Abstract
Medial tibial stress syndrome (MTSS) is a common overuse injury of the lower extremity predominantly observed in weight bearing activities. Knowledge about the pathological lesions and their pathophysiology is still limited. Only a single study was found to have investigated tibial bone density in the pain region, revealing lower density in athletes with long standing (range, 5-120 month) MTSS. In a follow-up study, bone density was determined to return to normal levels after recovery. The purpose of the present study was to investigate tibial bone density in athletes with shorter MTSS history (range, 3-10 weeks). A total of 11 athletes (7 males, 4 females) diagnosed with medial tibial stress syndrome were included in the study. The control group consisted of 11 regularly exercising individuals (7 males, 4 females). Tibial, femoral and vertebral bone densities were measured by dual energy x-ray absorptiometry. Total calcium intake was calculated by evaluating detailed nutrition history. No statistically significant differences were found in the tibial, femoral and vertebral bone densities between the groups. No statistically significant difference was found among groups, considering for calcium intake. Tibial bone densities were not lower in athletes with MTSS of 5.0 weeks mean duration (range, 3-10 weeks) compared to the healthy control group. Longitudinal studies with regular tibial bone density measurements in heavily trained athletes are necessary to investigate tibial density alterations in MTSS developing athletes during the course of the symptoms.
Key points.
Tibial, femoral and vertebral bone densities were measured by dual energy x-ray absorptiometry.
No differences were found between the MTSS group (MTSS history 3-10 weeks) and the healthy athletes group.
Key words: Medial tibial stress syndrome; tibial bone density; calcium intake, athletes; exercise; bone mineral density; overuse injury
Introduction
Medial tibial stress syndrome (MTSS) is a frequent repetitive- stress injury of the lower extremity (Clement et al., 1981; Yates et al., 2003). It is characterized with increasing pain in the distal 2/3rds of the posteromedial tibia. The aetiopathology of MTSS still remains unclear. Many factors contribute to the pathological pattern of tibial loading and the resulting strain (Anderson et al., 1997; Beck, 1998; Detmer, 1986; Fredericson et al., 1995; Michael and Holder, 1985). Increased scintigraphic uptake in the distal 2/3rds of the posteromedial tibial bone may be observed. This would support an increased bone remodelling in MTSS (Holder and Michael, 1984; Michael and Holder, 1985; Beck, 1998; Moen et al., 2009).
Likewise, some authors propose changes in the tibial bone features with MTSS (Beck, 1998; Franklyn et al., 2008; Magnusson et al., 2001). Magnusson et al., 2001 found lower tibial bone density in long-standing (average duration 31 months) MTSS patients. Bone density is reported to have returned to normal levels after recovery, in a follow-up study (Magnusson et al., 2003).
No further studies investigating tibial bone density in athletes with MTSS were found in the literature with the exception of the studies by Magnusson et al. Therefore it is not clear at which time of the developing MTSS injury the suggested decrease of tibial bone density starts.
The main purpose of this controlled, cross-sectional study was to compare tibial bone density with dual energy x-ray absorptiometry (DXA) measurements of athletes with MTSS in an early stage of the injury (average 5.0 weeks) with those of their healthy athletic counterparts. A possible difference in calcium intake between the two groups was also investigated in this study.
Methods
Eleven athletes (7 males, 4 females) with MTSS were included in the study. Their mean duration of medial tibial stress syndrome was 5.0 weeks (range, 3 - 10 weeks). Inclusion criteria for the patients were: to be between 18 - 23 years of age, be free of any systemic disease, and to be diagnosed with MTSS by two different physicians upon taking injury history, presence of pain at the junction of the middle and distal thirds of the medial tibia, tenderness with palpation in a diffuse area for at least 5 centimeters in the distal 2/3 of the posteromedial tibia, a positive one leg hop test, and the absence of any other additional pathology (Yates and White, 2004; Moen et al., 2009). Patients with anterior or posterior cruciate ligament injury, or with previous lower extremity surgery or fracture, or with neurological or vascular pathologies in the lower extremities, or females with amenorrhea were excluded from the study.
The control group consisted of 11 (7 males, 4 females) subjects regularly exercising in weight bearing activities (4 badminton players, 2 athletes, 2 dancers, 3 soccer players). They were free of any history of previously diagnosed MTSS injury, of any previous surgery, ligament injury or fracture in the lower extremities, and the female subjects were free of amenorrhea. Anthropometric data and weekly training durations of the two groups are given in Table 1.
Table 1.
Anthropometric data and training program of the subjects. Data are means (±SD).
| MTSS group (n=11) |
Control group (n=11) |
|
|---|---|---|
| Age (year) | 21.0(1.9) | 23.3 (3.0) |
| Height (m) | 1.79 (.12) | 1.72 (.08) |
| Body weight (kg) | 72.4 (14.8) | 65.5 (13.5) |
| BMI (kg·m-2) | 22.4 (2.6) | 21.9 (3.1) |
| Weekly training days | 4.1 (1.8) | 4.6 (1.4) |
| Training session duration (h) | 3.4 (2.5) | 3.2 (2.4) |
| Weekly training (h) | 16.9 (17.8) | 16.5 (15.9) |
BMI: body mass index.
A survey about nutritional habits concerning calcium-rich foods was applied to the subjects. They were asked about their average monthly consumptions of milk, yoghurt, cheese, nuts (sunflower seeds, pistachios, almonds), legumes (dried beans, lentils, chickpeas, pinto beans). Any food supplements or multivitamin intakes were also recorded.
The cumulative monthly consumption (milk in liters, the other nutrients in kilograms) of the above mentioned nutrients with the exception of cheese was multiplied with a factor of 1200; and the total monthly cheese consumption was multiplied with a factor of 4000. The sum of these two scores gives the monthly calcium intake in milligrams (Duyff, 2000). In case of dietary supplement or multivitamin use, the calcium content was determined and added to the monthly calcium consumption. The final calcium score was assumed to be the monthly total calcium intake.
DXA measurements were made by using a bone densitometer (Hologic QDR 4500A, USA). The lumbar measurements were done in the lumbar spine scan mode for L1-L4. The femoral head and total femur measurements were done in the left hip mode.
Tibial measurements were taken at both sides from a subregion scan mode. The distance between the medial tibial plateau and the medial malleolus was recorded. The tibia was then marked at two points in order to divide it into three equal parts (Figure 1). A third point was marked at 1/9th of the tibial length proximal to the most prominent point of the medial malleolus. The bone mineral density (BMD) in the area between this third mark and the point 1/12th of the tibial length proximal to it was designated as R1.
Figure 1.
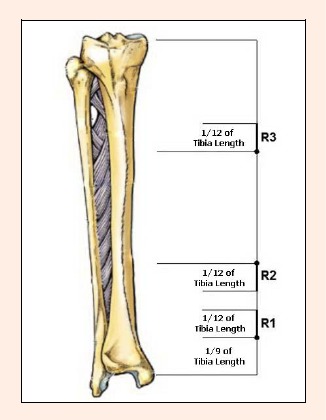
Regions of tibial dual energy x-ray absorptiometry measurement; R1, R2, R3.
The BMD in the area between the lower of the first marked two points and the point 1/12th of the tibial length distal to it was designated as R2. The BMD in the area between the upper of the first marked two points and the point 1/12th of the tibial length proximal to it was designated as R3.
Statistical analysis
Statistical analyses were made using the “SPSS v13.0 software package program”. Descriptive mean and standard deviation (SD) values for numerical data, and frequency values for relative data were calculated. Data not meeting the intra-group homogeneity precondition were classified as nonparametric, and their inter-group comparisons were made by using the Mann-Whitney U test. The remaining data were compared using independent samples T test.
Results
The anthropometric data (Table 1) of the two groups were similar (p > 0.05). Mean injury duration of the patient group was 5.0 ± 2.1 weeks (3-10 weeks). All patients had bilateral complaints. In three patients the symptoms were more severe on the right side, and in three patients on the left side.
No statistically significant differences were found between the groups’ training characteristics. Of the 11 patients 9 (81.8%) answered with “yes” to the question whether there was an increase in training duration or intensity in the time one month before the onset of the MTSS symptoms. No statistically significant difference was found between the two groups regarding the monthly intake of any calcium containing nutrients (p > 0.05), (Table 2).
Table 2.
Monthly calcium containing nutrients and total calcium intake. Data are means (±SD).
| MTSS group (n=11) |
Control group (n=11) |
|
|---|---|---|
| Milk (L) | 9.1 (6.3) | 7.9 (3.8) |
| Yoghurt (kg) | 5.1 (3.9) | 6.0 (4.0) |
| Cheese (kg) | 1.7 (1.1) | 2.6 (1.1) |
| Nuts (kg) | .9 (.8) | .5 (.4) |
| Legumes (kg) | 2.6 (1.3) | 2.9 (1.6) |
| Total calcium (g) | 24.4 (11.6) | 27.6 (7.1) |
The DXA measurements revealed no statistically significant differences between the groups (Table 3). Between the legs, tibial densities of the R1, R2 and R3 areas revealed no statistically significant differences among the two groups (Table 4), and no statistically significant differences in the groups.
Table 3.
Comparison of the DXA measurements of the groups. Data are means (±SD).
| MTSS group (n=11) |
Control group (n=11) |
|
|---|---|---|
| Femoral neck BMD (g·cm-2) | .976 (.134) | .964 (.134) |
| Total femur BMD (g·cm-2) | 1.114 (.180) | 1.084 (.149) |
| Total lumbar BMD (g·cm-2) | 1.046 (.097) | 1.107 (.095) |
| Femoral neck T score | .537 (.771) | .470 (.846) |
| Total femur T score | .793 (1.014) | .575 (.790) |
| Total lumbar T score | -.236 (.754) | .312 (.834) |
DXA (dual energy x-ray absorptiometry), BMD (bone mineral density)
Table 4.
The tibial R1, R2 and R3 BMDs of the groups. Data are means (±SD).
| MTSS group (n=11) |
Control group n=11) |
|
|---|---|---|
| right R1 (g·cm-2) | .211 (.049) | .217 (.072) |
| left R1 (g·cm-2) | .196 (.036) | .213 (.069) |
| right R2 (g·cm-2) | .305 (.088) | .321 (.097) |
| left R2 (g·cm-2) | .326 (.098) | .326 (.093) |
| right R3 (g·cm-2) | .317 (.080) | .333 (.073) |
| left R3 (g·cm-2) | .339 (.078) | .339 (.078) |
Discussion
In this study we did not find any statistically significant differences in the tibial BMDs between the athletes with MTSS (range duration symptoms, 3-10 weeks) and the control group.
Magnusson et al., 2001 had measured BMDs in five different tibial areas in athletes diagnosed with MTSS. The mean duration of symptoms was 31 months (range, 5-120 months). They compared BMD in MTSS patients with a healthy athletic group and a healthy non-athletic group. In the MTSS group, the BMD in the region corresponding to the pain was 15 ± 9% and 23 ± 8% lower than those observed in the non-athletic control and the athletic control groups, respectively. In the MTSS group, BMD measurements of the other regions were mostly higher than the measurements of the control group (proximal tibia: 16 ± 14%, femoral neck: 9 ± 9%), but lower than the measurements of the athletic control group (proximal tibia: 13 ± 11%, femoral neck: 11 ± 8%). They speculated that reduced accrual during growth and in response to exercise, or excessive bone loss after the onset of symptoms, or combinations of these mechanisms might contribute to the deficit in bone mineral density.
Three different areas of the tibia were measured in this study (Figure 1). These were not the same as in the Magnusson et al. study, which makes comparison between the studies hard. As the DXA device could not get a single shot for the whole tibia, we used metal marks for the before-mentioned three points and measured them separately. Magnusson et al., 2001 did not explain how they determined their five regions on the tibia. According to the figure in their study, our painful R2 area corresponds to theirs R4, and our R3 area corresponds to their R2 area. Our R1 area did not match any of the five regions of the Magnusson et al. study.
Mechanical loading is known to stimulate bone formation (Carter, 1982; Waldorff et al., 2010). Frost, 2004 stated in his excellent review about bone physiology that repeated bone strains cause microdamage in the bone. This microdamage has an operational threshold strain range that lies above the bone’s modeling threshold. Normally, load bearing bones can detect and repair the little microdamage caused by strains that stay belove the microdamage threshold; remodeling basic multicellular units provide that repair and osteocytes may provide this detection.
The diversity and daily repetition of stress stimulates bone remodeling up to a limit (Lanyon et al., 1982). During bone remodeling, while osteoclastic activity is completed within 2-3 weeks, the osteoblastic activity can continue up to 3 months. In our study, we did not find any statistically significant difference between the MTSS group and the control group regarding tibial BMD measurements. The average injury duration of the MTSS group was 5.0 weeks (range, 3-10 weeks). The mean injury duration in the Magnusson et al., 2001 study was 31 months. Probably due to repeated excessive stress for a long time, the repetitive osteoclastic stimulation and the slowly acting osteoblastic activity impair bone turnover during remodeling in favor to the destructive processes. This can be the reason why we could not find any change in the early period (5.0 ± 2.1 weeks), whereas Magnusson did measure significant differences in longstanding (31 month) MTSS patients.
In a follow-up study, Magnusson et al., 2003 reported that after an average of 5.7 years, when the MTSS patients were totally free of complaints, bone mineral density in the former painful tibia area increased by 19 ± 11%. No statistically significant difference in BMD with the healthy athletes group was found in the follow-up measurements. They proposed that the deficit in bone density was in conjunction with the symptoms.
Another finding of Magnusson et al., 2001 was that the femoral neck BMD and the lumbar BMD in the MTSS group were significantly lower than in the healthy athletes group. Myburgh et al., 1990 found a similar result in their study. They compared lumbar and femoral neck BMDs of 25 patients with stress fracture with those of healthy athletes. The stress fracture group displayed significantly lower BMD measurements. This may have various causes like an attainment of lower peak bone mineral density, a lower response to comparable exercise training, a marginally lower physical activity level, or bone loss associated with reduced activity during the MTSS (Magnusson et al., 2001). Our findings revealed no statistically significant differences between the groups regarding femoral and lumbar bone mineral densities. However, only with a controlled longitudinal study we would be able to assess whether it is developing later with MTSS.
As the purpose of this study was to investigate regional BMD in the painful localization, we also assessed a possible change in BMD due to calcium intake via a dietary survey, too. In order to calculate monthly calcium intake, we asked for the monthly consumption of foods with the highest calcium content. In the inquiry, monthly consumption of milk, yoghurt, cheese, legumes and nuts were noted. There was no significant difference between groups in terms of dietary total calcium intake. Therefore, we cannot discuss the effect of relative calcium deficiency in the development of MTSS or decrease in tibial BMD.
The subjects of both groups reported similar training characteristics. Many times, increases in training intensity, duration, or content have been associated with MTSS (Clement et al., 1981; Marti et al., 1988; Rudzki, 1997). In fact, in 81.8% of the patients in our study, MTSS developed following an increase in training intensity, duration, or content.
Limitations of the study
This study and its experimental design have a few methodological limitations that should be considered when interpreting the present results. First, the group sizes are small and participants from both genders are included in the study. Second, it is a cross-sectional study. Longitudinal studies may provide a better insight in temporal changes in tibial BMD. Third, three dimensional pQCT technology would provide a better assessment of BMD measurements.
Conclusion
Studies investigating tibial BMD measurements in athletes with MTSS are very limited in the literature, only one study reporting lower tibial BMD in athletes with long-standing MTSS. Our study revealed no differences in tibial BMD in athletes with shorter (3-10 weeks) history of MTSS. Longitudinal studies with regular tibial BMD measurements in athletes carrying out intensive training programs are needed. This would be the most accurate approach to investigate temporal changes in tibial BMD and complaints in athletes developing MTSS.
Biographies
Cengizhan Özgürbüz
Employment
Assoc. Prof., Ege University School of Medicine, Sports Medicine Department, Izmir, Turkey
Degree
MD
Research interests
Sports traumatology, exercise physiology
E-mail: cengizhan.ozgurbuz@ege.edu.tr
Oğuz Yüksel
Employment
Ege University School of Medicine, Sports Medicine Department, IzmirTurkey
Degree
MD
Research interests
Sports traumatology
Metin Ergün
Employment
Assoc. Prof., Ege University School of Medicine, Sports Medicine Department, Izmir, Turkey
Degree
MD
Research interests
Sports traumatology
Çetin İşlegen
Employment
Prof., Ege University School of Medicine, Sports Medicine Department, IzmirTurkey
Degree
MD
Research interests
Exercise physiology
Emin Taskiran
Employment
Prof., Ege University School of Medicine, Orthopedics and Traumatology Department, IzmirTurkey
Degree
MD
Research interests
Knee surgery, sports traumatology, arthroscopy
Nevzad Denerel
Employment
Ege University School of Medicine, Sports Medicine Department, IzmirTurkey
Degree
MD
Research interests
Sports traumatology
Oğuz Karamizrak
Employment
Prof.,Dr., Head, Ege University School of Medicine, Sports Medicine Department, Izmir, Turkey
Degree
PhD
Research interests
Exercise biochemistry, nutrition
References
- Anderson M., Ugalde V., Batt M., Gacayan J. (1997) Shin splints: MR appearance in a preliminary study. Radiology 204, 177-180 [DOI] [PubMed] [Google Scholar]
- Beck B. (1998) Tibial stress injuries: an aetiological review for the purposes of guiding management. Sports Medicine 26(4), 265-279 [DOI] [PubMed] [Google Scholar]
- Carter D.R. (1982) The relationship between in vivo strains and cortical bone remodelling. CRC Critical Reviews in Biomedical Engineering 8, 1-28 [PubMed] [Google Scholar]
- Clement D.B., Taunton J.E., Smart G.W., McNicol K.L. (1981) A survey of overuse running injuries. Physician and Sportsmedicine 9, 47-58 [DOI] [PubMed] [Google Scholar]
- Detmer D. (1986) Chronic shin splints. Classification and management of medial tibial stress syndrome. Sports Medicine 3(6), 436-446 [DOI] [PubMed] [Google Scholar]
- Duyff R.L. (2000) The American Dietetic Association’s Complete Food and Nutrition Guide. Chronimed Publishing, Minneapolis [Google Scholar]
- Franklyn M., Oakes B., Field B., Wells P., Morgan D. (2008) Section modulus is the optimum geometric predictor for stress fractures and medial tibial stress syndrome in both male and female athletes. American Journal of Sports Medicine 36(6), 1179-1189 [DOI] [PubMed] [Google Scholar]
- Fredericson M., Bergman G., Hoffman K., Dillingham M. (1995) Tibial stress reaction in runners: correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. American Journal of Sports Medicine 23, 427-481 [DOI] [PubMed] [Google Scholar]
- Frost H.M. (2004) A 2003 update of bone physiology and Wolff’s law for clinician. Angle Orthodontist 74(1), 3-15 [DOI] [PubMed] [Google Scholar]
- Holder L.E., Michael R.H. (1984) The specific scintigraphic pattern of “shin splints in the lower leg”: concise communication. Journal of Nuclear Medicine 25, 865-869 [PubMed] [Google Scholar]
- Lanyon L.E., Rubin C.T., O’Connor J.A. (1982) The stimulus for mechanically adaptive bone remodeling. In: Osteoporosis. Eds: Menczel J., Robin G.C. New York: Wiley; 135-147 [Google Scholar]
- Magnusson H.I., Westlin N.E., Nyqvist F., Gärdsell P., Seeman E., Karlsson M.K. (2001) Abnormally decreased regional bone density in athletes with medial tibial stress syndrome. American Journal of Sports Medicine 29(6), 712-715 [DOI] [PubMed] [Google Scholar]
- Magnusson H.I., Ahlborg H.G., Karlsson C., Nyquist F., Karlsson M.K. (2003) Low regional tibial bone density in athletes with medial tibial stress syndrome normalizes after recovery from symptoms. American Journal of Sports Medicine 31(4), 596-600 [DOI] [PubMed] [Google Scholar]
- Marti B., Vader J.P., Minder C.E., Abelin T. (1988) On the epidemiology of running injuries: the 1984 Bern Grand-Prix study. American Journal of Sports Medicine 16, 285-294 [DOI] [PubMed] [Google Scholar]
- Michael R.H., Holder L.E. (1985) The soleus syndrome. A cause of medial tibial stress (shin splints). American Journal of Sports Medicine 13(2), 87-94 [DOI] [PubMed] [Google Scholar]
- Moen M.H., Tol J.L., Weir A., Steunebrink M., de Winter T.C. (2009) Medial tibial stress syndrome. A critical review. Sports medicine 39, 523-546 [DOI] [PubMed] [Google Scholar]
- Myburgh K.H., Hutchins J., Fataar A.B., Hough S.F., Noakes T.D. (1990) Low bone density is an etiologic factor for stress fractures in athletes. Annals of Internal Medicine 113(10), 754-759 [DOI] [PubMed] [Google Scholar]
- Rudzki S.J. (1997) Injuries in Australian Army recruits. Part I: decreased incidence and severity of injury seen with reduced running distance. Military Medicine 162, 472-476 [PubMed] [Google Scholar]
- Waldorff E.I, Christenson K.B., Cooney L.C., Goldstein S.A. (2010) Microdamage repair and remodeling requires mechanical loading. Journal of Bone and Mineral Research 25(4), 734-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates B., Allen M.J., Barnes M.R. (2003) Outcome of surgical treatment of medial tibial stress syndrome. Journal of Bone and Joint Surgery-American Volume 85-A(10), 1974-1980 [DOI] [PubMed] [Google Scholar]
- Yates B., White S. (2004) The incidence and risk factors in the development of medial tibial stress syndrome among naval recruits. American Journal of Sports Medicine 32, 772-780 [DOI] [PubMed] [Google Scholar]


