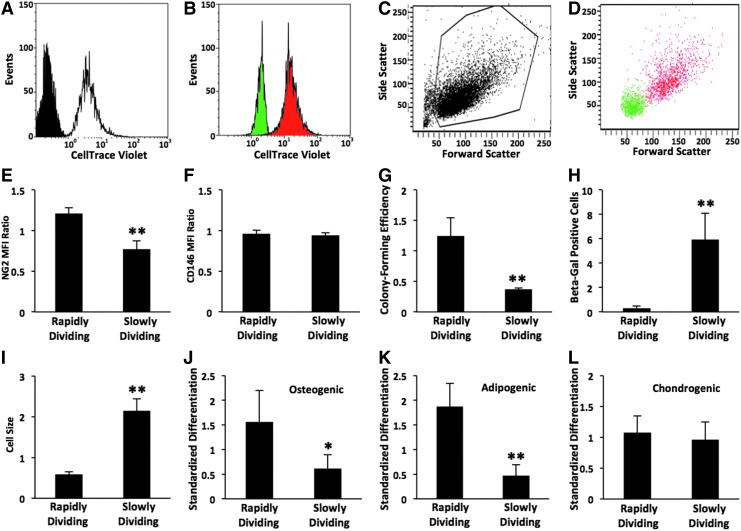
Variation in surface expression of NG2 and CD146 upon confluence (Figs. 2 and 3) and during serial passage (Fig. 4) of OACs suggests a potential relationship between proliferation and antigen expression, which we explored by sorting P2 MSCs into rapidly and slowly dividing groups (Fig. 5). Heterogeneous parental MSCs were labeled with CellTrace Violet and amplified for 4 days (Fig. 5A) to detect cell division through loss of fluorescence. Viable cells with the highest and lowest 10% of fluorescence intensity were sorted by FACS into slowly and rapidly dividing groups, respectively (Fig. 5B). Rapidly dividing MSCs exhibit a lower forward and side scatter than the slowly dividing group (Figs. 5C, D). Comparisons between the two groups in Figures 5E–L are presented relative to the postsort parental control. NG2 surface expression (Fig. 5E) is upregulated in rapidly dividing MSCs, with an MFI ratio that is 50% higher on average as compared with the slowly dividing group (n=3; p<0.01). This trend is evident among multiple MSC donors (Supplementary Fig. S8A). In contrast, no significant difference in CD146 surface expression was detected between the two MSC groups (Fig. 5F and Supplementary Fig. S8B). We validated our sorted groups by evaluating colony-forming efficiency as a standard measure of proliferative potential (Fig. 5G), and β-galactosidase expression and cell size (Fig. 5H, I) as indicators of senescence. Colony-forming efficiency (Fig. 5G) of rapidly dividing MSCs is >2.5-fold higher than that of the slowly dividing group (n=4; p<0.01). β-Galactosidase activity (Fig. 5H) is negligible in the rapidly dividing group. In agreement with the low scatter properties of rapidly dividing MSCs (Fig. 5D), they are greater than three times smaller in size (Fig. 5I) than slowly dividing cells (n=3; p<0.01). Consistent with the loss of potency during serial passage (Fig. 4), osteo- and adipogenesis is significantly impaired in slowly dividing MSCs (Fig. 5J, K). In conclusion, sorting early passage MSCs into rapidly and slowly dividing cells revealed a disparity between the two groups in NG2 surface expression, scatter properties, colony-forming efficiency, senescence, and potency, but not in CD146 surface expression. This suggests that NG2 may be a more effective marker of proliferation potential than CD146 in heterogeneous MSC cultures during ex vivo expansion.
FIG. 5.
Characterization of MSCs sorted into rapidly and slowly dividing populations. Heterogeneous parental MSCs (P2) were labeled with CellTrace Violet and amplified for 4 days. Viable cells with the highest and lowest 10% of fluorescence intensity were sorted by fluorescence-activated cell sorting (FACS) into slowly and rapidly dividing populations, respectively. Representative histograms (A–D) from FACS analysis of 10,000 cells. (A) Parental MSCs labeled with CellTrace Violet (white) are shown relative to unlabeled cells (black). (B) Purity of sorted populations was evaluated by reanalysis of rapidly (green) and slowly (red) dividing cells. Scatter properties of parental MSCs (C, viable gate shown) and sorted populations (D). Surface expression of NG2 (E) and CD146 (F) reported as an MFI ratio as in Figure 1 (n=3–4). (G) Efficiency of sorted cells to form colonies when plated at clonogenic levels (n=4). (H) Senescence-associated β-galactosidase activity as in Figure 4 (n=3). (I) Cell size determined by analysis of 30 images of n=3 subconfluent cultures. Cell potency to exhibit osteo- (J), adipo-, (K) and chondrogenesis (L) as in Supplementary Figure S1 (n=4–5). Threshold values of positive differentiation are given in Supplementary Figure S1. Data are expressed as a mean±standard deviation relative to the sorted parental control with the following phenotype: 240±10 NG2 MFI ratio, 42±6 CD146 MFI ratio, 46%±10% colony-forming efficiency, 9%±2% positive for β-galactosidase, 2900±300 μm2 cell size, and standardized score of 30±7, 170±60, and 8.0±0.8 for osteo-, adipo-, and chondrogenesis, respectively. *p<0.05 and **p<0.01 versus rapidly dividing MSCs.
Enrichment of heterogeneous MSCs for a proliferative phenotype
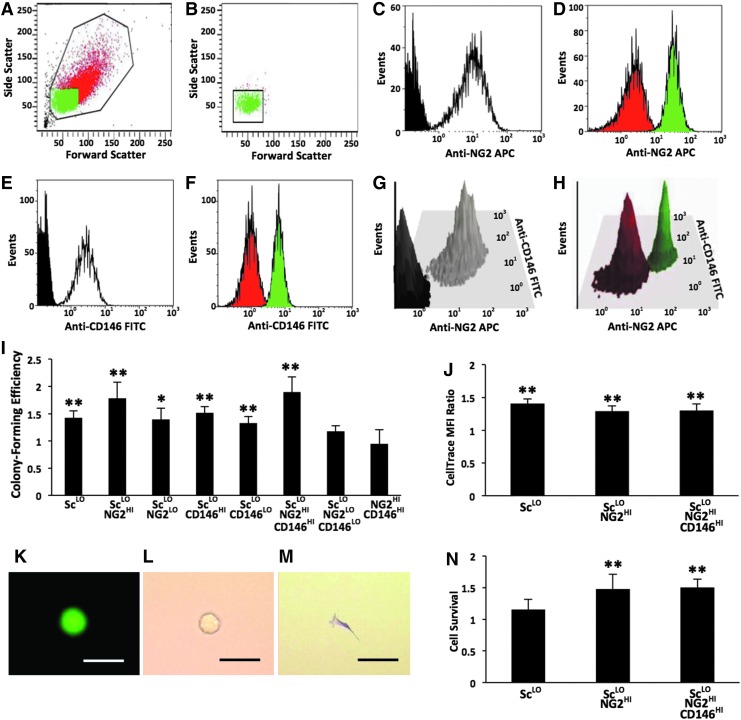
As demonstrated in Figure 5, rapidly dividing MSCs display low scatter and high NG2 surface expression. Figure 6 explores with FACS whether the inverse is true. We examined the proliferative potential of heterogeneous P2 MSCs sorted based on scatter properties and surface expression of NG2 and CD146. A low scatter gate (ScLO) was selected to include 95%±2% (n=3) of rapidly dividing cells labeled with CellTrace Violet (Fig. 6A, B). ScLO MSCs were sorted into the highest 10% (HI) or lowest 10% (LO) of the positive fluorescence intensities for NG2 and/or CD146 (Fig. 6C–H). Analysis of proliferative potential (Fig. 6I, J, N) is presented relative to the postsort parental control. ScLONG2HI and ScLONG2HICD146HI MSCs exhibit the highest colony-forming efficiencies among the groups examined (Fig. 6I), with values that are 1.5–2.2 times the colony-forming efficiency of the parental control (n=3; p<0.01). This trend is evident among multiple MSC donors (Supplementary Fig. S9) that were selected to have similar colony-forming efficiencies for the parental control, 36%±3% (n=3). Interestingly, the ScLONG2LOCD146LO phenotype is the only ScLO group that has a colony-forming efficiency that is comparable with that of the parental control (Fig. 6I). Sorting based on NG2 and CD146 alone does not improve colony-forming efficiency due to elevated autofluorescence of MSCs with high scatter (Fig. 6I). All ScLO groups have a similar morphology and are multipotent with trilineage potential between that of ScLO MSCs and the parental control (data not shown).
FIG. 6.
Proliferative potential of MSCs sorted based on scatter properties and surface marker expression. Heterogeneous parental MSCs (P2) were sorted by FACS to select for low scatter (ScLO) MSCs with either the highest 10% (HI) or lowest 10% (LO) of positive fluorescence intensities from anti-NG2 APC and/or anti-CD146 FITC. Representative histograms (A–H) from FACS analysis of 10,000 cells. (A) Parental MSCs were gated based on viability to eliminate debris (black) and sorted to select viable ScLO cells (green) from cells with higher scatter (red). (B) ScLO gate (shown) was selected to include ∼95% of rapidly dividing MSCs (green) labeled with CellTrace Violet as in Figure 5. Surface expression of NG2 (C), CD146 (E), and both markers (G) was determined for ScLO MSCs (white) relative to isotype controls (black). Purity of sorted populations for NG2 (D), CD146 (F), and both markers (H) was evaluated by reanalysis of the high (green) and low (red) expressing cells. (I) Colony-forming efficiency of sorted cells (n=3). (J) Cell division was measured as the MFI ratio of sorted MSCs immediately upon labeling with CellTrace Violet divided by the corresponding value 4 days after labeling (n=6). (K–N) Survival of sorted MSCs 24 h after plating at clonogenic levels. Fluorescence (K) and phase-contrast (L) micrographs of one sorted MSC/well labeled with CellTracker Green upon inoculating 96-well microplates. (M) Adherent cells after 24 h stained with Crystal Violet. Scale bars (K–M): 50 μm. (N) Cell survival for each MSC population (n=4). Data are expressed as the mean±standard deviation relative to the sorted parental control with the following phenotype: 35%±5% colony-forming efficiency, 29±7 CellTrace Violet MFI ratio, and 53%±15% cell survival. *p<0.05 and **p<0.01 versus parental control.
To further investigate colony formation in select MSC groups, we next examined cell survival at clonogenic levels and subsequent cell division. Relative cell division was analyzed with CellTrace Violet and is reported as the MFI ratio of sorted MSC groups on the day of labeling divided by the corresponding value 4 days after labeling (Fig. 6J). The ScLO, ScLONG2HI, and ScLONG2HICD146HI groups all divide faster than the parental control (n=6; p<0.01), but no difference in the rate of cell division is evident among the three ScLO groups. Cell survival was measured in 96-well microplates inoculated at one cell/well with sorted MSCs labeled with CellTracker Green, followed by Crystal Violet staining 24 h later (Fig. 6K–M). ScLONG2HI and ScLONG2HICD146HI MSCs exhibit a 50% increase in survival (Fig. 6N) on average relative to the parental control (n=4; p<0.01). In contrast, survival of ScLO MSCs and the parental control are similar. For the sorted groups examined, ScLONG2HI and ScLONG2HICD146HI MSCs have the highest proliferative potential as measured by colony-forming efficiency. The ScLO gate enriches for rapidly dividing cells in heterogeneous MSC cultures. Addition of the NG2HI gate increases cell survival to 1.5 times the parental control. Further addition of the CD146HI gate does not significantly improve cell division or survival.
Discussion
MSC heterogeneity
This study provides insight into the complex heterogeneity of MSC cultures. There are inconsistencies in the literature regarding the ability of the established MSC markers CD73, CD90, and CD10512 to detect cell populations in heterogeneous MSC cultures. Higher surface expression in rapidly growing MSCs has been reported for these markers in some cases,33,34 but not in others.35,36 In our laboratory, OACs and parental MSCs have similar fluorescence intensity histograms for the three biomarkers, suggesting that other means to isolate MSC populations are warranted.
In addition to the cell-surface antigens discussed below, we utilized a proliferation dye and scatter properties to enrich MSC populations. Historically, the earliest methods to enrich hematopoietic stem cell populations used forward and side scatter as selection criteria.37 When we applied a low scatter gate to MSCs, the sorted cells proliferated more rapidly than the parental MSC control. Proliferation dye has been employed to measure the doubling time of MSC subsets with different size and granularity.38 We built upon this initial work by demonstrating the value of a proliferation dye, CellTrace Violet, as a tool to sort heterogeneous MSCs into two groups with distinct phenotypes. The rapidly dividing population of CellTrace-labeled MSCs are small, agranular, and multipotent; whereas, slowly dividing cells are larger, and more granular, senescent, and lineage-committed. These two subsets are consistent with the recycling stem cells and mature cells in MSC cultures that were originally identified by Colter et al.8
Our findings also provide insight into the heterogeneity of MSC potency. In previous work with single-cell derived MSC colonies, we detected all eight possible categories of trilineage potential to exhibit osteo-, adipo-, and chondrogenesis.10 Mutipotent MSC colonies were in the majority, while more lineage-committed colonies with an adipo-chondrogenic or strictly adipogenic phenotype were in the minority. Likewise, others have detected these adipogenic potencies infrequently, if at all, in MSC cultures.9,39 The present study demonstrates that the adipogenic potential of MSCs is rapidly lost upon serial passage in growth medium. This kinetic profile is consistent with the rarity with which MSCs committed to an adipogenic lineage occur in vitro. In addition, the slower rate at which MSCs lose chondrogenic potential than adipogenic potential during serial passage may contribute to the broader stochastic variation in proliferation potential that we previously observed among single osteo-chondrogenic cell-derived MSC colonies with an osteochondrogenic phenotype than among osteo-adipogenic colonies.11
NG2
NG2 shows promise in this study as a cell-surface marker to enrich MSCs with increased proliferative potential as measured by colony-forming efficiency. We reported for multiple donors that ScLONG2HI cells in heterogeneous MSC cultures have a higher colony-forming efficiency than ScLO MSCs and the parental control. Similarly, we observed that sorting MSCs based on the fluorescence intensity of CellTrace Violet yields a rapidly dividing population with higher surface expression of NG2 relative to a slowly dividing population. While there are no data in the literature on NG2-sorted MSCs, our experiments with OACs provide supportive results. Surface expression of NG2 is higher on OACs than parental MSCs and lineage-committed MSCs, and we previously reported that OACs have the highest colony-forming efficiency and fastest growth rates of the various MSC subsets in culture.11 Likewise, maximum surface expression of NG2 is concurrent with minimum doubling time during serial passage. Moreover, we found that NG2 expression is downregulated upon contact-induced growth arrest in confluent OAC cultures. In aggregate, these results provide compelling evidence of a correlation between NG2 expression and the proliferative potential of MSCs.
FACS results suggest that the ScLO gate enriches rapidly dividing cells in heterogeneous MSC cultures. Addition of the NG2HI gate selects enhanced cell survival. These results are consistent with our selection of the ScLO gate to include ∼95% of rapidly dividing MSCs labeled with CellTrace Violet. Our data on the survival of ScLONG2HI MSCs agree with published reports of NG2-mediated chemoresistance in several cancers.40 For example, NG2-transfected U251 glioma cells are resistant to doxorubicin and other chemotherapeutic drugs; whereas, parental U251 cells are more sensitive to these agents.41 The involvement of NG2 in MSC survival is an important finding in our study given that cell survival is a basic prerequisite to any application with MSCs.
When we compare our findings with the limited data available on NG2 expression in heterogeneous MSCs, there is agreement with a previous report of downregulation of NG2 expression during adipogenesis.24 We extend this study by demonstrating that confluence alone is sufficient to rapidly downregulate NG2 surface expression. This implies that the use of NG2 as a MSC marker be limited to subconfluent cultures. Our findings are in apparent conflict with a report that the percentage of NG2-positive cells, but not their mean fluorescent intensity, changes with culture passage of heterogeneous MSCs.24 In contrast, we found fluorescent intensity for NG2 to be highly dependent on passage number. The discrepancy may reflect differences in the inocula from the two studies: heterogeneous MSCs versus OACs. For example, population dynamics in heterogeneous cultures cause change in the relative concentration of cell subsets,42 which would be mitigated with a well-defined inoculum of OACs. As an indicator of this, the percentage of NG2+ MSCs diminished monotonically over 9 passages in heterogeneous MSC cultures from the previous study;24 whereas, the NG2 expression profile is concave in our OAC cultures over a total of 10–15 passages.
Our serial passaging experiments revealed limitations to the use of NG2 as a potency biomarker inasmuch as its expression is insensitive to some changes in potency. Downregulation of NG2 at late passage corresponds to commitment of MSCs to an osteogenic lineage; however, upregulation of NG2 at early passage is accompanied by a loss of potency as well. The limitation of a proliferation marker to detect some changes in potency is consistent with the similarity of proliferation potentials for different intermediate potency groups that we detected in previous research with MSCs.11 Stallcup and Huang40 proposed NG2 as a marker of cell activation to describe patterns of NG2 expression during the development of connective tissue and the central nervous system. NG2 is expressed in mitotic progenitors that retain some developmental plasticity and downregulated in quiescent cells that are terminally differentiated.23 For instance, NG2 was first detected in progenitors with mixed glial and neuronal properties.43 The description of NG2 as a marker of MSC activation accurately characterizes NG2 expression in this study—both at early passage when proliferating MSCs lose potency and at late passage when lineage-committed MSCs become quiescent. Further, it is consistent with NG2 as a marker of cell survival of MSCs as described above.
As a perivascular marker,18 the level of NG2 surface expression could possibly be predictive of the propensity of MSCs to differentiate into pericytes. If so, the ScLONG2HI fraction of MSCs may select rapidly proliferating progenitors with increased capacity to assemble and remodel vascular structures. This possibility merits investigation.
CD146
For cultures inoculated with OACs, the pattern of CD146 surface expression is similar to those described above for NG2 and is consistent with published reports of CD146 as a marker for a proliferative fraction of MSCs.21,22 In contrast, the expression pattern for CD146 and NG2 is different when heterogeneous MSCs are sorted into rapidly and slowly dividing cells. The MFI ratio is higher for NG2 in rapidly dividing MSCs; whereas, it is comparable for CD146 in both groups. This finding is in apparent conflict with the correlation between higher CD146 expression and enhanced proliferation observed in our cultures inoculated with well-defined OACs. We attribute this discrepancy to the complex cell composition in heterogeneous MSC cultures, which convolutes results. As supporting evidence, the difference in the MFI ratio between cell groups with high and low NG2 expression is smaller in this study for cultures inoculated with heterogeneous MSCs than OACs. Moreover, the MFI ratio for CD146 is less responsive to perturbations in OAC proliferation potential and potency than the MFI ratio for NG2. Our inability to detect differences in CD146 expression between rapidly and slowly dividing cells in heterogeneous MSC cultures is consistent with this decrease in sensitivity.
Likewise, the colony-forming efficiency of ScLONG2HICD146HI MSCs is not significantly greater than the corresponding value for the ScLONG2HI fraction. Previous research showed that CD146 can isolate clonogenic cells from unfractioned bone marrow.21 Once this initial purification step is complete, our results suggest that further sorting cultured MSCs based on CD146 expression makes only modest improvements in proliferative potential by the methods employed in this study. While both ScLONG2HI and ScLONG2HICD146HI MSCs have high colony-forming efficiencies, it is possible that they may differ in other properties yet to be explored, such as tissue vascularization as both NG2 and CD146 are perivascular markers.
Our results provide insight into the influence of culture conditions on CD146 expression in MSCs. There are conflicting data about CD146 expression during adipogenesis in heterogeneous MSC cultures: the antigen is unaffected by differentiation in one report44 and downregulated in another.45 The discrepancy may reflect a combination of the extent of MSC heterogeneity, which could have obscured the phenomenon, and duration of differentiation (7 vs. 10 days). Using cultures inoculated with well-defined OACs, we detected a two- to three-fold decrease in MFI ratio for CD146 during the same period of adipogenesis. In another study, CD146 was expressed at high levels in nearly all clonal MSCs, but only a minority of nonclonal MSCs plated at high density were positive for this marker.21 The authors noted the presence of putatively mature (CD146−) osteoblasts in the nonclonal cultures. It is not possible to discern the role of confluence on CD146 expression from this earlier study because both culture heterogeneity and cell density were variables. Our results with well-defined OACs demonstrate that confluence alone is sufficient to downregulate CD146. Nor is it possible to evaluate the temporal change in marker expression from a single time point as in the previous example.21 Our kinetic experiment over multiple time points shows that the rate at which CD146 is downregulated is not affected by the addition of adipogenic agents to the culture medium. Previous research with heterogeneous MSCs showed a slight decrease in percentage of CD146+ cells during serial passage.44,46 In contrast, we observed a concave profile for surface expression of CD146 while well-defined cultures inoculated with OACs lost potency over 10–15 passages. MSC heterogeneity in prior studies could have obscured this complex change in marker expression. Our findings provide unique insight into possible limitations in the use of CD146 as a potency marker.
Applications
This study identified the combination of low scatter and high NG2 surface expression as a promising selection criterion to enrich a proliferative phenotype during ex vivo expansion of heterogeneous MSCs. Our finding that NG2 selects enhanced MSC survival is particularly significant because cell survival is essential to any application with MSCs. For basic research applications, we envision that our sort strategy may aid in standardizing the composition of MSC cultures to facilitate comparison of research results among laboratories. It has been proposed that multiple cell populations within heterogeneous MSC cultures participate in tissue repair through diverse mechanisms.47 The ability to isolate specific MSC populations will enable researchers to resolve their regenerative properties and examine their interactions. In addition, this sorting strategy may help researchers to elucidate the disparity in the response of MSC populations to regulatory factors that signal tissue repair.48 Our observation that confluence downregulates NG2 surface expression should not impede application of our sort strategy to MSC cultures because these cells are routinely cultivated under subconfluent conditions to mitigate loss of proliferation potential and potency during expansion.
Potentially, our findings may have a significant impact on the manufacturing of MSC therapies. MSCs must be expanded ex vivo for most clinical applications49 due to their rarity in tissues.50 Our sort strategy may aid in isolating MSCs with the ability to expand sufficient quantities of cells ex vivo for MSC therapies. Moreover, our results may enable manufacturers to monitor and enrich MSC populations during ex vivo expansion as a means to standardize the composition of MSC therapies. FACS greatly reduces manufacturing time and cost over clonal methods for large-scale enrichment of an MSC population.10 This improvement in efficiency is essential to the production of MSC therapies for time-critical conditions. Our sort strategy could possibly remove senescent cells from ex vivo MSC cultures during manufacturing of MSC therapies. This is especially relevant to autologous MSC therapies for the elderly where accumulation of senescent cells is likely.51
In vivo research is required in the future to determine if our sort strategy could improve the efficacy of MSC therapies. Proliferative potential and, in particular, colony formation has been shown in select cases to predict the clinical outcome of tissue repair with adult stem cells.52 Given the prognostic value of proliferative potential, our sort strategy could possibly help select donor MSCs from a cell bank or to evaluate the quality of autologous MSCs harvested from a patient. Lastly, rapidly proliferating MSCs preferentially engraft in tissue relative to slowly proliferating MSCs.6 Our sort strategy could potentially aid in the enrichment of MSCs that readily survive and engraft during tissue repair.
Summary
An initial comparison of cell-surface expression of perivascular and MSC markers in OACs and heterogeneous parental MSCs detected differential expression of NG2 and CD146 in the two groups. The use of well-defined OAC cultures provides unique insights into the relationship of NG2 and CD146 surface expression to proliferation and potency. This study helped to resolve discrepancies in the literature about the effect of adipogenesis on CD146 expression, discern the role of confluence in downregulating CD146 without the confounding variable in previous research, and reveal some limits previously unreported to the use of CD146 as a potency marker. This is the first detailed study of NG2 expression in MSC cultures. Cell-surface expression of NG2 is particularly responsive to changes in the proliferation potential of OACs during cultivation, but not to some changes in potency.
Based on these findings, NG2 and CD146 were evaluated as potential proliferation markers by sorting heterogeneous MSC cultures with FACS. Amidst the complex composition of a heterogeneous culture, differential surface expression was detected for NG2 when MSCs were sorted based on the rate of cell division, but not for CD146. Through a combinatorial approach of gating on scatter properties and surface expression of NG2 and CD146, we developed a novel sort strategy of low scatter and high NG2 surface expression, which shows promise for enrichment of proliferative cells during ex vivo expansion of heterogeneous MSCs. ScLONG2HI MSCs have a colony-forming efficiency in vitro that is at least 1.5 times greater than the parental controls. The ScLO gate removes cells with high autofluorescence and enriches rapidly dividing cells. Addition of a NG2HI gate increases cell survival to 1.5 times the parental control. Further addition of a CD146HI gate does not significantly improve cell division or survival. Given the importance of cell proliferation and survival, in particular, to the utilization of MSCs, this sort strategy has potentially numerous applications.
Supplementary Material
Acknowledgments
We are grateful for technical assistance for processing samples in the core flow cytometry facilities at the Center for Stem Cell Biology and Regenerative Medicine and Louisiana Cancer Research Consortium. This publication is based upon work supported in part by a grant from the National Science Foundation (CBET-1066167) to KOC, an IDEA award from Tulane University to KOC, and grants from the National Institutes of Health (CA-55164, CA-16672, CA-100632, and CA-136411) to MA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Barrilleaux B.L. Phinney D.G. Prockop D.J. O'Connor K.C. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 2.Salem H.K. Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phinney D.G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 4.Ho A.D. Wagner W. Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 5.Pevsner-Fischer M. Levin S. Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev. 2011;7:560. doi: 10.1007/s12015-011-9229-7. [DOI] [PubMed] [Google Scholar]
- 6.Lee R.H. Hsu S.C. Munoz J. Jung J.S. Lee N.R. Pochampally R. Prockop D.J. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S. Ge J. Sun A. Xu D. Qian J. Lin J. Zhao Y. Hu H. Li Y. Wang K. Zou Y. Comparison of various kinds of bone marrow stem cells for the repair of infarcted myocardium: single clonally purified non-hematopoietic mesenchymal stem cells serve as a superior source. J Cell Biochem. 2006;99:1132. doi: 10.1002/jcb.20949. [DOI] [PubMed] [Google Scholar]
- 8.Colter D.C. Sekiya I. Prockop D.J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muraglia A. Cancedda R. Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 10.Russell K.C. Phinney D.G. Lacey M.R. Barrilleaux B.L. Meyertholen K.E. O'Connor K.C. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 11.Russell K.C. Lacey M.R. Gilliam J.K. Tucker H.A. Phinney D.G. O'Connor K.C. Clonal analysis of the proliferation potential of human bone marrow mesenchymal stem cells as a function of potency. Biotechnol Bioeng. 2011;108:2716. doi: 10.1002/bit.23193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F.C. Krause D.S. Deans R.J. Keating A. Prockop D.J. Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Hachisuka H. Mochizuki Y. Yasunaga Y. Natsu K. Sharman P. Shinomiya R. Ochi M. Flow cytometric discrimination of mesenchymal progenitor cells from bone marrow-adherent cell populations using CD34/44/45(-) and Sca-1(+) markers. J Orthop Sci. 2007;12:161. doi: 10.1007/s00776-006-1098-6. [DOI] [PubMed] [Google Scholar]
- 14.Quirici N. Soligo D. Bossolasco P. Servida F. Lumini C. Deliliers G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 15.Jones E.A. Kinsey S.E. English A. Jones R.A. Straszynski L. Meredith D.M. Markham A.F. Jack A. Emery P. McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 16.Shih I.M. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Ouhtit A. Gaur R.L. Elmageed Z.Y.A. Fernando A. Thouta R. Trappey A.K. Abdraboh M.E. El-Sayyad H.I. Rao P. Raj M.G. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009;1795:130. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Covas D.T. Panepucci R.A. Fontes A.M. Silva W.A., Jr. Orellana M.D. Freitas M.C.C. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Crisan M. Yap S. Casteilla L. Chen C.-W. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.-N. Traas J. Schugar R. Deasy B.M. Badylak S. Bűhring H.-J. Giacobino J.-P. Lazzari L. Huard J. Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Shi S. Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 21.Sacchetti B. Funari A. Michienzi S. Di Cesare S. Piersanti S. Saggio I. Tagliafico E. Ferrari S. Robey P.G. Riminucci M. Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Sorrentino A. Ferracin M. Castelli G. Biffoni M. Tomaselli G. Baiocchi M. Fatica A. Negrini M. Peschle C. Valtieri M. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp Hematol. 2008;36:1035. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Stallcup W.B. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- 24.Kozanoglu I. Boga C. Ozdogu H. Sozer O. Maytalman E. Yazici A.C. Sahin F.I. Human bone marrow mesenchymal cells express NG2: possible increase in discriminative ability of flow cytometry during mesenchymal stromal cell identification. Cytotherapy. 2009;11:527. doi: 10.1080/14653240902923153. [DOI] [PubMed] [Google Scholar]
- 25.Sekiya I. Larson B.L. Smith J.R. Pochampally R. Cui J.-G. Prockop D.J. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 26.Barrilleaux B.L. Phinney D.G. Fischer-Valuck B.W. Russell K.C. Wang G. Prockop D.J. O'Connor K.C. Small-molecule antagonist of macrophage migration inhibitory factor enhances migratory response of mesenchymal stem cells to bronchial epithelial cells. Tissue Eng A. 2009;15:2335. doi: 10.1089/ten.tea.2008.0434. [DOI] [PubMed] [Google Scholar]
- 27.Blanch H.W. Clark D.S. Biochemical Engineering. New York: Marcel Dekker; 1997. [Google Scholar]
- 28.Roederer M. Compensation in Flow Cytometry. Curr Protoc Cytom. 2002;Chapter 1(Unit 1.14) doi: 10.1002/0471142956.cy0114s22. [DOI] [PubMed] [Google Scholar]
- 29.Song H. David O. Clejan S. Giordano C.L. Pappas-Lebeau H. Xu L. O'Connor K.C. Spatial composition of prostate cancer spheroids in mixed and static cultures. Tissue Eng. 2004;10:1266. doi: 10.1089/ten.2004.10.1266. [DOI] [PubMed] [Google Scholar]
- 30.Paradis H. Liu C.-Y. Saika S. Azhar M. Doetschman T. Good W.V. Nayak R. Laver N. Kao C.W.-C. Kao W.W.-Y. Gendron R.L. Tubedown-1 in remodeling of the developing vitreal vasculature in vivo and regulation of capillary outgrowth in vitro. Dev Biol. 2002;249:140. doi: 10.1006/dbio.2002.0757. [DOI] [PubMed] [Google Scholar]
- 31.Traktuev D.O. Merfeld-Clauss S. Li J. Kolonin M. Arap W. Pasqualini R. Johnstone B.H. March K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 32.Zannettino A.C.W. Paton S. Arthur A. Khor F. Itescu S. Gimble J.M. Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 33.Majore I. Moretti P. Hass R. Kasper C. Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun Signal. 2009;7:6. doi: 10.1186/1478-811X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haasters F. Prall W.C. Anz D. Bourquin C. Pautke C. Endres S. Mutschler W. Docheva D. Schieker M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214:759. doi: 10.1111/j.1469-7580.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markov V. Kusumi K. Tadesse M.G. William D.A. Hall D.M. Lounev V. Carlton A. Leonard J. Cohen R.I. Rappaport E.F. Saitta B. Identification of cord blood-derived mesenchymal stem/stromal cell populations with distinct growth kinetics, differentiation potentials, and gene expression profiles. Stem Cells Dev. 2007;16:53. doi: 10.1089/scd.2006.0660. [DOI] [PubMed] [Google Scholar]
- 36.Mareddy S. Crawford R. Brooke G. Xiao Y. Clonal isolation and characterization of bone marrow stromal cells from patents with osteoarthritis. Tissue Eng. 2007;13:819. doi: 10.1089/ten.2006.0180. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro H.M. Practical Flow Cytometry. 4th. Hoboken, NJ: Wiley-Liss; 2003. [Google Scholar]
- 38.Urbani S. Caporale R. Lombardini L. Bosi A. Saccardi R. Use of CFDA-SE for evaluating the in vitro proliferation pattern of human mesenchymal stem cells. Cryotherapy. 2006;8:243. doi: 10.1080/14653240600735834. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto T. Aoyama T. Nakayama T. Nakamata T. Hosaka T. Nishijo K. Nakamura T. Kiyono T. Toguchida J. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002;295:354. doi: 10.1016/s0006-291x(02)00661-7. [DOI] [PubMed] [Google Scholar]
- 40.Stallcup W.B. Huang F.-J. A role for the NG2 proteoglycan in glioma progression. Cell Adh Migr. 2008;2:192. doi: 10.4161/cam.2.3.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chekenya M. Krakstad C. Svendsen A. Netland I.A. Staalesen V. Tysnes B.B. Selheim F. Wang J. Sakariassen P.Ø. Sandal T. Lønning P.E. Flatmark T. Enger P.Ø. Bjerkvig R. Sioud M. Stallcup W.B. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H. O'Connor K.C. Papadopoulos K.D. Jansen D.A. Differentiation kinetics of in vitro 3T3-L1 preadipocyte cultures. Tissue Eng. 2002;8:1070. doi: 10.1089/107632702320934164. [DOI] [PubMed] [Google Scholar]
- 43.Wilson S. Baetge E. Stallcup W.B. Antisera specific for cell lines with mixed neuronal and glial properties. Dev Biol. 1981;83:146. doi: 10.1016/s0012-1606(81)80017-6. [DOI] [PubMed] [Google Scholar]
- 44.Gharibi B. Hughes F.J. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1:771. doi: 10.5966/sctm.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delorme B. Ringe J. Gallay N. Le Vern Y. Kerboeuf D. Jorgensen C. Rosset P. Sensebé L. Layrolle P. Häupl T. Charbord P. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 46.Halfon S. Abramov N. Grinblat B. Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cell Dev. 2011;20:53. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 47.Phinney D.G. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007;6:2884. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- 48.Bianchi G. Banfi A. Mastrogiacomo M. Notaro R. Luzzatto L. Cancedda R. Quarto R. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287:98. doi: 10.1016/s0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 49.Ringdén O. Uzunel M. Rasmusson I. Remberger M. Sundberg B. Lönnies H. Marschall H.U. Dlugosz A. Szakos A. Hassan Z. Omazic B. Aschan J. Barkholt L. Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 50.Veryrat-Masson R. Boiret-Dupré N. Papatel C. Descamps S. Guillouard L. Guérin J.J. Pigeon P. Boisgard S. Chassagne J. Berger M.G. Mesenchymal content of fresh bone marrow: a proposed quality control method for cell therapy. Br J Haematol. 2007;139:312. doi: 10.1111/j.1365-2141.2007.06786.x. [DOI] [PubMed] [Google Scholar]
- 51.Wagner W. Bork S. Horn P. Krunic D. Walenda T. Diehlmann A. Benes V. Blake J. Huber F.X. Eckstein V. Boukamp P. Ho A.D. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ezhkova E. Fuchs E. An eye to treating blindness. Nature. 2010;446:567. doi: 10.1038/466567a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.