Abstract
The purpose of the study was to determine the effects of cold water immersion (CWI) performed immediately or 3 h after a high intensity interval exercise session (HIIS) on next-day exercise performance. Eight male athletes performed three HIIS at 90%VO2max velocity followed by either a passive recovery (CON), CWI performed immediately post-exercise (CWI(0)) or CWI performed 3 h post-exercise (CWI(3)). Recovery trials were performed in a counter balanced manner. Participants then returned 24 h later and completed a muscle soreness and a totally quality recovery perception (TQRP) questionnaire, which was then followed by the Yoyo Intermittent Recovery Test [level 1] (YRT). Venous blood samples were collected pre-HIIS and pre-YRT to determine C-Reactive Protein (CRP) levels. Significantly more shuttles were performed during the YRT following CWI(0) compared to the CON trial (p=0.017, ES = 0. 8), while differences between the CWI(3) and the CON trials approached significance (p = 0.058, ES = 0.5). Performance on the YRT between the CWI(0) and CWI(3) trials were similar (p = 0.147, ES = 0. 3). Qualitative analyses demonstrated a 98% and 92% likely beneficial effect of CWI(0) and CWI(3) on next day performance, compared to CON, respectively, while CWI(0) resulted in a 79% likely benefit when compared to CWI(3). CRP values were significantly lower pre-YRT, compared to baseline, following CWI(0) (p = 0.0.36) and CWI(3) (p = 0.045), but were similar for CON (p = 0.157). Muscle soreness scores were similar between trials (p = 1.10), while TQRP scores were significantly lower for CON compared to CWI(0) (p = 0.002 ) and CWI(3) (p = 0.024). Immediate CWI resulted in superior next-day YRT performance compared to CON, while delayed (3 h) CWI was also likely to be beneficial. Qualitative analyses suggested that CWI(0) resulted in better performance than CWI(3). These results are important for athletes who do not have immediate access to CWI following exercise.
Key points.
Performance of cold water immersion as a recovery procedure following exercise is better than performing no recovery procedure
Athletes, coaches and sport trainers should implement cold water immersion post-exercise irrespective of the time of administration.
Where possible, cold water immersion should be performed immediately post-exercise to gain maximal recovery benefits.
Key words: Recovery, cold water immersion, C-Reactive Protein, yoyo test
Introduction
Recovery, the return of the body to its pre-exercise state (Tomlin and Wenger, 2001), is an integral component of every athlete's training programme (Cochrane, 2004). Recovery is particularly relevant to athletes due to an implied desire by these individuals to perform at optimal capacity during subsequent sporting events. While many athletes may participate in intermittent sporting events, such as an individual track event, there may be times when performance events are performed over a series of days or even on the same day, such as when an athlete enters into a number of events or when carnivals are held (Montgomery et al., 2008). This makes the recovery process between one sporting fixture and the next an extremely important process.
To date, numerous passive and active recovery methods have been trialed, such as carbohydrate replenishment, stretching and massage, with water immersion representing a popular application (Cochrane, 2004; Halson et al., 2008). Of relevance, a recent study by Ingram et al., 2009 reported that cold water immersion (CWI), as opposed to contrast (hot and cold) water therapy and a control condition, resulted in a more rapid return to baseline exercise (running) performance in male athletes. Benefits of CWI on exercise recovery have been attributed to both the hydrostatic pressure of water, as well as the water temperature, with both these factors reported to evoke several mechanisms that can attenuate the physical consequences of exercise (i.e., delayed onset of muscle soreness), and in particular, reduce inflammation and oedema (Wilcock et al., 2006). This is important, as inflammation and oedema cause stiffness, reduced range of movement, loss of force generation and pain (Wilcock et al., 2006), which in turn negatively affects physical movement and hence exercise. Of relevance, an analgesic effect is produced during CWI (Vaile et al., 2008), which may further contribute to the benefits of its use as a recovery modality.
Currently, there have been only a few studies that have investigated the effects of CWI on exercise performed on a subsequent day (Bosak et al., 2006; Ingram et al., 2009: Lane and Wenger, 2004; Vaile et al., 2008). This is an important issue for athletes involved in events that require exercise to be performed over a couple of days. Furthermore, only one study is known to the authors that examined the effects of a delayed recovery intervention following exercise on subsequent exercise performed 24 h after the initial exercise session (Lum et al., 2009). These investigators reported benefit of next day exhaustive exercise performance associated with a swimming based recovery period (compared to a passive control) that was performed 10 h following an initial high intensity exercise session. Further studies are needed to assess the effect of delayed CWI, as it can be a number of hours before an athlete has access to recovery procedures following exercise performance. This is demonstrated when athletes compete in events that are a reasonable distance from their home-base, as occurs in pennant tennis or team-sport games, particularly those played in rural areas.
Therefore, the aim of this study was to investigate the effects of CWI performed 3 h post a high intensity exercise session, compared to immediate CWI or passive recovery, on next-day running performance. It was hypothesised that immediate CWI would produce the most beneficial results in relation to next day exercise performance due to the immediate consequences of CWI on the inflammatory process. It was also hypothesised that CWI performed 3 h post-exercise would result in better next day performance than a passive recovery trial.
Methods
Eight well trained male athletes (Australian Rules football, n = 7, hockey, n = 1), competing at the highest amateur level of their chosen sport, were recruited for participation in this study. Mean (± standard deviation) age, height, body mass and VO2max were 20.9 ± 1.2 y, 1.84 ± 0.05 m, 79.4 ± 6.0 kg and 56.1 ± 4.6 ml·kg-1·min-1, respectively. Participants were in the mid-season phase of competition and were injury free at the time of testing. Ethics approval was obtained from the Human Research Ethics Committee of the University of Western Australia (UWA). Participants were informed of the risks and benefits associated with the study and provided written informed consent prior to participation.
Participants attended the human performance laboratory at UWA on seven separate occasions, for a partial familiarisation session and three trials, with each trial requiring participants to return the next day for testing. The three experimental trials consisted of a high intensity interval session (HIIS), followed by one of three recovery conditions, presented in a counter-balanced order so to reduce any potential order effect. The next day (24 h later), participants returned to undertake the Yoyo Intermittent Recovery test [Level 1] (YRT) in order to assess how well they had recovered from the previous day’s exercise.
All trials were performed at the same time of day, but separated by at least one week. Participants were asked to abstain from alcohol, caffeine and strenuous exercise for the 24 h period prior to each testing session. Participants were also required to complete a food diary in the 24 h period prior to, and for the duration of, each trial and requested to replicate dietary intake as closely as possible for each trial. The food diary was monitored weekly by the researchers to ensure compliancy, with this resulting in no concerns.
The partial familiarisation session involved a graded exercise test (GXT) to determine each participants’ VO2max and suitable exercise intensities for subsequent sessions. The GXT was conducted on a motorised treadmill (Nury Tec VR3000, Germany) in a step-like fashion, using 3 min work periods and 1 min rest periods. An initial speed of 12 km·h-1 was used, with 1 km·h-1 increases for each 3 min work period until volitional exhaustion occurred. The treadmill was set at a 1% gradient to replicate outdoor conditions (Jones and Doust, 1996). Heart rate (HR) was recorded before testing and at the completion of each work period (Polar Heart Rate Monitor, Polar Electro, Finland), while capillary blood samples were taken from the earlobe during each 1 min rest break so to assess plasma blood lactate. Samples were subsequently analysed using a blood-gas analyser (ABL 625, Radiometer Medical A/S, Copenhagen, Denmark). Expired air concentrations of O2 and CO2 were analysed continuously during the GXT (Ametek Gas Analysers, Applied Electrochemistry, SOV S-3A/1 and COV CD-3A, Pittsburgh, PA). Gas analysers were calibrated using gases of known concentrations (BOC Gases, Chatswood, Australia) before and after each testing session. Ventilation was recorded at 15 s intervals via a turbine ventilometer (Morgan, 225 A, Kent, England), which was calibrated before and after exercise using a 1 L syringe, in accordance with the manufacturer’s specifications. The sum of the four highest consecutive 15 s values of V O2 recorded during the GXT was used to determine each participant’s VO2max. The GXT was followed by 15 min of cold water immersion (CWI) in order to familiarise participants to this procedure.
Approximately a week after the familiarisation session, participants arrived at the UWA exercise laboratory at 1200 and were seated for 10 min so to allow for postural changes in blood plasma to occur before a venous blood sample was collected. Venous blood was drawn from the median cubital vein of the forearm in 8.5 ml serum separator collection tubes (SST II Advance, BD Vacutainer, UK) for the measurement of C-reactive protein (CRP) concentration. CRP is an acute-phase serum protein that plays a regulatory role in inflammation and is deposited at sites in the body where acute inflammation occurs (Du Clos and Mold, 2004). CRP was subsequently measured at a local hospital pathology laboratory using a Roche Cobas Integra 800 analyser (Roche Diagnostics Australia) and a particle enhanced immunoturbidimetric assay kit. Absorbance was measured at 552 nM. The analytical coefficient of variation for CRP determination at 14.85 and 27.15 mg·L-1 was 1.76% and 2.19% respectively.
Immediately following the drawing of blood for CRP assessment, participants then performed a WUP prior to the HIIS. The WUP consisted of participants running on a treadmill for 5 min at 60% of their VO2max velocity, followed by 5 min of range of motion stretching. A capillary blood sample was taken immediately prior to the commencement of the high intensity interval session (HIIS). The HIIS was performed in the laboratory and consisted of eight intervals, each 3 min long, performed at 90% VO2max velocity. One minute passive rest periods were performed between each set, while capillary blood samples were taken upon completion of the 4th and 8th repetitions. Heart rate was constantly monitored, with readings taken at rest and at the end of each exercise interval.
The recovery trials consisted of a control condition [CON] performed immediately after the HIIS, cold water immersion [CWI(0)] undertaken immediately after the HIIS, or cold water immersion performed 3 h [CWI(3)] following the HIIS. The CON trial required participants to be seated for 15 min in laboratory conditions (~23°C, 43% relative humidity). Both CWI trials consisted of submerging the body to the mid-sternum in water at a temperature of 15°C (± 1°C) for 15 min. Heart rate was recorded immediately prior to, and following, each condition.
Participants returned to the laboratory the next day, 24 h after the HIIS. On arrival, participants were seated for 10 min prior to a venous blood sample being taken for a second assessment of CRP levels. The percentage changes in CRP from baseline levels (pre HIIS) to pre YRT (24 h later) were calculated so to allow comparison between the three trials.
The exercise session began with a standardised 10 min warm up that consisted of 200 m of jogging at a self-selected pace, followed by two sets (20 m per set) of high knee lifts, buttock kicks and sideways running. Five 20 m run-throughs were then performed at progressively increasing speeds, with this pace self-selected by the participant. Participants then rated themselves on the Total Quality Recovery Perception (TQRP) scale (Kentta and Hassmen, 1998) and a 7-point Likert scale for muscle soreness. Following this, participants undertook the YRT (Veale et al., 2010). The test was performed indoors on a wooden gymnasium floor.
The YRT is a shuttle run that is similar to the beep test and involves high intensity running that is interspersed with recovery periods, making it applicable to track athletes as well as team sport athletes (Bangsbo et al., 2008). The YRT consists of 2 x 20 m shuttles (20 m out and 20 m back designates one shuttle) performed at a speed designated by the Yo-Yo compact disk (Helle Thompson, Dopenhagen, Denmark), with an active recovery undertaken between each shuttle. The active recovery requires participants to walk or jog (their wish) 2 x 5 m (out and back) and then to wait at the starting line for the next signal to run again (10 s recovery time all up). The YRT (level 1) starts at 10 km·h-1 with levels progressively increasing in speed throughout the test, with the test considered complete when the participant misses performing two consecutive shuttles within the required time. Upon completion of the test, the participants’ HR was recorded and plasma blood lactate concentrations were measured. Test- retest of the YRT (level 1) has been previously performed by Thomas et al., 2006 in 16 team sport and recreational athletes and resulted in an ICC of 0.95 (p < 0.01), a typical error of 0.26 (approximately 2 shuttles) and a CV% of 1.9%, respectively.
Statistical analyses
The mean ± standard deviations (SD) for all variables were calculated. Repeated- measures ANOVAs were used to compare next-day performance, blood parameters and psychological outcomes between the three recovery conditions. Post-hoc, pair wise comparisons were applied in the event of a main effect so to accurately determine where differences existed. The alpha level was set at p ≤ 0.05. Cohen’s d effect sizes (ES) and thresholds (< 0.5, small; 0.5 - 0.79, moderate; ≥ 0.8, large) were also used to compare the magnitude of the difference in change scores between the three trials (Cohen, 1988). Only moderate to large effects are reported. Further analysis was conducted to identify the smallest worthwhile change in YRT performance scores between the three recovery trials, using the method outlined by Batterham and Hopkins, 2006. This approach represents a contemporary method of data analysis that uses confidence intervals in order to calculate the probability that an effect is clinically beneficial, trivial or harmful (Batterham and Hopkins, 2006). The smallest worthwhile value of change was set at a Cohen’s effect size of 0.2, representing the hypothetical, smallest change in next day YRT performance that would benefit the athlete. Where the chance of benefit and harm were both calculated to be ≥ 5%, the true effect was deemed unclear (Batterham and Hopkins, 2006). When clear interpretation was definitively possible, a qualitative descriptor was assigned to the following quantitative chances of benefit: 25-75%, benefit possible; 75-95%, benefit likely; 95- 99%, benefit very likely; > 99%, benefit almost certain (Batterham and Hopkins, 2006).
Results
Environmental conditions were similar between trials for the HIIS and the recovery conditions (23.5 ± 1.2°C, RH = 40.7 ± 6.1 %), which were both performed in a performance laboratory. Furthermore, environmental conditions were similar between trials for the YRT (16.3 ± 1.5°C; RH 50. 1 ± 5.9%), which was performed outdoors.
Results showed that physiological measures of effort for the HIIS were similar between trials, in that there were no significant differences in final exercise values for HR (p = 0.206) and plasma blood lactate concentrations (p = 0.353). Final HR and plasma blood lactate values were 188 ± 8, 186 ± 5 and 189 ± 7 bpm and 8.0 ± 2, 8.2 ± 2.3, and 8.7 ± 2.2 mmol·L-1 for the CWI(0), CWI(3) and the CON trials, respectively.
Table 1 illustrates the difference in the number of shuttles completed in the YRT following the CON, CWI(0) and CWI(3) trials. There was a significant main effect of trial on YRT performance (p = 0.010), with the average number of completed shuttles following CWI(0) being significantly greater than the CON trial (p = 0.017). Further, CWI(0) resulted in the completion of 5.5 additional shuttles. This outcome was supported by qualitative analysis that resulted in a large ES and a 98% ‘very likely’ beneficial effect associated with CWI(0), compared to CON (Table 2). In addition, the difference in next-day YRT performance scores between the CWI(3) and the CON trials approached significance (p = 0.058), with qualitative analyses resulting in a 92% ‘likely benefit’ associated with CWI(3), as well as a moderate ES (Table 2). However, there was no significant difference in shuttles completed (p = 0.147) between the CWI(0) and CWI(3) trials, qualitative analysis suggested that CWI(0) had a 79% more beneficial effect. Furthermore, plasma blood lactate and HR were similar between all three trials at the conclusion of the YRT (BLa, p = 0.956, HR, p = 0.578; Table 1).
Table 1.
Yoyo Intermittent Recovery Test (level 1) results for completed shuttles, blood lactate and heart rate following the control (CON), cold water immersion performed immediately after exercise (CWI(0)) and cold water immersion performed 3 h following exercise (CWI(3)) trials. Values are means (±SD, n = 8).
| CON | CWI(0) | CWI(3) | |
|---|---|---|---|
| Shuttles completed | 32.4 (5.0) | 37.9 (8.6) * | 35.7 (7.8) |
| Plasma blood lactate (mmol∙L-1) | 7.4 (1.6) | 7.3 (1.4) | 7.5 (1.2) |
| Heart rate (bpm) | 185 (9) | 187 (10) | 186 (7) |
* significantly (p < 0.05) different from CON
Table 2.
Qualitative analysis of shuttles completed following the control trial (CON), cold water immersion performed immediately after exercise (CWI(0)) and cold water immersion performed three hours after the exercise trial (CWI(3)) (n = 8).
| CON vs CWI(0) | CON vs CWI(3) | CWI(0) vs CWI(3) | |
|---|---|---|---|
| Cohen’s d Effect Size | .80 | .50 | .30 |
| Mean Change ± 90% confidence limits | 5.5 (.7) | 3.3 (.6) | 2.2 (.5) |
| % chance that effect is beneficial (trivial/harmful) | 98 (2/0) | 92 (7/1) | 79 (18/2) |
Scores for perceived recovery (as defined by the TQRP) exhibited a significant main effect of trial (p = 0.003). Ratings were significantly lower after the CON trial compared to both the CWI(0) and CWI(3) trials (p = 0.002 and p = 0.024, respectively). Scores for the TQRP were 12.6 ± 1. 7, 15.2 ± 2.0 and 14.2 ± 1.6 for the CON, CWI(0) and CWI(3) trials, respectively. In respect to muscle soreness, no significant main effect of trial was apparent (p = 0.110). Scores for muscle soreness were 4.2 ± 1.7, 5.6 ± 1.3 and 5.0 ± 1.2, for the CON, CWI(0) and CWI(3) trials, respectively.
Figure 1 shows the percentage change in CRP levels from immediately prior to the HIIS (baseline) to 24 h later prior to YRT for CON, CWI(0) and CWI(3). A main effect was revealed (p = 0.046), with post hoc testing indicating that CWI(0) was significantly different to the CON trial (p = 0.018) 24 h after the HISS. The difference between the CON and CWI(3) approached significance (p = 0.073), while there was no significant difference between the two CWI trials (p = 0.464). Within trials CRP values were significantly lower following HIIS than baseline levels following CWI (CWI(0), p = 0.036 and CWI(3), p = 0.045), whereas no changes were noted between the CRP levels in the CON trial (p = 0.157).
Figure 1.
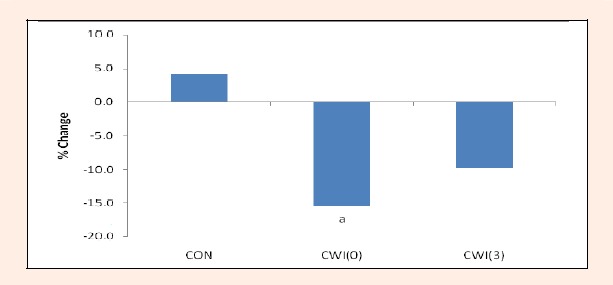
Percentage change for levels of C-Reactive Protein (CRP) pre the high intensity interval training session (baseline) and 24 h later prior the Yoyo Intermittent Recovery test in the control (CON: 4.1 ± 10.6 %), cold water immersion performed immediately after exercise (CWI(0): -15.5 ± 18.8 %) and cold water immersion performed three hours after the exercise (CWI(3): -9.8 ± 14.1 %) trials. N = 8. a Denotes significant difference from CON (p < 0.05).
Discussion
The aim of this study was to investigate the effects of CWI performed either immediately or 3 h after a HIIS on next-day YRT performance, compared to a passive control condition. Results showed that immediate CWI significantly improved next-day YRT performance when compared to the CON trial, while differences between delayed (3 h) CWI and the CON trial approached significance. These results were supported by qualitative analyses that suggested a ‘very likely benefit’ and a ‘likely benefit’ associated with CWI[0] and CWI[3], respectively, compared to CON, as well as moderate to large ES. Furthermore, significantly lower CRP levels measured pre-YRT compared to pre-HIIS (baseline) following CWI, provide evidence for benefit associated with this form of recovery. While there was no significant difference observed in YRT performance following CWI(0) and CWI(3), qualitative analysis indicated that CWI(0) had a 79% more beneficial effect. Furthermore, similar HR and plasma blood lactate values recorded at the end of the YRT between trials (Table 2), despite improved exercise performance following both CWI trials, further supports benefits associated with either immediate or delayed (3 h) CWI.
Similar to the current study, other researchers have reported that CWI performed immediately post-exercise resulted in benefit to subsequent exercise performed ~1 h (compared to active recovery; Vaile et al. , 2010) and 24 h (compared to passive control; Bosak et al., 2006; Lane and Wenger, 2004) post immersion. In addition, Ingram et. al. (2009) reported improved repeated sprint performance performed 48 h after CWI that was performed both immediately and 24 h following exhaustive exercise (compared to contrast water immersion and a control condition), while Vaile et al., 2008, found improved cycle performance undertaken over five consecutive days that was immediately followed by CWI, compared to passive recovery and hot water immersion.
Of importance, this current study also demonstrated that delayed (3 h) CWI may have provided a benefit to next day exercise performance compared to a control trial. These results are consistent with Lum et al., 2009, who required participants to perform a high intensity interval exercise session, which was followed 10 h later by either a swimming-based recovery session or a passive recovery session, with a time to fatigue run performed 24 h later. Results showed that the swimming recovery session resulted in a significantly longer time to fatigue in next day exercise performance (830 ± 198 s vs 728 ± 183 s, p = 0.005). Benefits associated with subsequent exercise performance following the swim recovery session were proposed to be a result of the hydrostatic pressure of water that resulted in a decreased inflammatory response, as determined by a significant reduction in circulating CRP levels measured 24 h following the initial exercise session (Lum et al., 2009). According to Wilcock et al., 2006, hydrostatic pressure can reduce the severity of exercise-induced oedema, as well as the infiltration of monocytes and leukocytes into the cell, resulting in reduced levels of inflammatory cells/muscle enzymes into the blood. It is also likely that the temperature of the water had a positive effect on subsequent exercise performance. Cold water immersion is proposed to reduce inflammation by evoking vasoconstriction (Peiffer et al., 2009) and decreasing peripheral blood flow (Sramek et al., 2000; Vaile et al., 2010). Vasoconstriction reduces the permeability of cellular, lymphatic and capillary vessels, which slows fluid diffusion into the interstitial space, therefore limiting oedema (Eston and Peters, 1999). In addition, CWI has also been reported to induce an increase in stroke volume and cardiac output (Gabrielsen et al., 2002; Lollgen et al., 1981; Sramek et al., 2000), with the resulting increased blood flow assisting in the maintenance of core temperature. This process has been proposed to attenuate blood flow to the traumatised muscle (Thorsson et al., 1985; Vaile et al., 2010), resulting in reduced inflammation, and hence improved recovery. Therefore, the application of CWI should attenuate oedema (Arnheim and Prentice, 1993). Importantly, reduced inflammation and hence swelling, also results in reduced pain and the loss of force generation that is associated with inflammation (Wilcock et al., 2006).
Similar to the study by Lum et al., 2009, a decrease in circulating CRP levels 24 h after the initial exercise session was also seen in the current study following CWI, suggesting that both the positive effects of hydrostatic pressure and cold water resulted in a reduced inflammatory response, with this most likely resulting in improved subsequent exercise performance. These effects of CWI provide some insight into the small difference (ns) in next day exercise performance between the CWI(0) and CWI(3) trials, as CWI performed straight after HIIS would have acted to limit oedema almost immediately, whereas delayed CWI would have resulted in some inflammation and hence oedema occurring in the 3 h period between the HIIS and the immersion.
A reduced inflammatory response in the current study (as determined by lower circulating CRP levels following both CWI trials) may also explain the significantly higher perceived recovery scores (TQRP) recorded following the CWI(0) and CWI(3) trials, compared to the control trial. This result is similar to that by Suzuki et al., 2004 who reported improved psychological ratings (Profile of Mood State questionnaire) related to an aquatic exercise recovery compared to complete rest, following a game of rugby. In contrast, Lum et al., 2009 found no significant difference in perceived recovery scores as determined by the TQRP between a swim and a passive recovery trial that followed high intensity interval exercise. However this result may be due to the longer delay in performing the swim-based recovery session compared to the current study (10 h vs 3 h),which may have been perceived by the participants to be too long to result in benefit. Furthermore, as few publications have used the TQRP scale to date, it is difficult to identify trends between studies.
In agreement with several previous investigations using CWI after exercise (Bosak et al., 2006; Eston and Peters, 1999; Sellwood et al., 2007), muscle soreness ratings did not differ significantly between trials in the current study. These results differ to other studies that reported reduced sensations of muscle soreness following CWI (Bailey et al., 2007; Ingram et al., 2009). Differences in results between studies may be due to use of different muscle soreness scales (i.e., varying visual analogue scales), the different initial exercise protocols used (i.e., various durations, modes and major muscle groups employed), the temperature of the water (range of 5 to 15°C), body parts exposed to immersion (i.e., elbows or legs as opposed to immersion to the umbilicus or to the level of the anterior superior spines), as well as the duration of immersion (range of 3 x 1 min sessions to a continuous 15 min session). Of relevance, results from the current study demonstrate that sensations of muscle soreness have minimal effect on subsequent exercise performance.
Conclusion
In summary, quantitative and qualitative analyses demonstrated that immediate CWI performed after a HIIS resulted in better next day running performance (YRT), while delayed (3 h) CWI was also likely to result in improved YRT performance, compared to no CWI. Importantly, greater benefit was associated with immediate CWI. This information is pertinent to athletes, particularly if they do not have immediate access to recovery facilities following exercise performance.
Acknowledgement
We wish to acknowledge the financial support we received from UWA.
Biographies
Ned Brophy-Williams
Employment
PSchool of Sport Science, Exercise & Health, the University of Western Australia, Australia.
Degree
Bachelor in Science (Hons)
Research interests
Exercise and recovery modalities.
Grant Landers
Employment
Ass. Prof., School of Sport Science, Exercise & Health, the University of Western Australia, Au.
Degree
PhD.
Research interests
Exercise and recovery modalities.
Karen Wallman
Employment
Prof., School of Sport Science, Exercise & Health, the University of Western Australia, Australia.
Degree
PhD.
Research interests
Ergogenic aids, recovery modalities and exercise in chronic illnesses.
E-mail: karen.wallman@uwa.edu.au
References
- Arnheim D.D., Prentice W.E. (1993) Therapeutic modalities. In: Principles of athletic training. 8thedition Missouri, Mosby Year Book [Google Scholar]
- Bailey D.M., Erith S.J., Griffin P.J., Dowson A., Brewer D.S., Gant N., Williams C. (2007) Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running. Journal of Sports Science 25(11),1163-1170 [DOI] [PubMed] [Google Scholar]
- Bangsbo J., Iaia M., Krustrup P. (2008) The yoyo intermittent recovery test: a useful tool for evaluation of physical performance in intermittent sports. Sports Medicine 38(1), 37-51 [DOI] [PubMed] [Google Scholar]
- Batterham A.M., Hopkins W.G. (2006) Making meaningful inferences about magnitudes. International Journal of Sports Physiology & Performance 1(1), 50-57 [PubMed] [Google Scholar]
- Bosak A., Bishop P., Smith J., Green M., Richardson M., Iosia M. (2006) Impact of cold water immersion on 5km racing performance. Medicine & Science in Sport & Exercise 38 (5), S233 [Google Scholar]
- Cochrane D.J. (2004) Alternating hot and cold water immersion for athlete recovery: a review. Physical Therapy in Sport 5(1), 26-32 [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences. 2ndedition New Jersey, Lawrence Earlbaum Associates [Google Scholar]
- Du Clos T.W., Mold C. (2004) C-Reactive Protein: An activator of innate immunity and a modulator of adaptive immunity. Immunologic Research 30(3), 261-267 [DOI] [PubMed] [Google Scholar]
- Eston R., Peters D. (1999) Effects of cold water immersion on the symptoms of exercise-induced muscle damage. Journal of Sports Science 17(3), 231-238 [DOI] [PubMed] [Google Scholar]
- Gabrielsen A., Pump B., Bie P., Christensen N.J., Warberg J., Norsk P. (2002) A trial distension, haemodilution, and acute control of renin release during water immersion in humans. Acta Physiology Scandinavia 174(2), 91-99 [DOI] [PubMed] [Google Scholar]
- Halson S.L., Quod M.J., Martin D.T., Gardner A.S., Ebert T.R., Laursen P.B. (2008) Physiological responses to cold water immersion following cycling in the heat. International Journal of Sports Physiological Performance 3(3), 331-346 [DOI] [PubMed] [Google Scholar]
- Ingram J., Dawson B., Goodman C., Wallman K. and, Beilby J. (2009) Effect of water immersion methods on post-exercise recovery from simulated team sport exercise. Journal of Science and Medicine in Sport 12(3), 417-421 [DOI] [PubMed] [Google Scholar]
- Jones A.M., Doust J.H. (1996) A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. Journal of Sports Science 14(4), 321-327 [DOI] [PubMed] [Google Scholar]
- Kentta G., Hassmen P. (1998) Overtraining and recovery. A conceptual model. Sports Medicine 26(1), 1-16 [DOI] [PubMed] [Google Scholar]
- Lane K.N., Wenger H.A. (2004) Effect of selected recovery conditions on performance of repeated bouts of intermittent cycling separated by 24 hours. Journal of Strength & Conditioning Research 18(4), 855-860 [DOI] [PubMed] [Google Scholar]
- Lum D., Landers G., Peeling P. (2009) Effects of a recovery swim on subsequent running performance. International Journal of Sports Medicine 30(1), 1-5 [DOI] [PubMed] [Google Scholar]
- Lollgen H., Nieding G., Koppenhagen K., Kersting F., Just H. (1981) Hemodynamic response to graded water immersion. Journal of Molecular Medicine 59(12), 623-628 [DOI] [PubMed] [Google Scholar]
- Montgomery P.G., Pyne D.B., Cox A.J., Hopkins W.G., Minahan C.L., Hunt P.H. (2008) Muscle damage, inflammation, and recovery interventions during a 3-day basketball tournament. European Journal of Sport Science 8(5), 241-250 [Google Scholar]
- Peiffer J.J., Abbiss C.R., Nosaka K., Peake J.M., Laursen P.B. (2009) Effect of cold water immersion after exercise in the heat on muscle function, body temperatures, and vessel diameter. Journal of Science and Medicine in Sport 12(1), 91-96 [DOI] [PubMed] [Google Scholar]
- Sellwood K., Brukner P., Williams D., Nicol A., Hinman R. (2007) Ice-water immersion and delayed-onset muscle soreness: a randomised controlled trial. British Journal of Sports Medicine 41, 392-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sramek P., Simeckova M., Jansky L., Savlikova J., Vybiral S. (2000) Human physiological responses to immersion into water of different temperatures. European Journal of Applied Physiology 81(5), 436-442 [DOI] [PubMed] [Google Scholar]
- Suzuki M., Umeda T., Nakaji S., Shimoyama T., Mashiko T., Sugawara K. (2004) Effect of incorporating low intensity exercise into the recovery period after a rugby match. British Journal of Sports Medicine 38, 436-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., Dawson B., Goodman C. (2006) The yo-yo test: reliability and association with a 20-m shuttle run and VO2max. International Journal of Sports Physiology and Performance 1, 137-149 [DOI] [PubMed] [Google Scholar]
- Thorsson O., Lilja B., Ahlgren L., Hemdal B., Westlin N. (1985) The effect of local cold application on intramuscular blood flow at rest and after running. Medicine & Science in Sports & Exercise 17, 710-713 [DOI] [PubMed] [Google Scholar]
- Tomlin D.L., Wenger H.A. (2001) The relationship between aerobic fitness and recovery from high intensity intermittent exercise. Sports Medicine 31(1), 1-11 [DOI] [PubMed] [Google Scholar]
- Vaile J., O’Hagan C., Stefanovic B., Walker M., Gill N., Askew C. (2010) Effect of cold water immersion on repeated cycling performance and limb blood flow. British Journal of Sports Medicine 45, 825-829 [DOI] [PubMed] [Google Scholar]
- Vaile J., Halson S., Gill N., Dawson B. (2008) Effect of hydrotherapy on recovery from fatigue. International Journal of Sports Medicine 29(7), 539-544 [DOI] [PubMed] [Google Scholar]
- Veale J., Pearce A., Carlson J. (2010) The yo-yo intermittent recovery test (level 1) to discriminate elite junior Australian football players. Journal of Science & Medicine in Sport 13(3), 329-331 [DOI] [PubMed] [Google Scholar]
- Wilcock I.M., Cronin J.B., Hing W.A. (2006) Physiological response to water immersion. Sports Medicine 36(9), 747-765 [DOI] [PubMed] [Google Scholar]


