Abstract
The aim of this study was to investigate the effect of two different tapering period lengths on the concentration of plasma interleukin- 6 (IL-6), interleukin (IL-1β) and tumor necrosis factor-α (TNF-α) and performance in elite male cyclists. To this end, after completing 8 weeks progressive endurance exercise, twenty four high-level endurance cyclists were randomly assigned to one of two groups: a control group of cyclists (n = 12) continued performing progressive weekly training volume for 3 weeks while a taper group of cyclists (n = 12) proceeded with a 50% reduction in weekly training volume relative to the control group. A simulated 40 min time trial (40TT) performance ride was used as the criterion index of performance before and after the tapering period to evaluate the physiological and performance effects of each protocol. Blood samples were collected immediately post-40TT from all participants at the beginning of week 1, and the end of weeks 4, 8, 9 and 11. IL-1β, IL-6 and TNFα were assayed using a standard commercial ELISA kits (Quantikine; R & D Systems, Minneapolis, MN). The mean time to complete the 40TT in the taper group decreased significantly (p < 0.01) after both 1 and 3 weeks with reduced training volume relative to the control group. There were significant reductions in (p < 0.001) IL-1β, IL-6 and TNFα concentrations in the taper group relative to the control group at the end of the 3 week tapering period, but not at the end of the 1 week tapering period. These results demonstrate that both a 1 and a 3 week taper period will result in improved physical performance in trained cyclists but only a 3 week taper period will result in attenuation of post-exercise pro- inflammatory cytokines when compared to those continuing a more intense training regimen.
Key points.
The excessive endurance exercise-induced elevations in pro-inflammatory cytokines would, in turn, stimulate the release of anti-inflammatory cytokines.
Elevations in pro-inflammatory cytokines indicate athletes are highly susceptible to infections.
1and 3-week taper periods will reduce circulating pro-inflammatory cytokine levels thereby possibly limiting the chances of infection and potentially reducing the effects of these cytokines in inducing fatigue-like symptoms in athletes.
Key words: Endurance training, immune, performance, interleukin-1β, interleukin-6, Tumor necrosis factor α.
Introduction
Several epidemiological studies examining endurance exercise have found that there are interactive effects of exercise on immune function (Scharhag et al., 2006). Moderate exercise training improves immune system function, but sustained and prolonged bouts of endurance training cause a decrease in various indicators of immune function which can last between 3-24 hours following exercise cessation (Scharhag et al., 2006). Post- exercise immune function is most strongly suppressed when the exercise is prolonged, without interruption (>1.5 h), of at least moderate to high intensity (at least 55-75% maximum O2 uptake), and performed without sufficient recovery time. Increasing exercise volume and intensity in addition to decreasing recovery time may cause athletes to develop symptoms of overtraining and/or overreaching and compromise immune function. Training programs which can optimize training responses while minimizing the possibilities for overtraining and compromised immune function in athletes would benefit performance and reduce the likelihood of athlete illness. In order to improve endurance performance and to decrease symptoms of overreaching and overtraining including immune system suppression, many athletes reduce their training load for 6-21 days before the major competitions (a training procedure known as tapering). Planned tapering generally consists of high intensity exercise, with low volumes (Neary et al., 2003a). After a period of tapering, improved performance times have been reported in numerous athlete groups including swimmers (Mujika et al., 2002), runners (Shepley et al., 1992) and cyclists (Neary et al., 2003a). However, some coaches and athletes still equate tapering with detraining. They presume that the tapering period has negative effects on endurance performance (Houmard et al., 1994). Nevertheless, tapering has been consistently demonstrated to improve endurance performance as noted in the aforementioned studies.
As a positively stressful stimulus, physical training induces important physiological changes. These changes can readily be seen in the neuro-endocrine, cardiovascular and immune systems among others (Goldshy et al., 2003). The immune system is the primary physiological system that mediates resistance and response to noxious exogenous agents (e.g., endotoxin), endogenous agents (e.g., tumor cells) and pathogens (e. g., viruses) (Goldshy et al., 2003).
Cytokines are polypeptides originally discovered within the immune system. However, it appears that many cell types produce cytokines and that the biological roles of cytokines extend beyond regulation of immune function. Recent data suggests that several cytokines have important metabolic functions and that they may either manifest their effects locally or work systemically in a hormone like fashion (Pedersen and Febbraio, 2008). Many studies on cytokines come from sepsis research. In sepsis models, the cytokine cascade comprises increased plasma levels of tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), interleukin-6 (IL-6) which can all act as pro-inflammatory cytokines.
Most studies indicate that prolonged exercise induces systemic pro-inflammatory responses and that pro- inflammatory cytokines such as TNFα increase and remain elevated in the plasma for at least about 2 hours (Peake et al., 2004; 2005). They have further demonstrated that increased circulating levels of TNFα are positively related to the rate of infection (Pedersen and Hoffman-Goetz, 2000). Rokitzki and colleagues (1994) found that plasma TNFα levels increased immediately after endurance training in 14 well trained endurance athletes. Ostrowski et al., 1998 also showed 2.5 fold increases in the plasma TNFα levels after 2.5 hours of endurance training at moderate to high intensity. They suggested that muscle injury consequent to prolonged endurance exercise might play an important role in this response. It is difficult to determine the exact magnitude of physical training stress needed to induce a significant increase in circulating levels of pro-inflammatory cytokines since athlete responses are not homogenous and are affected by many physiological psychological and training related factors. Tuan et al., 2008 reported that one week of decreasing of training volume and/or sessions, resulted in plasma TNFα concentrations returning to normal pre-training values in previously intensely trained athletes. Studies demonstrate up to 100 fold increases in IL-6 after endurance exercise events (Ostrowski et al., 1999). This increase in circulating IL-6 concentration in athletes is similar to that seen in patients with infections (Bruunsgaard et al., 1999). Although some physiological roles of TNFα, IL-6 and IL-1β overlap, they also exhibit some distinctly different functions.
The primary purpose of this study was to investigate the effect of shorter (7 day) and longer (21 day) taper periods on the concentration of post-exercise plasma levels of IL-6, IL-1β and TNFα in athletes. We were interested in determining if longer taper periods could improve indicators of athletes’ acute immune function without compromising the taper induced benefits to their performance capacity. We were particularly interested in the post-exercise values as these are reflective of the acute stress of the exercise on cytokine production. While monitoring of chronic non-exercise induced or resting changes in plasma cytokine levels would have further informed this study, we were not able to perform these measures in the study design.
Methods
Subjects
Twenty four cyclists participating in the Iran national cycling league volunteered to participate in this study as subjects. After receiving oral and written information about the study plans and procedures, subjects signed an informed consent form. The experimental protocol was approved by the local university ethics committee (Tabriz petrochemical ethic committee) and all subjects were informed of the risks and purposes of the study before their written consent was obtained. After completing 8 weeks progressive endurance training exercise, the subjects were randomly assigned to two groups (the first: control and the second: taper). Table 1 gives the physical and training characteristics of the subjects at the start of the study.
Table 1.
Demographic, anthropometric and sports activity data for the control and taper group cyclists at the start of the study. Data are means (±SD).
| Variable | Taper | Control |
|---|---|---|
| Height (cm) | 1.73 (.05) | 1.76 (.06) |
| Weight (kg) | 65.7 (7.2) | 68.1 (9.1) |
| Age (years) | 26.1 (3.6) | 24.8 (2.9) |
| VO2max (ml.kg-1.min-1) | 66.7 (.8) | 66.5 (.9) |
| Cycling experience (years) | 5.8 (3.8) | 6.7 (3.7) |
Determination of Endurance Exercise Capacity and Vo2max Assessment
To create a realistic training and reduced training scenario, 24 well trained elite cyclists were fully trained as if preparing for a competition season. The training status of every subject over the preceding 8 weeks and their training histories were obtained by questionnaire, training records, and personal interview. We considered the subjects to be well trained elite cyclists if they had trained for at least 3 years, 2 hours a day, 4 to 5 times a week. Only subjects who met these criteria were included in the study.
Three days before the start of the 11 week training period, each subject performed an incremental VO2max test on a calibrated Monark cycle ergometer (Monark, Sweden). Following a 2-min rest period of sitting stationary on the cycle ergometer, each cyclist began pedaling at an initial work rate of 80 W for 2 min, followed by 45 W increments every minute up to 260 W. Thereafter, work rate was increased each minute by 20 W increments to volitional fatigue. This test lasted approximately 10-14 min.
Expired gases were collected and analyzed by open circuit spirometry by using an automated metabolic analysis system. The data were averaged in 20 sec intervals. The gas analyzer was calibrated with primary standard gas (16.0% O2, 4.0% CO2, balance N2) before each test.
Cycling Performance Measurement
A simulated 40 km time trial (40TT) performance ride was used as the standard test (i.e., index of performance) at the beginning of week 1 and after week 4, 8, 9 and 11 (before and after tapering period) to evaluate the physiological and performance effects of training and of the length of the taper protocol. Each cyclist was asked to complete the ride as fast as possible with no information provided on how well he was performing until the end of the test. The description of this test has been reported in detail previously (Kuipers et al., 1985; Rietjens et al., 2001). Briefly, each cyclist used his own bicycle attached to a set of aluminum cast wind-loaded cycling rollers fitted with a bar connected to the handle bar of the bicycle for safety. The air pressure of the tyres was checked before and after each ride to ensure that maximum pressure was preserved. The same set of cycling rollers was used for all simulated 40 TT rides with the rollers being connected with a computer to record velocity, distance, and cycling time. This device was calibrated by measuring the circumference of the rollers and thus the distance was a product of the circumference and rpm, which was recorded by the computer.
Exercise Program
All subjects completed an 8-wk progressive high-intensity endurance training period. After 8 wks progressive training and before the taper period began, each cyclist was randomly assigned to one of two protocols: the control group cyclists continued performing the intense progressive weekly training volume (n=12) for 3 more weeks. The taper group cyclists proceeded with a 50% reduction in training volume relative to the weekly training volume performed by the control group (n=12) for this 3 week period. Training volume in this study was defined as a combination of the distance and the frequency. Table 2 shows the distance cycled for taper and control groups throughout the study.
Table 2.
Distance cycled at one session exercise training.
| Week | Distance (km) at 50% Vo2max |
Distance (km) at 60% Vo2max | Distance (km) at 70% Vo2max |
Sessions per week |
|
|---|---|---|---|---|---|
| Training program for control and taper groups before tapering period | |||||
| First | 10 | 70 | - | 6 | |
| Second | 10 | 80 | - | 6 | |
| Third | 10 | 90 | - | 6 | |
| Forth | 10 | 100 | - | 6 | |
| Fifth | 10 | 100 | 2 × 5 | 6 | |
| Sixth | 10 | 110 | 2 × 5 | 6 | |
| Seventh | 10 | 120 | 2 × 5 | 6 | |
| Eighth | 10 | 130 | 2 × 5 | 6 | |
| Training program for control group | |||||
| Ninth | 10 | 134 | 2 × 5 | 6 | |
| Tenth | 10 | 138 | 2 × 5 | 6 | |
| Eleventh | 10 | 142 | 2 × 5 | 6 | |
| Training program for control group | |||||
| Ninth | 5 | 67 | 2 × 2.5 | 6 | |
| Tenth | 5 | 69 | 2 × 2.5 | 3 | |
| Eleventh | 5 | 71 | 2 × 2.5 | 3 | |
Blood Sampling
Blood samples were collected from 24 participants at the beginning of week 1, and the end of weeks 4, 8, 9 and 11, immediately after 40 min TT. Venous blood samples were drawn from a forearm vein into sodium heparin tubes chilled on ice. Blood was centrifuged at 700 rpm at 23°C for 10 min. Plasma was separated and stored at -70°C.
Plasma Cytokine Measurements
TNFα, IL-1β and IL-6 levels were analyzed in duplicate with all values expressed as a mean of the two determinations, using validated ELISA kits (Quantikine; R & D Systems, Minneapolis, MN). The minimum detectable dose (MDD) of TNFα, IL-1β and IL-6 respectively ranged from 0.038 pg·mL-1 to 0.191 pg·mL-1, less than 1.0 pg·mL-1 and from 0.016 to 0.110 pg·mL-1. A standard curve was made by using standards provided in the kits, and the cytokine concentrations were appointed from the standard curves by use of linear regression analysis. The assays were a two-step “sandwich ”enzyme immunoassay in which samples and standards were incubated in a 96-well microtiter plate coated with polyclonal antibodies for the test cytokine as the capture antibody. After the proper incubation time, the wells were washed and a second detection antibody conjugated to either alkaline phosphatase (IL-6 high sensitivity) or horseradish peroxidase (TNFα and IL-1β) was added. The plates were incubated and washed, and the amount of bound enzyme-labeled detection antibody was measured by adding a chromogenic substrate. The plates were then read at the proper wave length (450 minus 570 nm for TNFα and IL-1β; 490 minus 650 nm for IL-6 high sensitivity). The minimum detectable dose of IL-1β, IL-6 and TNFα are typically less than 1.0, 0.016 - 0.110 and 0.038 - 0.191 pg·mL-1 respectively.
Statistical Analysis
Means and standard deviations were used to describe quantitative variables. Two-way repeated measures analysis of variance (ANOVA) was used to determine significant differences between taper and control plasma IL-6, IL-1β and TNFα concentrations and 40TT performance time means. Also the significant differences between the 1 week and 3 week taper periods for the performance, IL-6, IL-1β and TNFα concentrations were determined using two-way repeated measures analysis of variance (ANOVA). Data in text and figures are given as the mean ± sem. P ≤ 0.05 considered statistically significant. All data was analyzed by using SPSS for windows software version 16.0 (SPSS Inc, Chicago, IL).
Results
There were no physical, training experience or VO2max differences between the control or taper group subjects (see Table 1). All 24 subjects completed the 11 week training period and the data from all subjects were included in the analyses. Figure 1 indicates the comparison of the means (SD) of plasma IL-6, IL-1β and TNFα concentrations and their performance time in the control and taper groups.
Figure 1.
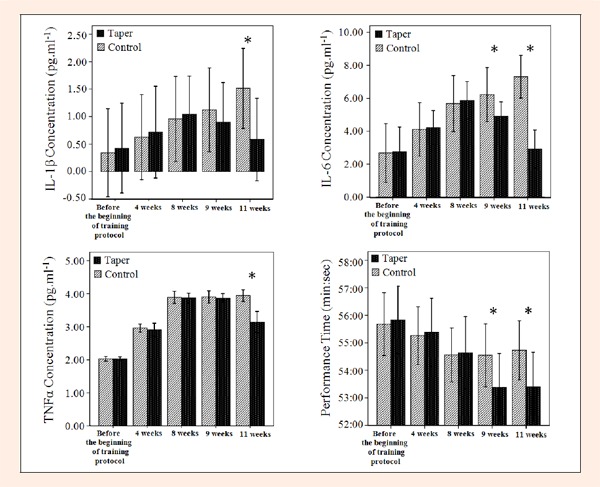
The comparison of the means (SD) of the plasma IL-6, IL-1β and TNFα concentrations in the control and taper groups immediately after the 40TT at various time points.
The IL-1β concentrations between two training groups were significantly different (P=0.01). In the taper group alone there were also significant differences between one and three week taper period (weeks 9 and 11) at specific time points (p < 0.01). Means at the end of week 8 were 148% greater than those at the day before beginning the training protocol (p ≤ 0.01). The mean IL-1β concentrations at the end of weeks 9 and 11 were respectively 13% and 44% less than those at the end of week 8 (p ≤ 0.01 and p ≤ 0.01). 36% decreases were observed when comparing levels of IL-1β at the end of week 9 with those at the end of week 11 in the taper group (p ≤ 0.01).
IL-6 levels between the two training groups were also significantly different at various collecting periods (p < 0.05). In the taper group alone there were also significant differences at various time points (p < 0.05). The mean IL-6 concentrations at the end of week 8 were 113% more than those at the day before the beginning of the training protocol (p ≤ 0.01). Mean IL-6 concentrations in the tapering group at the end of weeks 9 and 11 were 17% and 50% less than those at the end of week 8 (p ≤ 0.01 and p ≤ 0.01). Additionally, 41% decreases were observed when comparing levels of IL-6 at the end of week 9 with those at the end of week 11 in the taper group (p ≤ 0.01).
Plasma TNFα levels between the two training groups also showed significant differences at several collecting periods (p < 0.05). Plasma TNFα levels in the taper group alone showed that there were significant differences at various time points (p < 0.01). We found that the mean TNFα concentrations at the end of week 8 were 91% more than those at the day before beginning of the training protocol (p ≤ 0.01). The mean TNFα concentrations at the end of week 9 were 25% less than those at the end of week 8, but they were not significantly increased (p ≤ 0.054). However, the mean TNFα concentrations at the end of week 11 were 19% less than those at the end of week 8 (p ≤ 0.01). Significant differences were observed when comparing levels of TNFα at the end of week 9 with those at the end of week 11 in the taper group (p ≤ 0.01). The mean TNFα concentrations at the end of week 11 were 19% less than those at the end of week 9. See Figure 1 for all of the above cytokine data.
The 40TT performance time between the two training groups were significantly different at several time points (p < 0.05). Additionally the mean of the 40TT time at the end of week 8 was 2.1% less than at the day before the beginning of training protocol (p ≤ 0.01). The 40TT performance times at the end of weeks 9 and 11 were both less than those at the end of week 8 (p ≤ 0.01 and p ≤ 0.01). Non-significant differences were observed when comparing the 40TT performance time at the end of week 9 with those at the end of week 11 in the taper group (p > 0.05). The performance data is also depicted in Figure 1.
Discussion
This study demonstrates that post-exercise plasma elevations of several pro-inflammatory cytokines can be attenuated by a three week taper period and that both a one and three week taper period will elicit approximately equal improvements in cycling performance in elite cyclists relative to those who continue intense training. This study examined the effects of 1 and 3 weeks of tapered training in which training volume was reduced (50% decrease in training volume). Mean 40TT performance time in the taper group decreased (i.e. improved) significantly over 7 and 21 day periods of reduced training volume. Our findings support previous studies which have reported improved performance time was maintained during taper periods of 10-28 days in trained endurance athletes (Houmard, 1991; Neary et al., 1992; Mujika et al., 2002).
These findings also indicated that continuous training sessions performed in this study can induce increases in circulating pro-inflammatory cytokines immediately post-exercise suggesting that intense training performed by cyclists in this study may have temporarily compromised their immune system (Ronsen et al., 2002). Previous studies have reported that 10 to 30 percent of professional athletes exhibited negative physiological signs indicative of increased training volume during final preparation for championships events (Tessetore et al., 2005). These symptoms have included the changes in hypothalamus-pituitary-adrenal axis (Nijs et al., 2005). Our findings support suggestions that for a period of weeks before competition, athletes should significantly reduce their training levels. Other studies have also demonstrated that limiting cycling training to one hour per day for four days a week reversed the fatigue and infection symptoms induced by the prolonged endurance training (Meyer et al., 2004). In our results, there were significant reductions in plasma IL-1β, IL-6 and TNFα concentrations of the taper relative to the control groups following exercise at the end of week 11 of training. While after one week of the tapering program (at the end of week 9), there were no significant differences between the IL-1β and TNFα concentrations between the two groups. Post-exercise elevation of IL-1β, IL-6 and TNFα concentrations in the control group which continued heavy training at through week 11 may be directly due to their higher training volume and that in turn may make these athletes more prone to infection (Shepley et al., 1992). Since our measures were only performed immediately following time trials, it is difficult to attribute how long these elevations may have lasted or if they also resulted in chronic resting elevations. Nevertheless, significant increases in the post-exercise IL-1β, IL-6 and TNFα concentrations after 8 weeks of the endurance training in both groups and the continued elevation of these cytokines at 9 and 11 weeks in the control group, suggesting a greater pro-inflammatory profile in these athletes. Previous studies have also reported that there were significant increases in IL-1β, IL-6 and TNFα post-exercise plasma concentrations after the prolonged endurance training (Brenner et al., 1999; Camus et al., 1997) that may be an indicator of acute inflammation. Elevation of these plasma cytokines is related to increase susceptibility to infection (Gleeson et al., 2004). Smith, 2000 has suggested that prolonged endurance exercise training may induce a long-term inflammatory state and may also indicate muscle damage. Previous studies have also noted that the cytokine responses to the training are similar to the cytokine responses to the injuries. Clinical research has found that the cytokines also play an important role in the initiating of fatigue in disease states (Nishimoto et al., 2000) and in the prolonged fatigue syndrome (Arnold et al., 2002). Elite athletes often suffer from the excessive and chronic fatigue and upper respiratory tract infection (URTI) and their exercise performance is consequently compromised (Metz, 2003). Following sustained endurance training, the elevation of plasma IL-1β, IL-6 and TNFα concentrations might be factors in elevating the muscular proteolysis. Some studies have also suggested that exercise associated leakage of intestinal endotoxins may also directly cause increased plasma IL-6, IL-1β and TNFα levels (Ostrowski et al., 1998). Elevated IL-6 and TNFα concentrations may also decrease cellular glucose metabolism and thereby also possibly limiting optimal athletic performance (Plomgard, 2005). One of the important effects of systemic inflammation is induction of disease like symptoms including sleepiness, weakness and tiredness, which by limiting the work and effort an athlete can produce can also serve to protect athletes from exhaustion and excessive injuries and thereby help heal damaged tissues. Hence if elevated pro-inflammatory cytokine levels are present in athletes prior to a major competition this could indicate possible negative effects on maximum physical performance, a greater susceptibility to post-competition infection and possibly a slower and longer post-competition recovery period. Our findings of increases in acute elevations of post-exercise cytokine levels support such contentions, however further study is needed to determine if a longer taper period will also affect prolonged and resting plasma cytokine levels in these athletes.
Conclusion
Our findings indicate that prolonged intense training for 8 weeks in elite male cyclists can significantly elevate post exercise plasma levels of several pro-inflammatory cytokines and that a taper period of 1-3 weeks will essentially reverse these elevations while at the same time improving cycling performance. Continuation of the more intense training for 3 more weeks will not result in improved cycling performances nor will it attenuate pro-inflammatory cytokine levels in the blood as was seen in the taper group. While both a 1 and 3 week taper period will enhance cycling performance, these results highlight the importance of a 3 week taper period in proper preparation of athletes for competition since this taper period will reduce circulating pro-inflammatory cytokine levels thereby possibly limiting the chances of infection and potentially reducing the effects of these cytokines in inducing fatigue-like symptoms in athletes. These reductions in pro-inflammatory cytokines can be achieved with a 3 week taper period without any compromise in the performance benefits afforded by the taper. Therefore, coaches should seek to optimize rest between training sessions and apply approximately 3 week tapering periods, prior to important competitions in order to properly prepare endurance cyclists of optimal performance and recovery.
Biographies

Negin Farhangimaleki
Employment
Physical Education & Sport science Department, Islamic Azad University - Tabriz Branch.
Degree
MSc
Research interests
Exercise immunology.
E-mail: ngn_farhangi@yahoo.com

Farzad Zehsaz
Employment
Physical Education & Sport science Department, Islamic Azad University - Tabriz Branch.
Degree
PhD
Research interests
Exercise immunology.
E-mail: f-zehsaz@iaut.ac.ir

Peter M. Tiidus
Employment
Faculty of Science, Wilfrid Laurier University, Waterloo ON Canada.
Degree
PhD
E-mail: ptiidus@wlu.ca
References
- Arnold M.C., Papanicolaou D.A., O’Grady J.A., Lotsikas A., Dale J.K., Straus S.E., Grafman J., (2002) Using an interleukin-6 challenge to evaluate neuropsychological performance in chronic fatigue syndrome. Psychological Medicine 32, 1075-1089 [DOI] [PubMed] [Google Scholar]
- Brenner I.K.M., Natale V.M., Vasiliou P., (1999) Impact of three different types of exercise on components of the inflammatory response. European Journal of Applied Physiology 80, 452-460 [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H., Skinhoj P., Qvist J., Pedersen B.K., (1999) Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. Journal of Infectious Diseases 180, 551-554 [DOI] [PubMed] [Google Scholar]
- Camus G., Poortmans J., Nys M.I., Deby-Dupont G., Duchateau J., Deby C., Lamy M., (1997) Mild endotoxemia and the inflammatory response induced by a marathon race. Clinical Science 92, 415-422 [DOI] [PubMed] [Google Scholar]
- Gleeson M., Nieman D.C., Pedersen B.K., (2004) Exercise, nutrition and immune function. Journal of Sports Sciences 22, 115-125 [DOI] [PubMed] [Google Scholar]
- Goldshy R.A., Kindt T.J., Osborne B.A., (2003) Immunology. NewYork: Freeman; 1-8 [Google Scholar]
- Houmard J., (1991) Impact of reduced training on performance in endurance athletes. Sports Medicine 12, 380-393 [DOI] [PubMed] [Google Scholar]
- Houmard J.A., Scott B.K., Justice C.L., (1994) The effects of taper on performance in distance runners. Medicine and Science in Sports and Exercise 26, 624-631 [PubMed] [Google Scholar]
- Kuipers H., Verstappen F.T.J., Keizer H.A., (1985) Variability of aerobic performance in the laboratory and its physiologic correlates. Sports Medicine 6, 197-201 [DOI] [PubMed] [Google Scholar]
- Metz J., (2003) Upper respiratory tract infections: who plays, who sits? Current Sports Medicine Reports 2(84), 84-90 [DOI] [PubMed] [Google Scholar]
- Meyer T., Faude O., Urhausen A., Scharhag J., Kindermann W., (2004) Different effects of two regeneration regimens on immunological parameters in cyclists. Medicine and Sciences in Sports and Exercise 36(10), 1743-1749 [DOI] [PubMed] [Google Scholar]
- Mujika I., Goya A., Ruiz E., Grijalba A., Santisteban J., Padilla S., (2002) Physiological and performance responses to a 6-day taper in middle-distance runners: influence of training frequency. International Journal of Sports Medicine 23, 367-373 [DOI] [PubMed] [Google Scholar]
- Neary J.P., Martin T.P., Reid D.C., (1992) The effects of a reduced exercise duration taper programme on performance and muscle enzymes of endurance cyclists. European Journal of Applied Physiology 65, 30-36 [DOI] [PubMed] [Google Scholar]
- Neary J.P., Bhambhani Y.N., McKenzie D.C., (2003a) Effects of different stepwise reduction taper protocols on cycling performance. Canadian Journal of Applied Physiology 28(4), 576-587 [DOI] [PubMed] [Google Scholar]
- Nijs J., Meeus M., Mc Gegor N.R., (2005) Chronic fatigue syndrome: exercise performance related to immune dysfunction. Medicine and Science in Sports and Exercise 37(10), 1647-1654 [DOI] [PubMed] [Google Scholar]
- Nishimoto N., Sasai M., Shima Y., Nakagawa M., Matsumoto T., Shirai T., Kishimoto T., Yoshizaki K., (2000) Improvement in Castleman’s disease by humanized anti-interleukin-6 recep tor antibody therapy. Blood 95, 56-61 [PubMed] [Google Scholar]
- Ostrowski K., Rohde T., Asp S., Schjeriing P., Pedersen B.K., (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. Journal of Physiology 515, 287-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K., Rohde T., Zacho M., Asp S., Pedersen B.K., (1998) Evidence that interieukin-6 is produced in human skeletal muscle during prolonged running. Journal of Physiology 508, 949-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake J.M., Suzuki K., Wilson G., Hordern M., Yamaya K., Nosaka K., Mackinnon L., Coombes J.S., (2005) Exercise-induced muscle damage, plasma cytokines and markers of neutrophil activation. Medicine and Science in Sports and Exercise 37, 737-745 [DOI] [PubMed] [Google Scholar]
- Peake J.M., Wilson G., Hordern M., Suzuki K., Nosaka K., Yamaya K., Mackinnon L., Coombes J., (2004) Changes in neutrophil receptor expression, degranulation and respiratory burst activity after moderate and high intensity exercise. Journal of Applied Physiology 97, 612-618 [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Febbraio M., (2008) Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiological Review 88, 1379-1406 [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Hoffman-Goetz L., (2000) Exercise and the immune system: regulation, integration and adaptation. Physiological Review 80, 1055-1081 [DOI] [PubMed] [Google Scholar]
- Plomgard P., Bouzakri K., Krogh-Madsen R., Mittendorf B., Zierath J.R., Pedersen B.K., (2005) Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54, 2939-2945 [DOI] [PubMed] [Google Scholar]
- Rietjens G.J.W.M., Keizer H.A., Kuipers H., Saris W.H.M., (2001) A reduction in training volume and intensity for 21 days does not impair performance in cyclists. British Journal of Sports Medicine 35, 431-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokitzki L., Logemann E., Keul J., (1994) Interleukin-6, tumor necrosis factor-alpha and malondialdehyde serum concentration during a marathon-run. International Journal of Sports Medicine 15, 360 [Google Scholar]
- Ronsen O., Kjeldsen-Kragh J., Haug E., (2002) Recovery time affects immunoendocrine responses to a second bout of endurance exercise. American Journal of Physiology - Cell Physiology 283, C1612-C1620 [DOI] [PubMed] [Google Scholar]
- Scharhag J., Meyer T., Gabriel H.H.W., Schlick B., Faude O., Kindermann W., (2006) Does prolonged cycling of moderate intensity affect immune cell function? British Journal of Sports Medicine 39, 171-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepley B., MacDougall J.D., Cipriano N., Sutton J.R., Tarnopolsky M.A., Coates G., (1992) Physiological effects of tapering in highly trained athletes. Journal of Applied Physiology 72(2), 706-711 [DOI] [PubMed] [Google Scholar]
- Smith L.L., (2000) Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Medicine and Science in Sports and Exercise 32, 317-331 [DOI] [PubMed] [Google Scholar]
- Tuan TC, Hsu T.G., Fong M.C., Hsu C.F., Tsai K.K., Lee C.Y., Kong C.W., (2008) Deleterious effects of short-term high-intensity exercise on the immune function: evidence from leukocyte mitochondrial alternations and apoptosis. British Journal of Sports Medicine 42(1), 11-15 [DOI] [PubMed] [Google Scholar]
- Tessetore A., Meeusen R., Tiberi M., Cortis C., Pagano R., Capranica L., (2005) Aerobic and anaerobic profiles, heart rate and match analysis in older soccer players. Ergonomics 48(11-14), 122-129 [DOI] [PubMed] [Google Scholar]


