Abstract
Bone response to a single bout of exercise can be observed with biochemical markers of bone formation and resorption. The purpose of this study was to examine the response of bone biochemical markers to a single bout of exhaustive high-impact exercise. 15 physically active young subjects volunteered to participate. The subjects performed continuous bilateral jumping with the ankle plantarflexors at 65 % of maximal ground reaction force (GRF) until exhaustion. Loading was characterized by analyzing the GRF recorded for the duration of the exercise. Venous blood samples were taken at baseline, immediately after, 2h and on day 1 and day 2 after the exercise. Procollagen type I amino terminal propeptide (P1NP, marker of bone formation) and carboxyterminal crosslinked telopeptide (CTx, marker of bone resorption) were analyzed from the blood samples. CTx increased significantly (32 %, p = 0.015) two days after the exercise and there was a tendensy towards increase seen in P1NP (p = 0.053) one day after the exercise. A significant positive correlation (r = 0.49 to 0.69, p ≤ 0.038) was observed between change in P1NP from baseline to day 1 and exercise variables (maximal slope of acceleration, body weight (BW) adjusted maximal GRF, BW adjusted GRF exercise intensity and osteogenic index). Based on the two biochemical bone turnover markers, it can be concluded that bone turnover is increased in response to a very strenuous single bout of exhaustive high-impact exercise.
Key points.
Studies on bone acute biochemical response to loading have yielded unequivocal results.
There is a paucity of research on the biochemical bone response to high impact exercise.
An increase in bone turnover was observed one to two days post exercise.
Key words: Bone biochemical marker, jumping, bone turnover, osteogenic index
Introduction
It has been shown that physical exercise has osteogenic effect. Specifically high-impact exercises, activities causing high vertical ground reaction forces including maximal jumping and leaping to vertical and onward directions (Nikander et al., 2006), have been shown to have osteogenic effects (Heinonen et al., 1996; Karinkanta et al., 2007; Vainionpaa et al., 2005). However, it takes several months before exercise-induced changes in bone structural properties can be observed (Christenson, 1997). The state of the skeleton can be evaluated by many techniques, of which biochemical markers exhibit the fastest response to intervention while being minimally invasive (Watts, 1999). The skeletal response to exercise can be measured from blood samples (Virtanen et al., 1993) and the markers can be used to estimate the bone remodeling rate (Ebeling and Akesson, 2001;Weisman and Matkovic, 2005) by comparing the resorption markers to formation markers (Christenson, 1997). Biochemical markers of bone remodeling can be measured from the serum or urine. These markers fall into two main categories: markers of bone formation and markers of bone resorption (Weisman and Matkovic, 2005). The serum concentration of procollagen type I amino terminal propeptide (P1NP) reflects changes in the synthesis of new collagen. P1NP appears to be a dynamic and sensitive marker of changes in bone formation. Carboxy terminal crosslinked telopeptide (CTx) represents the rate of bone resorption and it can also be measured in serum or urine (Ebeling and Akesson, 2001).
Observations of short-term responses of bone turnover to an acute bout of exercise in young adults indicate that the biochemical marker responses are exercise and intensity specific, and are consequently non-uniform (Brahm et al., 1997b; Guillemant et al., 2004; Kristoffersson et al., 1995; Maimoun et al., 2006; Wallace et al., 2000; Welsh et al., 1997; Whipple et al., 2004; Zittermann et al., 2002). With untrained men an intense bout of resistance training has been show to lead to decreased concentrations of bone resorption and formation markers, and to an increased amount of urinary calcium excretion (Ashizawa et al., 1998). In case of moderate resistance training in untrained men the decrease in resorption is higher leading into positive change in formation to resorption ratio (Whipple et al., 2004). Endurance-type exercises have produced mixed results. An acute bout of endurance-type exercise has been shown to stimulate bone resorption (Guillemant et al., 2004) and formation markers in aerobically trained men (Maimoun et al., 2006;Wallace et al., 2000), to have no effect on bone markers with male and female subjects with varying fitness levels (Brahm et al., 1997a) or to reduce the concentration of bone resorption markers in trained and untrained men (Welsh et al. , 1997;Whipple et al., 2004;Zittermann et al., 2002). Similarly, short-term anaerobic endurance exercise has been demonstrated to elevate biochemical bone markers in untrained male and female subjects (Brahm et al., 1997b) and to have no effect (Kristoffersson et al., 1995) on biochemical bone markers in trained men.
Previous studies have shown equivocal acute short-term bone responses measured from blood or urine samples after a single bout of loading (Brahm et al., 1997b; Guillemant et al., 2004; Kristoffersson et al., 1995; Maimoun et al., 2006; Wallace et al., 2000; Welsh et al., 1997; Whipple et al., 2004; Zittermann et al., 2002). Differences in bone responses to short-term exercise are most likely related to the various exercise types, intensities and protocols that have been used. Furthermore, most of the studies in this area have not used high-impact exercises, which are most likely to provoke exercise-induced bone responses (Turner, 1998). Therefore, the purpose of this study was to examine the response of bone biochemical markers to a single bout of high-impact exercise.
Methods
A sample of 15 young male students (age 25 (3) years, height 177 (6) cm, weight 75 (10) kg) served as the subjects for the study. Inclusion criteria were: willing to participate, age from 20 to 35, full understanding of the study protocol and no history of any illness contraindicated to exhaustive loading protocol. The subjects represented a wide range of physical activity levels ranging from sedentary to amateur athletes (exercising up to 6 times/week) from a wide variety of sporting events (ballgames, running, bicycling, rock climbing and resistance exercise). The studies were conducted according to the declaration of Helsinki with the approval of the ethics committee of the University of Jyväskylä. The subjects gave their informed written consent to participate.
Protocol
The subjects first warmed up on a bicycle ergometer for 10 - 15 minutes with a freely chosen intensity. A fatiguing bilateral jumping exercise was then performed until exhaustion. The subjects were instructed to jump with minimal knee flexion so that the ankle plantar flexors were the targeted muscle group in the exercise. Ten to twenty straight-legged continuous jumps were performed with increasing intensity until a steady maximal ground reaction force (GRF) level, corresponding to maximal performance, was achieved. The maximal GRF was used to determine a submaximal target level of 65 % of maximal GRF. The subjects were instructed to reach the target GRF level with each successive jump during exercise. Exercise was continued until the target level of 65 % of maximal GRF could not be reached for 10 successive jumps or until the subject refused to continue. During exercise an experimenter monitored the instantaneous ground reaction force and provided the subject with verbal instructions to jump higher or lower. The jumps were conducted continuously with no intervening breaks. Verbal encouragement was provided, especially during the late phases of the exercise.
Blood samples
A venous blood sample was taken prior to the warm-up (baseline) and immediately after (after) the exercise. The subjects returned to the laboratory for further venous blood sample collections two hours after the exercise (2 hours) and for two successive days (day 1 and day 2) after the exercise. The follow-up blood samples were taken close to the same time of day as the pre-exercise measurement. Subjects were instructed to avoid strenuous exercise for two days prior to the pre-exercise measurement and during the two-day follow- up period. The subjects were not asked to fast prior to blood sampling.
Exercise quantification
Bilateral jumping was performed on a custom-made force plate (natural frequency 140 ± 10 Hz in the vertical direction, 90 ± 10 Hz in the horizontal direction, linearity ≤ 1%, cross-talk ≤ 1%, University of Jyväskylä, Finland). The number of jumps was calculated manually. Exercise intensity was expressed in multiples of BW determined from the average ground reaction force peaks of each jump. Both the intensity determination and the fatiguing exercise ground contacts were included in this average. The ground reaction force curves of each jump were time scaled to the average length of the ground contact time and then averaged to produce a single ground reaction force curve to represent the loading. The time normalization was performed by taking the fast Fourier transformation of a ground contact reaction force and then recomposing the transformed data to consist of an appropriate number of data points. This representative ground reaction force was then divided by the weight of the subject to produce an presentative acceleration curve. From the representative average acceleration curve maximum slope of acceleration (slope) and the area of acceleration (area) were calculated. Another fast Fourier transformation was then taken from the representative acceleration curve and osteogenic index was calculated as follows:
Fourier series, fl= l:th frequency in the Fourier series and N = the number of loading cycles. Frequency content up to 50 Hz was included in the analysis.
Ground reaction force analysis was conducted with MATLAB® (MATLAB® the language of technical computing, version 7.0.1.24704 (R14) service pack 1, The MathWorks, Inc.) software.
Biochemical bone markers
CTx (β- CrossLaps/serum, ECLIA assay, Roche, CV = 4.3 %) and P1NP (total P1NP, ECLIA assay, Roche, CV = 2.4 %) were analyzed from the venous blood samples with automatic immunoassay (Elecsys 2010, Roche) methods. The blood samples were stored at -80°C until analysis. The time in storage was 6 - 18 months. Bone marker results were adjusted for changes in plasma volume as follows:
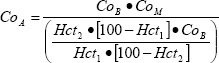 |
[adapted from (van Beaumont et al., 1973)]
where CoA= adjusted concentration, CoB = concentration before exercise, CoM = measured concentration, Hct2 = hematocrit after exercise and in the follow up measurements, Hct1 = hematocrit before exercise. The ratio of bone formation to bone resorption markers (Christenson, 1997) was calculated as P1NP divided by CTx. Values adjusted for changes in plasma volume were used for all calculations and statistical analyses and the adjusted values are reported.
Statistical analysis
The data values are reported as mean (standard deviation). The data normality was tested with Shapiro-Wilk normality test. The distribution was not normal on the bone markers and thus non-parametric tests were selected for further analysis. A non-parametric Friedman test was used to determine whether bone marker differences existed between repeated measurements. When differences did exist, the follow-up measurements were compared with the measurements taken before using a non-parametric Wilcoxon test. The p-values were not adjusted for multiple Wilcoxon comparisons. Correlations between exercise variables (number of jumps, maximal GRF, maximal GRF in multiples of body weight, exercise intensity in multiples of body weight, osteogenic index, maximum slope of acceleration and the area of acceleration) and the largest change in bone marker variables were calculated using the one-tailed Spearman rank correlation coefficient. The significance level was set at P ≤ 0.05 for all statistical analyses. Power calculations indicated that at the chosen 0.05 significane level with a standard deviation of 15 % for the difference between before and after measurements, a sample size of 15 would give 80 % power for the study to detect a 14 % increase or decrease within a given variable (Vincent, 1995). For correlation analysis a correlation coefficient of r = 0.60 could be detected at a power of 80 % with the chosen sample size of 15 (Cohen, 1988).
Results
The average number of jumps in the exhaustive stretch shortening cycle exercise was 1250 (570) (range 520 to 2278). The average maximal ground reaction force was 4400 (900) N in maximal bilateral jumping, which was 6.0 (0.7) times body weight. The intensity of the fatiguing exercise was 4.2 (0.5) times body weight in terms of peak reaction force (Figure 1). The average osteogenic index reached the value of 110 (26). The average maximum slope of acceleration during increasing force was 660 (150) m/s3and the area of the acceleration during ground contact was 4.6 (0.7) m·s-1. Bone marker results adjusted for changes in plasma volume are reported in Table 1.
Figure 1.
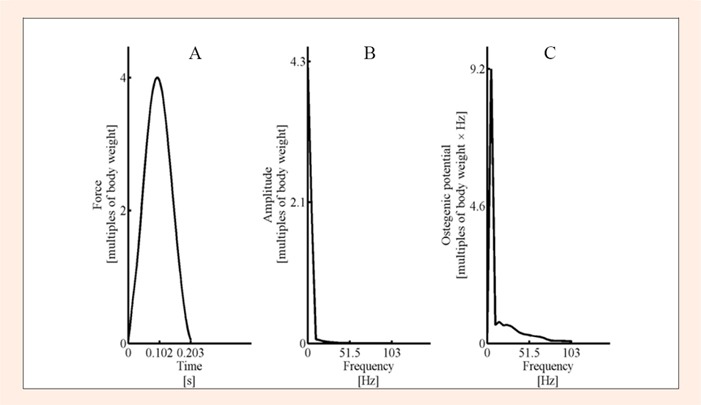
A) Representative fatiguing exercise ground reaction force in multiples of body weight (which is the same as acceleration in multiples of gravitational acceleration) from one subject. B) The corresponding amplitudes of frequency components obtained with fast Fourier transformation from the representative ground reaction force curve. C) Osteogenic potential components of frequency components of the representative ground reaction force curve. Frequency content up to 50 Hz was included in the osteogenic index calculations.
Table 1.
Biochemical bone markers before and after the fatiguing exercise and reference values obtained from literature (Szulc et al., 2001). Bone marker values were adjusted with plasma volume changes. Data are mean (±standard deviation).
| P1NP [ng·ml-1] | CTx [ng·ml-1] | P1NP/CTx [ratio] | |
|---|---|---|---|
| Reference value | 74 (29) (Szulc et al., 2001) | .6 (.2) (Szulc et al., 2001)§ | 123 (45) (calculated from (Szulc et al., 2001) D calculatedas the RMS of relative SDs) |
| Baseline | 78 (47) | .51 (.23) | 157 (54) |
| After | 85 (50) | .42 (.16) | 197(69) |
| 2 hours | 78 (45) | .42 (.19) | 190 (55) |
| Day 1 | 86 (46) | .59 (.31) | 166 (79) |
| Day 2 | 85 (54) | .67 (.36)* | 145 (71) |
§ Szulc et al. (2001) reported CTx results in mmol·L-1. The units have been converted to ng·ml-1with the following equation taken from (Crofton et al., 2002):x (ng·L-1) = y (pmol·L-1) · 138]/7.75.
* signifies significant difference from baseline, p ≤ 0.05. P1NP = procollagen type I amino terminal propeptide, CTx = carboxyterminal crosslinked telopeptide.
The P1NP values at different sampling points differed from each other (p = 0.013) as well as the CTx (p = 0. 008) values at different sampling points. In multiple comparisons a significant increase was seen from the baseline to the day 2 measurement (p = 0.015) in the CTx bone resorption marker. The average value of the increase was 32 %. Even though the repeated measures differed significantly, no significant differences in P1NP were observed between baseline and any of the follow up measurements. The biggest difference was seen on day 1 after the exercise. However, this difference was not statistically significant (p = 0.053) (Table 1). The repeated measures of ratios of bone formation to resorption differed significantly (p = 0.047). In multiple comparisons there was no significant difference from the baseline (p ≥ 0.078) (Table 1).
Correlations were calculated between changes in the bone marker values and exercise variables. The largest differences from the baseline values were seen at day 1 for P1NP and at day 2 for CTx; these values were used to quantify the changes in bone markers. There was significant positive correlation between the percentage change in P1NP from baseline to day 1 and body weight adjusted maximal ground reaction force (r = 0.49, p = 0.038), body weight adjusted ground reaction force exercise intensity (r = 0. 6, p = 0.012), slope of acceleration (r = 0.68, p = 0.004) and osteogenic index (r = 0.69, p = 0. 003). There was a significant negative correlation between the change in CTx from baseline to 2 days after the exercise and maximal GRF (r = -0.49, p = 0.039) (Figure 2). There was no significant correlation between the number of jumps and change in P1NP (r = 0. 26, p = 0.174) or change in CTx (r = 0.26, p = 0.174). The scatterplots in Figure 2 seem to indicate markedly higher change in P1NP in one of the subjects. There was no methodological reason to eliminate this potential outlier. However, if this subject was removed, the correlation coefficients and P-values for the aforementioned correlations were as follows: ∆P1NP% to BW adjusted maximal GRF (r = 0.36, p = 0.112), to BW adjusted ground reaction force exercise intensity (r = 0.52, p = 0.032), to slope of acceleration (r = 0.73, P = 0.003), to OI (r = 0.73, p = 0.002), and to number of jumps (r = 0. 55, p = 0.020). ∆CTx% to BW adjusted maximal GRF (r = 0.12, p = 0.354) and to number of jumps (r = 0. 10, p = 0.368). No other significant correlations were observed between exercise variables and changes in P1NP or CTx (r ≤ ±0.41, p ≥ 0.075).
Figure 2.
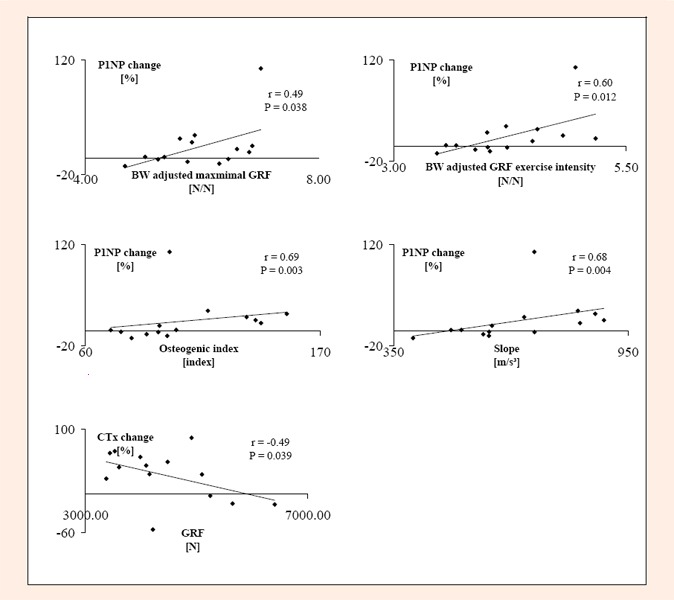
Correlations between ground reaction force variables and bone biochemical marker changes. GRF = maximal ground reaction force in bilateral jumping. P1NP = Procollagen type I amino terminal propeptide. CTx = Carboxyterminal crosslinked telopeptide.
Discussion
The primary finding of the current study was that bone resorption marker CTx (Carboxyterminal crosslinked telopeptide) was elevated 2 days after the exhaustive high-impact exercise. In addition, body size independent exhaustive exercise variables (body weight adjusted maximal GRF, BW adjusted GRF exercise intensity and osteogenic index) and P1NP (procollagen type I amino terminal propeptide) bone formation marker responses were positively associated. The primary findings support the results found in some of the endurance exercise and short lasting dynamic activity studies (Brahm et al., 1997a; 1997b; Guillemant et al., 2004; Maimoun et al., 2006; Wallace et al., 2000). However, the timing of increased bone resorption marker is delayed in comparison to the aforementioned endurance exercise studies where increment in resorption markers was seen within two hours of cessation of the exercise, whereas in this study the marker increase was observed two days after the exercise. These findings are not completely in agreement with resistance training studies (Ashizawa et al., 1998; Whipple et al., 2004). In resistance training studies, both formation and resorption markers have decreased acutely (Ashizawa et al., 1998; Whipple et al., 2004). Instead a somewhat contrasting non-significant trend of increase was observed in bone resorption in the present study. In agreement with Whipple et al., 2004 the trend in change of the ratio of bone formation to bone resorption was in favour of formation whilst not statistically significant in this study.
Strain rate, strain distribution and strain magnitude play major (Turner and Robling, 2003; Turner, 1998) roles in determining the bone response to loading. Therefore, the differences in loading characteristics most likely explain the apparent discrepancy between the current results and previous results from resistance training studies. In the present study the loading characteristics might have been closer to, for example, running than the loading characteristics of resistance exercise. Even though the magnitude of ground reaction force, and presumably muscular force production was relatively high, which is the case in resistance exercises, osteogenic loading is characterized by both the magnitude and rate of force change. The rate of force change in conventional resistance training does not match the rate of force change in jumping. It thus appears that division according to just the loading magnitude or exercise type (endurance training vs. resistance training) might not be appropriate.
There was a positive relationship observed between the loading characteristics and the bone formation marker change between the baseline and day 1 follow up. No dependency was seen between the number of jumps and the bone marker response. These results are in line with the suggestion that strain rate and magnitude play a bigger role than number of loading cycles in intiating a bone response (Turner and Robling, 2003; Turner, 1998).
Some recent studies have suggested that in middle aged women, the minimal effective loading intensity to achieve gains in bone strength is around five times body weight (Jamsa et al., 2006; McKay et al., 2005; Vainionpaa et al., 2006) and the minimal number of loading cycles needed around 60 (Vainionpaa et al., 2006). In additition, the area of the acceleration peak has been shown to be associated with positive bone characteristic change, when the area of the peak is ≥ 4 m·s-1 (Heikkinen et al., 2007). The osteogenic index of the exercise in the current study, imposed during a single bout of loading, is comparable to the osteogenic index of walking 20 minutes for 5 days a week, which appears to be osteogenic in early postmenopausal women (Asikainen et al., 2004). Osteogenic index caused by walking 20 minutes for 5 days a week was estimated to consist of 800 loading cycles (Turner and Robling, 2003) and the ground reaction force pattern was reproduced from the results by Schneider and Chao, 1983 (Schneider and Chao, 1983). However, even though the osteogenic index accounts for the loading frequency, magnitude and rate, it is unclear if comparison between a single bout and several bouts of exercise is reasonable. Furthermore achieving higher loading magnitude than the one obtained in the current study cannot be achieved without impact forces or external weights. The highest Achilles tendon forces have been recorded during continuous submaximal bilateral jumping (Komi et al., 1992), in which case there is no impact spike as can be seen from figure 1. The Achilles tendon forces in submaximal hopping exceed the forces measured during maximal sprinting and jumping activities (Komi et al., 1992; Kyrolainen et al., 2003). Even though there is some evidence from cross-sectional studies that impacts are not needed for bone gains (e.g. high bone strength in slalom skiers in lower limbs (Nikander et al., 2008), high bone strength in swimmers in upper limbs (Nikander et al., 2006)) it is not clear if impact forces are required for osteogenic effects. The loading characteristics and the volume of the exhausting exercise in the current study were such that the exercise can be assumed to have been osteogenic.
Limitations
The ratio of bone formation to resorption markers, which can be considered to reflect the balance between formation and resorption (Christenson, 1997), did not change significantly during the follow up period. However, it can not be ruled out that there might have been a change, which was not detected because of insufficient statistical power. The non-significant change from baseline to 2 hours after exercise was 19 %, which is above the change threshold of the preliminary statistical power calculations. The observed variation was, however, markedly higher (observed variations in change were 210 % and 125 % for P1NP and CTx respectively) than what was used for power calculations and thus the possibility of insufficient statistical power cannot be ruled out. Furthermore, bone biochemical markers were only followed two days post exercise and the bone remodeling cycle takes 2 to 3 months (Watts, 1999), so the amount of overall change in bone turnover, and the resulting overall change in bone characteristics could not be determined from the results of the present study. Nevertheless it appears that a single bout of loading is effective in causing changes in bone turnover, and that this activation of bone turnover can be observed from blood samples.
Biochemical bone markers at baseline were in line with values previously measured from young healthy males (Guillemant et al., 2004; Maimoun et al., 2006; Szulc et al., 2001). Possible changes in bone marker basal level or the diet of the subjects were not controlled in the current study. However, although circadian variation of up to 50 % exists in bone markers (Ebeling and Akesson, 2001), no significant day-to-day variation has been seen in CTx (Zittermann et al., 2002), or P1NP (Munday et al., 2006) levels when the samples have been collected at the same time of day. In the aforementioned studies the diet was controlled for. It is also known that loading history affects bone remodelling (Kato et al., 2006; Rubin and Lanyon, 1984; Turner and Robling, 2003; Umemura et al., 2002) and could thus affect the observed biochemical response. In the present study, however, long-term loading history of the subjects was not controlled. To minimize the effect of loading history, the exercise bout was continued until exhaustion, to have relatively comparable loading independent of the training status of the subject. One additional limitation is that the subjects did not exercise at the same time of the day, which might have affected the observed response even, when the follow up samples were then collected corresponding to the exercise time of day of the individual.
Conclusion
In conclusion, considering the two biochemical bone resorption and formation markers that were assessed, it can be concluded that bone turnover is increased in response to a very strenuous single bout of high-impact exercise.
Acknowledgements
This study was funded by the Academy of Finland and in part by the TBGS National Graduate School of Musculoskeletal Disorders and Biomaterials.
Biographies
Timo Rantalainen
Employment
Ph.D. student, University of Jyväskylä.
Degree
MSc
Research interests
Bone.
E-mail: timo.j.rantalainen@jyu.fi
Ari Heinonen
Employment
Professor of Physiotherapy, University of Jyväskylä.
Degree
PhD
Research interests
Physiotherapy.
Vesa Linnamo
Employment
Professor of Biology of Physical Activity, University of Jyväskylä.
Degree
PhD
Research interests
Biomechanics.
Paavo V. Komi
Employment
Professor emeritus of Biomechanics, University of Jyväskylä.
Degree
PhD
Research interests
Biomechanics.
Timo E. S. Takala
Employment
Oulu Deaconess Institute.
Degree
PhD
Research interests
Exercise Physiology.
Heikki Kainulainen
Employment
Professor of Exercise Physiology, University of Jyväskylä.
Degree
PhD
Research interests
Exercise Physiology.
References
- Ashizawa N., Ouchi G., Fujimura R., Yoshida Y., Tokuyama K., Suzuki M. (1998) Effects of a single bout of resistance exercise on calcium and bone metabolism in untrained young males. Calcified Tissue International 62, 104-108 [DOI] [PubMed] [Google Scholar]
- Asikainen T.M., Kukkonen-Harjula K., Miilunpalo S. (2004) Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials. Sports Medicine 34, 753-778 [DOI] [PubMed] [Google Scholar]
- Brahm H., Piehl-Aulin K., Ljunghall S. (1997a) Bone metabolism during exercise and recovery: the influence of plasma volume and physical fitness. Calcified Tissue International 61, 192-198 [DOI] [PubMed] [Google Scholar]
- Brahm H., Piehl-Aulin K., Saltin B., Ljunghall S. (1997b) Net fluxes over working thigh of hormones, growth factors and biomarkers of bone metabolism during short lasting dynamic exercise. Calcified Tissue International 60, 175-180 [DOI] [PubMed] [Google Scholar]
- Christenson R.H. (1997) Biochemical markers of bone metabolism: an overview. Clinical Biochemistry 30, 573-593 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum, Hillsdale, N.J.567 [Google Scholar]
- Crofton P.M., Evans N., Taylor M.R., Holland C.V. (2002) Serum CrossLaps: pediatric reference intervals from birth to 19 years of age. Clinical Chemistry 48, 671-673 [PubMed] [Google Scholar]
- Ebeling P.R., Akesson K. (2001) Role of biochemical markers in the management of osteoporosis. Best Practice & Reserach Clinical Rheumatology 15, 385-400 [DOI] [PubMed] [Google Scholar]
- Guillemant J., Accarie C., Peres G., Guillemant S. (2004) Acute effects of an oral calcium load on markers of bone metabolism during endurance cycling exercise in male athletes. Calcified Tissue International 74, 407-414 [DOI] [PubMed] [Google Scholar]
- Heikkinen R., Vihriala E., Vainionpaa A., Korpelainen R., Jamsa T. (2007) Acceleration slope of exercise-induced impacts is a determinant of changes in bone density. Journal of Biomechanics 40, 2967-2974 [DOI] [PubMed] [Google Scholar]
- Heinonen A., Kannus P., Sievanen H., Oja P., Pasanen M., Rinne M., Uusi-Rasi K., Vuori I. (1996) Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet 348, 1343-1347 [DOI] [PubMed] [Google Scholar]
- Jamsa T., Vainionpaa A., Korpelainen R., Vihriala E., Leppaluoto J. (2006) Effect of daily physical activity on proximal femur. Clinical Biomechanics 21, 1-7 [DOI] [PubMed] [Google Scholar]
- Karinkanta S., Heinonen A., Sievanen H., Uusi-Rasi K., Pasanen M., Ojala K., Fogelholm M., Kannus P. (2007) A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporosis International 18, 453-462 [DOI] [PubMed] [Google Scholar]
- Kato T., Terashima T., Yamashita T., Hatanaka Y., Honda A., Umemura Y. (2006) Effect of low-repetition jump training on bone mineral density in young women. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 100, 839-843 [DOI] [PubMed] [Google Scholar]
- Komi P.V., Fukashiro S., Jarvinen M. (1992) Biomechanical loading of Achilles tendon during normal locomotion. Clinics in Sports Medicine 11, 521-531 [PubMed] [Google Scholar]
- Kristoffersson A., Hultdin J., Holmlund I., Thorsen K., Lorentzon R. (1995) Effects of short-term maximal work on plasma calcium, parathyroid hormone, osteocalcin and biochemical markers of collagen metabolism. International Journal of Sports Medicine 16, 145-149 [DOI] [PubMed] [Google Scholar]
- Kyrolainen H., Finni T., Avela J., Komi P.V. (2003) Neuromuscular behaviour of the triceps surae muscle-tendon complex during running and jumping. International Journal of Sports Medicine 24, 153-155 [DOI] [PubMed] [Google Scholar]
- Maimoun L., Manetta J., Couret I., Dupuy A.M., Mariano-Goulart D., Micallef J.P., Peruchon E., Rossi M. (2006) The intensity level of physical exercise and the bone metabolism response. International Journal of Sports Medicine 27, 105-111 [DOI] [PubMed] [Google Scholar]
- McKay H., Tsang G., Heinonen A., MacKelvie K., Sanderson D., Khan K.M. (2005) Ground reaction forces associated with an effective elementary school based jumping intervention. British Journal of Sports Medicine 39, 10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday K., Ginty F., Fulford A., Bates C.J. (2006) Relationships between biochemical bone turnover markers, season, and inflammatory status indices in prepubertal Gambian boys. Calcified Tissue International 79, 15-21 [DOI] [PubMed] [Google Scholar]
- Nikander R., Sievanen H., Heinonen A., Karstila T., Kannus P. (2008) Load-specific differences in the structure of femoral neck and tibia between world-class moguls skiers and slalom skiers. Scandinavian Journal of Medicine & Science in Sports 18, 145-153 [DOI] [PubMed] [Google Scholar]
- Nikander R., Sievanen H., Uusi-Rasi K., Heinonen A., Kannus P. (2006) Loading modalities and bone structures at nonweight-bearing upper extremity and weight-bearing lower extremity: A pQCT study of adult female athletes. Bone 39, 886-894 [DOI] [PubMed] [Google Scholar]
- Rubin C.T., Lanyon L.E. (1984) Regulation of bone formation by applied dynamic loads. Journal of Bone and Joint Surgery (American volume) 66, 397-402 [PubMed] [Google Scholar]
- Schneider E., Chao E.Y. (1983) Fourier analysis of ground reaction forces in normals and patients with knee joint disease. Journal of Biomechanics; Journal of Biomechanics 16, 591-601 [DOI] [PubMed] [Google Scholar]
- Szulc P., Garnero P., Munoz F., Marchand F., Delmas P.D. (2001) Cross-sectional evaluation of bone metabolism in men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 16, 1642-1650 [DOI] [PubMed] [Google Scholar]
- Turner C.H., Robling A.G. (2003) Designing exercise regimens to increase bone strength. Exercise and Sport Sciences Reviews 31, 45-50 [DOI] [PubMed] [Google Scholar]
- Turner C.H. (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23, 399-407 [DOI] [PubMed] [Google Scholar]
- Umemura Y., Sogo N., Honda A. (2002) Effects of intervals between jumps or bouts on osteogenic response to loading. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 93, 1345-1348 [DOI] [PubMed] [Google Scholar]
- Vainionpaa A., Korpelainen R., Leppaluoto J., Jamsa T. (2005) Effects of high-impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporosis International 16, 191-197 [DOI] [PubMed] [Google Scholar]
- Vainionpaa A., Korpelainen R., Vihriala E., Rinta-Paavola A., Leppaluoto J., Jamsa T. (2006) Intensity of exercise is associated with bone density change in premenopausal women. Osteoporosis International 17, 455-463 [DOI] [PubMed] [Google Scholar]
- van Beaumont W., Strand J.C., Petrofsky J.S., Hipskind S.G., Greenleaf J.E. (1973) Changes in total plasma content of electrolytes and proteins with maximal exercise. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 34, 102-106 [DOI] [PubMed] [Google Scholar]
- Vincent W.J. (1995) Statistics in kinesiology. Human Kinetics, Champaign (IL), 256 [Google Scholar]
- Virtanen P., Viitasalo J.T., Vuori J., Vaananen K., Takala T.E. (1993) Effect of concentric exercise on serum muscle and collagen markers. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 75, 1272-1277 [DOI] [PubMed] [Google Scholar]
- Wallace J.D., Cuneo R.C., Lundberg P.A., Rosen T., Jorgensen J.O., Longobardi S., Keay N., Sacca L., Christiansen J.S., Bengtsson B.A., Sonksen P.H. (2000) Responses of markers of bone and collagen turnover to exercise, growth hormone (GH) administration, and GH withdrawal in trained adult males. The Journal of Clinical Endocrinology and Metabolism 85, 124-133 [DOI] [PubMed] [Google Scholar]
- Watts N.B. (1999) Clinical utility of biochemical markers of bone remodeling. Clinical Chemistry 45, 1359-1368 [PubMed] [Google Scholar]
- Weisman S.M., Matkovic V. (2005) Potential use of biochemical markers of bone turnover for assessing the effect of calcium supplementation and predicting fracture risk. Clinical Therapeutics 27, 299-308 [DOI] [PubMed] [Google Scholar]
- Welsh L., Rutherford O.M., James I., Crowley C., Comer M., Wolman R. (1997) The acute effects of exercise on bone turnover. International Journal of Sports Medicine 18, 247-251 [DOI] [PubMed] [Google Scholar]
- Whipple T. J., Le B.H., Demers L.M., Chinchilli V.M., Petit M.A., Sharkey N., Williams N.I. (2004) Acute effects of moderate intensity resistance exercise on bone cell activity. International Journal of Sports Medicine 25, 496-501 [DOI] [PubMed] [Google Scholar]
- Zittermann A., Sabatschus O., Jantzen S., Platen P., Danz A., Stehle P. (2002) Evidence for an acute rise of intestinal calcium absorption in response to aerobic exercise. European Journal of Nutrition 41, 189-196 [DOI] [PubMed] [Google Scholar]


