Significance
This study describes a unique form of cell death induced by a cytosolic flagellin that does not require NLRC4 (nucleotide oligomerization domain-like receptor family, caspase activation recruitment domain domain-containing 4), ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), or caspase-1/11 but is regulated by lysosomal cathepsins B and D. Inflammasome-independent cell death is characterized by loss of membrane integrity and IL-1α secretion and participates in the clearance of Salmonella Typhimurium by macrophages. Lysosomal pathway activated by flagellin also regulates inflammasome-dependent responses such as IL-1β and IL-1α secretion and pyroptosis. Together, our data identify a pathway induced by cytosolic flagellin that cooperates with inflammasomes to the clearance of infections.
Abstract
NAIP5/NLRC4 (neuronal apoptosis inhibitory protein 5/nucleotide oligomerization domain-like receptor family, caspase activation recruitment domain domain-containing 4) inflammasome activation by cytosolic flagellin results in caspase-1–mediated processing and secretion of IL-1β/IL-18 and pyroptosis, an inflammatory cell death pathway. Here, we found that although NLRC4, ASC, and caspase-1 are required for IL-1β secretion in response to cytosolic flagellin, cell death, nevertheless, occurs in the absence of these molecules. Cytosolic flagellin-induced inflammasome-independent cell death is accompanied by IL-1α secretion and is temporally correlated with the restriction of Salmonella Typhimurium infection. Despite displaying some apoptotic features, this peculiar form of cell death do not require caspase activation but is regulated by a lysosomal pathway, in which cathepsin B and cathepsin D play redundant roles. Moreover, cathepsin B contributes to NAIP5/NLRC4 inflammasome-induced pyroptosis and IL-1α and IL-1β production in response to cytosolic flagellin. Together, our data describe a pathway induced by cytosolic flagellin that induces a peculiar form of cell death and regulates inflammasome-mediated effector mechanisms of macrophages.
Flagellin, the monomeric subunit of flagella present in Gram-negative and Gram-positive bacteria, is one of the few protein structures that can activate both transmembrane and cytosolic pattern recognition receptors of the innate immune system. Extracellular flagellin is recognized by the transmembrane Toll-like receptor (TLR)5 (1). On the other hand, flagellin can be directly delivered into the cytosol by transport systems, such as the type III secretion system (T3SS) of Salmonella (2) and the type IV secretion system (T4SS) of Legionella (3). Once in the cytosol, flagellin is sensed by the inflammasome complex comprised of the NOD-like receptor (NLR) proteins neuronal apoptosis inhibitory protein (NAIP)5 and NLRC4 [NLR family, caspase activation recruitment domain (CARD) domain-containing 4] (2–5).
Both TLR5 and NAIP5/NLRC4 receptors recognize conserved regions of flagellin. TLR5 is thought to detect a region of flagellin located in the D1 domain (6), whereas a sequence of three leucine residues that is present in the C-terminal D0 domain of flagellin is required to activate the NAIP5/NLRC4 inflammasome (7). Despite some redundant roles that are attributed to NLRC4 and NAIP5 in flagellin-mediated macrophage activation (7), a new model for NAIP5/NLRC4 inflammasome activation in response to flagellin was recently proposed (8, 9). In this model, NAIP5 acts as an immune sensor protein that specifically binds to flagellin (9). The interaction between NAIP5 and flagellin promotes the recruitment of NLRC4 through the NOD domain. The formation of this protein complex leads to the association of NLRC4 with procaspase-1 via CARD-CARD interactions. Additionally, NLRC4 can recruit the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), which also contains a CARD domain and is able to recruit and process procaspase-1.
Caspase-1 activation results in the cleavage and secretion of biologically active forms of the inflammatory cytokines interleukin (IL)-1β and IL-18 (10) and the induction of a form of cell death named pyroptosis (11). The activation of caspase-1 in response to cytosolic flagellin by the NAIP5/NLRC4 inflammasome complex can also induce other effector mechanisms to restrict infections, such as caspase-7–dependent phagosome maturation (4, 12) and the activation of inducible nitric oxide synthase (iNOS) by macrophages (13). Both of these effector mechanisms lead to the inhibition of Legionella pneumophila replication. Importantly, caspase-1–induced IL-1β and IL-18 are not involved in phagosome maturation (4, 12), induction of pyroptosis (14), or iNOS activation (13), suggesting that caspase-1 mediates independent effects that cooperate to clear infections.
Although the NAIP5/NLRC4 inflammasome complex is involved in the control of many bacterial infections, such as infection with Salmonella Typhimurium (2, 5), Shigella flexneri (15), Pseudomonas aeruginosa (16, 17), L. pneumophila (3, 4), and Listeria monocytogenes (18), the precise effector mechanism mediated by these receptors is not completely understood. Among the NAIP5/NLRC4 inflammasome-mediated effector mechanisms that have been implicated with intracellular bacterial replication control, pyroptosis has received great attention.
Pyroptosis has been described as a programmed cell death pathway that uniquely depends on caspase-1 (19). Recently, it was demonstrated that the enteric pathogenic bacteria Escherichia coli, Citrobacter rodentium, and Vibrio cholerae and the cholera toxin B subunit can trigger the activation of a noncanonical inflammasome that targets caspase-11 (also known as caspase-4 in humans and related to caspase-1) (20). These stimuli induce cell death in a caspase-11–dependent fashion, but the process is not dependent on ASC, NLRC4, or caspase-1. Interestingly, this process of cell death (also named pyroptosis) is accompanied by the secretion of IL-1α but not by the secretion of IL-1β (which requires caspase-1). Importantly, the 129 mouse strain that was used to generate the first caspase-1−/− mutants (21, 22) harbors a mutation in the caspase-11 locus that impairs caspase-11 function. Because of the close proximity in the genome between the caspase-1 and caspase-11 genes, the two proteins cannot be segregated by recombination. Therefore, these caspase-1−/− mice are also defective for caspase-11 (20).
Importantly, although pyroptosis is regulated by caspase activation, similarly to apoptosis, inhibition of or genetic deficiency in apoptotic caspase does not rescue cells from pyroptosis (11, 23). In addition, pyroptosis and apoptosis provide distinct outcomes for the immune response, which may be explained by the different morphological and biochemical changes that are observed in cells undergoing these forms of cell death (24, 25). Activation of caspase-1/11 results in the rapid formation of pores in the plasma membrane that dissipate cellular ionic gradients. This process allows the influx of water into the cells, resulting in cell swelling, osmotic lysis, and the release of intracellular contents (25, 26). The loss of plasma membrane integrity and the secretion of inflammatory mediators during pyroptosis, including IL-1β and IL-18, results in the induction of a strong inflammatory response (27). The inflammatory milieu produced by pyroptosis could result in the recruitment of effector cells to the site of infection as a mechanism of pathogen clearance. Recently, it was demonstrated that the ectopic expression of the Salmonella flagellin protein FliC during the intracellular phase of infection triggers pyroptosis of infected cells in vivo (14). The bacteria released by the pyroptotic macrophages were controlled by infiltrating neutrophils through a reactive oxygen species-dependent mechanism.
Despite the evidence implicating pyroptosis as an important host defense mechanism to clear intracellular pathogens, the molecular regulation of pyroptosis is poorly understood. Here, we analyzed the regulation of macrophage death using purified flagellin as a single, death-inducing stimulus. Our data demonstrate that cytosolic flagellin is able to induce cell death in the absence of caspase-1/11. Although displaying some apoptotic features, such as cell shrinkage and the formation of membrane blebs, cytosolic flagellin-induced caspase-1/11–independent cell death does not require apoptotic caspases but depends on lysosomal events. Similar to pyroptosis, cytosolic flagellin-induced caspase-1/11–independent cell death results in the release of intracellular inflammatory contents. Caspase-1/11–independent cell death also contributes to the control of Salmonella enterica serovar Typhimurium (Salmonella Typhimurium) infection by macrophages, supporting the existence of an effector mechanism important to restrict bacterial infection. Finally, our data provide evidences that lysosomal cathepsins also regulate IL-1β secretion and pyroptosis in response to cytosolic flagellin. Taken together, our results suggests lysosome events as a central regulator of both inflammasome-dependent and inflammasome-independent macrophage responses induced by cytosolic flagellin.
Results
Purified Flagellin Protects both Wild-Type and TLR5-Deficient Mice from Flagellin-Deficient Salmonella Typhimurium Infection.
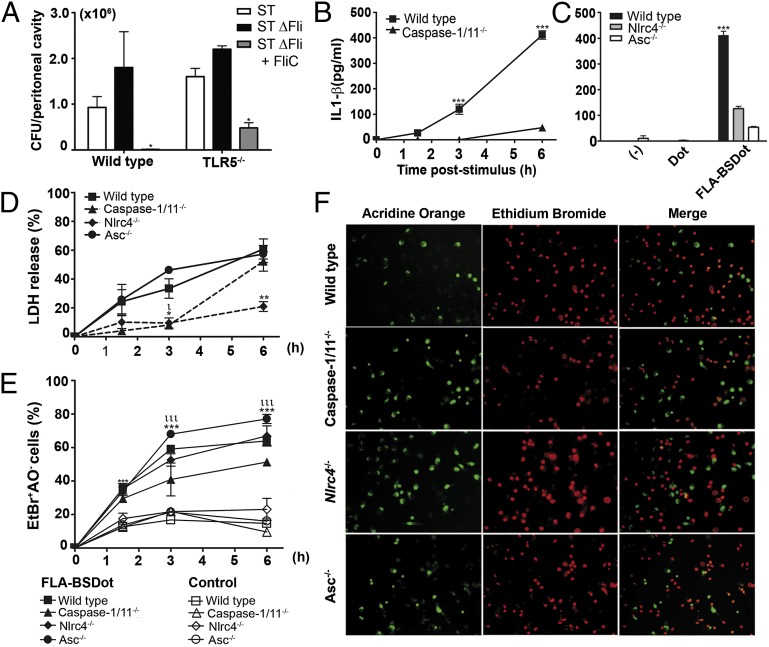
Recent studies have suggested that flagellin-induced pyroptosis controls the infection of virulent bacteria, such as L. pneumophila and Salmonella Typhimurium (14). To investigate this phenomenon in more detail, we inoculated the Salmonella Typhimurium flagellin-expressing 1412 strain (ST) and flagellin-deficient 2157 strain (ST ΔFli) in wild-type (WT) and TLR5−/− mice and evaluated the number of colony-forming units (CFUs) recovered after 6 h from the peritoneal cavity (PEC). Recognition of flagellin was involved in the protection, since a higher bacterial load was found when mice were infected with ST ΔFli strain (Fig. 1A, black boxes) in comparison with ST (Fig. 1A, white boxes). Surprisingly, the i.p. inoculation of purified flagellin from Salmonella Typhimurium, 3 h after infection with ST ΔFli strain, significantly reduced the bacterial load in PECs of both WT and TLR5−/− mice, suggesting that a mechanism dependent on flagellin but independent of TLR5 can operate to clear Salmonella Typhimurium infection in mice (Fig. 1A, gray boxes).
Fig. 1.
Cell death induced by cytosolic flagellin occurs in the absence of the NLRC4/ASC-caspase-1/11 axis. (A) CFU numbers recovered from the peritoneal cavity of C57BL/6 and TLR5-deficient mice infected with the flagellin-proficient Salmonella Typhimurium 1412 strain (ST WTFli) or the flagellin deficient Salmonella Typhimurium 2157 strain (ST ΔFli) (5× 103 bacteria per mouse) or the ST ΔFli strain plus purified FliC flagellin (5 µg per mouse) (ST ΔFli + FliC). Numbers represent the means ± SEM (n = 3 per group). *P < 0.05 compared with St ΔFli-infected group. (B and C) IL-1β levels from supernatants of PMs stimulated with purified B. subtilis flagellin trapped into DOTAP (FLA-BSDot) (3 μg/mL) for 6 h were determined by ELISA (C). Numbers represent the means ± SEM (n = 2). ***P < 0.001 compared with knockout groups. (D) LDH levels quantified in culture supernatants after 6 h using a LDH kit. Numbers represent the means ± SEM (n = 2). ιP < 0.05; **P < 0.01 compared with WT group. (E) Cytotoxicity assessed as the percentage of EtBr single-positive cells in fluorescence micrographs according to AO/EtBr staining. Numbers represent the means ± SEM of at least seven images per treatment. ιιιP < 0.001 compared with control group; ***P < 0.001 compared with WT group. (F) Representative micrographs of FLA-BSDot-stimulated PMs at 6 h. AO-stained PMs (green) show viable cells in each field, and EtBr (red) indicates permeabilized PMs. At right are merged images. Micrographs represent an original magnification of 400×. All data are representative of four (C57BL/6:00 PMs vs. caspase-1/11−/− PMs) or two independent experiments.
Because inoculation of mice with purified flagellin potentially stimulates both TLR5 and NLRC4-dependent responses (28), we decided to investigate the relative contribution of these receptors to macrophage responses to flagellin. Peritoneal macrophages (PMs) were stimulated with 1, 3, or 6 μg/mL (correspondent to 0.3, 1, or 2 × 10−7 M) of purified flagellin from Bacillus subtilis (FLA-BS) or with FLA-BS inserted into cationic lipid vesicles, which permits the delivery of the flagellin into the cellular cytosol (FLA-BSDot). Free flagellin is thought to activate TLR5, whereas cytosolic flagellin is believed to activate NAIP5/NLRC4 receptors (2, 13). As expected, only treatment with cytosolic flagellin induced the secretion of IL-1β by macrophages (Fig. S1A), whereas IL-6 was only observed when macrophages were treated with free flagellin (Fig. S1B). Additionally, death in PM was also only observed when cells were treated with cytosolic flagellin, as assessed by LDH release (Fig. S1C), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay (Fig. S1D), and loss of vital acridine orange (AO) dye in combination with ethidium bromide (EtBr) incorporation (Fig. S1E). All of these responses occurred in a dose-dependent manner. Being derived from a Gram-positive bacterial species devoid of lipopolysaccharide (LPS) in the cell envelope, flagellin from B. subtilis was used to prevent any interference of LPS in treated cells. Moreover, similar levels of IL-1β were found in WT and TLR2−/− PMs culture after stimulation with cytosolic flagellin, discarding the interference of any contamination with TLR2 ligands in flagellin preparations (Fig. S2A). Cell death (Fig. S2B) and IL-1β production (Fig. S2C) were also observed when cells were treated with purified Salmonella Typhimurium FliC flagellin (FliCDot) but not when cells were treated with the peptide of Plasmodium vivax VK210 circumsporozoite protein (pepVK210Dot) trapped into lipid vesicles. Similarly to purified native Salmonella Typhimurium FliC flagellin, a recombinant flagellin produced in an E. coli strain (BL21DE) (rFliCDot) also induced macrophage death (Fig. S2B) and IL-1β production (Fig. S2C). In contrast, these responses were not obtained with a recombinant p24 protein from HIV (rp24Dot), produced in the same E. coli strain and purified by the same nickel-affinity chromatography, discarding the influence of nonspecific cytotoxic effects of flagellin preparations. Of note, the purity of flagellin preparations is similar (Fig. S2D), and all proteins were inserted into N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP) and used in similar concentrations. These results indicate that only intracellular flagellin is capable of inducing macrophage responses, suggesting a role for inflammasome activation in cell viability. Finally, MyD88−/− and WT PMs displayed similar morphology (Fig. S2E) and similar levels of LDH liberation (Fig. S2F) when stimulated with cytosolic flagellin. These data eliminate the possibility that the MYD88 pathway participates in cytosolic flagellin-induced cell death.
Caspase-1/11, NLRC4, and ASC Are Not Required for Cytosolic Flagellin-Induced Cell Death.
Currently, it is thought that after exposure to cytosolic flagellin, caspase-1 is recruited to the NAIP5/NLRC4 inflammasome complex, where it is activated. The ASC adaptor molecule may or may not participate in this process (5, 7, 29). IL-1β production by PM from C57BL/6 WT mice in response to cytosolic flagellin increased over time in culture, peaking at 6 h (Fig. 1B). Using caspase-1/11–deficient (Fig. 1B), NLRC4-deficient, and ASC-deficient (Fig. 1C) cells, we observed that the cytosolic flagellin-mediated secretion of IL-1β was dramatically reduced in the absence of these molecules, thus confirming their requirement for the secretion of IL-1β in response to cytosolic flagellin. Low levels of LDH were released by NLRC4−/− and caspase-1/11−/− macrophages in comparison with WT and ASC−/− macrophages 1–3 h after cytosolic flagellin stimulation (Fig. 1D). However, the levels of LDH released from cytosolic flagellin-stimulated WT and caspase-1/11−/− PM was similar 6 h after stimulation (Fig. 1D). Surprisingly, although less pronounced compared with the cell death observed in WT and ASC−/− cultures, a high frequency of macrophage death after cytosolic flagellin stimulation was also observed in the absence of NLRC4 and caspase-1/11, as assessed by loss of vital AO dye in combination with EtBr incorporation (Fig. 1 E and F). Similar results were observed when 6 μg/mL of cytosolic flagellin was used for stimulation (Fig. S3 A–C) and with bone marrow-derived macrophages (BMDMs) (Fig. S3 D and E). It is noteworthy that BMDM are not able to release IL-1β in response to cytosolic flagellin in the absence of priming with TLR ligands, in contrast to observed with starch-elicited PMs (Fig. 1 B and C). Elicited PMs are composed mainly by newly recruited macrophages originated from inflammatory monocytes, which could present basal levels of pro-IL-1β because they hold a constitutively activated form of caspase-1 (30, 31). Taken together, these data indicate that whereas IL-1β secretion is dependent on NLRC4, ASC, and caspase-1/11, cytosolic flagellin-induced cell death occurs in the absence of these molecules.
Flagellin Induces Inflammasome-Dependent Pyroptosis and Inflammasome-Independent Cell Death, both Contributing to the Clearance of Salmonella Typhimurium.
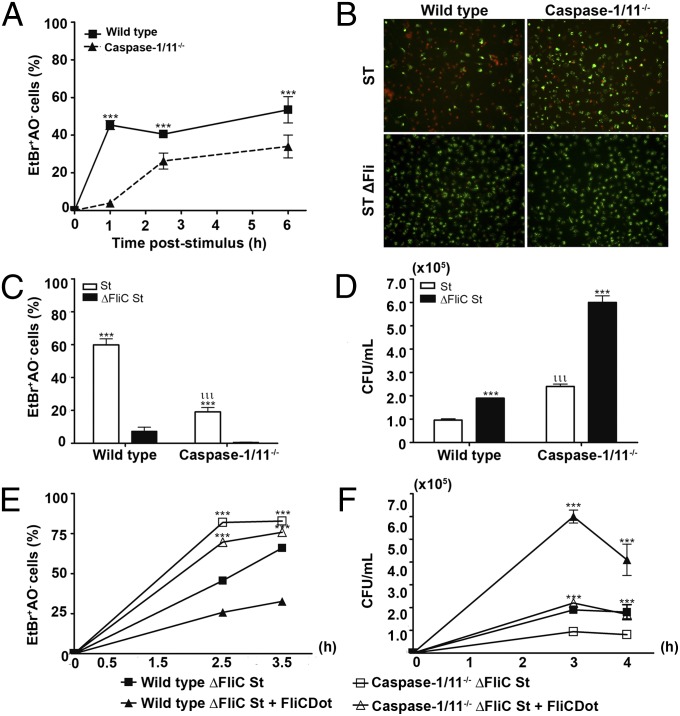
We then evaluated cell death in WT and caspase-1/11−/− PMs that were infected with the ST and ST ΔFli. Of note, the plates were centrifuged at 290 × g for 10 min to enable the same rate of ingestion of the two Salmonella Typhimurium strains by the macrophages, overcoming the absence of flagellum in ST ΔFli, as described previously (2). At 1 h postinfection, a higher frequency of cell death was observed in WT PMs compared with caspase-1/11−/− PM (Fig. 2A). The early caspase-1/11–dependent cell death appeared to be dependent on the presence of flagellin because infection of WT macrophages with the ST induced a higher frequency of PMs death compared with infection with ST ΔFli strain (Fig. 2 B and C). Flagellin-dependent death was reduced and displayed slower kinetics after infection ST in caspase-1/11−/− PMs (Fig. 2 A–C), as demonstrated previously by cytosolic flagellin stimulation. Early caspase-1/11–dependent cell death was correlated with a reduction in the number of CFUs observed in WT PMs compared with caspase-1/11−/− PMs (Fig. 2D). These data confirm the role of early caspase-1/11–dependent pyroptosis in the control of Salmonella Typhimurium infection by macrophages. Importantly, however, recognition of flagellin also conferred protection in a caspase-1/11–independent manner, because significantly higher numbers of CFUs were found in caspase-1/11−/− macrophages infected with the ST ΔFli strain with regard to PMs infected with the ST strain (Fig. 2D). Moreover, adding cytosolic flagellin during infection with ST ΔFli strain resulted in a marked increase in cell death (Fig. 2E) and a decrease in the numbers of CFUs (Fig. 2F) found in macrophages from caspase-1/11−/−, as well as WT mice, corroborating our previous in vivo data (Fig. 1A). Altogether, these data indicate that both flagellin-induced caspase-1/11–dependent pyroptosis and caspase-1/11–independent cell death participate in the clearance of Salmonella Typhimurium by macrophages.
Fig. 2.
Flagellin-induced caspase-1/11–independent cell death contributes to the control of Salmonella Typhimurium infection. (A) Cytotoxicity assessed in WT and caspase-1/11–deficient PMs infected with Salmonella Typhimurium 1412 strain (St WTFli) or Salmonella Typhimurium 2157 strain (St ΔFli) [multiplicities of infection (MOI), 5:1], as the percentage of EtBr single-positive cells in fluorescence micrographs according to AO/EtBr staining. Numbers represent the means ± SEM (n = 3). ***P < 0.001 compared with the caspase-1/11−/− group. Data are representative of three independent experiments. (B) Representative micrographs of stimulated PMs at 3 h. AO-stained PMs (green) show viable cells in each field, and EtBr (red) indicates permeabilized PMs. Micrographs represent an original magnification of 400×. (C) Cytotoxicity assessed as the percentage of EtBr single-positive cells in fluorescence micrographs according to AO/EtBr staining of St WTFli-stimulated PMs at 3 h. ***P < 0.001 compared with the St ΔFli strain-infected group. Data are representative of three independent experiments. ***P < 0.001 compared with the St WTFli-infected group; ιιιP < 0.001 compared with WT group. Data are representative of three independent experiments. (D) CFUs recovered from PMs infected with St WTFli or St ΔFli (MOI, 5:1) at 3 h. ***P < 0.001 compared with the ST ΔFliC-infected group. Data are representative of three independent experiments. (E) Cytotoxicity assessed as the percentage of EtBr single-positive cells in fluorescence micrographs according to AO/EtBr staining of PMs infected with the St ΔFliC strain (MOI, 5:1) concomitantly treated (open symbols) or not with FliCDot (closed symbols). Data are representative of two independent experiments. (F) The number of CFUs recovered from the peritoneal cavity of mice infected with ΔFliC strain (MOI, 5:1) in the presence or absence of FliCDot. Numbers represent the means ± SEM (n = 3). Data are representative of two independent experiments.
Flagellin-Induced, Caspase-1/11–Independent Cell Death Displays Apoptotic Features but Does Not Rely on Caspase Activity.
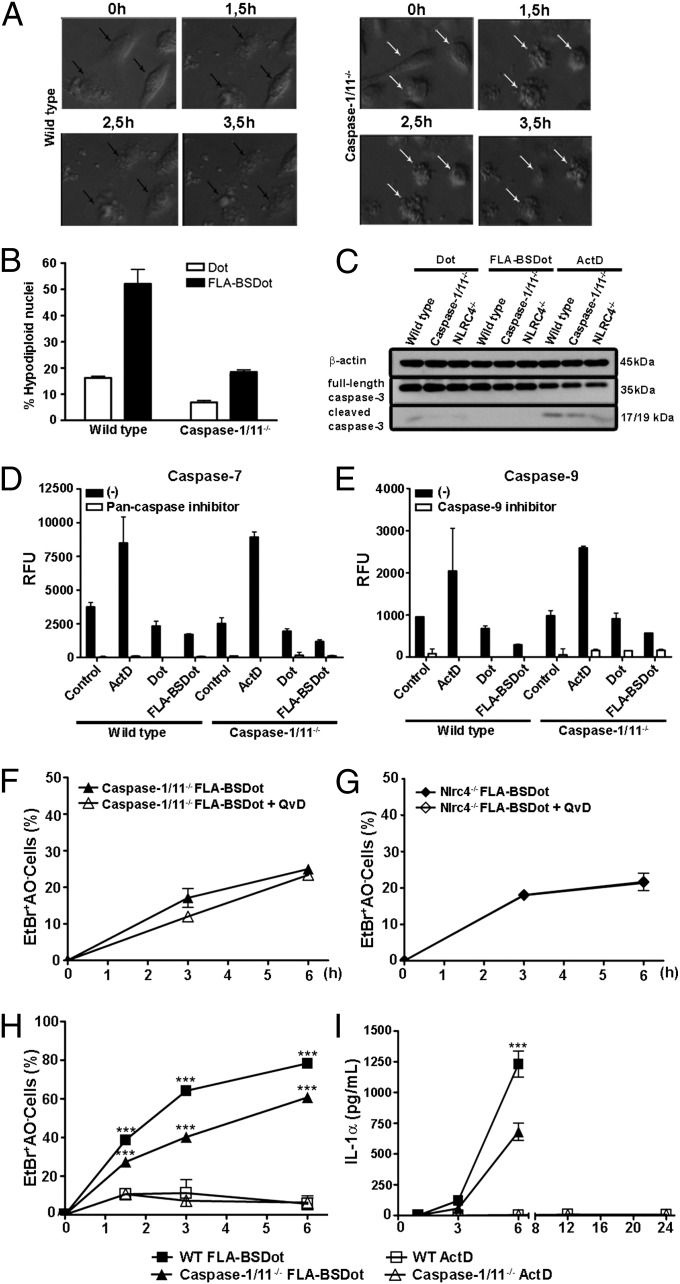
To evaluate the morphological and biochemical features of caspase-1/11–independent macrophage death, cell morphology after stimulation with cytosolic flagellin was assessed. One hour after stimulation, WT PMs were permeable to EtBr before the loss of vital AO staining (Fig. S4A). These data are indicative of the formation of pores in the plasma membrane (26, 32). Double staining with AO/EtBr was not observed in caspase-1/11−/− macrophages (Fig. S4A). Giemsa-stained slides indicated that the majority of WT PM appeared to be swelled compared with caspase-1/11−/− PMs that seemed to have a reduction in volume (Fig. S4B). Moreover, time-lapse, phase-contrast images indicated a higher frequency of swollen cells in WT PM that increase in size over time, resulting in the accumulation of cellular debris (Fig. 3A). Despite the presence of cells with a similar morphology compared with WT PMs, we found that the majority of caspase-1/11−/− PMs died with apoptotic characteristics, which included the loss of cell volume and the formation of membrane blebs (Fig. 3A). The flagellin-induced caspase-1/11–independent cell death closely resembled apoptotic cells (Fig. S4C). To determine whether macrophages stimulated with cytosolic flagellin are biased toward apoptotic cell death in the absence of NLRC4 or caspase-1/11, we compared the flagellin-induced caspase-1/11–independent cell death to actinomycin D (ActD)-induced apoptosis. Of note, ActD treatment induced the same frequency of cell death (Fig. S4D, black bars) and hypodiploid nuclei (Fig. S4E, center graphs, and Fig. S4F, black bars) in both WT and caspase-1/11−/− PMs. These data demonstrate that the lack of caspase-1/11 did not interfere with the apoptotic program of cell death. The frequency of hypodiploid nuclei observed 3 h after stimulation with cytosolic flagellin is strongly reduced in the absence of caspase-1/11 (Fig. 3B), demonstrating the involvement of caspase-1/11 in DNA fragmentation that occurs in response to cytosolic flagellin. However, a decrease in full-length caspase-3, which is associated with the appearance of the active, cleaved 17/19-kDa caspase-3, was observed only in ActD-treated WT, caspase-1/11−/−, and NLRC4−/− macrophages but not in any of cytosolic flagellin-treated WT, caspase-1/11−/−, and NLRC4−/− cells (Fig. 3C). The involvement of cytosolic flagellin in the activation of apoptotic caspase-7 and caspase-9 were also investigated using a fluorimetric assay. ActD-treated WT or caspase-1/11−/− macrophages displayed caspase-7 (Fig. 3D) and caspase-9 (Fig. 3E) activation that was abrogated by the addition of z-VAD-fmk and LEHD-fmk (pan-caspase and caspase-9 inhibitors, respectively). In contrast, activation of caspase-7 or caspase-9 was not observed in response to cytosolic flagellin stimulation (Fig. 3 D and E), suggesting that the apoptotic machinery is not involved in cytosolic flagellin-induced cell death. According to these data both, AO loss (Fig. S4B, white bars) and the formation of hypodiploid nuclei (Fig. S4C, right graphs, and Fig. S4D, white bars) induced by ActD were strongly inhibited by Q-VD-fmk, a pan-caspase inhibitor. In contrast, Q-VD-fmk did not interfere with cell death in cytosolic flagellin-stimulated caspase-1/11−/− (Fig. 3F) and NLRC4−/− macrophages (Fig. 3G), as measured by AO/EtBr staining. Thus, the absence of apoptotic caspase activation and the lack of caspase interference in cytosolic flagellin-induced cell death indicate that caspase-1/11−/− PMs did not have a bias toward the classical apoptotic program of cell death.
Fig. 3.
Cell death induced by cytosolic flagellin is independent of caspase activity and culminates in IL-1α release. (A) The cell morphology of FLA-BSDot-stimulated PMs (3 μg/mL) accompanied in phase contrast microscopy for 3.5 h. Micrographs represent an original magnification of 400× . Data are representative of three independent experiments. (B) Percentage of hypodiploid nuclei evaluated by flow cytometry according to PI incorporation in FLA-BSDot– or empty DOTAP-treated PMs at 3 h. Numbers represent the means ± SEM (n = 3). ***P < 0.001 compared with the WT group. (C) Western blot analysis for caspase-3 activation in BMDMs stimulated with FLA-BSDot (6 μg/mL) or ActD (5 μg/mL) at 6 h. Activation of caspase-3 was determined by decrease in the full-length caspase-3 (35 kDa) associated with the appearance of the cleaved 17/19-kDa caspase-3. (D and E) Fluorimetric assay for caspase-7 (D) and caspase-9 (E) activity in FLA-BSDot or ActD-treated PMs at 4 h. Cell-free extracts were incubated with caspase-7 or caspase-9 fluorimetric substrates in the presence or absence of specific caspase inhibitors. Numbers represent the means ± SEM (n = 3). Results represent relative fluorescence units (RFU). (F and G) Cytotoxicity assessed as the percentage of EtBr single-positive cells in fluorescence micrographs according to AO/EtBr staining in PMs stimulated with FLA-BSDot (3 μg/mL). Cells were treated or not with Q-VD-fmk (20 µM) 1 h prior to stimulation. Numbers represent the means ± SEM (n = 3). (H) Cytotoxicity in PMs stimulated with FLA-BSDot (6 μg/mL) or ActD (5 μg/mL). Numbers represent the means ± SEM of at least five images per treatment. ***P < 0.001 compared with the Act-D group. (I) IL-1α levels from supernatants of PMs stimulated with FLA-BSDot (3 μg/mL) or ActD (5 μg/mL) were determined by ELISA ***P < 0.001 compared with the knockout group. Data are representative of two independent experiments.
Caspase-1/11–Independent Cell Death Induced by Cytosolic Flagellin Retains an Inflammatory Outcome Characterized by the Release of IL-1α.
Despite displaying some apoptotic features, almost 40% of caspase-1/11−/− PMs that were stimulated with cytosolic flagellin were permeable to EtBr at 3 h, whereas almost all ActD-treated cells remained impermeable (Fig. 3H). In comparison, up to 12 h after ActD treatment, a high frequency of ActD-treated PM from both WT and caspase-1/11−/− mice lost vital AO but remained impermeable to EtBr, indicating that these cells died but did not lose plasma membrane integrity. Importantly, even less than WT cells, cytosolic flagellin-stimulated caspase-1/11−/− PMs released large amounts of IL-1α (Fig. 3I), which is a known damage signal released by necrotic cells (33). In contrast, apoptotic cell death induced by ActD did not result in IL-1α release, even after 24 h of stimulation (Fig. 3I), when almost all cells were already dead, consistent with the idea that apoptotic cells retain these molecules within apoptotic bodies. These data demonstrate that cytosolic flagellin-induced caspase-1/11–independent cell death retains the same inflammatory outcome as the classical caspase-1/11–dependent pyroptosis, both characterized by the loss of cell integrity and release of intracellular proinflammatory contents, corroborating with the idea that cytosolic flagellin does not activate the classic apoptotic machinery in the absence of caspase-1/11.
Lysosomal Cathepsins Regulate Caspase-1/11–Dependent and –Independent Macrophage Responses to Cytosolic Flagellin.
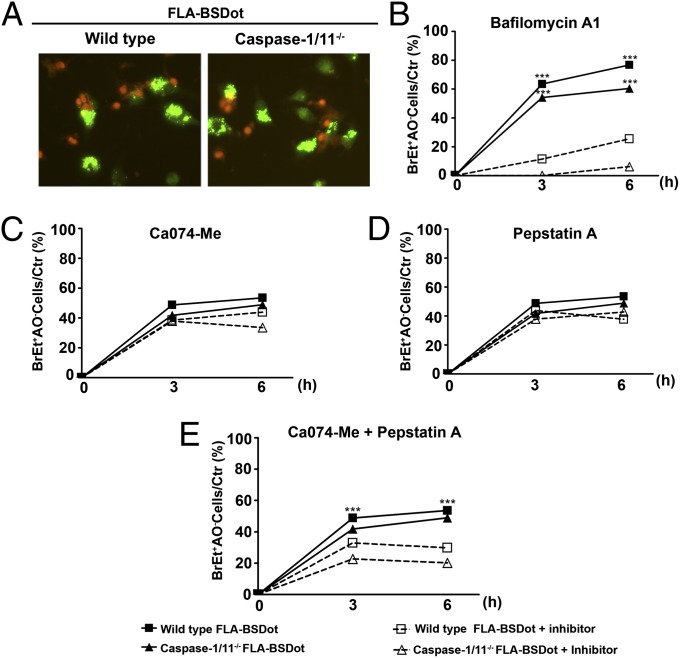
Given the peculiar features displayed by caspase-1/11–deficient dying cells after cytosolic flagellin stimulation, we investigated the participation of alternative cell death mechanisms. Lysosomal membrane permeabilization (LMP) induced by a variety of stimuli activates a programmed cell death process independent of the classical apoptotic machinery (34–36). This unusual form of cell death induces a mixed necrotic or subapoptotic semblance (37), similar to what we observed in caspase-1/11–independent cell death induced by cytosolic flagellin. To investigate the ability of cytosolic flagellin to induce loss of lysosomal acidity, a hallmark of lysosomal damage, we analyzed macrophages in accordance to AO staining upon flagellin stimulation. AO is a cell permeable dye with lysosomotropic properties that accumulate within acidic lysosomes. When elicited by blue light, the fluorescence emitted by AO varies from red (at high concentrations into the intact acidic lysosomes) to green (under low concentrations in the cytosol or nucleus) (37–39). A large number of spots with bright green fluorescence were observed in both WT and caspase-1/11−/− PMs a few minutes after stimulation with cytosolic flagellin (Fig. 4A) but not with empty DOTAP or medium (Fig. S5A). Because the appearance of a high green AO fluorescence indicates the disruption of lysosomes filled with AO, which then disperse diffusely through the cell cytosol (37–39), our data suggest that cytosolic flagellin can induce loss of lysosomal membrane integrity and leakage of the lysosomal contents into the cell cytosol. The lysosomal damage was likely to be a result of the transfection with cytosolic flagellin but not the transfection of any protein, because a similar staining pattern was observed with FliC flagellin from Salmonella Typhimurium inserted into DOTAP but not with other proteins such as VK210 and rp24 also inserted into DOTAP (Fig. S5A). These results indicate that different preparations of flagellin induced the same effect on macrophages death, in contrast to that observed with nonrelated proteins.
Fig. 4.
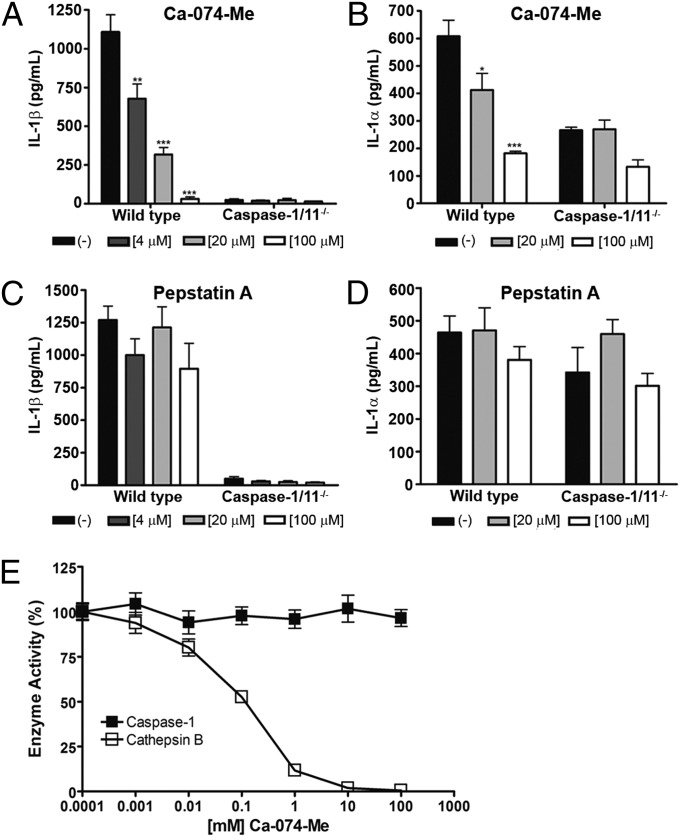
Major role for lysosomal permeability in flagellin-induced macrophage death. (A) Representative fluorescence micrographs of PMs stimulated with FLA-BSDot (3 μg/mL) and stained with AO/EtBr. (Left) Cells with high cytoplasmic green fluorescence. (Center and Right) AO-stained PMs with decreased intensity in cytoplasmic green fluorescence. Micrographs represent an original magnification of 400× . (B–E) Cytotoxicity assessed as the percentage of EtBr single-positive cells in fluorescence micrographs in PMs stimulated with FLA-BSDot (3 μg/mL). Cells were pretreated or untreated with the lysosomal acidification inhibitor bafilomycin A1 (20 nM) (B), cathepsin B inhibitor CA074-Me (25 μM) (C), cathepsin D inhibitor pepstatin A (25 μM) (D) or both CA074-Me and pepstatin A (E), 1.5 h prior stimulation. Numbers represent the means ± SEM of at least 15 images per treatment. ***P < 0.001 compared with the pretreated group. Data are representative of two independent experiments.
The presence of bafilomycin A1, which specifically inhibits a vacuolar H+-ATPase (V-ATPase), preventing lysosomal acidification, abolished cytosolic flagellin-induced cell death in caspase-1/11−/− macrophages (Fig. 4B). Moreover, the presence of bafilomycin also had a significant inhibitory effect on cell death in WT macrophages stimulated with cytosolic flagellin, which suggests the involvement of lysosomal pathways in both pyroptosis and caspase-1/11–independent cell death induced by cytosolic flagellin. To further examine the role of lysosomal pathways in the regulation of cell death induced by cytosolic flagellin, the involvement of lysosomal cathepsins B and D was evaluated by the addition of the selective inhibitors, N-(L-3-trans-propylcarbonyl-oxirane-2-carbonyl)-L-isoleucyl-L-proline methyl ester (CA074-Me) and pepstatin A, respectively. No significant effect on the levels of cell death induced by cytosolic flagellin in both WT and caspase-1/11−/− macrophages was observed in the presence of CA074-Me (Fig. 4C) or pepstatin A alone (Fig. 4D). However, when added together, cell death was halved in both WT and caspase-1/11−/− macrophages (Fig. 4E), indicating a redundant role for cathepsins B and D during cytosolic flagellin-induced cell death. Despite lysosomal disruption and cathepsin B release are associated with NLRP3 inflammasome activation (40, 41), cytosolic flagellin-stimulated NLRP3−/− macrophages displayed similar frequencies of dead cells (Fig. S5D) and IL-1α release (Fig. S5E) compared with WT macrophages, ruling out the participation of NLRP3 in lysosomal-mediated inflammatory cell death induced by cytosolic flagellin.
Interestingly, the presence of CA074-Me during cytosolic flagellin stimulation significantly inhibited caspase-1–dependent IL-1β production (Fig. 5A). The inhibition of cathepsin B also reduced IL-1α production by WT macrophages, at levels similar to those released by caspase-1/11−/− cells (Fig. 5B), suggesting that cathepsin B might regulate caspase-1/11 activities in response to cytosolic flagellin. In contrast to the effect of CA074-Me, the presence of pepstatin A did not affect IL-1β (Fig. 5C) or IL-1α (Fig. 5D) production in response to the stimulation with cytosolic flagellin, which suggests that cathepsin B may have a major role in the regulation of caspase-1–dependent IL-1β production and partially caspase-1/11–dependent IL-1α secretion in response to cytosolic flagellin. It is noteworthy that CA074-Me inhibitor is broadly accepted as a ”specific” inhibitor of cathepsin B (40, 42, 43). However, some findings obtained with CA074-Me are not reproduced in cathepsin B knockout cells (41). In addition, because it has been demonstrated that caspase inhibitors can interact with cathepsin B, thus inhibiting its activity (44), we tested the possibility of the cross-reactivity between CA074-Me and recombinant caspase-1. As shown in Fig. 5E, CA074-Me was not able to directly inhibit caspase-1 activity, whereas inhibited cathepsin B activity in a dose-dependent manner, discarding the nonspecific effect of CA074-Me in the inhibition of caspase-1 and suggesting that the inhibition of caspase-1–dependent macrophages responses to cytosolic flagellin was a result of cross-talk between cathepsin B and caspase-1 and not an off-target effect of CA074-Me. Together, these data indicate that lysosomal events, particularly the activated cathepsins, participate in the regulation of caspase-1/11–dependent and –independent responses after cytosolic flagellin stimulation.
Fig. 5.
Lysosomal cathepsins regulate cytokine production in response to cytosolic flagellin. IL-1β (A and C) or IL-1α (B and D) levels from supernatants of PMs stimulated with 3 μg/mL cytosolic flagellin from B. subtilis (FLA-BSDot) were determined by ELISA. Cells were pretreated or not with the cathepsin B inhibitor CA074-Me (A and B), cathepsin D inhibitor pepstatin A (C and D), with the indicated concentrations, 1.5 h prior to stimulation with FLA-BSDot. Data obtained in A and B are representative of two independent experiments. Numbers represent the means ± SEM (n = 2). Data obtained in C and D are shows as the mean of two independent experiments. Numbers represent the means ± SEM (n = 4). *P < 0.05; *P < 0.01; ***P < 0.001 compared with the untreated group. (E) Recombinant cathepsin B and caspase-1 were pretreated with CA074-Me and their residual activity measured and represented as means ± SEM (n = 3).
Discussion
Caspase-1–mediated cellular signaling induced by flagellin stimulation seems to be strictly coordinated. Activation of caspase-1 by the NAIP5/NLRC4 inflammasome complex promotes rapid formation of pore in the plasma membrane that culminates in cell swelling, osmotic lysis, and the release of the proinflammatory cytokines IL-1β and IL-18 (11, 25). Taken together, these caspase-1–dependent events characterize the pyroptosis process. However, we show here that cytosolic flagellin also induces cell death in the absence of caspase-1/11. Cytosolic flagellin-induced caspase-1/11–independent cell death occurs with slower kinetics in comparison with pyroptosis, and dying cells have some apoptotic-like features, including loss of cell volume and the formation of structures that resemble membrane blebs. Despite the initial events that distinguish it from pyroptosis, cytosolic flagellin-induced caspase-1/11–independent cell death seems to result in the same inflammatory outcome, culminating in cell lysis with the release of intracellular contents including the proinflammatory cytokine IL-1α. Moreover, cytosolic flagellin-induced caspase-1/11–independent cell death strongly correlated with the restriction of Salmonella Typhimurium infection by macrophages and could explain the reduction of in vivo infection found in caspase-1/11−/− mice after the inoculation of flagellin. Importantly, the inhibition of cathepsin B and cathepsin D reduced cytosolic flagellin-induced cell death by half, in both WT and caspase-1/11−/− macrophages. In addition, IL-1β production in response to cytosolic flagellin was abrogated in the absence of active cathepsin B. Taken together, our data indicate that cytosolic flagellin activates the lysosomal pathway that, in its turn, regulate both caspase-1/11–dependent and caspase-1/11–independent macrophages responses.
Despite the fact that flagellin may be recognized by both TLR5 and NAIP5/NLRC4 inflammasome, their individual activation results in nonredundant cellular responses. Caspase-1 activation, cytotoxicity, and release of IL-1β have been demonstrated to proceed normally in TLR5-deficient BMDM during Salmonella Typhimurium infection (5). In agreement with this study, our results demonstrated that the introduction of purified flagellin from B. subtilis directly into the macrophage cytosol using transfection lipid vesicles (DOTAP) was sufficient to induce caspase-1–dependent IL-1β secretion, the formation of membrane pores and the early LDH release by macrophages. These same effects were not observed when macrophages were stimulated with purified flagellin in its free form. Stimulation with free flagellin only resulted in IL-6 production because of its extracellular recognition by TLR5. Moreover, the LDH release and cell death that was induced by cytosolic flagellin were still observed when MyD88 was absent, suggesting that the TLR5, IL-1R, and IL-18R signaling pathways are not required for macrophage death.
Because flagellin-induced pyroptosis is accompanied by cell permeabilization, most studies have assessed this peculiar program of cell death by analyzing early liberation of LDH, which is indicative of a loss of cell integrity. In contrast to C57BL/6 WT PMs, LDH was not identified in NLRC4−/− and caspase-1/11−/− macrophage cultures early after stimulation with cytosolic flagellin. Using distinct approaches to characterize cell death, we observed that LDH liberation did not necessarily correlate with cell death. Stimulation of macrophages with a low dose of cytosolic flagellin was sufficient to induce LDH liberation but was not sufficient to induce cell death. Moreover, caspase-1/11−/− macrophages lost AO dye and incorporated EtBr at 3 h after stimulation with cytosolic flagellin, but no LDH release was identified in these cultures. These data suggest that the release of cellular inflammatory contents and cell death, which are two important consequences of the recognition of cytosolic flagellin, possibly represent two independent events. The disagreement observed between the cytotoxicity assays can be explained by the nature of each assay (45). LDH release is predicted on the release and accumulation of the enzyme into the culture supernatant after a loss in plasma membrane integrity. Conversely, the EtBr/AO assay is based on the loss of acidic compartments, specifically the acidity of nuclei, in combination with an increased cell membrane permeability that permits the EtBr diffusion. Therefore, it is possible that some temporary membrane damage could lead to the release of LDH without inducing cell death. Conversely, other intracellular events could culminate in a loss of AO staining before any cell membrane damage that would allow the liberation of LDH. Therefore, the techniques that are used to characterize cell death can be misinterpreted when used alone. Here, we determined that caspase-1/11 is not required for cell death in response to cytosolic flagellin stimulation as proposed previously (19, 24).
Caspase-1/11–dependent pyroptosis has been described as an important effector mechanism to clear bacterial infections (19, 46). It is believed that the death of infected macrophages by this pathway results in the loss of a replication niche for the infecting bacteria and exposes the bacteria to an inflammatory microenvironment that is optimized for the control of bacterial replication. Miao and his collaborators (14) provided the first in vivo evidence to suggest that pyroptosis could be a host defense mechanism to clear intracellular pathogens. They demonstrated that the induction of flagellin expression during Salmonella Typhimurium infection led to NLRC4-dependent pyroptosis, which resulted in the exposure of the released bacteria to infiltrating neutrophils. The bacteria were then killed by the production of reactive oxygen species. Although there was not a direct correlation between cell death and neutrophils-mediated killing in caspase-1/11−/− mice, the authors showed that caspase-1/11−/− mice were more permissive to Salmonella Typhimurium infection compared with WT mice (14). Our experiments demonstrated that the i.p. inoculation of flagellin significantly reduces the bacterial load in WT and TLR5−/− mice, indicating that flagellin induces the control of in vivo infection by a mechanism independent of TLR5. Then, we hypothesize that the flagellin-dependent control of Salmonella infection by caspase-1/11−/− macrophages is correlated with caspase-1/11–independent cell death. In fact, caspase-1/11−/− macrophages underwent flagellin-dependent cell death in vitro during infection with Salmonella Typhimurium. The slow kinetics of caspase-1/11–independent cell death was likely related to the higher number of CFUs observed in caspase-1/11−/− macrophages compared with WT cells. However, caspase-1/11−/− macrophages were more susceptible to flagellin-deficient Salmonella Typhimurium compared with flagellin-sufficient Salmonella Typhimurium. Finally, the stimulation of both WT and caspase-1/11−/− macrophages with cytosolic flagellin significantly reduces the infection of flagellin-deficient Salmonella Typhimurium in both macrophages. Most importantly, this reduction is temporally correlated with the induction of cell death, which corroborates our hypothesis.
In contrast to proIL-1β, proIL-1α is not a caspase-1 substrate and does not require processing to allow receptor binding (47). However, the role of caspase-1 in the regulation of IL-1α release is still a matter of debate (21, 33, 48). Recently, it was demonstrated that for most NLRP3 soluble activators, including ATP and nigericin, the release of IL-1α was dependent on NLRP3, ASC, and caspase-1 (49). However, the ability of caspase-1 in controlling IL-1α secretion occurs independently of its protease activity. In contrast, some particles or crystals such as silica and MSU induced processing and secretion of IL-1α in a manner independently of inflammasome. In our hands, the secretion of IL-1α in response to cytosolic flagellin seems to be partially dependent on caspase-1 because casapse-1/11−/− macrophages is able to release IL-1α, but with levels significantly lower in comparison with that found in WT macrophages cultures. The secretion of IL-1α, in vivo, was equally or more important than the secretion of IL-1β to induce IL-6 production and neutrophil recruitment during model of peritonitis induced by MSU (49). Therefore, cell injury that is concomitant to the release of IL-1α could be an important consequence of flagellin-induced caspase-1/11–independent cell death that contributes to the inflammatory milieu at the site of bacterial infection.
Interestingly, cytosolic flagellin-stimulated macrophages displayed some apoptotic characteristics in the absence of caspase-1/11 and NLRC4, such as loss in cell volume and the membrane blebbing. These results suggest the possibility that, in the absence of inflammasome activation, macrophages could have a bias toward apoptotic program of cell death. In fact, it was demonstrated that in the absence of caspase-1, dying Salmonella-infected macrophages could exhibit apoptotic or even autophagic features (50, 51). Chromatin condensation and DNA breakage observed in the absence of caspase-1 is mediated by caspase-2 and requires the SipB protein from Salmonella. However, cytosolic flagellin-induced caspase-1/11–independent cell death seems to be regulated by a pathway distinct from classical apoptosis. Conversely to ActD-induced apoptosis, caspase-1/11–independent cell death induced by cytosolic flagellin resulted in the loss of cell integrity, observed by the entry of EtBr and in the release of intracellular contents few hours after stimulation, thus maintaining a necrotic outcome (33). Moreover, cytosolic flagellin did not activate apoptotic caspases 3, 7, and 9 and the broad pharmacological inhibition of caspases did not modify the kinetics or outcome of cytosolic flagellin-induced cell death in the absence of caspase-1/11. Despite the independency of caspases, cytosolic flagellin-induced caspase-1/11–independent cell death is abrogated in the presence of bafilomycin, which suggests the involvement of the lysosomal pathway. Concomitant inhibition of cathepsin B and cathepsin D have a significant negative effect on cytosolic flagellin-induced cell death, which indicates a redundant role of these two proteins and supports the involvement of lysosomal damage-mediated program of cell death. Thus, our work adds a flagellin-mediated mechanism by which Salmonella Typhimurium can kill the infected macrophage in the absence of caspase-1 and caspase-11.
It is well established that moderate lysosomal damage with slow liberation of lysosomal hydrolases into the cell cytosol can culminate in controlled lysosome-mediated apoptotic cell death (37). Once in cytosol, cathepsins B and D can induce apoptosis in a synergistic manner by simultaneously cleavage of the proapoptotic protein Bid, and the antiapoptotic members of Bcl-2 family, like Bcl-2, Bcl-xL, Ncl-1e, and XIAP (52–56). According to this, the early cell shrinkage and formation of membrane blebs observed in cytosolic flagellin-stimulated caspase-1/11−/− macrophages and that resembles apoptosis could be a result of the activation of the apoptotic machinery by lysosomal cathepsins into cell cytosol. However, different from apoptotic cells, cytosolic flagellin-stimulated caspase-1/11−/− macrophages undergo rapid loss of membrane integrity, which is consistent with a massive induction of lysosomal membrane permeability and consequent loss of organelle membrane integrity (37). This lysosomal damage-mediated cell death program commonly results in uncontrolled necrotic cell death without apoptotic caspase activation where macrophages exhibit promiscuous features of apoptosis and necrosis, consistent with our observations. The mechanism by which flagellin induces massive loss of lysosome membrane integrity has yet to be elucidated. Therefore, the fact of distinct flagellin preparations but not other nonrelated proteins were able to induce lysosomal cell death, discard nonspecific effects of possible contaminants in preparations, and point out the unique lysosomotropic propriety of flagellin.
In our model, the lysosomal pathway has a central role in flagellin-induced cell death because the inhibition of either lysosome acidification or cathepsin B and D activation, but not the absence of caspase-1/11, suppresses cell death. In addition, because cell death occurs less prominently and with distinct characteristics in the absence of caspase-1/11, it is possible that these two proinflammatory caspases act as accessory/catalytic factors rather than being the central effector mechanism of cytosolic flagellin-induced cell death. This is reminiscent to what occurs during apoptosis, particularly after insults that triggers the intrinsic pathway, where the apoptotic effector caspases -3, -6, and -7 are also not required for cell death, but they are essential for imprinting apoptotic characteristics, such as the appearance of “eat me” of “find me” signals, DNA degradation, and inactivation of DAMPs, in cells that are already destined to die (57–59). Likewise, we propose that pyroptosis, as defined as a caspase-1/11–dependent cell death, is rather a program of cell demise capable of imprinting particular characteristics to the cells committed to die, such as the secretion of IL-1β/IL-18.
There are few and conflicting data about the relationship between inflammasomes and lysosomal damage-mediated cell death. In some instances, LMP, a hallmark characteristic of lysosomal-mediated cell death, can occur upstream or downstream of inflammasome activation (42, 60). In other circumstances, LMP is an independent event that occurs simultaneously with caspase-1 activation, thus contributing to cell death (38). It has been described that the inhibition of cathepsin B activation abolishes caspase-1–dependent IL-1β and IL-18 production in addition to caspase-1–independent pyronecrosis that are mediated by NLRP3 inflammasome (42). Despite the similarities of the cathepsin B effects, we clearly show here that cytosolic flagellin-induced lysosomal cell death is not mediated by NLRP3. However, it is important to note that it is still possible that NLRP3-dependent responses may compensate for the lack of Nlrc4 in Nlrc4 knockout mice and, similarly, Nlrc4-dependent responses may be sufficient in the absence of NLRP3. Future studies could address this question using Nlrc4/Asc and Nlrc4/NLRP3 double knockouts. One of the few studies that have attempted to investigate the regulation of this pathway suggested that phosphorylated tyrosine kinase Syk is physically associated with ASC, and Syk is required to activate both the NLRP3 inflammasome and cathepsin B in macrophages that were stimulated with hemozoin from Plasmodium, but not with uric acid or silica (60). Because IL-1β production is abolished in the absence of active cathepsin B or Syk, these data suggest that the phosphorylation of Syk and the activation of cathepsin B are upstream of caspase-1 activation. However, the mechanisms involved in the activation of cathepsin B and NLRP3 by phosphorylated Syk remain to be elucidated. Recently, cathepsin B was demonstrated to be involved in pyroptosis induced by Bacillus anthracis lethal toxin (LT) (38). Interestingly, it was observed that LMP could be upstream or downstream of the NLRP1b inflammasome activity. LMP required a NLRP1 LT-responsive allele of NLRP1b and caspase-1 activation, but LT-induced mitochondrial outer membrane permeabilization seems to be sufficient to induce LMP and can function as a positive regulator of NLRP1b inflammasome activities. Here, we demonstrated evidences of the regulation of NAIP5/NLRC4 inflammasome activities by lysosomal pathway, because inhibition of cathepsin B impacts cytosolic flagellin-induced inflammasome-mediated effects.
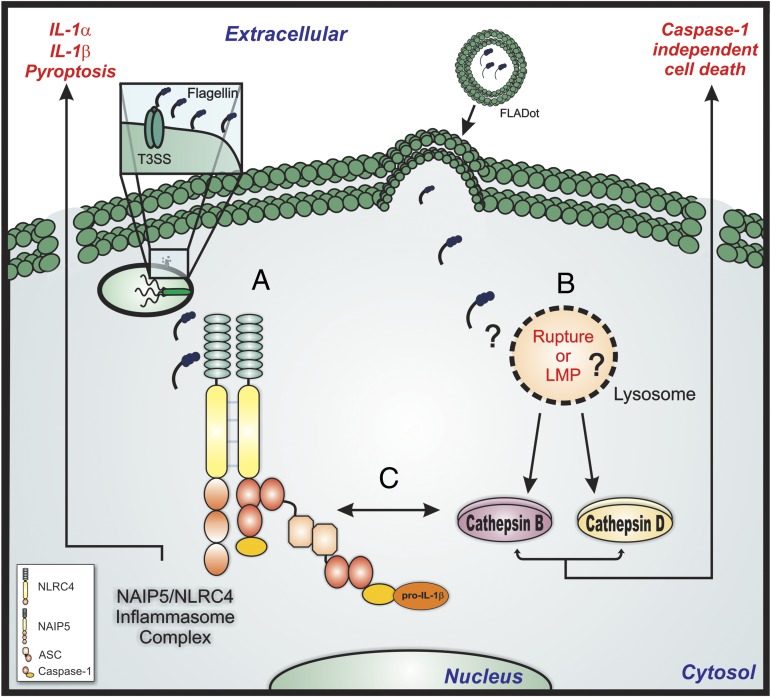
Taken together, our results indicate that NAIP5/NLRC4 inflammasome (labeled “A” in Fig. 6) and lysosomal pathway (labeled “B” in Fig. 6) are both activated after cytosolic flagellin stimulation. Both NAIP5/NLRC4 inflammasome and lysosomal pathway seem to synergistically contribute to cell death/pyroptosis, IL-1β production and IL-1α secretion (labeled “C” in Fig. 6). Moreover, lysosomal cathepsins regulate flagellin-induced cell death in the absence of NAIP5/NLRC4 inflammasome. Given that this peculiar form of cell death is also involved in the clearance of bacterial infection, our results provide further understanding of the molecular mechanisms that coordinate the activation and death of macrophages in response to flagellin and its biological significance to host resistance against infections with flagellated bacteria.
Fig. 6.
The relationship between inflammasome and lysosomal pathway for cytokine production and cell death. Bacterial flagellin inserted into macrophage cytosol through T3SS/T4SS secretion systems or by lipid vesicles can induce the activation of both NAIP5/NLRC4 inflammasome complex (labeled A) and lysosomal pathway (labeled B). Cytosolic flagellin could induce lysosome rupture or LMP that permits the release of active cathepsins into cell cytosol. Inflammasome activation results in caspase-1/11–mediated pyroptosis and IL-1β production and IL-1α secretion. However, the inflammasome-mediated macrophages responses seem to be a result of the cross-talk between inflammasome complex and lysosomal pathway, where cathepsin B could regulate the activation of NAIP5/NLRC4 inflammasome or caspase-1 activities, because it is involved with IL-1β production (labeled C). On the other hand, the lysosomal pathway orchestrates the cytosolic flagellin induced caspase-1/11–independent inflammatory cell death.
Methods
Animal studies were all performed using protocols that were approved by the Federal University of São Paulo Committees on the Use and Care of Animals (CEP 0159/11). Details on methods and any associated references are available in SI Methods. Briefly, PMs were obtained by peritoneal lavage 4 d after i.p. treatment with starch solution (1%) (Sigma Aldrich). Purified flagellins from B. subtilis (FLA-BS) and Salmonella Typhimurium (FliC) were purchased from Invivogen. The recombinant Salmonella Typhimurium FliC flagellin and the HIV p24 protein were expressed in the E. coli BL21 DE strain, and the recombinant proteins were purified by nickel affinity chromatography, as described previously (61, 62). VK210 peptide of P. vivax protein was purchased from GenScript. PMs were cultured in the presence of purified flagellin from B. subtilis or Salmonella Typhimurium in its free form (FLA-BS or FliC) or inserted into DOTAP (FLA-BSDot or FliCDot) (Roche Diagnostics), a cationic lipid formulation that permits its delivery to cell cytosol (63). DOTAP was used accordingly to the manufacturer’s instructions, and protein concentration was calculated to maintain the same molarity. Cytokines were measured in culture supernatant with ELISA kits from BD (OptEIA), following the manufacturer’s instructions. Pore formation and cytotoxicity was assessed using EtBr incorporation in combination with AO (Sigma) staining as described previously (64).
Supplementary Material
Acknowledgments
We thank Dr. Richard Flavell for kindly providing ASC−/−, caspase-1/11−/−, and NLRC4−/− mice and Dr. Vishva Dixit for kindly providing NLRP3−/− mice. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo and the Brazilian Research Council.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305316110/-/DCSupplemental.
References
- 1.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 2.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7(6):576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 3.Zamboni DS, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7(3):318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 4.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281(46):35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 5.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7(6):569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 6.Smith KD, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4(12):1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 7.Lightfield KL, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9(10):1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lightfield KL, et al. Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun. 2011;79(4):1606–1614. doi: 10.1128/IAI.01187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477(7366):596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 10.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: Two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19(1):1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 11.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38(1):31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 12.Akhter A, et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5(4):e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzzo CL, et al. A novel pathway for inducible nitric oxide synthase activation through inflammasomes. J Biol Chem. 2010;285(42):32087–32095. doi: 10.1074/jbc.M110.124297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki T, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3(8):e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchi L, et al. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37(11):3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 17.Sutterwala FS, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204(13):3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180(11):7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 21.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 22.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80(3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Smith MR, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15(15):3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 24.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 26.Silveira TN, Zamboni DS. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun. 2010;78(3):1403–1413. doi: 10.1128/IAI.00905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8(5):372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 28.Vijay-Kumar M, Carvalho FA, Aitken JD, Fifadara NH, Gewirtz AT. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur J Immunol. 2010;40(12):3528–3534. doi: 10.1002/eji.201040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8(6):471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosn EE, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci USA. 2010;107(6):2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassado AdosA, et al. Cellular renewal and improvement of local cell effector activity in peritoneal cavity in response to infectious stimuli. PLoS ONE. 2011;6(7):e22141. doi: 10.1371/journal.pone.0022141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGahon AJ, et al. The end of the (cell) line: Methods for the study of apoptosis in vitro. Methods Cell Biol. 1995;46:153–185. doi: 10.1016/s0091-679x(08)61929-9. [DOI] [PubMed] [Google Scholar]
- 33.Eigenbrod T, Park JH, Harder J, Iwakura Y, Núñez G. Cutting edge: Critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol. 2008;181(12):8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23(16):2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 35.Hitomi J, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreuzaler PA, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol. 2011;13(3):303–309. doi: 10.1038/ncb2171. [DOI] [PubMed] [Google Scholar]
- 37.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 38.Averette KM, et al. Anthrax lethal toxin induced lysosomal membrane permeabilization and cytosolic cathepsin release is Nlrp1b/Nalp1b-dependent. PLoS ONE. 2009;4(11):e7913. doi: 10.1371/journal.pone.0007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nascimento FD, et al. Crotamine mediates gene delivery into cells through the binding to heparan sulfate proteoglycans. J Biol Chem. 2007;282(29):21349–21360. doi: 10.1074/jbc.M604876200. [DOI] [PubMed] [Google Scholar]
- 40.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dostert C, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS ONE. 2009;4(8):e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hentze H, Lin XY, Choi MS, Porter AG. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 2003;10(9):956–968. doi: 10.1038/sj.cdd.4401264. [DOI] [PubMed] [Google Scholar]
- 43.Duncan JA, et al. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182(10):6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schotte P, et al. Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem Biophys Res Commun. 1998;251(1):379–387. doi: 10.1006/bbrc.1998.9425. [DOI] [PubMed] [Google Scholar]
- 45.Lage SL, Amarante-Mendes GP, Bortoluci KR. Evaluation of pyroptosis in macrophages using cytosolic delivery of purified flagellin. Methods. 2013;61(2):110–116. doi: 10.1016/j.ymeth.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: Role of TLR and NLR. Cell Mol Life Sci. 2010;67(10):1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 48.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24(3):317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Gross O, et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36(3):388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Jesenberger V, Procyk KJ, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: A novel mechanism in pathogen-mediated apoptosis. J Exp Med. 2000;192(7):1035–1046. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez LD, Pypaert M, Flavell RA, Galán JE. A Salmonella protein causes macrophage cell death by inducing autophagy. J Cell Biol. 2003;163(5):1123–1131. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Repnik U, Turk B. Lysosomal-mitochondrial cross-talk during cell death. Mitochondrion. 2010;10(6):662–669. doi: 10.1016/j.mito.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Kågedal K, Johansson U, Ollinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001;15(9):1592–1594. doi: 10.1096/fj.00-0708fje. [DOI] [PubMed] [Google Scholar]
- 54.Kågedal K, Zhao M, Svensson I, Brunk UT. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem J. 2001;359(Pt 2):335–343. doi: 10.1042/0264-6021:3590335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foghsgaard L, et al. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol. 2001;153(5):999–1010. doi: 10.1083/jcb.153.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamashima T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog Neurobiol. 2000;62(3):273–295. doi: 10.1016/s0301-0082(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 57.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11(7):725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 58.Pereira WO, Amarante-Mendes GP. Apoptosis: A program of cell death or cell disposal? Scand J Immunol. 2011;73(5):401–407. doi: 10.1111/j.1365-3083.2011.02513.x. [DOI] [PubMed] [Google Scholar]
- 59.Amarante-Mendes GP, et al. Anti-apoptotic oncogenes prevent caspase-dependent and independent commitment for cell death. Cell Death Differ. 1998;5(4):298–306. doi: 10.1038/sj.cdd.4400354. [DOI] [PubMed] [Google Scholar]
- 60.Shio MT, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5(8):e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bargieri DY, et al. New malaria vaccine candidates based on the Plasmodium vivax Merozoite Surface Protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine. 2008;26(48):6132–6142. doi: 10.1016/j.vaccine.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 62.Bargieri DY, et al. Immunogenic properties of a recombinant fusion protein containing the C-terminal 19 kDa of Plasmodium falciparum merozoite surface protein-1 and the innate immunity agonist FliC flagellin of Salmonella typhimurium. Vaccine. 2010;28(16):2818–2826. doi: 10.1016/j.vaccine.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Simberg D, Weisman S, Talmon Y, Barenholz Y. DOTAP (and other cationic lipids): Chemistry, biophysics, and transfection. Crit Rev Ther Drug Carrier Syst. 2004;21(4):257–317. doi: 10.1615/critrevtherdrugcarriersyst.v21.i4.10. [DOI] [PubMed] [Google Scholar]
- 64.Kasibhatla S, et al. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. 2006 doi: 10.1101/pdb.prot4493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.