Abstract
Interstitial pneumonitis as an adverse effect of mesalamine therapy is a rare but potentially serious complication. Patients typically have a mild disease course with no documented cases of respiratory failure in published literature. Given its variable latent period and non-specific signs and symptoms, it may be difficult to diagnose. We present the case of a 65-year-old man who presented with symptoms of fever, shortness of breath and a non-productive cough, 2 weeks after initiation of therapy with mesalamine. His hospital course was complicated by acute respiratory failure requiring intubation and mechanical ventilation. Radiographic studies revealed bilateral lower lobe infiltrates and bronchosopy with bronchoalveolar lavage and transbronchial biopsy were consistent with a diagnosis of drug-induced interstitial pneumonitis. The aim of this paper is to highlight the importance of considering a diagnosis of mesalamine-induced lung injury in patients presenting with respiratory symptoms while on mesalamine therapy and to review relevant literature.
Background
Ulcerative colitis (UC) is a disease of unknown aetiology characterised by the chronic, recurrent mucosal and submucosal inflammation of the gastrointestinal tract. In the USA, it has a prevalence of 238 cases per 100 000 and is most commonly seen in individuals between the ages of 15 and 40 years. Higher rates are seen in well developed areas of the western world among individuals of Caucasian and Ashkenazi Jewish backgrounds, while environmental factors such as cigarette smoking and a history of prior appendectomy have been reported as having a protective effect.1 Patients present with symptoms of bloody diarrhoea, abdominal pain and rectal urgency manifesting in a relapsing and remitting nature.2 3 The medical approach for management of UC includes drugs to achieve a clinical response and remission (induction therapy) and those used to maintain remission (maintenance therapy). Commonly used drugs include 5-aminosalicylic acid (5-ASA) compounds, corticosteroids, antitumour necrosis factor (anti-TNF) agents and thiopurines.4
Extraintestinal manifestations such as uveitis, episcleritis, erythema nodosum, primary sclerosing cholangitis, pyoderma gangrenosum, ankylosing spondylitis and pancreatitis are seen in 21–41% of patients with UC.5 6 Pulmonary involvement is uncommon and is mostly secondary to drug-induced lung disease. Pulmonary toxicity secondary to sulfasalazine has been well documented, with the most common patterns being broncholitis obliterans organising pneumonia and pulmonary granulomatosis.7 The sulfapyridine moiety in sulfasalazine has been implicated as the cause of these side effects. Mesalamine, which lacks this sulfa moiety, very rarely produces lung-related toxicity. Brimblecombe8 reported a 3% side effect rate related to mesalamine use in 1700 patients and no single patient presenting with lung manifestations. Scattered case reports suggest that interstitial lung disease is the most common finding in cases of mesalamine-related lung injury and most of them have had a mild course with no documented cases of respiratory failure.9 Here we report the case of a patient who presented with features suggestive of mesalamine-induced interstitial pneumonitis requiring subsequent intubation, 2 weeks after initiation of treatment.
Case presentation
A 65-year-old man presented to the emergency department with fever and shortness of breath. The patient described the fever as being intermittent for the last 1 week, of low grade, without diurnal variation and not associated with chills, rigours and night sweats. He also had coinciding symptoms of difficulty breathing over the same period. He described the shortness of breath as being mainly related to exertion but had however noticed worsening of symptoms over the past couple of days. He had symptoms of a non-productive cough during the same period, not associated with haemoptysis. The patient denied symptoms of orthopnoea, paroxysmal nocturnal dyspnoea, chest pain, palpitations and swelling of his extremities. Review of systems was unremarkable for nausea, vomiting, headaches, abdominal pain, weight loss, skin rashes and joint pains. He also denied any recent travel, sick contacts, occupational or household exposure to chemical fumes and dust. The patient had been diagnosed with histologically proven UC 2 weeks prior during an outpatient evaluation for chronic diarrhoea which was occasionally bloody in nature. He was started on therapy with mesalamine 1.2 g four times daily. His medical history was also significant for gout for which he was treated with non-steroidal anti-inflammatory drugs. The patient was not allergic to any medications and he denied the use of tobacco, alcohol or any illicit drugs. His family and surgical history was unremarkable.
On physical examination the patient appeared anxious, pale and in mild respiratory distress. Initial vital signs revealed a blood pressure of 115/55 mm Hg, pulse rate of 102 bpm, respiratory rate of 24 breaths/min and a temperature of 38.4° C. The patient had no conjunctival pallor or scleral icterus and examination revealed a supple neck with no evidence of jugular venous distention. Examination of his lungs revealed decreased breath sounds in both lung bases with end inspiratory crackles. No clubbing, cyanosis or peripheral oedema was noted. Cardiovascular and abdominal examinations were unremarkable.
Investigations
Laboratory investigations revealed haemoglobin to be 13.1 g/dL and haematocrit 37.3%, platelet count of 129 000/µL and white cell count of 11 000/µL with 83% neutrophils, 11.5% lymphocytes, 2.2% eosinophils and 3.3% monocytes. Blood urea nitrogen levels were measured at 8.6 mmol/L and creatine was 132 µmol/L. Liver-related tests revealed an alanine aminotransferase of 29 IU/L, aspartate aminotransferase of 41 IU/L, alkaline phosphatase of 75 IU/L, total bilirubin level of 30.7 µmol/L and direct bilirubin of 10.3 µmol/L. Arterial blood gas values revealed a pH of 7.50, pCO2 of 20 mm Hg, pO2 of 70.7 mm Hg and bicarbonate level of 19 mmol/L on 2 L oxygen via nasal cannula. A chest radiograph and CT of the chest showed bilateral lower lobe infiltrates with trace pleural effusions (figure 1). On admission, mesalamine was discontinued and intravenous antibiotics were initiated. However, the patient developed worsening respiratory distress and was intubated later on the same day (figure 2). Further tests included a bronchosopy with bronchoalveolar lavage which was negative for malignant cells and a transbronchial biopsy which revealed interstitial lymphocytic infiltrates, few histiocytes and mild fibrosis. Tuberculin skin test as well as sputum and blood cultures were negative.
Figure 1.
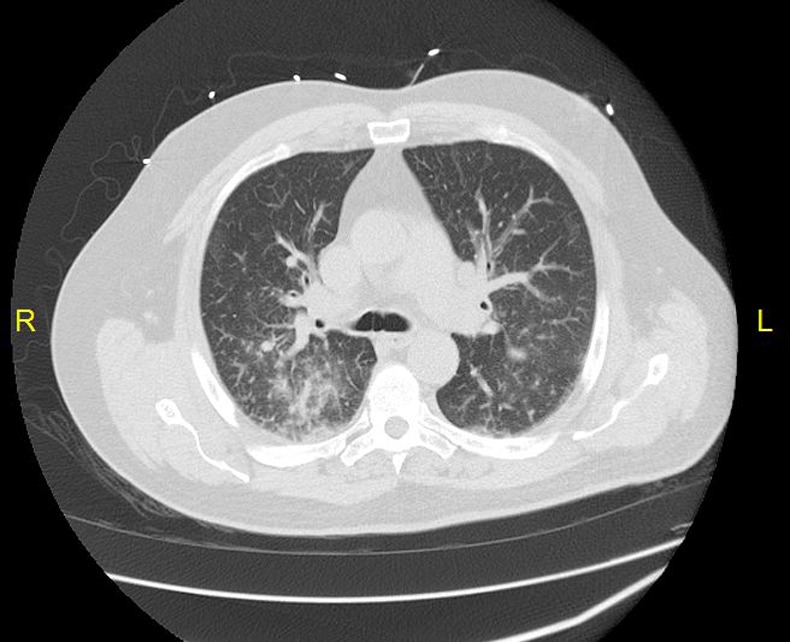
CT of the chest with intravenous contrast showing the presence of bilateral lower lobe infiltrates and trace pleural effusion.
Figure 2.
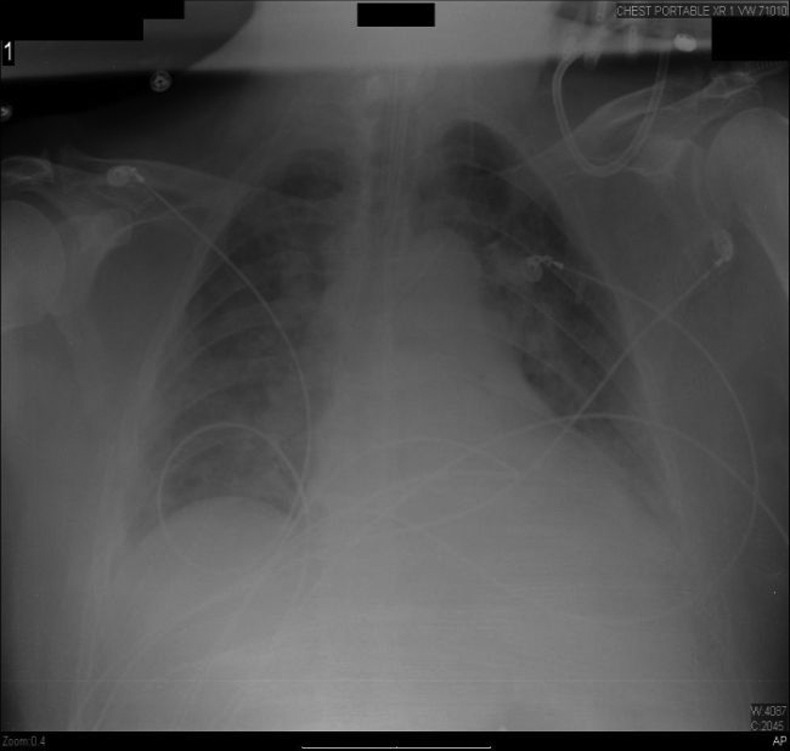
Chest radiograph showing interval placement of the endotracheal tube and bilateral patchy infiltrates.
Treatment
A diagnosis of drug-induced interstitial pneumonitis was made and intravenous corticosteroids were started. The patient showed gradual clinical improvement and 4 days after initial presentation he was extubated. Eight days after initial presentation, the patient's blood work showed normalisation of his complete blood count and metabolic panel including liver-related tests and renal function.
Outcome and follow-up
The patient was discharged upon clinical resolution of symptoms with oral prednisone. At a routine follow-up 6 months after admission the patient has been symptom free and continues to do well.
Discussion
Mesalamine, which is a 5-ASA compound, is recommended as a first-line agent for the treatment of UC. It is the active component of sulfasalazine which in addition contains an inactive sulfapyridine moiety bound to mesalamine by an azo bond. Owing to the absence of the sulfapyridine group which has been implicated as the cause of most adverse effects and hypersensitivity reactions, mesalamine is well tolerated by most patients. The most frequently reported side effects include headaches, nausea, vomiting, abdominal pain, diarrhoea and rashes.10 More serious but rare adverse effects include drug-induced pancreatitis, interstitial nephritis and drug-induced liver injury.11 Mesalamine-induced lung injury is very rare and manifests in various forms including interstitial and eosinophilic pneumonitis, broncholitis obliterans and eosinophilic pleural effusion.12 The exact pathophysiology of mesalamine-induced lung injury is not known. Some authors have suggested that mesalamine causes an immune mediated dose-independent inflammation of the alveolus.13 However, some data suggest that mesalamine may cause a dose-dependent direct toxic insult or oxidant injury to the pulmonary epithelium.9 14
Symptoms related to mesalamine-induced lung injury are insidious and progressive and can range from an ‘asymptomatic stage with radiographic findings’ to fever, cough, fatigue and shortness of breath.7 9 10 15 Available data suggest that mesalamine-induced lung injury follows a mild course; however, our patient presented with severe symptoms requiring intubation and mechanical ventilation. Sossai et al9 reviewed previously published case reports of 5-ASA-related pulmonary toxicity and states the mean age of presentation to be 46 years, with an age range of 15–72 years. Women were also reported to be affected more often than men with most cases involving mesalamine used for the treatment of UC as opposed to Crohn's disease. The time for appearance of symptoms since initiation of therapy varied greatly and ranged from 2 days to 8 years. The reasons for this are poorly understood, but could depend upon host and environmental factors including the mode of mesalamine delivery used.
The diagnosis of mesalamine-induced lung toxicity can be challenging to physicians. Most patients do not present with significant leucocytosis; however, peripheral eosinophilia has been occasionally reported.15 Arterial blood gas may show hypoxia that may be mild to severe; however, none of the earlier reported patients has required mechanical ventilation. Chest radiographs are non-specific and can reveal pleural effusions, bilateral or unilateral upper or lower lobe interstitial infiltrates or consolidation. CT may aid in diagnosis by excluding other lung and mediastinal pathology. Pulmonary function tests if performed reveal a restrictive picture with decreased diffusing capacity.7 16 Bronchoscopy and bronchoalveolar lavage shows increased lymphocytes and/or macrophages with an inversion of the CD4+/CD8+ ratio.9 Transbronchial biopsy may reveal interstitial infiltrates, alveolar fibrinous exudates and non-necrotising granulomas.7
It is important to distinguish pulmonary manifestations in patients with inflammatory bowel disease (IBD) secondary to drug-related toxicity as opposed to the disease process itself. Unlike mesalamine-related lung injury, large airway abnormalities are more common in patients with IBD, with bronchiectasis being the single most common lung pathology. Other less commonly reported abnormalities include diseases of the upper airways such as epiglottitis and tracheobronchitis, small airway diseases such as broncholitis obliterans, interstitial diseases and autoimmune disease processes such as pulmonary vasculitis and Wegner granulomatosis.6 17 A detailed history and the above-mentioned laboratory and diagnostic studies can help make that distinction. Most cases of IBD-related pulmonary disease follow the progression of the underlying gastrointestinal syndrome and occur during a period of exacerbation. Moreover, 70% of these patients experience an additional extraintestinal manifestation in combination with their lung involvement. Thus in our patient, in addition to the positive biopsy findings, a diagnosis of mesalamine-induced interstitial pneumonitis was substantiated by the following: appearance of respiratory symptoms during drug therapy, absence of extraintestinal manifestations of UC, absence of other medications and clinical improvement upon discontinuation of mesalamine. Our patient was not subject to rechallenge with the drug given the severity of his adverse reaction.
The most important aspect of treatment involves the safe discontinuation of therapy with mesalamine. It is imperative however that this should be followed by documented improvement in both the clinical and radiographic picture of the patient. The role of steroids for mesalamine-induced interstitial pneumonitis is unclear. Nonetheless some authors suggest initiating corticosteroid therapy at 1 mg/kg to facilitate rapid recovery, especially if stopping mesalamine does not produce the expected improvement.7 The prognosis of mesalamine-induced lung injury is good, which in contrast to sulfasalazine-induced pulmonary toxicity has no reported deaths.
In conclusion, pulmonary toxicity is a rare but increasingly reported complication related to mesalamine therapy. This adverse effect may be difficult to diagnose but should be suspected in those patients being administered 5-ASA who develop respiratory symptoms and variable radiographic changes. Given the complexities in diagnosis and management, it is essential that a joint evaluation be carried out by a multidisciplinary team comprising gastroenterologists, pulmonologists, radiologists and pathologists.
Learning points.
Mesalamine is well tolerated by most patients due to the absence of the sulfapyridine group present in sulfasalzine.
Interstitial pneumonitis as an adverse effect of mesalamine therapy is an extremely rare but potentially serious complication.
The management of mesalamine-induced lung injury can be challenging and requires a multidisciplinary approach.
Footnotes
Contributors: AA carried out writing of this manuscript and review of the literature. AK was involved in patient management and carried out review of the literature.
Competing interests: None.
Patient consent : Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Loftus EV. Clincal epidemiology of inflammatory bowel disease: incidence, prevalence and enviornmental influences. Gastroenterology 2004;2013:1504–17 [DOI] [PubMed] [Google Scholar]
- 2.Loftus CG, Loftus EV, Harmsen WS, et al. Update on the incidence and prevalence of Chron's disease and ulcerative colitis in Olmstead County, Minnesota 1940–2000. Inflamm Bowel Dis 2007;2013:254–61 [DOI] [PubMed] [Google Scholar]
- 3.Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol 2011;2013:3204–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanda K, Moss A. Update on the management of ulcerative colitis: treatment and maintenance approaches focused on MMX mesalamine. Clin Pharmacol 2012;2013:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricart E, Panaccione R, Loftus EV, Jr,et al. Autoimmune disorders and extraintestinal manifestations in first degree familial and sporadic inflammatory bowel disease: a case- control study. Inflamm Bowel Dis 2004;2013:207–14 [DOI] [PubMed] [Google Scholar]
- 6.Schleiermacher D, Hoffmann J. Pulmonary abnormalities in inflammatory bowel disease. J Crohns Colitis 2007;2013:61–9 [DOI] [PubMed] [Google Scholar]
- 7.Foster R, Zander D, Mergo P, et al. Mesalamine related lung disease: clinical, radiographic and pathologic manifestations. Inflamm Bowel Dis 2003;2013:308–15 [DOI] [PubMed] [Google Scholar]
- 8.Brimblecombe R. Mesalamine: a global safety evaluation. Scand J Gastroenterol Suppl 1990;2013:66. [DOI] [PubMed] [Google Scholar]
- 9.Sossai P, Cappellato M, Stefani S. Can a drug induced pulmonary hypersensitivity reaction be dose dependent? A case with mesalamine. Mt Sinai J Med 2001;2013:389–95 [PubMed] [Google Scholar]
- 10.Moss A, Peppercorn A. The risks and benefits of meslamine as a treatment for ulcerative colitis. Expert Opin Drug Saf 2007;2013:99–107 [DOI] [PubMed] [Google Scholar]
- 11.Ransford R, Langman M. Sulphasalazine and mesalazine: serious adverse reactions reevaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002;2013:536–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cilloniz R, Chesrown S, Gonzalez-Peralta R. Asymptomatic presentation of mesalamine induced lung injury in an adolescent with Crohn's disease. BMJ Case Rep. Published Online: 20 Mar 2000. doi:10.1136/bcr.09.2008.0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain N, Petruff C, Bandyopadhyay T. Mesalamine lung toxicity. Conn Med 2010;2013:265–7 [PubMed] [Google Scholar]
- 14.Matsuno O. Drug induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 2012;2013:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bitton A, Peppercorn M, Hanrahan J, et al. Mesalamine induced lung toxicity. Am J Gastroenterol 1996;2013:1039–40 [PubMed] [Google Scholar]
- 16.Reinoso M, Schroeder K, Pisani R. Lung disease associated with orally administered mesalamine for ulcerative colitis. Chest 1992;2013:1469–71 [DOI] [PubMed] [Google Scholar]
- 17.Camus P, Piard F, Ashcroft T, et al. The lung in inflammatory bowel disease. Medicine 1993;2013:151–80 [PubMed] [Google Scholar]


