Abstract
Tradeoffs provide a rationale for the outcome of natural selection. A prominent example is the negative correlation between the growth rate and the biomass yield in unicellular organisms. This tradeoff leads to a dilemma, where the optimization of growth rate is advantageous for an individual, whereas the optimization of the biomass yield would be advantageous for a population. High-rate strategies are observed in a broad variety of organisms such as Escherichia coli, yeast, and cancer cells. Growth in suspension cultures favors fast-growing organisms, whereas spatial structure is of importance for the evolution of high-yield strategies. Despite this realization, experimental methods to directly select for increased yield are lacking. We here show that the serial propagation of a microbial population in a water-in-oil emulsion allows selection of strains with increased biomass yield. The propagation in emulsion creates a spatially structured environment where the growth-limiting substrate is privatized for populations founded by individual cells. Experimental evolution of several isogenic Lactococcus lactis strains demonstrated the existence of a tradeoff between growth rate and biomass yield as an apparent Pareto front. The underlying mutations altered glucose transport and led to major shifts between homofermentative and heterofermentative metabolism, accounting for the changes in metabolic efficiency. The results demonstrated the impact of privatizing a public good on the evolutionary outcome between competing metabolic strategies. The presented approach allows the investigation of fundamental questions in biology such as the evolution of cooperation, cell–cell interactions, and the relationships between environmental and metabolic constraints.
Keywords: metabolic engineering, group selection, r/K selection, droplets, microbial diversity
Although the existence of tradeoffs in evolution seems to be undisputable, experimental evidence obtained under controlled conditions is scarce. Several examples failed to show tradeoffs (1–4), whereas others could find them (5, 6) or found general but not universal tradeoffs (7, 8). A tradeoff between growth rate and growth yield in microbes (9–11) has direct implications for experiments carried out in liquid cultures. This is especially of importance during prolonged cultivations such as laboratory evolution experiments. In suspension, fast-growing variants outcompete slower growing ones at the cost of biomass yield (5). The yield versus rate optimization is governed by a dilemma where fast growth is advantageous from the perspective of an individual cell, whereas slow growth, and therefore high yield, is advantageous from the perspective of a population. This dilemma is consistent with a concept termed the tragedy of the commons (12). It is well described that spatial structure is essential for the selection of high-yield strategies (13–15). The yield/rate tradeoff of microbial growth has been linked to metabolic strategies (11) such as the switch between respiration and fermentation in yeast (9). It is suggested that the underlying cause of this tradeoff is based on thermodynamic principles, which describe that the rate of an isolated metabolic reaction is driven by free-energy dissipation and hence is negatively correlated with the product yield (9). However, it remains unclear if this is also valid for growth of an organism where near-equilibrium thermodynamics do not necessarily apply. Several studies that tried to address the yield/rate tradeoff experimentally failed. Luckinbill compared strains that were either adapted by serial propagation during exponential growth or in stationary phase (16). Velicer and Lenski compared strains that evolved in either carbon-limited chemostat cultures or in batch cultures (2). Both studies assumed that during the exponential phase of a batch culture, there was excess of substrate and therefore fast growing mutants should become dominant, whereas in either stationary phase or a carbon-limited chemostat, nutrients are limited and mutants that use their substrate efficiently should become dominant. We think that the reason why these and other studies (4, 6) failed to identify a yield/rate tradeoff over the course of experimental evolution is that in all cases the experiments were carried out in suspension cultures. In such an unstructured environment resources are a public good and accessible to every individual. In the case of batch cultures, this will select for the fastest growing organism, whereas in the case of a chemostat, selection will favor mutants with a higher affinity to the limiting substrate (17).
In theory, the selection of cells with increased metabolic efficiency but potentially slower growth rates is only possible if there is no or only limited substrate competition between fast and slow growing cells. We showed that such criteria are met in an emulsion-based serial propagation protocol, which favored the selection of strains with an increased number of offspring but decreased growth rates. The mechanism underlying those phenotypic changes was a shift in metabolic efficiency caused by altered glucose transport.
Results
Selection During Serial Compartmentalization in Emulsion.
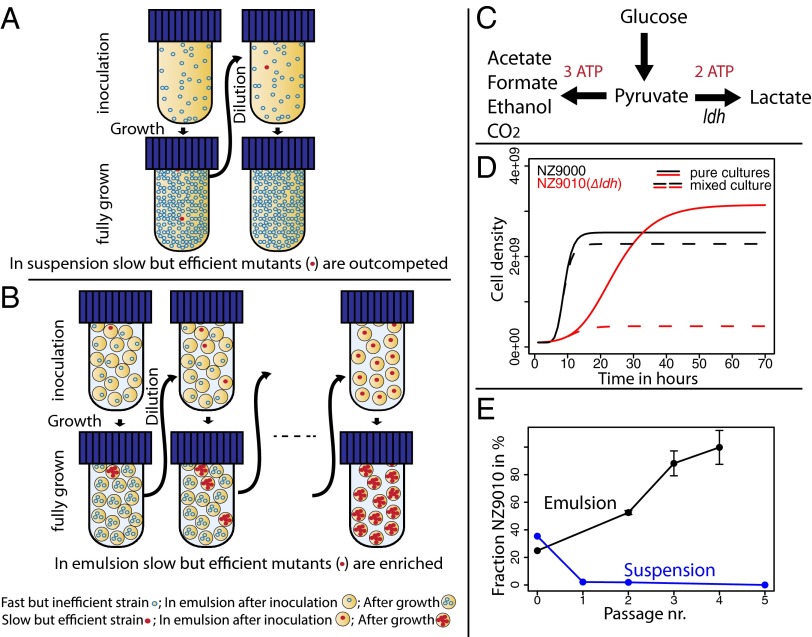
To select mutants with an increased number of offspring rather than increased growth rate, competition between individual cells needs to be eliminated through e.g., compartmentalization of single cells. Additionally, the number of compartments has to be high to guarantee a large enough population size for selection to act on. We reasoned that these criteria are met in a water-in-oil (w/o) emulsion. The water phase consists of the growth medium of an organism while the droplets in the emulsion are surrounded by an oil phase that prevents diffusion of molecules between individual droplets (Fig. 1). Following a Poisson distribution (e.g., λ = 0.1), droplets can be inoculated with single cells that are the founders of new populations in each droplet (SI Appendix, Fig. S1). Given sufficient time, populations in each droplet can grow to the maximum carrying capacity of the given medium. At the end of the incubation, the fraction of mutants that are producing a higher number of offspring will have increased compared with the fraction of these mutants at the time of inoculation. After breaking the emulsion, the cells are pooled in the medium (water) phase. At this stage, cells can be enumerated, diluted, and used again for the inoculation of the following emulsion. By repeating such a transfer regime in emulsion, cells with an increased number of offspring will be selected (Fig. 1 A and B). We validated this concept by competing a Lactococcus lactis strain with its lactate dehydrogenase (ldh) negative derivative. In the ldh mutant, the glycolytic flux is diverted from lactate toward acetate (Fig. 1C), which results in a higher ATP- and biomass yield but also a decreased growth rate (Fig. 1D). Our experiments showed that in suspension, the wild-type strain rapidly won the competition, whereas in emulsion, the slow growing ldh mutant became dominant (Fig. 1E). This demonstrates a fundamental difference in selection pressure between the two culturing systems.
Fig. 1.
Serial propagation in suspension leads to the selection of mutants with increased growth rates (A). If droplets in emulsions are initially occupied with a single cell, mutants with a higher number of offspring will be able to grow to a higher cell density compared with the wild-type strain even if such mutants grow slower. If the emulsion is subsequently broken, diluted, and used to inoculate a new emulsion, one can enrich for strains with a higher number of offspring (B). In lactic acid bacteria catabolization of glucose to lactate yields two molecules of ATP and it is faster than the conversion to acetate, which yields three molecules of ATP (C). This leads to a yield/rate tradeoff, which is demonstrated by deleting the lactate dehydrogenase (ldh) of L. lactis. In a pure culture strain NZ9000 is inefficient but fast (solid black line in D), whereas the ldh negative variant NZ9010 is efficient but slow (solid red line in D) (21). In coculture the fast strain is expected to reach the maximum carrying capacity quicker than the slow growing strain and subsequently overall growth will cease (dashed lines in D). This will lead to the loss of the slow growing variant from the culture. However, growth of individual cells in emulsion rather reflects many individual growth curves of pure cultures. Therefore, serial propagation in emulsion should allow the enrichment of slow growing but efficient cells, whereas in suspension the opposite is expected. This concept was demonstrated in a competition experiment of L. lactis NZ9000 and its ldh negative derivative NZ9010 (E). See SI Appendix for details.
Exploring the Yield/Rate Tradeoff During Serial Propagation in Emulsion.
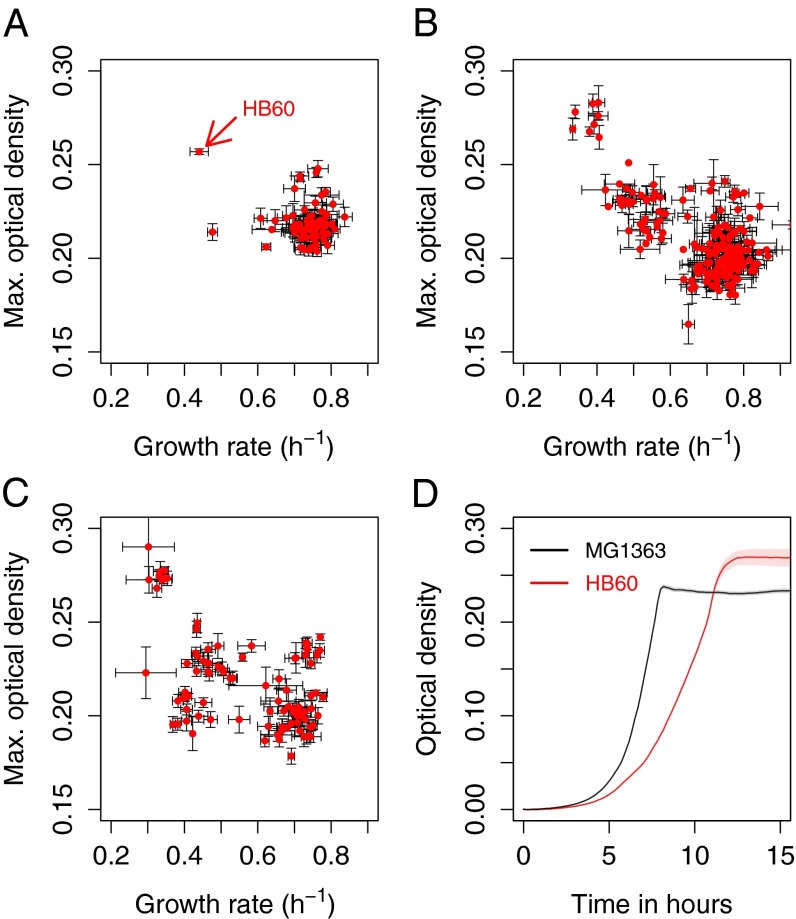
We then explored the yield/rate tradeoff in isogenic L. lactis cells through serial propagation of chemically mutagenized populations in emulsions. Bacteria were allowed to grow for 2 d in the individual droplets and after 22 propagation cycles 88 individual colonies from two independent cultures were isolated. Growth rates and the maximum optical densities, which were used as a proxy for the total biomass yield, were determined for the individual cultures. One of the 88 strains (designated HB60) showed an 25% increase in maximal optical density, a 26% increase in culture dry weight, a 47% decrease in growth rate (MG1363 µ = 0.79 h−1, HB60 µ = 0.42 h−1), a 38% decrease in cell volume (MG1363 = 3.97 µm3, HB60 = 2.45 µm3), and a 71% increase in the total number of cells per culture volume (Fig. 2 and SI Appendix, Fig. S2). The results furthermore showed that after 22 propagation steps, most isolates cluster closely together in the yield/rate plot (Fig. 2A), whereas after 28 and 31 transfers multiple populations with more distinct properties arise. Interestingly also variants arise that seem to be slower and have a lower optical density compared with other variants in the same population. This indicates that optical density measurements might not always be representative for the number of offspring or that selection is also influenced by properties such as survival in stationary phase. Although we only characterized one strain of the above experiment in depth we are confident that the enrichment of strain HB60 was no coincidence. This was evident from the continuation of the original propagation experiment (Fig. 2) but also from the proof-of-principle experiment (Fig. 1) and the invasion of HB60 in the wild-type population from a low frequency (SI Appendix, Fig. S3). Taken together, the results are consistent with the idea that propagation in a compartmentalized environment would enrich for strains with an increased number of offspring. In the case of HB60, the increased number of offspring also coincides with an increased biomass yield and a decreased growth rate indicating a yield/rate tradeoff.
Fig. 2.
Enrichment of strains with increased maximum optical densities (y axis) and decreased growth rates (x axis) throughout propagation in emulsion. Strain HB60 was isolated after 22 transfers in emulsion (A). Further propagation of this culture in emulsion up to 28 (B) and 31 (C) transfers resulted in an increased fraction of strains with increased optical densities and/or decreased growth rates. The data also indicate that several clusters of new phenotypes emerge. SEs are indicated (n = 4). D shows growth curves of the wild-type M1363 (black line) and isolate HB60 (red line), which grows slower but reaches a higher cell density.
Major Metabolic Shift Caused Decreased Growth Rate but Increased Biomass Yield.
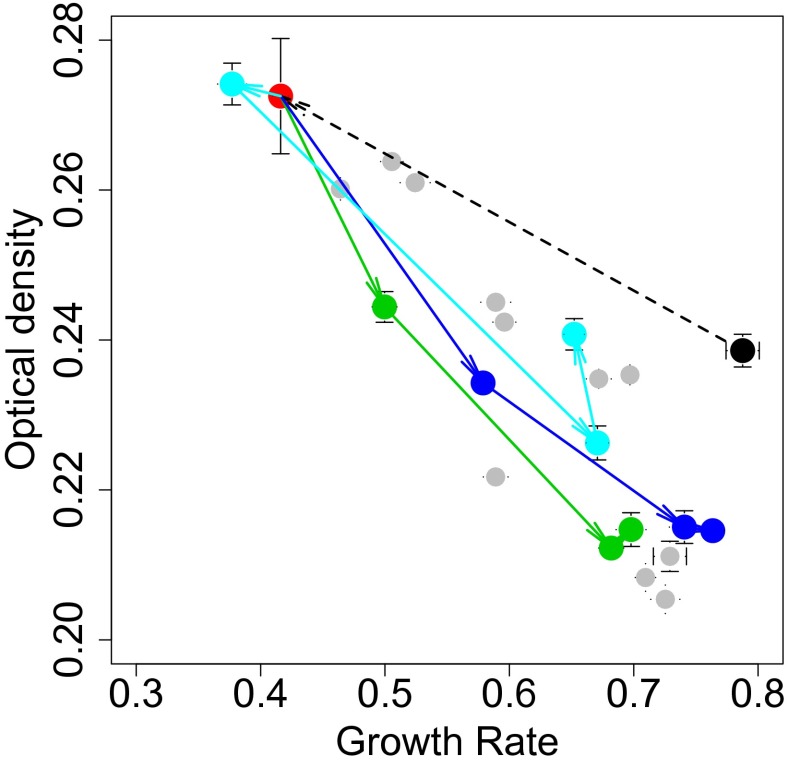
Whereas the wild-type strain MG1363 produced mainly lactate as a metabolic end product, HB60 produced mainly acetate, formate, and ethanol (SI Appendix, Fig. S4). This represents a metabolic shift in fermentation products, which is a major determinant of the ATP yield per glucose molecule (metabolic efficiency). We subsequently tested the stability of this metabolic strategy during cell propagation in suspension. Three independently propagated cultures of HB60 showed that within 90 generations, the cultures were dominated again by fast growing variants (designated HB61, HB62, and HB63) with decreased yield, increased cell size, and lactate as the major metabolic end product (Fig. 3 and SI Appendix, Fig. S5). These results suggest a strong selective pressure of the culturing conditions on the metabolic shift and its effect on growth yield and growth rate.
Fig. 3.
Yield/rate trajectories during selection in emulsion (dotted black line) and in suspension (solid lines). L. lactis MG1363 (black dot) was propagated in emulsion, which led to the isolation of HB60 (red dot). Subsequently HB60 was propagated in suspension for 160 generations. The solid arrows show the trajectories followed by three individual cultures of HB60 after 60, 100, and 160 generation in suspension. The dots after 100 generations are from single colonies isolated from the respective cultures, designated HB61 (green), HB62 (blue), and HB63 (turquoise). Gray dots show other isogenic isolates of MG1363 (SI Appendix, Fig. S10). SEs are indicated (n = 11).
Altered Glucose Transport Caused the Metabolic Shift.
Genome resequencing of strain HB60 revealed a point mutation in ptnC (SI Appendix, Fig. S6), which encodes a component of the main glucose transport system of L. lactis. This phosphoenolpyruvate:phosphotransferase system (designated PTSMan because of its initial description as a mannose transporter) is the only high-affinity glucose transporter in this strain (Km = 13 µM), whereas the other glucose transport systems, the glucose permease glcU and the PTSCel system (a PTS that is also known to transport cellobiose) have Km values of 2.4 mM and 8.7 mM, respectively (18). The sugar concentration at the beginning of our batch cultures was 5 mM and therefore mutations in the high-affinity system are expected to have the largest effects on glucose transport in our selection medium. In L. lactis, the acidification rate is a measure of the glycolytic flux. We found that HB60 had a 41% decreased acidification rate compared with the wild type, whereas the three fast growing revertants HB61, HB62, and HB63 showed acidification rates that were, respectively, 27%, 45%, and 32% increased relative to the wild type (SI Appendix, Fig. S7). Alterations from a high to a low glycolytic flux lead to a shift from the lactate toward the acetate branch and vice versa, changing the ATP yield and explaining the observed alterations in biomass yields. Genome resequencing of fast growing revertant strains showed that strain HB61 and HB63 have the transposable element IS905 inserted in the promoter region of PTSMan, encoding ptnABCD. Additionally HB63 has IS905 also inserted upstream of the glucose permease glcU. IS905 contains an outward facing perfect −35 promoter element at its 3′ end. Upon transposition upstream of ptnABCD and glcU new consensus promoter sequences are formed with −10 promoter elements located in the appropriate spacing on the integration locus (SI Appendix, Figs. S8 and S9). An increase in transcription levels upon transposition of IS905 into other promoter regions of MG1363 has been described earlier (19, 20). The transpositions of IS905 can therefore explain the enhanced acidification rate phenotype of the revertant strains HB61 and HB63.
Fast Growth Rates Were Strongly Linked to Lactate Production.
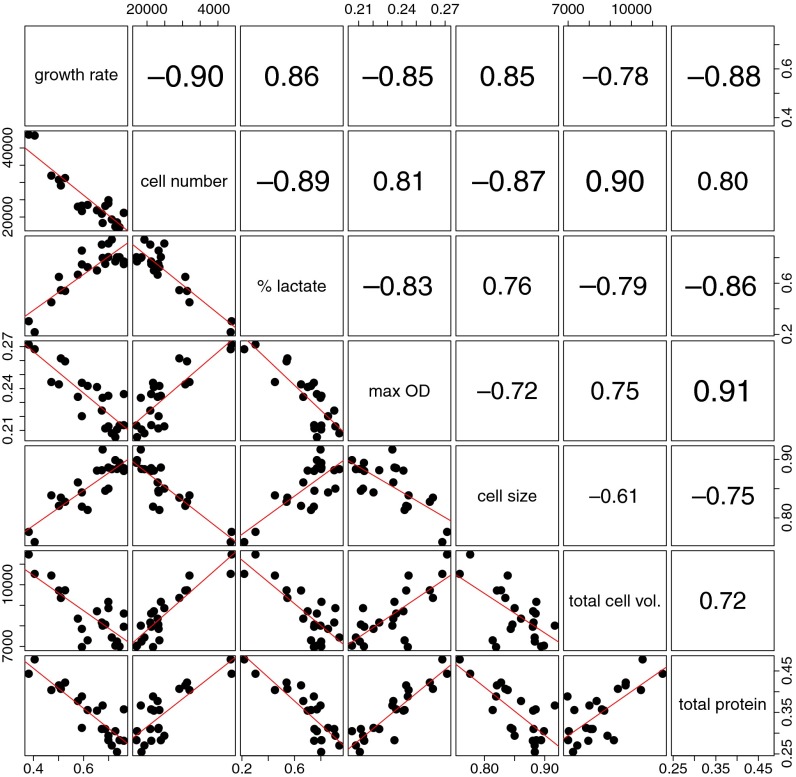
A comparison of all strains evolved in this study, including two mutant strains that were deficient in two glucose transport systems, revealed a significant negative correlation of growth rate and growth yield over all 22 strains (adjusted R2 = 0.71, P < 0.0001) (Fig. 4 and SI Appendix, Fig. S10). The growth rate was also positively correlated with the fraction of carbon that was catabolized through the lactate branch (Fig. 4). The data suggest that there is a limit, also termed Pareto front, where improvement in growth rate can be achieved only at the expense of growth yield or vice versa. As neither selection in suspension nor emulsion allowed the isolation of strains that crossed the apparent yield/rate Pareto front, we challenged this front by serially propagating L. lactis NZ9020 in suspension. In NZ9020 (21) both known lactate dehydrogenase genes are disrupted and it is therefore unable to produce lactate as a fermentation end product. Three independent serial propagations of NZ9020 in suspension for 240 generations as well as 96 independent propagations for 100 generations resulted in no significant alterations of the growth rate or metabolic end products (organic acids were determined for nine cultures), indicating that an increase in growth rate, while metabolizing substrate efficiently through the acetate branch, is not very likely (SI Appendix, Fig. S11).
Fig. 4.
Correlation matrix of parameters that relate to the yield/rate tradeoff. Growth rate and the maximum optical density (max OD) were obtained from growth experiments, cell number, cell size, and total cell volume from Coulter Counter measurements. The fraction of consumed substrate diverted toward lactate (percent lactate) as metabolic end product is a measure for the metabolic strategy. Throughout this paper we use optical density measurements as a proxy for biomass yield. Total cell volume (cell count × cell volume) and total protein are independent parameters for biomass yield and they confirm the validity of this assumption for the presented strains. A positive correlation of growth rate and cell size as shown here is consistent with earlier results from adaptation experiments (45). (Lower) Individual data points and a linear regression line (red). (Upper) Corresponding Pearson correlation coefficient. All correlations are significant (P < 0.01). The 22 data points in each plot are individual strains, which are all derivatives of L. lactis MG1363 and they correspond to the strains shown in SI Appendix, Fig. S10.
Discussion
The described work is relevant to several related concepts in evolutionary biology. Serial propagation in emulsion describes the periodic isolation and propagation of single individuals, which underlies the concept of group selection (22) and has previously been described with the haystack model (23). Each droplet in the emulsion would be the equivalent of a haystack in the model and emulsion propagation resembles migration and colonization of haystacks with single individuals. Whereas the relevance of group selection in nature was the subject of intense discussions (23, 24), we show that periodic isolation does lead to a fundamentally different evolutionary outcome if compared with populations with continuously interacting individuals.
The described yield/rate tradeoff is also necessary to understand the mechanism of r/K selection. In r/K selection theory, high growth rate (r) is beneficial at low population densities and high food availability. However, at high population densities, close to the carrying capacity (K), food is scarce and its economic use is favored (25). It is assumed that selection acts reciprocally on the parameters r and K of a population (26). The demonstrated yield/rate tradeoff suggests the fundamental nature of this prerequisite for r/K selection theory. However, we would like to note that in our system faster growing cells not only waste energy by producing lactate, they also spent longer periods under starvation conditions in stationary phase. The latter by itself potentially favors the selection of slow growing cells in the presented propagation system.
The evolution of restrained growth phenotypes was shown earlier in systems that combined limited dispersal and/or spatial structure with ecological feedback. Examples include experiments with a community of three competitors that are engaged in a nontransitive (rock–paper–scissors) relationship (27) or the evolution of bacterial cross-feeding, which is costly for the individual but benefits the community (28). Compared with those studies, we show that the evolution of restraint is also possible without ecological feedback if the selection regime directly aims at an increased number of offspring. Another example for the evolution of restraint is described for bacteriophages of Escherichia coli. Two phage strategies lead to a “tragedy of the commons” by either being fast in infecting host bacteria but with low productivity when alone or vice versa. The selection of either phage strategy is determined by spatial restrictions in migration patterns (29, 30). The presented approach permits the variation of cells/phages per droplet in the emulsion, which will allow the investigation of interactions in microbial communities, which hitherto could not be addressed because of the limited number of compartments available in standard culturing techniques.
Cooperative traits often relate to extracellular molecules that confer a benefit to the total population but are a burden for the producing cell. Because of this burden the expression of extracellular proteases in lactococci (31, 32), invertase expression in yeast (33), or siderophore production in the pathogen Pseudomonas aeruginosa (34) are described as unstable. Such cooperative traits are expected to behave fundamentally differently in a suspension compared with an emulsion-based culturing system. The described approach should therefore open new possibilities for the investigation of the evolution of cooperation as well as facilitate the selection of strains with increased production levels of industrially relevant biomolecules such as extracellular substrate degrading enzymes.
Other industrial applications of the described selection procedure include the increase of biomass yield or the reduction of unwanted metabolic side products that are associated with fast growth such as acetate production in E. coli cultures (35) or ethanol production in respiring yeast (36). Furthermore, metabolic engineering strategies are increasingly based on genome-scale metabolic models, in particular through flux balance analysis (FBA). Such stoichiometric models optimize the molar yield of metabolic networks, which is discrepant with experimentally evolved organisms that are mostly optimized for growth rate (37). The selection of cells with optimized biomass yield therefore provides a direct correspondence between model predictions and evolutionary outcomes, allowing in silico design in metabolic engineering (38).
Our selection scheme for microbial biomass yield is similar to compartmentalized self-replication (CSR), a technique used for the directed evolution of DNA polymerases (39). Polymerases self-replicate their encoding DNA and can be enriched through iterative cycles of compartmentalization following dilution. These two approaches, which are based on polydisperse emulsion samples (droplets vary in diameter), have the advantage that sample preparation is straightforward. By mixing aqueous samples together with a suitable surfactant-containing oil phase, millions of compartments are generated in minutes, not requiring any specialized equipment. Although monodisperse emulsion samples (uniform droplet size) produced with microfluidic droplet generators (40) allow for a high degree of control in encapsulating single cells in droplets (41), the additional technological complexity as well as the difficulty in handling a multitude of samples in parallel in our opinion outweighs the benefit of a likely only moderate acceleration of this passive selection process. However, future advances in microfluidic droplet sorting (42), for example, sorting based on optical density of droplet contents, could well result in experimental protocols capable of directly selecting cells with a high-yield phenotype and therefore greatly facilitate such evolution campaigns.
In conclusion, our results experimentally confirmed theoretical predictions that selection of increased biomass yield is possible with a protocol that selects for an increased number of offspring through the compartmentalization of cells. Moreover, we demonstrated how the yield/rate tradeoff affects the evolutionary outcome in structured and unstructured environments. The evolutionary solution of walking along a yield/rate Pareto front through alterations in sugar uptake systems is intriguingly simple.
Materials and Methods
Strains and Media.
L. lactis MG1363 (43) and derivatives were used throughout this study. Experiments were carried out at 30 °C in a chemically defined medium (CDM) (44), which was supplemented with 5 mM glucose (GCDM) with the exception of the competition experiment of NZ9000 and NZ9010 (proof-of-principle experiment) where 25 mM glucose was added. Bacterial plating was done on the rich medium M17 (Oxoid) supplemented with 25 mM glucose (GM17).
Propagation in Emulsion.
Three hundred microliters of a freshly inoculated L. lactis culture was used to make an emulsion by shaking it for 3–4 min with 700 µL HFE7500 (3M Novec) on a vortex mixer. The oil was supplemented with 0.5% vol/vol surfactant. Shaking was carried out at 2,200–3,200 rpm in capped 10-mL tubes (Greiner Bio-One; 164161). After shaking an emulsion separated in the tube from the surplus of oil within a few minutes and 650 µL of the oil phase was removed from the bottom of the tube. Cells were allowed to grow in emulsion at 30 °C for 1 or 2 d and subsequently the emulsion was broken. The breaking was done by adding 300 µL of 1H,1H,2H,2H-perfluorooctanol (Alfa Aesar) and frequent light shaking of the tube over a period of up to 15 min. This resulted in the separation of an oil and a water phase. After the separation of the two phases 700 µL of GCDM was added and 900 µL of medium with the bacterial cells was transferred to a sterile tube. The number of bacteria in this solution was then determined using a Coulter Counter (Beckman-Coulter). Subsequently, the cells were diluted in GCDM to give a concentration of 2 × 106 cells per milliliter and a new emulsion was prepared as described. This process was repeated serially for up to 31 times. Throughout the experiments, stock solutions of cells from broken emulsions supplemented with 15% (vol/vol) glycerol were prepared and stored at −80 °C in regular intervals.
Isolation of Strains with Increased Final Optical Density.
The serial propagation of mutagenized L. lactis MG1363 in emulsion was carried out in duplicate. After 22 propagation steps, dilutions from each broken emulsion were prepared and plated on GM17. A total of 44 single colonies from each propagated emulsion were isolated and transferred to a 96-well microplate filled with 200 µL GCDM. After growth for 2–3 h, these cultures were propagated in fourfold into a 384-well microplate filled with 100 µL of GCDM. Cells in the original 96-well plate were allowed to grow for 20–24 h, and subsequently glycerol was added to each well to a final concentration of 15% (vol/vol) upon which the plate was frozen at −80 °C. The inoculated 384-well microplate was placed in a microplate reader and the optical densities at 600 nm were recorded for each well every 10 min for 48 h.
Supplementary Material
Acknowledgments
The authors thank Jim Bull for critically reading the manuscript. H.B. was funded by Technologiestichting STW Project 10619. F.B.d.S. is supported by the Netherlands Organisation for Scientific Research by VENI Grant 863.11.019. This work took part in the Kluyver Centre for Genomics of Industrial Fermentation and the Netherlands Consortium for Systems Biology, both funded by the Netherlands Genomics Initiative (NGI).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Sequence reads have been deposited in the NCBI Sequence Read Archive (SRA), www.ncbi.nlm.nih.gov/sra (accession nos. SRP017852 and SRS385784).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308523110/-/DCSupplemental.
References
- 1.Beldade P, Koops K, Brakefield PM. Developmental constraints versus flexibility in morphological evolution. Nature. 2002;416(6883):844–847. doi: 10.1038/416844a. [DOI] [PubMed] [Google Scholar]
- 2.Velicer GJ, Lenski RE. Evolutionary trade-offs under conditions of resource abundance and scarcity: Experiments with bacteria. Ecology. 1999;80:1168. [Google Scholar]
- 3.Frankino WA, Zwaan BJ, Stern DL, Brakefield PM. Natural selection and developmental constraints in the evolution of allometries. Science. 2005;307(5710):718–720. doi: 10.1126/science.1105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzsimmons JM, Schoustra SE, Kerr JT, Kassen R. Population consequences of mutational events: Effects of antibiotic resistance on the r/K trade-off. Evol Ecol. 2009;24:227–236. [Google Scholar]
- 5. Jasmin JN, Dillon MM, Zeyl C (2012) The yield of experimental yeast populations declines during selection. Proc Biol Sci 279(1746):4382–4388. [DOI] [PMC free article] [PubMed]
- 6.Novak M, Pfeiffer T, Lenski RE, Sauer U, Bonhoeffer S. Experimental tests for an evolutionary trade-off between growth rate and yield in E. coli. Am Nat. 2006;168(2):242–251. doi: 10.1086/506527. [DOI] [PubMed] [Google Scholar]
- 7.Bennett AF, Lenski RE. An experimental test of evolutionary trade-offs during temperature adaptation. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8649–8654. doi: 10.1073/pnas.0702117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper TF, Lenski RE. Experimental evolution with E. coli in diverse resource environments. I. Fluctuating environments promote divergence of replicate populations. BMC Evol Biol. 2010;10:11. doi: 10.1186/1471-2148-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 10.MacLean RC. The tragedy of the commons in microbial populations: insights from theoretical, comparative and experimental studies. Heredity (Edinb) 2008;100(3):233–239. doi: 10.1038/sj.hdy.6801073. [DOI] [PubMed] [Google Scholar]
- 11.Molenaar D, van Berlo R, de Ridder D, Teusink B. Shifts in growth strategies reflect tradeoffs in cellular economics. Mol Syst Biol. 2009;5:323. doi: 10.1038/msb.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science. 1968;162(3859):1243–1248. [PubMed] [Google Scholar]
- 13.Kreft JU. Biofilms promote altruism. Microbiology. 2004;150(Pt 8):2751–2760. doi: 10.1099/mic.0.26829-0. [DOI] [PubMed] [Google Scholar]
- 14.Nowak MA, May RM. Evolutionary games and spatial chaos. Nature. 1992;359:826–829. [Google Scholar]
- 15.Hauert C, De Monte S, Hofbauer J, Sigmund K. Volunteering as Red Queen mechanism for cooperation in public goods games. Science. 2002;296(5570):1129–1132. doi: 10.1126/science.1070582. [DOI] [PubMed] [Google Scholar]
- 16.Luckinbill LS. r and K selection in experimental populations of Escherichia coli. Science. 1978;202(4373):1201–1203. doi: 10.1126/science.202.4373.1201. [DOI] [PubMed] [Google Scholar]
- 17.Brown CJ, Todd KM, Rosenzweig RF. Multiple duplications of yeast hexose transport genes in response to selection in a glucose-limited environment. Mol Biol Evol. 1998;15(8):931–942. doi: 10.1093/oxfordjournals.molbev.a026009. [DOI] [PubMed] [Google Scholar]
- 18.Castro R, et al. Characterization of the individual glucose uptake systems of Lactococcus lactis: Mannose-PTS, cellobiose-PTS and the novel GlcU permease. Mol Microbiol. 2009;71(3):795–806. doi: 10.1111/j.1365-2958.2008.06564.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar P, et al. Engineering Lactococcus lactis for production of mannitol: High yields from food-grade strains deficient in lactate dehydrogenase and the mannitol transport system. Appl Environ Microbiol. 2004;70(3):1466–1474. doi: 10.1128/AEM.70.3.1466-1474.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodd HM, Horn N, Gasson MJ. Characterization of IS905, a new multicopy insertion sequence identified in lactococci. J Bacteriol. 1994;176(11):3393–3396. doi: 10.1128/jb.176.11.3393-3396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bongers RS, et al. IS981-mediated adaptive evolution recovers lactate production by ldhB transcription activation in a lactate dehydrogenase-deficient strain of Lactococcus lactis. J Bacteriol. 2003;185(15):4499–4507. doi: 10.1128/JB.185.15.4499-4507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynne-Edwards VC Intergroup selection in the evolution of social systems. Nature. 1963;200:623–626. [Google Scholar]
- 23.Maynard-Smith J. Group selection and kin selection. Nature. 1964;201:1145–1147. [Google Scholar]
- 24.Eldakar OT, Wilson DS. Eight criticisms not to make about group selection. Evolution. 2011;65(6):1523–1526. doi: 10.1111/j.1558-5646.2011.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pianka ER. On r- and K-selection. Am Nat. 1970;104:592. [Google Scholar]
- 26.Luckinbill L. Selection and the r / K continuum in experimental populations of protozoa. Am Nat. 1979;113:427–437. [Google Scholar]
- 27.Nahum JR, Harding BN, Kerr B. Evolution of restraint in a structured rock-paper-scissors community. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10831–10838. doi: 10.1073/pnas.1100296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harcombe W. Novel cooperation experimentally evolved between species. Evolution. 2010;64(7):2166–2172. doi: 10.1111/j.1558-5646.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 29.Kerr B, Neuhauser C, Bohannan BJM, Dean AM. Local migration promotes competitive restraint in a host-pathogen ‘tragedy of the commons’. Nature. 2006;442(7098):75–78. doi: 10.1038/nature04864. [DOI] [PubMed] [Google Scholar]
- 30.Eshelman CM, et al. Unrestricted migration favours virulent pathogens in experimental metapopulations: Evolutionary genetics of a rapacious life history. Philos Trans R Soc Lond B Biol Sci. 2010;365(1552):2503–2513. doi: 10.1098/rstb.2010.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachmann H, Molenaar D, Kleerebezem M, van Hylckama Vlieg JET. High local substrate availability stabilizes a cooperative trait. ISME J. 2011;5(5):929–932. doi: 10.1038/ismej.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann H, Starrenburg MJC, Molenaar D, Kleerebezem M, van Hylckama Vlieg JET. Microbial domestication signatures of Lactococcus lactis can be reproduced by experimental evolution. Genome Res. 2012;22(1):115–124. doi: 10.1101/gr.121285.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gore J, Youk H, van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459(7244):253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430(7003):1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 35.Eiteman MA, Altman E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 2006;24(11):530–536. doi: 10.1016/j.tibtech.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 36.van Dijken JP, Weusthuis RA, Pronk JT. Kinetics of growth and sugar consumption in yeasts. Antonie van Leeuwenhoek. 1993;63(3-4):343–352. doi: 10.1007/BF00871229. [DOI] [PubMed] [Google Scholar]
- 37.Schuster S, Pfeiffer T, Fell DA. Is maximization of molar yield in metabolic networks favoured by evolution? J Theor Biol. 2008;252(3):497–504. doi: 10.1016/j.jtbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Pharkya P, Burgard AP, Maranas CD. Exploring the overproduction of amino acids using the bilevel optimization framework OptKnock. Biotechnol Bioeng. 2003;84(7):887–899. doi: 10.1002/bit.10857. [DOI] [PubMed] [Google Scholar]
- 39.Ghadessy FJ, Ong JL, Holliger P. Directed evolution of polymerase function by compartmentalized self-replication. Proc Natl Acad Sci USA. 2001;98(8):4552–4557. doi: 10.1073/pnas.071052198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theberge AB, et al. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew Chem Int Ed Engl. 2010;49(34):5846–5868. doi: 10.1002/anie.200906653. [DOI] [PubMed] [Google Scholar]
- 41.Huebner A, et al. Development of quantitative cell-based enzyme assays in microdroplets. Anal Chem. 2008;80(10):3890–3896. doi: 10.1021/ac800338z. [DOI] [PubMed] [Google Scholar]
- 42.Agresti JJ, et al. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc Natl Acad Sci USA. 2010;107(9):4004–4009. doi: 10.1073/pnas.0910781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wegmann U, et al. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189(8):3256–3270. doi: 10.1128/JB.01768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goel A, Santos F, Vos WM, Teusink B, Molenaar D. Standardized assay medium to measure Lactococcus lactis enzyme activities while mimicking intracellular conditions. Appl Environ Microbiol. 2012;78(1):134–143. doi: 10.1128/AEM.05276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenski RE, Travisano M. Dynamics of adaptation and diversification: A 10,000-generation experiment with bacterial populations. Proc Natl Acad Sci USA. 1994;91(15):6808–6814. doi: 10.1073/pnas.91.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.