Abstract
The aim of the study was to investigate the association between the initial metabolic state and exercise-induced endotoxaemia on the appearance of gastrointestinal symptoms (GIS) during exercise. Eleven males (36.6 ± 4.9 yrs, 1.7 ± 0.1 m, 74.5 ± 7.7 kg, DEXA body fat % 17.2 ± 6.6, VO2max 57.4 ± 7.4 ml·kg-1·min-1) underwent two isoenergetic diets designed to change their initial metabolic status by either depleting or maintaining their hepatic and muscular glycogen content. These diets and accompanying exercise sessions were performed by each participant in the days before completing a laboratory-based duathlon (5-km run, 30-km cycling, 10-km run). Blood samples were obtained before, immediately and 1- and 2-h following the duathlon for determination of insulin (IN), glucagon (GL), endotoxin, aspartic aminotransferase (AST), and alanine aminotransferase (ALT) markers. GIS were assessed by survey before and after exercise. Diet content produced a different energy status as determined by macronutrient content and the IN/GL ratio (p < 0.05), and mild exercise-induced endotoxaemia was observed in both experimental duathlons. Regardless of the diet, the AST/ALT ratio following exercise and in the recovery phase indicated hepatocyte and liver parenchyma structural damage. In spite of GIS, no significant correlations between endotoxin levels and GIS were found. In conclusion, increased markers of endotoxaemia observed with the high-intensity exercise were unrelated to hepatic function and/or GIS before and after exercise.
Key points.
Gastrointestinal symptoms before, during, and after a competition are reported by approximately 20%-50% of the athletes participating in endurance events such as marathon, cycling and triathlon.
Energy status, exercise-induced endotoxaemia and liver structural damage might be related to gastrointestinal symptoms.
In this study, gastrointestinal symptoms observed before and after endurance exercise were unrelated to endotoxin levels or hepatic structural damage.
Key words: Liver structure, endurance, lipopolysaccharide, endotoxaemia, exercise
Introduction
Gastrointestinal symptoms (GIS) before, during, and after a competition are reported by approximately 20%-50% of the athletes participating in endurance events such as marathon, cycling and triathlon (Gil et al., 1998; Peters et al., 2001a). In spite of the high frequency of GIS such as bloating, side-pain, nausea, and diarrhea, the exact physiological mechanisms responsible for such disturbances are not fully- understood. Endotoxaemia has been suggested as an underlying cause responsible for the deleterious effect of strenuous physical exercise on athletes, who in extreme conditions might require medical attention (e.g., heat-stroke) (Hales and Sakurada, 1998; Ryan, 1993). Endotoxaemia is characterized by the presence of toxins in the blood, generally lipopolysaccharides (LPS) from gram-negative bacteria that bind to LPS-binding protein (LBP) to form the LPS-LBP complex (Triantafilou and Triantafilou, 2002). This complex is considered a marker of bacterial translocation and transport responsible for initiating a cell-mediated signal response (Nanbo et al., 1999; van Deventer et al., 1988; 1998; Camus et al., 1997).
Large amounts of endotoxins normally reside within the human intestines. Bacterial translocation from the intestines to the portal circulation towards the liver might occur when factors such as a reduction in splanchnic blood flow, intense physical exercise, long stays at altitude, or high body core temperature affect the integrity of the intestinal wall (Hales and Sakurada, 1998; Hall et al., 2001; Peters et al., 2001a; Wagenmakers, 1992). During high-intensity and/or prolonged exercise blood flow to the splanchnic areas decreases by approximately 50% from the resting state (Pals et al., 1997). This reduction in blood flow is correlated to the intensity and duration of the exercise (Otte et al., 2001). A reduction in visceral blood flow might elicit intestinal ischemia, compromising intestinal wall integrity and leading to even higher levels of circulating endotoxins. Furthermore, a reduction in visceral blood flow has been related to gastrointestinal problems such as side-pain, flatulence, nausea and vomiting (Peters et al., 2001b).
Thus, there is consistent evidence showing that endotoxaemia occurs during endurance exercise (Brock-Utne et al., 1988; Jeukendrup et al., 2000) and that there might be a link to GIS and other medical conditions such as heat-stroke (Peters et al., 2001b; Ryan, 1993). Although Jeukendrup et al., 2000, did not report a correlation between endotoxin and GIS; Brock-Utne et al., 1988, found a correlation between endotoxin levels and athletes suffering from nausea, vomiting, and diarrhea. Therefore, a potential role of endotoxins has been suggested (Gil et al., 1998; Peters et al., 2001b) that might partially explain the appearance of GIS during endurance exercise.
An exercise- induced endotoxaemia model similar to the one proposed by Hales and Sakurada, 1998 might be valid to assess the impact of high-intensity and long duration exercise on physiological variables. First, during exercise, endotoxins will translocate at a higher rate from a possibly compromised intestinal wall. Second, under these circumstances, the liver may be unable to clear endotoxins and therefore elevated concentrations of these bacteria may cause disturbances in cardiovascular function.
Based on this model, we hypothesized that the liver’s ability to clear endotoxins can be impaired if its energy level is low since this metabolic process is energy-dependent. In other words, we hypothesized that the enzymatic indicators of structural hepatic changes will prevent liver to clear endotoxins. Thus, endotoxins will be responsible for producing gastrointestinal distress (Hales and Sakurada, 1998), and might be a limiting factor for athletes exercising at high-intensity and for prolonged events. Therefore, the aim of the study was to investigate the association between the initial metabolic state and exercise-induced endotoxaemia on hepatic structure and the appearance of gastrointestinal symptoms during high-intensity endurance exercise.
Methods
Participants and study protocol
A crossover design was used and experimental conditions were randomly assigned to the participants. Thus, each athlete performed the experiment on two different occasions, separated by at least 7 days. The Institutional Review Board from Auburn University approved the study. Written informed consent was obtained from each subject. Eleven males 20 to 44 years of age who had a maximal oxygen consumption (VO2max) ≥ 50 ml·kg-1·min-1 as determined in a treadmill-based graded exercise test (GXT) were allowed to participate in the study. Volunteers were not allowed to participate if they reported or exhibited anemia, had any gastrointestinal disorders, and/or other chronic disorders, were current cigarette smokers, and/or were under any current nonsteroidal anti-inflammatory drug (NSAID) (Lambert et al., 2007).
Volunteers who apparently met the inclusion criteria arrived to the Exercise Technology Laboratory at Auburn University and underwent body composition assessment to determine body fat mass via dual energy x-ray absorptiometry (DEXA), and a GXT on a treadmill to determine VO2max via respiratory gas analysis. Indications to stop the GXT were to achieve at least two of the following criteria: a) a request to stop the test, b) respiratory exchange ratio (RER) ≥ 1.15, and/or c) a plateau of the VO2 curve < 2 ml·kg-1·min-1 with increased workload.
Initial metabolic state manipulation
Participants were instructed to record their food consumption over a three-day period (2 weekdays and 1 weekend-day) in a food log to determine food preferences and energy needs (Economos et al., 1993). A registered dietitian analyzed food logs and designed two individual diets; one high in fat and one high in carbohydrates (CHO). These diets met the participant’s daily energy needs; however, their nutrient content was different. A nutrient composition of 60% CHO, 25% fat, and 15% protein was the goal for the high-CHO diet; and 20% CHO, 65% fat, and 15% protein for the high-fat diet (Sherman et al., 1981).
Diets consisted of commercially-available pre-packaged foods provided by the researchers and were given to each participant 72-h before an exercise trial. Allotments for breakfast, lunch, dinner, and snacks 48-h before experimental exercise session were provided to each participant. Participants were instructed to rest (i.e., no exercise) the day before the exercise intervention and to avoid unusually consumed foods that might cause GIS such as fiber-rich and spicy foods, caffeinated drinks, and controlled drugs such as alcohol, tobacco, and/or non-steroidal anti- inflammatory drugs (NSAIDs) (Lambert et al., 2007; Van Nieuwenhoven et al., 1999; Ryan et al., 1996).
Exercise intervention
Two-days before completing a duathlon, participants on the high-CHO diet were instructed to return to the laboratory to complete a 60-min sub-maximal (70% VO2max) jog on the treadmill, and one-day before the duathlon, participants were instructed to rest. During the 48-h in which participants consumed the high-fat diet, they were required to run on a treadmill for 60-min (70% VO2max) two-days before the duathlon and 45-min at the same intensity on the day prior to the duathlon. The exercise regimens combined with the dietary manipulations were used to change the initial metabolic state of the participants prior to when they report for the duathlon (Sherman et al., 1981).
Experimental exercise session (duathlon)
On the day of the duathlon participants arrived at the laboratory, returned empty food packages, and were instructed to void their bladders before body weight was measured. Then, they were instructed to sit quietly for 5-min. During the 5- min rest period, participants completed a “gastrointestinal symptom survey”. This is a modified visual scale (Morton and Callister, 2002) used to obtain information about the anatomical location and the specific GIS felt. The 18 GIS list was obtained from the literature (belching, bloating, diarrhea, dizziness, flatulence/gas, headache, heartburn, intestinal cramps, muscle cramps, nausea, side-pain/side stitch, stomach cramps, stomach upset, urge to defecate, urge to urinate, urge to vomit, vomiting, and/or other). We did not ask the magnitude (i.e., how hard/light was felt) of the symptom, rather to indicate (in the visual scale) the anatomical area in which the symptom was experienced.
Next, a fasting blood sample was obtained. Following the initial blood draw, participants were provided with a standardized breakfast of two slices of white bread, one slice of American cheese, water and a small banana to eat while resting in a comfortable chair. This breakfast provided 1570.1 kJ (375 kcal) and was consumed in about 10-min. After a rest period of 60-min, participants had 10-min to warm up and then started the duathlon in the following order: a) treadmill run of 5-km (Run-1); b) 30-km stationary cycle (Bike); and c) 10-km treadmill run (Run- 2). The subjects ran at 0% grade and were allowed to modify only the treadmill speed. For the cycling part of the race, participants had previously attached their own bicycles to a CompuTrainerTM (Racer Mate, Inc., Seattle, WA). Total time was recorded for further analysis. During the duathlon the participants were given the opportunity to drink chilled water ad libitum; solid foods were avoided at all times. The environmental conditions of temperature and relative humidity in the laboratory during the duathlon were 20-21°C and 50-60%, respectively.
Participants were asked to give their best effort during the duathlon. Volume of oxygen (VO2), heart rate (beats·min-1), and GIS were monitored for at least two minutes during the duathlon. VO2 and heart rate were used to assure an exercise intensity ≥ 70% VO2max. Participants were instructed to increase their effort if exercise intensity was < 70% of their individual VO2max.
Participants completed the “gastrointestinal symptom survey ”along with each of the blood samples. Once the experimental session was completed, the subjects were provided with rehydration fluids, fruit, and an appointment for the next visit to the laboratory.
Blood sampling and analysis
Venous blood samples for each participant were collected before, immediately after, and 1- and 2-h following the duathlon. Two-7 ml serum tubes were obtained at each blood sampling time point. Only the blood samples for glucagon analyses were collected in chilled lavender-top tubes containing 100 μL of aprotinin (Sigma, Aprotinin, Cat# A6279). Immediately following the blood draw, a small portion of the sample was collected in three microcapillary tubes and spun to determine hematocrit (Hct). To determine hemoglobin (Hb) concentration another portion of fresh blood (10 μL) was also immediately transferred into a tube containing a previously prepared Hb reagent solution (2.5 mL Sodium Lauryl Sulfate). This mixture was transferred into a cuvette and read at 540 Nm in a spectrophotometer.
The remainder of the blood sample was allowed to clot and serum was separated by centrifugation at 1500 g for 15-min. Serum aliquots were prepared and stored at -80°C for further analysis of glucose (Glucose Flex® Dimension®, Dade Behring Inc., Deerfield, IL), AST (AST Flex® Dimension®, Dade Behring Inc., Deerfield, IL), and ALT (ALT Flex® Dimension®, Dade Behring Inc., Deerfield, IL). Enzyme linked-immuno-sorbent assay (ELISA) kits were used for determination of insulin (IN), glucagon (GL) (LINCO, St. Charles, MO), LPS-LBP complex as a measure of endotoxaemia (Cell Sciences, Inc., Canton, MA). Plasma concentration of LPS-LBP complex was corrected to take into consideration changes in plasma volume occurred during exercise (Dill and Costill, 1974).
Statistical analysis
Data were analyzed with the Statistical Package for the Social Sciences (SPSS®), version 15.0 for Windows. Data are presented as mean ± standard deviation (SD), and statistical significance was set a priori at p ≤ 0.05. Paired t-tests were used to determine significant mean differences between experimental conditions in the dependent variables IN/GL ratio, performance time in the duathlon, diet content composition, and hydration status. Factorial 2 (diets) x 4 (pre-exercise, immediately after exercise, 1-h post-exercise, 2-h post- exercise) repeated measures analyses of variance (ANOVA) were computed to analyze AST, ALT, the AST/ALT ratio, and LPS-LBP complex. Metabolic indicators (i.e., VO2, %VO2) were analyzed by a 2 (diets) x 3 (run-1, bike, run-2) factorial, repeated-measures ANOVA. For all ANOVA tests, appropriate follow-up analyses were computed if significant interactions and/or main effects were found (i.e., Bonferroni adjustment for multiple comparisons). Finally, a Chi2 (χ2) test was carried to analyze gastrointestinal problems and endotoxaemia.
results
Table 1 shows the physical characteristics of the eleven healthy athletes participating in the study. Participants trained for middle and long distance events such as triathlon and marathon. They trained on average 11 h·wk-1, including running on average 13 km·wk-1 and cycling 24 to 40 km·wk-1. Resting Hb and Hct values were within normal ranges for adult males.
Table 1.
Descriptive statistics for athletes (n = 11).
| Variable | Mean (±SD) |
|---|---|
| Age (years) | 36.6 (4.9) |
| Height (m) | 1.70 (.10) |
| Body mass (kg) | 74.5 (7.7) |
| Body fat mass (DEXA, %) | 17.2 (6.6) |
| VO2max (ml · kg-1 · min-1) | 57.4 (7.4) |
The fasting mean IN/GL ratio in the high-fat diet (0.27 ± 0.10) was lower (p ≤ 0.05) than the ratio on the high-CHO diet (0.39 ± 0.20), indicating participant’s compliance to the dietary regimen. Participants in the high-fat diet consumed 11.59 ± 1.58 MJ distributed in meals consisting of 21% CHO, 67% fat, and 11% protein. In the high-CHO diet, energy intake was 11.50 ± 1.48 MJ distributed in meals consisting of 63% CHO, 25% fat, and 11% protein. In the high-fat diet, the athletes consumed 2.8, 1.9, and 1.0 g·kg-1 body weight of fat, CHO, and protein, respectively. In the high-CHO diet, fat, CHO, and protein consumed were 0.8, 6.3, and 1.0 g·kg-1 body weight, respectively. Fiber content of each diet was 31.63 ± 16.41 g·d-1 for the high-fat diet and 42.02 ± 15.93 g·d-1 for the high-CHO diet (p = 0.292).
No differences in duathlon performance were observed between the high-fat trial (136.38 ± 20.09 min) and the high-CHO trial (134.88 ± 20.89 min). Regardless of the diet, subjects performed the duathlon at 71.07 ± 1.97% of their individually determined VO2max; achieving higher %VO2max in the run-1 (79.10 ± 2.59%) than in the cycle (64.20 ± 2.19%) and run-2 (69.90 ± 2.49%) (p ≤ 0.001). Heart rate response, estimated as a percentage of HRmax, was higher in the run-1 (88 ± 2%) compared to the bike (84 ± 2%) and run-2 (86 ± 2%) (p = 0.011).
Analysis of hydration status at the end of the duathlon indicated higher dehydration after the high-fat diet trial (-1.6 ± 1.2% body mass) compared to the high-CHO diet trial (-1.0 ± 1.4% body mass) (p = 0.007).
For hepatic integrity markers AST, ALT, and AST/ALT ratio, no significant combined effects were found between diet conditions and measurement time. Independently of measurement time, AST concentrations were higher in the high-fat diet trial compared to the high-CHO diet trial (39.23 ± 3.54 U/L vs. 29.40 ± 1.75 U/L) (p = 0.007). Regardless of the diet, AST levels increased from baseline (28.53 ± 2.03 U/L), to immediately following exercise (36.58 ± 2.52 U/L), and remained elevated 1- and 2-h following exertion (35.42 ± 2.50 U/L and 36.72 ± 2.81 U/L, respectively) (p ≤ 0.001). Also, regardless of the diet, ALT levels increased only from baseline (34.15 ± 1.74 U/L) to immediately following exercise (35.83 ± 2.05 U/L) (p = 0.010).
Independently of measurement time, AST/ALT ratio was higher in the low-CHO diet trial compared to the high-CHO diet trial (1.15 ± 0.12 vs. 0.89 ± 0.04) (p = 0.042). Regardless of the diet, AST/ALT ratios increased from baseline (0.84 ± 0.05), to immediately following exercise (1.03 ± 0.06), and remained elevated 1- and 2-h following exertion (1.08 ± 0.07 and 1.14 ± 0.11, respectively) (p ≤ 0.001) (Figure 1).
Figure 1.
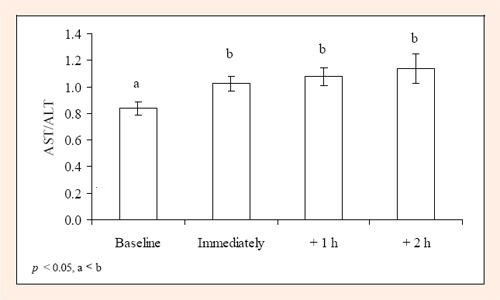
AST/ALT ratio for 11 males following a duathlon (mean ± SD).
Intestinal permeability and bacterial translocation as determined by the LPS-LBP complex increased from baseline regardless of the dietary manipulation (p ≤ 0.05). LPS-LBP complex concentrations increased from baseline to immediately following exercise (∆ = 16.34%) and decreased from baseline to 1- (∆ = - 0.79%) and 2-h (∆ = - 4.10%) following exercise.
Mild endotoxaemia was found at baseline in 27% of the participants (n = 3) who followed the high-fat diet compared to 9% (n = 1) who followed the high-CHO diet. These figures increased to 55% (n = 6) immediately following exercise in the high-fat diet, compared to 45% (n = 5) in the high-CHO diet. One-hour after exercise, 36% (n = 4) and 30% (n = 3) of the athletes in the high-fat and the high-CHO diets had endotoxaemia. Finally, 2-h following exercise, endotoxaemia was found only in 9% (n = 1) and 10% (n = 1) of the athletes in the high-fat and high-CHO diets, respectively. The highest measured value for a participant was 16.7 pg·ml-1, immediately after exercise in the high-fat diet condition.
Table 2 shows the incidence of GIS for both diet conditions. No significant associations were found between GIS and endotoxaemia in either diet (high-fat, p = 0.399 vs. high-CHO, p = 0.752). Three participants (27%) reported GIS under both dietary conditions; four participants (36%) did not report GIS in either dietary trial. Finally, 2 participants (18%) in the high-fat diet and 2 participants (18%) in the high-CHO diet trials reported GIS.
Table 2.
Frequency of participants with GI symptoms and exercise-induced endotoxaemia following exercise under a high-fat and a high-CHO diet (n = 11).
| Gastrointestinal Symptoms | Endotoxaemia immediately after exercise | ||
|---|---|---|---|
| LPS-LBP > 5 pg·ml-1 | LPS-LBP < 5 pg·ml-1 | ||
| High-fat diet | Yes | 2 | 3 |
| No | 4 | 2 | |
| High-CHO diet | Yes | 2 | 3 |
| No | 3 | 3 | |
High-fat χ2 = 0.711; p = 0.399, High-CHO χ2 = 0.100; p = 0.752.
We estimated a GIS/time indicator by taking the number of reported GIS and dividing them by the cumulative time subjects performed exercise. Thus, in general, there were 0.35 episodes/h-1 regardless of the diet, 0.51 episodes/h-1 in the high-fat diet, and 0.20 episodes/h-1 in the high-CHO diet. GIS reported were belching, flatulence, intestinal cramps, nausea, side-pain/stitch, stomach upset, urge to vomit, vomiting, and constipation. The anatomical region where participants felt a GIS following duathlon in the high-CHO diet is depicted in Figure 2. No specific anatomical regions for GIS were reported during the high-fat diet.
Figure 2.
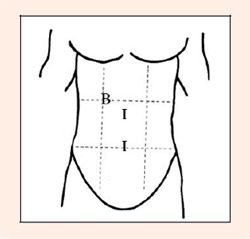
Anatomical region where participants felt GIS in the high-CHO diet trial. B= Resting; I= Immediately after duathlon.
Discussion
The aim of the study was to investigate the effect of the initial metabolic state and exercise-induced endotoxaemia on the appearance of GIS during high-intensity endurance exercise in males.
In this study, we tried to modify the initial metabolic state by short-term diet and exercise. We did not measure hepatic or muscular glycogen stores directly to determine metabolic state. Sherman et al., 1981, combined training runs (~60-min, 73% VO2max) and diet (104 g CHO/d-1 for 48-h), then directly measured muscle glycogen by biopsies, and found a dramatic reduction in glycogen levels. Similarly, Widrick et al., 1993, combined exercise (60-min, 70% VO2max) and diet (181 g CHO/d-1 for 48-h), and also found significant reductions in muscle glycogen content. In this study we followed an intermediate protocol; we provided athletes with ~141 g CHO/d-1 for 48-h and supervised treadmill training runs (60-min, 70% VO2max). Therefore we expected reduced muscle (i.e., diet + training) and liver (i.e., diet + training + overnight fast) glycogen stores. Both, the combination of a reduced CHO diet, treadmill exercise, and fasting allowed us to confidently assume that both, muscle and liver reserves, were low.
We also support the energy status change with the information provided by glucoregulatory hormones IN and GL. A subject in the absorptive state is expected to show higher IN concentrations than during post-absorptive state; while a subject in the post-absorptive state or fasting is expected to show higher GL than IN levels (Vander et al., 1998). Therefore, an elevated IN/GL ratio would indicate an absorptive state and a hypoglycemic state mediated by IN (i.e., high glycogen stores and high energy status). A reduced IN/GL ratio would indicate a post-absorptive state, meaning a higher glycogenolysis rate and gluconeogenesis in order to maintain normal blood glucose levels (i.e., low glycogen stores and low energy status) (Brooks et al., 2000). In addition, total liver glycogen content is dramatically reduced following a 12-h fasting and/or a low carbohydrate diet (Houston, 1995). We observed significant differences in the IN/GL ratio between dietary conditions, indicating a change in the initial energy status mediated by a combined effect of the diet and exercise regimen.
Even though a potential low energy level was achieved at baseline in the high-fat diet trial, as shown by a reduced IN/GL ratio, we did not find a significant association between LPS-LBP complex and hepatic markers AST and ALT following exercise. Our findings are similar to those reported when studying hepatocyte function in fasted and semifasted rats (Latour et al., 1999). In this study, the AST/ALT ratio was > 1.0 immediately following exercise and in the recovery phase (i.e., 1- and 2-h post-exercise), indicating hepatocyte and liver parenchyma structural damage. Indeed, ALT, a more specific marker of liver damage (Sherlock and Dooley, 2001), increased from baseline to immediately following exercise, suggesting hepatocyte damage (i.e., structure) possibly explained by the combined effect of exercise intensity and duration. Nevertheless, liver function did not appear to be jeopardized in our participants. Changes of liver structure observed in this study were similar to findings reported in highly trained competitive cyclists (Mena et al., 1996), marathoners (Smith et al., 2004), and other athletes performing a series of physical activities (Fojt et al., 1976; Schlang and Kirkpatrick, 1961).
Since endotoxaemia has been proposed as a potential mechanism explaining the appearance of GIS in athletes, we expected to find a significant correlation between endotoxaemia and GIS such as nausea, vomiting, and diarrhea (Brock-Utne et al., 1988; van Deventer et al., 1990). However, similar to Jeukendrup et al., 2000; we did not find a correlation between endotoxaemia and GIS. Indeed, GIS were virtually absent in the subjects participating in our study. We documented only one case of belching, dizziness, headache, stomach upset, nausea and vomiting 1-h after the duathlon in the high-fat diet. The participant remained in the laboratory for observation and symptoms resolved one hour after vomiting. Another subject complained of tightness in the upper abdominal area. However, this complaint was unrelated to our experimental intervention (i.e., diet, exercise). Finally, two participants reported having a transient side-stitch in the lower abdominal area during exercise that lasted less than 10-min and did not interfere with their performance.
We hypothesized that the combination of both, a high intensity and long duration exercise, would reduce splanchnic blood flow allowing bacteria to translocate from the intestines to the portal circulation to finally reach the liver (Gil et al., 1998; Pals et al., 1997; Otte et al., 2001; Nielsen et al., 2002). In the present study, the mean exercise intensity elicited by the subjects during the duathlon in both dietary conditions was high enough to cause intestinal permeability and bacterial translocation from baseline as demonstrated by the increased LPS-LBP complex values. Regardless of the dietary trial, the subjects performed the duathlon at approximately 70% of their individual VO2max and this exercise intensity caused bacterial translocation as measured after exercise. Similar results immediately after exercise have been previously reported in marathon and ultraendurance events (Jeukendrup et al., 2000; Øktedalen et al., 1992).
Although there were no direct correlations between endotoxaemia and GIS, we cannot rule out the endotoxaemia model for explaining at least some of the gastrointestinal distress felt by athletes. Several individual characteristics may explain the variation in how an athlete responds to exercise, especially as it relates to gastrointestinal distress and/or exercise-induced endotoxaemia. A list of psychological (e.g., pre-competitive anxiety), pre-exercise presentation (e.g., diet, rest, fitness), physiological function (e.g., buffering capacity, endotoxin clearance, blood flow redistribution to vital organs), and environment conditions (e.g., heat, cold, humidity), and variables that might explain gastrointestinal distress still deserve further investigation. Other factors might include, for instance, the fiber content of the diet before the trials might impact the orocecal transit time producing gastrointestinal distress. In addition, the fat and CHO content of the diet, as well as hydration status during the race might impact the gastrointestinal system. In this study, the fiber content was similar between diets (combined mean ~36.8 g·d-1), slightly above than the 20-35 g·d-1 recommended range for healthy adults (Marlett et al., 2002). Since it has been reported that physically-active people have rapid orocecal transit time (i.e., higher gastrointestinal system motility) (Harris et al., 1991), we assumed that the impact of the fiber content of the diets on the gastrointestinal system would be negligible. Thus, we did not find an association between fiber content and GIS before, during or after exercise even in the presence of higher dehydration levels in the high-fat diet. In addition, in spite of a having two significantly different diet composition (i.e., high-fat vs. high-CHO), the fat content did not influence the gastrointestinal system during the trials. We did not find evidence to support that fat content might have played a role in the few GIS reported during exercise. Finally, further studies need to be conducted to determine the influence of different levels of dehydration on the appearance of GIS.
In conclusion, hepatic structural damage after a duathlon was similar between athletes consuming a high-fat and a high-CHO diet. High-intensity (i.e., ~70% VO2max) and prolonged (i.e., ~ 130 min) exercise increased intestinal permeability to produce mild endotoxaemia; however, post-exercise endotoxin levels were unrelated to frequency of gastrointestinal symptoms and liver structural markers.
Acknowledgments
The Gatorade Sports Science Institute (GSSI), the United Nations Educational, Scientific, and Cultural Organization (UNESCO), and the Exercise Technology Laboratory at Auburn University supported this study. All the experiments performed in this study comply with the current laws of the United States of America.
Biographies
José Moncada-Jimènez
Employment
Doctoral student, director of the Laboratory of Human Movement Sciences at the School of Physical Education and Sports at the University of Costa Rica, Costa Rica.
Degree
MSc
Research interests
The effects of exercise on gastrointestinal and immune responses.
E-mail: jose.moncada@ucr.ac.cr
Eric P. Plaisance
Employment
Postdoctoral fellow at the Boshell Diabetes and Metabolic Diseases Research Program at Auburn University, Alabama, USA.
Degree
PhD
Research interests
The study of physiological and metabolic changes elicited by exercise and medications.
E-mail: plaisep@auburn.edu
Michael L. Mestek
Employment
Research associate at the Integrative Vascular Biology Laboratory at University of Colorado, Colorado, USA.
Degree
PhD
Research interests
Effects of aging, cardiometabolic risk factors, HIV-1, and physical activity on vascular endothelial function.
E-mail: michael.mestek@colorado.edu
Felipe Araya-Ramirez
Employment
Doctoral student and graduate assistant at the Exercise Technology Laboratory at Auburn University, Alabama, USA.
Degree
MSc
Research interests
Effects of exercise training in cardiovascular risk reduction in obesity.
E-mail: arayafe@auburn.edu
Lance Ratcliff
Employment
Department of Nutrition and Food Sciences, Auburn University, Alabama, USA.
Degree
PhD
Research interests
Effects of nutrition interventions on exercise performance.
E-mail: ratclla@auburn.edu
James K. Taylor
Employment
Division of Clinical Laboratory Science, Auburn University-Montgomery, Alabama, USA.
Degree
MSc
Research interests
Effects of exercise on metabolic syndrome.
E-mail: jtaylor@mail.aum.edu
Peter W. Grandjean
Employment
Associate professor at the Department of Kinesiology, Auburn University, Alabama, USA.
Degree
PhD
Research interests
Effects of exercise on lipid metabolism.
E-mail: grandpw@auburn.edu
Luis F. Aragonvargas
Employment
Professor at the School of Physical Education and Sports at the University of Costa Rica, Costa Rica.
Degree
PhD
Research interests
Sports nutrition, fasting, dehydration and rehydration in sports.
E-mail: luis.aragon@ucr.ac.cr
References
- Brock-Utne J.G., Gaffin S.L., Wells M.T., Gathiram P., Sohar E., James M.F., Morrell D.F., Norman R.J. (1988) Endotoxaemia in exhausted runners after long-distance race. South African Medical Journal 73, 533-536 [PubMed] [Google Scholar]
- Brooks G.A., Fahey T.D., White T.P., Baldwin K.M. (2000) Exercise physiology, human bioenergetics and its applications. Mayfield Publishing Co., Mountain View, CA [Google Scholar]
- Camus G., Poortmans J., Nys M., Deby-Dupont G., Duchateau J., Deby C., Lamy M. (1997) Mild endotoxaemia and the inflammatory response induced by a marathon race. Clinical Science 92, 415-422 [DOI] [PubMed] [Google Scholar]
- Dill D.B., Costill D.L. (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. Journal of Applied Physiology 37, 247-248 [DOI] [PubMed] [Google Scholar]
- Economos C.D., Bortz S.S., Nelson M.E. (1993) Nutritional practices of elite athletes. Practical recommendations. Sports Medicine 16, 381-399 [DOI] [PubMed] [Google Scholar]
- Fojt E., Ekelund L.G., Hultman E. (1976) Enzyme activities in hepatic venous blood under strenuous physical exercise. Pflügers Archives 361, 287-296 [DOI] [PubMed] [Google Scholar]
- Gil S.M., Yazaki E., Evans D.F. (1998) Aetiology of running-related gastrointestinal dysfunction. How far is the finishing line? Sports Medicine 26, 365-378 [DOI] [PubMed] [Google Scholar]
- Hales J.R.S., Sakurada S. (1998) Heat tolerance: A role for fever? Annals of The New York Academy of Sciences 856, 188-205 [DOI] [PubMed] [Google Scholar]
- Hall D.M., Buettner G.R., Oberley L.W., Xu L., Matthes R.D., Gisolfi C.V. (2001) Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. American Journal of Physiology 280, H509-H521 [DOI] [PubMed] [Google Scholar]
- Harris A., Lindeman A.K., Martin B.J. (1991) Rapid orocecal transit in chronically active persons with high energy intake. Journal of Applied Physiology 70, 1550-1553 [DOI] [PubMed] [Google Scholar]
- Houston M.E. (1995) Biochemistry primer for exercise science. Human Kinetics, Champaign, IL [Google Scholar]
- Jeukendrup A.E., Vet-Joop K., Sturk A., Stegen J.H.J.C., Senden J., Saris W.H.M., Wagenmakers A.J.M. (2000) Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clinical Science 98, 47-55 [PubMed] [Google Scholar]
- Lambert G.P., Boylan M., Laventure J.P., Bull A., Lanspa S. (2007) Effect of Aspirin and Ibuprofen on GI permeability during exercise. International Journal of Sports Medicine 28, 722-726 [DOI] [PubMed] [Google Scholar]
- Latour M.G., Brault A., Huet P.M., Lavoie J.M. (1999) Effects of acute physical exercise on hepatocyte volume and function in rat. American Journal of Physiology 276, R1258-R1264 [DOI] [PubMed] [Google Scholar]
- Marlett J.A., McBurney M.I., Slavin J.L. (2002) Position of the American Dietetic Association Health Implications of Dietary Fiber. Journal of the American Dietetic Association 102, 993-1000 [DOI] [PubMed] [Google Scholar]
- Mena P., Maynar M., Campillo J.E. (1996) Changes in plasma enzyme activities in profesional racing cyclists. British Journal of Sports Medicine 30, 122-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D.P., Callister R. (2002) Factors influencing exercise-related transient abdominal pain. Medicine & Science in Sports & Exercise 34, 745-749 [DOI] [PubMed] [Google Scholar]
- Nanbo A., Nishimura H., Muta T., Nagasawa S. (1999) Lipopolysaccharide stimulates HepG2 human hepatoma cells in the presence of lipopolysaccharide-binding protein via CD14. European Journal of Biochemistry 260, 183-191 [DOI] [PubMed] [Google Scholar]
- Nielsen H.B., Clemmensen J.O., Skak C., Ott P., Secher N.H. (2002) Attenuated hepatosplanchnic uptake of lactate during intense exercise in humans. Journal of Applied Physiology 92, 1677-1683 [DOI] [PubMed] [Google Scholar]
- Øktedalen O., Lunde O.C., Opstad P.K., Aabakken L., Kvernebo K. (1992) Changes in the gastrointestinal mucosa after long-distance running. Scandinavian Journal of Gastroenterology 27, 270-274 [DOI] [PubMed] [Google Scholar]
- Otte J.A., Oostveen E., Geelkerken R.H., Groeneveld A.B.J., Kolkman J.J. (2001) Exercise induces gastric ischemia in healthy volunteers: a tonometry study. Journal of Applied Physiology 91, 866-871 [DOI] [PubMed] [Google Scholar]
- Pals K.L., Chang R.T., Ryan A.J., Gisolfi C.V. (1997) Effect of running intensity on intestinal permeability. Journal of Applied Physiology 82, 571-576 [DOI] [PubMed] [Google Scholar]
- Peters H.P.F., Akkermans L.M.A., de Vries W.R. (2001a) Gastrointestinal symptoms during prolonged exercise: Incidence, etiology, and recommendations. American Journal of Medicine and Sports 3, 94-106 [Google Scholar]
- Peters H.P.F., de Vries W.R., Vanberge-Henegouwen G.P., Akkermans L.M.A. (2001b) Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 48, 435-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A.J. (1993) Heat stroke and endotoxaemia: Sensitisation or tolerance to endotoxins? In: Perspectives in Exercise Science and Sports Medicine, Vol. 6., Exercise, Heat, and Thermoregulation. Eds: Gisolfi C.V. Lamb D.R.and Nadel E.R. Carmel, IL: Cooper Publishing Group [Google Scholar]
- Ryan A.J., Chang R.T., Gisolfi C.V. (1996) Gastrointestinal permeability following aspirin intake and prolonged running. Medicine & Science in Sports & Exercise 28, 698-705 [DOI] [PubMed] [Google Scholar]
- Schlang H.A., Kirkpatrick C.A. (1961) The effect of physical exercise on serum transaminase. American Journal of Medicine and Science 242, 338-341 [DOI] [PubMed] [Google Scholar]
- Sherlock S., Dooley J. (2001) Diseases of the liver and biliary system. Chapter 2. Assessment of liver function. 11th Edition Blackwell Science; Oxford [Google Scholar]
- Sherman W.M., Costill D.L., Fink W.J., Miller J.M. (1981) Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. International Journal of Sports Medicine 2, 114-118 [DOI] [PubMed] [Google Scholar]
- Smith J.E., Garbutt G., Lopes P., Pedoe D.T. (2004) Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. British Journal of Sports Medicine 38, 292-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M., Triantafilou K. (2002) Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends in Immunology 23, 301-304 [DOI] [PubMed] [Google Scholar]
- van Deventer S.J., Knepper A., Landsman J., Lawson J., ten Cate J.W., Büller H.R., Sturk A., Pauw W. (1988) Endotoxins in portal blood. Hepatogastroenterology 35, 223-225 [PubMed] [Google Scholar]
- van Deventer S.J.H., Büller H.R., ten Cate J.W., Aarden L.A., Hack E., Sturk A. (1990) Experimental endotoxaemia in humans: Analysis of cytokine release and coagulation, fibrinolitic, and complement pathways. Blood 76, 2520-2526 [PubMed] [Google Scholar]
- van Deventer S.J.H., Büller H.R., ten Cate J.W., Sturk A., Pauw W. (1998) Endotoxaemia: An early predictor of septicaemia in febrile patients. Lancet 331, 605-608 [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhoven M.A., Brouns F., Brummer R.J.M. (1999) The effect of physical exercise on parameters of gastrointestinal function. Neurogastroenterology and Motility 11, 431-439 [DOI] [PubMed] [Google Scholar]
- Vander A.J., Sherman J.H., Luciano D.S. (1998) Human physiology: The mechanisms of body function. McGraw-Hill, New York [Google Scholar]
- Wagenmakers A.J. (1992) Amino acid metabolism, muscular fatigue and muscle wasting. Speculations on adaptations at high altitude. International Journal of Sports Medicine 13, s110-s113 [DOI] [PubMed] [Google Scholar]
- Widrick J.J., Costill D.L., Fink W.J., Hickey M.S., McConell G.K., Tanaka H. (1993) Carbohydrate feedings and exercise performance: Effect of initial muscle glycogen concentration. Journal of Applied Physiology 74, 2998-3005 [DOI] [PubMed] [Google Scholar]


