Abstract
Misfolded MHC class I heavy chains (MHC I HCs) are targeted for endoplasmic reticulum (ER)-associated degradation (ERAD) by the ubiquitin E3 ligase HRD1, and E2 ubiquitin conjugating enzyme UBE2J1, and represent one of the few known endogenous ERAD substrates. The mechanism by which misfolded proteins are dislocated across the ER membrane into the cytosol is unclear. Here, we investigate the requirements for MHC I ubiquitination and degradation and show that endogenous misfolded MHC I HCs are recognized in the ER lumen by EDEM1 in a glycan-dependent manner and targeted to the core SEL1L/HRD1/UBE2J1 complex. A soluble MHC I HC lacking its transmembrane domain and cytosolic tail uses the same ERAD components and is degraded as efficiently as wild-type MHC I. Unexpectedly, HRD1-dependent polyubiquitination is preferentially targeted to the ER luminal domain of full-length MHC I HCs, despite the presence of an exposed cytosolic C-terminal tail. MHC I luminal domain ubiquitination occurs before p97 ATPase-mediated extraction from the ER membrane and can be targeted to nonlysine, as well as lysine, residues. A subset of integral membrane proteins, therefore, requires an early dislocation event to expose part of their luminal domain to the cytosol, before HRD1-mediated polyubiquitination and dislocation.
The assembly and regulated expression of plasma membrane and secreted proteins is fundamentally reliant on effective endoplasmic reticulum quality control (ERQC). Endoplasmic reticulum (ER)-associated degradation (ERAD) is central to ERQC, selectively disposing of misfolded or surplus proteins to maintain ER homeostasis. ERAD involves recognition, ubiquitination, and retrotranslocation of substrates from the ER to the cytosol for proteasome-mediated degradation (1). Polyubiquitination of the substrate is critical, both for facilitating ER membrane extraction by the p97 ATPase-ubiquitin fusion degradation 1 (Ufd1)-nuclear protein localization 4 (Npl4) complex (2–4) and for delivery to the proteasome. However, the precise role of ubiquitin in the actual dislocation event is unclear.
The cellular ERAD machinery consists of multiprotein complexes, typically comprising a membrane-embedded ubiquitin E3 ligase, which engages substrates directly or via ER luminal adaptors (1). Different substrates degraded by the same E3 may use distinct ERAD cofactors that facilitate substrate delivery to the ligase and E2-conjugating enzyme for ubiquitination.
Attempts to delineate distinct degradation pathways according to the site of the defect in a misfolded protein have been made in yeast. Proteins with cytosolic defects (ERAD-C) require Doa10p, whereas proteins with transmembrane (ERAD-M) or luminal (ERAD-L) lesions use Hrd1p (5, 6). The expanded repertoire of ERAD E3s in mammalian cells makes developing analogous rules more challenging. 3-hydroxy-3-methylglutaryl-CoA reductase degradation protein 1 (HRD1) and its homolog gp78/autocrine motility factor receptor (AMFR) are the best-characterized mammalian ERAD ligases. The association of HRD1 with SEL1L in the ER membrane forms the core of a multimeric complex that interacts with ER luminal adaptors such as osteosarcoma amplified 9 (OS9), XTP3-B, endoplasmic reticulum degradation-enhancing α-mannosidase-like protein 1 (EDEM1), and p97 in the cytosol (7, 8). Because HRD1 substrates encompass soluble luminal, single-pass transmembrane, and polytopic proteins, a key mechanistic question is how substrates of different conformations are recognized and retrotranslocated across the lipid bilayer. This remains difficult to address in the absence of robust in vitro dislocation assays. However, identifying the ERAD cofactors and critical substrate characteristics, together with the target sites for ubiquitination, may provide some clues.
Although most studies have focused on a limited number of exogenously expressed model substrates, several endogenous ERAD substrates were described recently (9–11). We identified MHC class I heavy chain (MHC I HC) as an endogenous HRD1 substrate (10). This type I transmembrane glycoprotein assembles with β2-microglobulin (β2m) and its peptide ligand in the ER lumen and then traffics to the cell surface for display to cytotoxic T cells. Under physiological conditions, the failure of MHC I HCs to associate with β2m, or acquire peptide, leads to incorrect folding, recognition by the ERAD machinery, ubiquitination, and HRD1-mediated retrotranslocation to the cytosol (10, 12). This process is exaggerated in the absence of β2m (10, 12). HRD1 and the membrane-anchored E2 UBE2J1 are absolutely required for MHC I ubiquitination and retrotranslocation, and, in their absence, glycosylated MHC I HCs accumulate in the ER membrane (10). MHC I HC is, therefore, an endogenous ERAD substrate for which both the E2 and E3 enzyme are defined, providing a unique system to investigate the mechanism of HRD1-mediated dislocation of integral membrane proteins.
Here, we show that before p97-mediated extraction from the ER membrane, HRD1-dependent ubiquitination of MHC I HCs is preferentially targeted to residues in the ER luminal domain. This occurs despite the presence of exposed cytosolic lysine residues. We propose that a subset of integral membrane proteins require an early dislocation event to expose part of their luminal domain to the cytosol before HRD1-mediated polyubiquitination and retrotranslocation.
Results
Degradation of Soluble and Tailless MHC I HCs Is as Efficient as Wild-Type MHC I and Requires the Same ERAD Components.
To identify the requirements for HRD1-mediated dislocation, we expressed a series of N-terminal GFP-tagged MHC I HC mutants (GFP-HLA-A2) in β2m-deficient HeLa cells, thus targeting MHC I for ERAD (10). The MHC I cytosolic tail contains three lysine residues, of which the two most proximal are conserved across all MHC I alleles. These residues would be accessible to HRD1 and UBE2J1 and we predicted would be the most likely site of ubiquitination. Despite substituting all three lysine residues, GFP-HLA-A2 K335R/K340A/K364R was rescued similarly to wild-type GFP-HLA-A2 following siRNA-mediated depletion of HRD1 or UBE2J1 (Fig. S1). Furthermore, depletion of HRD1, UBE2J1, or SEL1L in cells expressing tailless or soluble (lacking the tail and transmembrane domain) GFP-HLA-A2 gave a similar gain in fluorescent signal to wild-type GFP-HLA-A2 (Fig. 1A). These effects were specific, as untagged tailless HLA-B51 and soluble HLA-A2 accumulated on depletion of HRD1, SEL1L, or UBE2J1 but not on depletion of other ERAD E3 ligases or E2 enzymes (Fig. 1 B and C and Fig. S2).
Fig. 1.
Degradation of soluble and tailless MHC I HC is as efficient as wild-type MHC I and requires the same ERAD components. (A–C) Degradation of wild-type, tailless, and soluble MHC I HC requires HRD1, SEL1L, and UBE2J1. (A) Cytofluorometric analysis of GFP levels in siRNA-treated (black line) vs. control (shaded) HeLa cells expressing wild-type, tailless, or soluble GFP-HLA-A2 plus β2m shRNA. (B and C) Immunoblot for MHC I on siRNA depletion of HRD1(H1), gp78, SEL1L(S1L), UBE2J1(J1), UBE2J2(J2), or UBE2G2(G2) in β2m-depleted HeLa cells expressing tailless HLA-B51 or soluble HLA-A2. (D) Soluble MHC I HC dislocation is HRD1- and SEL1L-dependent. β2m siRNA-treated cells expressing soluble HLA-A2 were depleted of SEL1L or HRD1 and pulse-labeled for 10 min, and MHC I HCs were immunoprecipitated from lysates at the indicated times. The graph represents mean plus SEM (vertical lines) from two independent experiments. (E) Degradation of wild-type and soluble MHC I HC involves OS9 and XTP3-B. GFP levels in HeLa wild-type or soluble GFP-HLA-A2 shβ2m cells treated with OS9 and/or XTP3-B siRNA (black line) vs. control siRNA (shaded). Bars represent means plus 95% confidence intervals (CI) (black lines) from three independent experiments.
To compare the rate of degradation of soluble MHC I HC with wild-type, β2m-depleted cells expressing soluble HLA-A2 were radiolabeled with [35S]methionine/cysteine, and MHC I HCs were immunoprecipitated from detergent lysates at the indicated times. Soluble HCs were degraded as efficiently as full-length MHC I, and both were effectively stabilized in the absence of either HRD1 or SEL1L (Fig. 1D). These data suggest the MHC I transmembrane domain and cytosolic tail are dispensable for retrotranslocation by SEL1L, HRD1, and UBE2J1.
Our finding that dependence on HRD1 and SEL1L is equivalent for degradation of soluble and wild-type (membrane-bound) MHC I HCs contrasts with a previous report that HRD1 and SEL1L, plus ER lectins OS9 and XTP3-B, are absolutely required for degradation of soluble but not membrane-anchored, ERAD substrates (13). We, therefore, asked whether OS9 and XTP3-B are involved in degradation of both wild-type and soluble MHC I HC. In β2m-depleted cells expressing wild-type or soluble GFP-HLA-A2, only combined depletion of OS9 and XTP3-B significantly rescued the GFP signal, suggesting involvement but partial redundance of these lectins in targeting MHC I for ERAD (Fig. 1E and Fig. S3). Importantly, the dependence on OS9 and XTP3-B was comparable for soluble and membrane-bound MHC I HCs.
In summary, soluble and full-length MHC I HCs are degraded with equivalent efficiency using the same ERAD components, implying that they may use similar mechanisms of recognition and retrotranslocation.
The MHC I Glycan Is Required for EDEM1-Dependent HC Degradation.
The lack of requirement for the MHC I HC transmembrane domain and cytosolic tail for HRD1-mediated dislocation implies that all of the determinants for ERAD recognition lie within the MHC I luminal domain. Substrate N-linked glycans are pivotal for ERAD regulation and progressive trimming of terminal mannose residues from maturing glycoproteins allows recognition by ERAD adaptors OS9 and XTP3-B (8).
MHC I has a single glycosylation site within its α1 domain and associates with calnexin before β2m binding. To determine whether the glycan is required for MHC I degradation, we mutated the serine in the HLA-A2 glycosylation site to arginine to generate a nonglycosylated MHC I HC (HLA-A2 S88R) (Fig. S4 A and B). HLA-A2 S88R (faster migrating band) was degraded at a similar rate to endogenous MHC I (upper two bands) and was similarly stabilized in the absence of HRD1 (Fig. 2A). To avoid potential confusion with deglycosylated endogenous MHC I on SDS/PAGE analysis, we generated a soluble form of HLA-A2 S88R. Soluble HLA-A2 S88R accumulated on siRNA depletion of either HRD1 or SEL1L in β2m-depleted cells (Fig. 2B), indicating that the MHC I glycan is not required for HRD1/SEL1L mediated dislocation.
Fig. 2.
The MHC I glycan is required for EDEM1-dependent HC degradation. (A and B) Nonglycosylated MHC I is degraded via HRD1 and SEL1L. (A) β2m siRNA-treated HeLa cells expressing HLA-A2 S88R were depleted of HRD1 or gp78 and pulse-labeled for 10 min, and MHC I HCs were immunoprecipitated at the indicated times. Graphs represent mean plus SEM (vertical lines) from two independent experiments. (B) MHC I immunoblot in β2m-depleted cells expressing soluble HLA-A2 S88R treated with indicated siRNA. (C and D) EDEM1 is required for degradation of glycosylated, endogenous MHC I. (C) GFP levels in HeLa GFP-HLA-A2wt shβ2m cells treated with EDEM1 or HRD1 siRNA (black line) vs. control siRNA (shaded). (D) β2m-depleted HeLa cells treated with EDEM1 or control siRNA were treated as in A. The graph represents mean plus SEM (vertical lines) from three independent experiments. (E and F) Degradation of nonglycosylated MHC I is EDEM1-independent but requires Herp. (E) MHC I immunoblot in β2m-depleted cells coexpressing soluble HLA-A2 and HLA-A2-S88R treated with indicated siRNA. (F) GFP levels in β2m-depleted cells expressing GFP-HLA-A2wt, S88R, or soluble S88R, treated with EDEM1 or Herp siRNA (black line) vs. control (shaded). Bars represent mean plus 95% CI (black lines) from three independent experiments.
Although both glycosylated and nonglycosylated MHC I HCs are targeted to HRD1/SEL1L, we predicted the glycan would regulate degradation of endogenous, glycosylated MHC I HCs upstream of the ligase complex. EDEM1 has been implicated in delivery of misfolded glycoproteins to SEL1L and is a putative ER mannosidase (7). Depletion of EDEM1 from β2m-depleted cells rescued GFP-HLA-A2 levels (Fig. 2C and Fig. S2D) and inhibited degradation of endogenous MHC I on pulse–chase analysis (Fig. 2D), indicating a requirement for EDEM1 for endogenous MHC I degradation. EDEM1 depletion rescued only glycosylated HLA-A2 and not HLA-A2 S88R following coexpression of soluble forms of both proteins (Fig. 2E). The N-linked glycan is, therefore, essential for EDEM1-dependent MHC I ERAD. Stabilization of endogenous MHC I was less effective following EDEM1 depletion than SEL1L or HRD1 depletion (Fig. 2D vs. Fig. 1D), which could reflect incomplete EDEM1 siRNA knockdown. Alternatively, some MHC I HCs may use additional recruitment factors or directly engage SEL1L, because nonglycosylated MHC I HCs are still degraded via HRD1/SEL1L despite being unable to use the EDEM1-dependent degradation pathway. Little is known about degradation pathways for nonglycosylated ER proteins, although Herp, an integral ER membrane protein that associates with HRD1, is implicated in the degradation of nonglycosylated BiP substrates (14). RNAi-mediated Herp depletion rescued the GFP signal in β2m-deficient cells expressing full-length or soluble GFP-HLA-A2 S88R, with no effect in cells expressing wild-type GFP-HLA-A2, whereas the converse was true for EDEM1 depletion, supporting the existence of a distinct Herp-dependent degradation pathway for nonglycosylated MHC I (Fig. 2F and Fig. S2C).
Full-Length MHC I HC Is Preferentially Ubiquitinated on ER Luminal Residues.
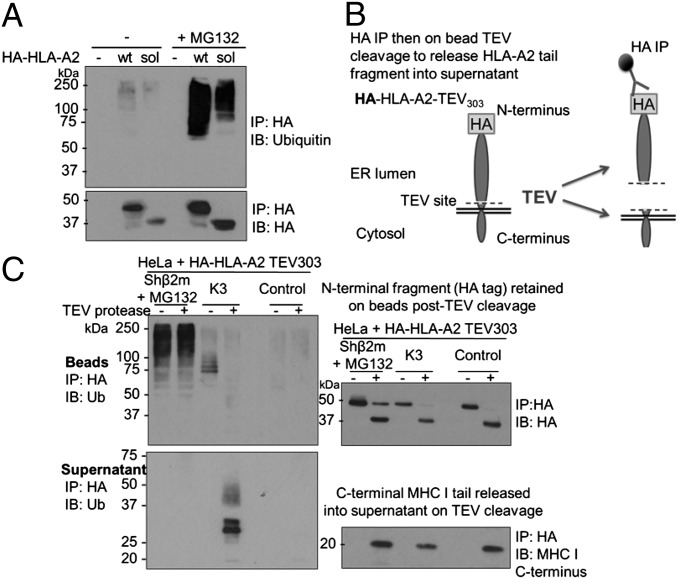
Ubiquitination of endogenous non–β2m-bound MHC I HC requires HRD1 and UBE2J1 and can be visualized on proteasome inhibition (10). To determine whether soluble MHC I HCs are ubiquitinated, HA-tagged soluble and wild-type HLA-A2 were immunoprecipitated from β2m-depleted cells and probed for ubiquitin (Fig. 3A). Proteasome inhibition revealed robust ubiquitination of soluble MHC I HCs demonstrating ubiquitination can be targeted to residues within the MHC I luminal domain (the HA tag contains no ubiquitinatable residues).
Fig. 3.
Full-length MHC I HC is preferentially ubiquitinated on ER luminal residues. (A) Soluble MHC I HC is ubiquitinated in β2m-depleted cells. HeLa shβ2m cells expressing wild-type (wt) or soluble (sol) HA-HLA-A2 were incubated with or without 50 μM MG132 for 5 h, before SDS lysis, immunoprecipitation with anti-HA, and immunoblot for polyubiquitin and HA. (B and C) Full-length MHC I HC is preferentially ubiquitinated on ER luminal residues in β2m-depleted cells. Lysates from HeLa cells expressing HA-HLA-A2 TEV303 with β2m shRNA (MG132-treated), K3 viral E3 ligase or alone (control), were immunoprecipitated with anti–HA-agarose beads. On-bead incubation with TEV protease released the MHC I C terminus into the supernatant, and the residual HA-tagged N-terminal (luminal) fragment was eluted from the beads. Bead and supernatant fractions were probed for ubiquitin, HA, and MHC I C terminus (R.A3e7).
Because substrates of the HRD1 ligase encompass soluble and integral membrane proteins, HRD1 may have the flexibility to alter the target site for ubiquitination as substrate conformation changes. However, soluble MHC I must be ubiquitinated on luminal residues and, compared with wild-type MHC I HCs, showed equivalent degradation rates and requirements for OS9, XTP3-B, SEL1L, and HRD1, suggesting a similar dislocation process. This prompted us to question whether the luminal domain of full-length MHC I HCs is the preferred site for ubiquitination, despite the presence of exposed cytosolic lysine residues.
We used mass spectrometry to determine where full-length MHC I HCs are preferentially ubiquitinated. MHC I HCs were immunoprecipitated from lysates of β2m-depleted HeLa cells treated with a proteasome inhibitor. After trypsinization and reverse-phase liquid chromatography, the resulting peptides were analyzed by mass spectrometry. We achieved 64% coverage of the HLA-A heavy chain and identified two ubiquitin(diglycine)-modified lysine residues at positions 200 and 267 of the MHC I HC, located in the α2 and α3 domain, respectively (Fig. S5). In addition, after subcellular fractionation, MHC I HCs with diglycine-modification of lysine 200 were detected in both membrane and soluble fractions (Fig. S5, experiment 2). Peptides containing two of the three lysine residues from the MHC I (HLA-A) cytosolic tail were isolated, neither of which were modified with ubiquitin. However, because no peptides containing the membrane proximal lysine residue from the stop-transfer region were isolated, likely because of the hydrophobic nature of this perimembrane region, we could not exclude ubiquitin modifications on this conserved lysine residue. We, therefore, inserted a tobacco etch virus (TEV) protease cleavage site into HA-tagged HLA-A2 between the α3 domain and the transmembrane domain (HA-HLA-A2 TEV303). HA-HLA-A2 TEV303 assembles with β2m and peptide and is expressed at the cell surface in wild-type cells (Fig. S4C). HA-HLA-A2 TEV303 was isolated from cell lysates using anti-HA antibody-conjugated beads, which were then incubated with TEV protease to release the ∼15-kDa HLA-A2 transmembrane domain and cytosolic tail into the supernatant (Fig. 3 B and C). Bead (Fig. 3C, Upper Left and Upper Right) and supernatant fractions (Fig. 3C, Lower Left and Lower Right) were separated by SDS/PAGE and probed for ubiquitin. In β2m-depleted cells treated with MG132, the polyubiquitin signal was entirely retained on the MHC I N-terminal (luminal) domain despite cleavage of at least 80% of the MHC I tail (Fig. 3C, Upper Right), and no ubiquitin was detected on the tail fragment (Fig. 3C, Lower Left, lanes 1 and 2). To ascertain that we could detect ubiquitinated MHC I species released into the supernatant, we examined cells expressing the viral E3 ligase K3, which attaches a polyubiquitin chain to lysine 340 in the MHC I cytosolic tail (15). In the presence of K3, TEV cleavage dramatically reduced ubiquitin signal on the MHC I N-terminal fragment and released the polyubiquitinated cytosolic tail into the supernatant (Fig. 3C, Lower Left, lanes 3 and 4). Therefore, a substantial proportion, if not all of the polyubiquitin chains attached to non–β2m-bound, full-length MHC I HCs targeted by HRD1 and UBE2J1 appear to be conjugated to residues within the MHC I luminal domain.
Ubiquitination of Luminal MHC I Residues Precedes p97-Mediated Extraction from the ER Membrane.
Following dislocation from the ER to the cytosol, the MHC I HC might be further modified by ubiquitinating enzymes. We, therefore, examined whether luminal ubiquitination of MHC I is an early event, preceding ER membrane extraction. Polyubiquitinated ERAD substrates are recognized by the p97 ATPase, which associates with its cofactors Ufd1 and Npl4 to drive ER membrane extraction (2–4). Following proteasome inhibition, deglycosylated, intact, MHC I HC species are visualized in the cytosol, implying the entire MHC I HC is fully extracted from the ER lipid bilayer before proteasome-mediated degradation (10, 12). To disrupt the p97 complex, we depleted cells of Ufd1 (Fig. S2E), which, in yeast, leads to accumulation of polyubiquitinated ERAD substrates in the ER membrane (3).
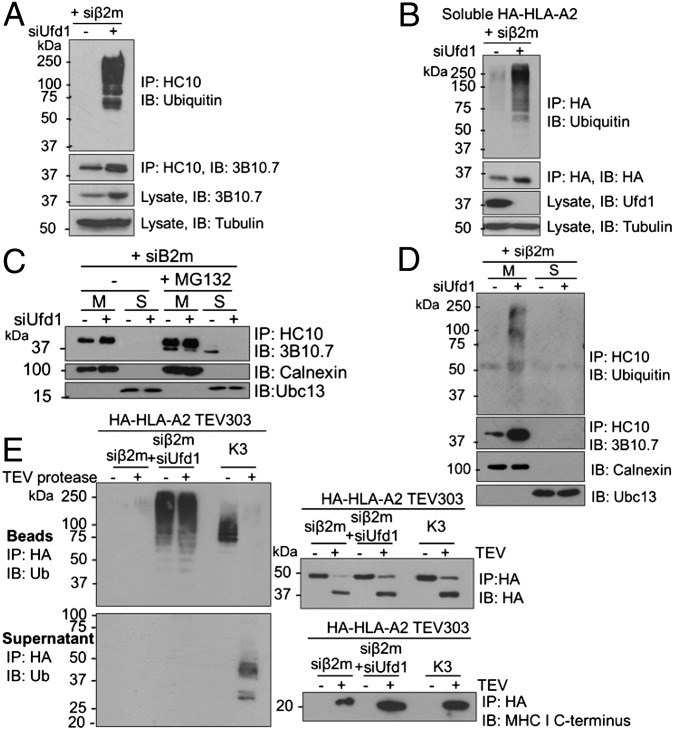
The loss of Ufd1 from β2m-depleted cells rescued endogenous MHC I levels, implying a role for Ufd1 in MHC I degradation (Fig. 4A). Strikingly, Ufd1 depletion allows visualization of polyubiquitinated full-length and soluble MHC I HC species in the absence of proteasome inhibition (Fig. 4 A and B and Fig. S6). Because HRD1 or UBE2J1 depletion prevents MHC I ubiquitination (10), Ufd1 must function downstream of HRD1 and UBE2J1 but before the proteasome. To ensure ubiquitinated MHC I HCs are retained in the ER membrane upon Ufd1 depletion, we immunoprecipitated MHC I HCs from membrane and soluble fractions of β2m-depleted cells. Ufd1 depletion prevented the appearance of the faster migrating (deglycosylated) dislocated MHC I HC in the soluble fraction (Fig. 4C) and stabilized polyubiquitinated MHC I in the membrane fraction (Fig. 4D), consistent with inhibition of p97 function.
Fig. 4.
Ubiquitination of luminal MHC I residues precedes p97-mediated extraction from the ER membrane. (A and B) Ubiquitinated full-length and soluble MHC I HCs accumulate in the absence of a proteasome inhibitor on depletion of Ufd1. (A) Lysates from β2m siRNA-treated HeLa cells, treated with Ufd1 or control siRNA were immunoprecipitated with HC10 (MHC I) and probed for MHC I and ubiquitin. Lysates were directly probed for MHC I and tubulin control. (B) β2m-depleted cells expressing soluble HA-HLA-A2 treated with Ufd1 or control siRNA were immunoprecipitated with anti-HA and probed for ubiquitin and HA. (C and D) Ubiquitinated MHC I accumulates in the ER membrane in the absence of Ufd1. (C) β2m-depleted HeLa cells were treated with or without 50 μM MG132 for 5 h and fractionated into membrane (M) and soluble fractions (S). MHC I HCs were immunoprecipitated from each fraction and visualized with 3B10.7. On depletion of Ufd1, MHC I HC is retained in the membrane with no soluble, deglycosylated MHC I detected. Lysates were probed for calnexin and Ubc13 (fractionation controls). (D) Cells treated as in C. Immunoprecipitates additionally probed for ubiquitin. (E) Polyubiquitinated full-length MHC I HC stabilized on depletion of Ufd1 is ubiquitinated on ER luminal residues. Lysates from HA-HLA-A2 TEV303-expressing HeLa cells treated with β2m siRNA plus Ufd1 or control siRNA, or expressing K3 viral E3 ligase, were immunoprecipitated with anti–HA-agarose. On-bead incubation with TEV protease released the MHC I C terminus into the supernatant, and the residual HA-tagged N-terminal (luminal) fragment was eluted from the beads. Bead and supernatant fractions were probed for ubiquitin, HA, and MHC I C terminus.
To localize the site of ubiquitin-modification of MHC I HCs stabilized in the absence of Ufd1, β2m-depleted cells expressing HA-HLA-A2 TEV303 were depleted of Ufd1. After release of the MHC I transmembrane domain and cytosolic tail with TEV protease, polyubiquitin was retained on the ER luminal domain (Fig. 4E). The ubiquitin signal was preserved when immunoprecipitates were denatured in SDS and reprecipitated with an anti-HA antibody, confirming direct ubiquitin conjugation to luminal MHC I residues (Fig. S7). Preferential ubiquitination on luminal residues, therefore, precedes extraction of MHC I HC into the cytosol. Because p97’s function is compromised in the absence of Ufd1, the ubiquitinated MHC I HC must be trapped in a partially dislocated state with at least part of the luminal domain exposed to the cytosol with an attached polyubiquitin chain. Our results imply that dislocation and polyubiquitination of the MHC I luminal domain are early events, upstream of p97 activity, mediated by the core HRD1/SEL1L/UBE2J1 complex targeting a membrane-bound MHC I HC.
HRD1- and UBE2J1-Dependent Ubiquitination of MHC I Can Be Targeted to Nonlysine Residues.
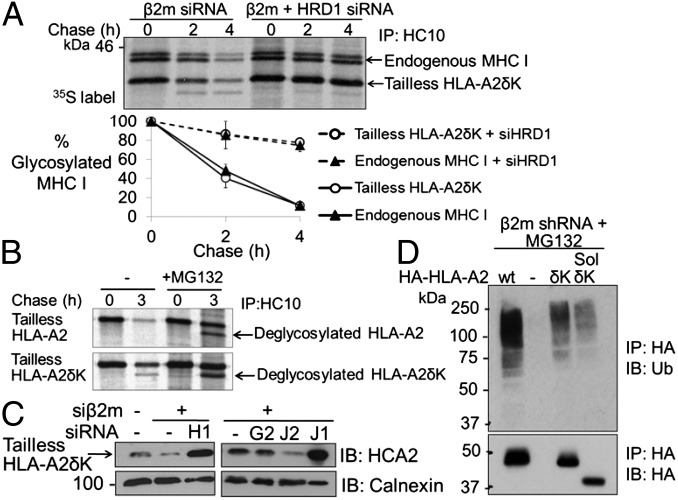
Having identified ubiquitin-modified lysine residues within the MHC I luminal domain by mass spectrometry (Fig. S5), we investigated whether lysine mutations in the MHC I HC would prevent its degradation. Unexpectedly, an HLA-A2 mutant with all lysine residues substituted to arginine (HLA-A2δK) was degraded in β2m-depleted cells. By pulse–chase analysis, tailless HLA-A2δK was dislocated as efficiently as endogenous MHC I (assessed by loss of the glycosylated form) and was stabilized on depletion of HRD1 (Fig. 5A). Unusually, at later time points, a faster migrating species representing dislocated, deglycosylated tailless HLA-A2δK (Fig. 5A and Fig. S4E) was visible. Unlike wild-type deglycosylated MHC I, which is only detected with a proteasome inhibitor, deglycosylated HLA-A2δK is readily detectable in untreated cells, although it accumulates on addition of MG132, suggesting it is ultimately degraded by the proteasome (Fig. 5B). Therefore, in the absence of lysine residues, the MHC I HC continues to be efficiently dislocated via HRD1, but proteasomal degradation is delayed, leading to stabilization of a deglycosylated intermediate not normally seen in the absence of proteasome inhibition.
Fig. 5.
HRD1- and UBE2J1-dependent ubiquitination of MHC I can be targeted to nonlysine residues. (A and B) Lysineless MHC I undergoes efficient HRD1-dependent dislocation but delayed proteasomal degradation. (A) β2m-depleted HeLa cells expressing tailless HLA-A2δK were treated with HRD1 siRNA and pulse-labeled, and MHC I HCs were immunoprecipitated from lysates at the indicated times. The graph represents mean plus SEM (vertical lines) from two independent experiments. (B) β2m-depleted cells expressing tailless HLA-A2 or HLA-A2δK were pulse-labeled for 10 min and chased in the presence or absence of 20 μM MG132. Lysates were immunoprecipitated for MHC I. Deglycosylated tailless HLA-A2δK is detected in the cytosol in the absence of proteasome inhibition. (C) Degradation of lysineless MHC I requires UBE2J1. Immunoblot for MHC I in β2m-depleted cells expressing tailless HLA-A2δK treated with HRD1(H1), UBE2J1(J1), UBE2J2(J2), or UBE2G2(G2) siRNA. (D) Lysineless MHC I HCs are ubiquitinated in β2m-depleted cells. MG132-treated HeLa shβ2m cells expressing HA-HLA-A2wt (wild type), HA-HLA-A2δK, and soluble (sol) HA-HLA-A2δK were immunoprecipitated with anti-HA and probed for ubiquitin and HA.
E2 enzymes are critical in determining the target residue for ubiquitination and, as with wild-type MHC I, depletion of UBE2J1, but not UBE2J2 or UBE2G2, rescued HLA-A2δK in β2m-depleted cells (Fig. 5C). HRD1 depletion may prevent dislocation through disruption of the retrotranslocation complex; however, the requirement for UBE2J1 implies that HLA-A2δK dislocation is ubiquitin-dependent. Furthermore, HLA-A2δK is itself polyubiquitinated (Fig. 5D and Fig. S8), suggesting that ubiquitin can be conjugated to nonlysine (cysteine, serine, or threonine) residues in the MHC I HC. The oxyester bond formed between serine/threonine residues and ubiquitin is sensitive to mild alkaline hydrolysis (16). We, therefore, incubated HA-HLA-A2δK with 0.1 M NaOH to release serine/threonine-conjugated polyubiquitin chains, followed by reprecipitation of HLA-A2δK. This treatment markedly reduced the ubiquitin signal from HLA-A2δK (Fig. S9 A and C), without substantially affecting MHC I ubiquitination in K3-expressing cells (Fig. S9B). Ubiquitination of wild-type HLA-A2 was moderately reduced after NaOH treatment, suggesting the presence of serine/threonine ubiquitin modifications (Fig. S9C). However, most of the ubiquitin signal was resistant to alkaline hydrolysis implying that ubiquitin is predominantly conjugated to lysine residues in wild-type MHC I HCs.
Taken together, these data indicate that HRD1/UBE2J1-mediated ubiquitination can be targeted to both lysine and nonlysine residues, although lysines are the dominant ubiquitin acceptor site. Whereas ubiquitination of nonlysine residues is sufficient for efficient HRD1-mediated dislocation, proteasomal degradation of deglycosylated HLA-A2δK is delayed (Fig. 5 A and B), suggesting differential handling by the cytosolic ERAD machinery.
Discussion
Our results suggest that endogenous misfolded MHC I HCs are recognized in the ER lumen by EDEM1 in a glycan-dependent manner and targeted to the core SEL1L/HRD1/UBE2J1 complex via OS9 and XTP3-B. Unexpectedly, polyubiquitination is preferentially targeted to luminal residues on full-length MHC I HC, and, in keeping with this, the MHC I transmembrane and cytosolic tail are dispensable for efficient HRD1-mediated degradation. How diverse misfolded proteins are dislocated across the ER membrane to the cytosol remains unclear. We envisaged the requirements for integral membrane proteins to be different from soluble proteins residing entirely within the ER lumen. Transmembrane proteins could access the E3 ligase via lateral diffusion in the membrane, leaving the exposed cytosolic domain accessible for ubiquitination, whereas soluble ERAD substrates must partially traverse the lipid bilayer before ubiquitination (1). However, our data imply that MHC I HC uses a similar mechanism of retrotranslocation to soluble ERAD substrates, with part of the luminal domain crossing the ER membrane to be exposed to the cytosol, either by leading with the N terminus or as a loop (17). The exposed luminal segment is then likely to be polyubiquitinated by HRD1 and UBE2J1 and trapped on the cytosolic side of the membrane, allowing subsequent extraction by the p97 ATPase.
We show that MHC I can be ubiquitinated on nonlysine residues, although luminal lysines are the dominant targets because the ubiquitin signal on wild-type HC is largely resistant to alkaline hydrolysis. The flexibility to ubiquitinate serine or threonine residues might benefit an ERAD ligase complex that handles diverse substrates where lysines may be inaccessible or absent. Although the ubiquitination and HRD1/UBE2J1-mediated dislocation of a lysineless MHC I HC was unexpected, it was not without precedent. Other HRD1 substrates, TCRα (18) and NS-1 Ig light chain (19), can be ubiquitinated on serine/threonine residues, although the requisite E2 enzymes were not identified. Intriguingly, murine UBE2J2, the closest homolog of UBE2J1, cooperates with murine γ-herpesvirus K3 ligase to ubiquitinate serine and lysine residues on murine MHC I HCs (20). As orthologs of yeast Ubc6p, shared features of UBE2J1 and J2 may allow them to conjugate ubiquitin to serine, threonine and possibly cysteine residues. UBE2J1 and J2 are therefore likely candidate E2s for ubiquitinating ERAD substrates on nonlysine residues.
MHC I HCs are targeted for ERAD by the human cytomegalovirus US2 and US11 proteins; however, rather than coopt the cellular MHC I ERAD pathway, the virus employs different E3 ligases within distinct dislocation complexes. US2-mediated MHC I dislocation requires TRC8 (21), whereas the E3 ligase for US11-mediated MHC I dislocation is not reported, but is neither HRD1 nor TRC8 (10). Our findings suggest the cellular mechanism of MHC I dislocation is different from that used by these viral proteins. In contrast to HRD1-mediated dislocation, the MHC I cytosolic tail is essential for US11-mediated dislocation and tailless HLA-A2 is stabilized in US11-expressing cells, despite the interaction with US11 being preserved (22). US11-mediated MHC I dislocation does not require lysine residues (23, 24), so ubiquitination may be targeted to nonlysine residues in the MHC I tail. Similar to HRD1-mediated MHC I dislocation, lysineless MHC I HCs are dislocated in US11-expressing cells with delayed kinetics of degradation (24). US2 interacts with the luminal domain of conformational, β2m-associated MHC I HC, but requires the MHC I transmembrane domain, and possibly tail residues, to mediate dislocation (22, 25). Mutation of cytosolic tail lysines results in a marked loss of US2-induced ubiquitination of membrane-associated MHC I HCs, implying that TRC8 preferentially targets lysine residues in the MHC I tail (26). The mutant HC is, however, still dislocated, suggesting US2 may use alternative mechanisms of dislocation in the absence of accessible cytosolic lysines (26).
The only integral membrane protein targeted by the cellular ERAD machinery for which sites of ubiquitination are defined is TCRα, a type I transmembrane glycoprotein targeted by both HRD1 and gp78, and ubiquitinated on its cytosolic tail (18, 27). In contrast to MHC I HC, in which the folding defect (degron) appears to be located within the luminal domain, unpaired charged residues in the transmembrane domain of TCRα are essential for ERAD (28), suggesting that location of the degron may be important for determining the site of ubiquitination and ligase involved.
Our finding that dislocation of full-length, endogenous MHC I HCs is strictly dependent on HRD1 and SEL1L, with no requirement for gp78, contrasts with a report that anchoring of soluble HRD1 substrates to the ER membrane allows additional targeting by gp78 (13). Deletion of the transmembrane region and tail from CD3δ and β-secretase 476, type I membrane proteins targeted by gp78, converted them to obligate HRD1/SEL1L substrates (13). The transmembrane region and cytosolic tail of CD3δ and β-secretase 476, but not MHC I, may, therefore, contain determinants which allow recognition and ubiquitination by gp78. This is consistent with the premise that specific features within either the transmembrane or cytosolic domain, rather than simply attachment to the ER membrane (13), determine the ligase complex, and potentially the site of ubiquitination, for membrane-bound ERAD substrates. Our data suggest that transmembrane glycoproteins that are exclusively degraded via HRD1 and SEL1L and contain a purely luminal degron use a similar mechanism of retrotranslocation to soluble ERAD substrates, with polyubiquitination targeted to the ER luminal domain. This is analogous to yeast ERAD-L substrates, which use the same dislocation complex regardless of whether they are soluble or membrane-bound (6). Future work may determine whether distinct substrate retrotranslocation pathways can be defined within the complex mammalian ERAD network.
Materials and Methods
Details of experimental procedures, constructs, and reagents can be found in SI Materials and Methods.
TEV Cleavage.
After immunoprecipitation of HA-HLA-A2 with anti-HA agarose, beads were washed and incubated with 0.8 μg/μL TEV protease in 0.1% Triton X-100/TEV buffer (50 mM Tris-HCl pH 7.4, 1 mM PMSF, 0.5 mM EDTA, 1 mM DTT) for 18 h at 4 °C. Supernatant was removed and beads washed in 0.1% Triton X-100/TEV buffer. Bead and supernatant samples were heated in reducing SDS sample buffer, separated by SDS/PAGE and analyzed by immunoblotting.
Supplementary Material
Acknowledgments
We thank all members of the P.J.L. laboratory. This work was supported by the Wellcome Trust and the National Institute for Health Research Cambridge Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303380110/-/DCSupplemental.
References
- 1.Hegde RS, Ploegh HL. Quality and quantity control at the endoplasmic reticulum. Curr Opin Cell Biol. 2010;22(4):437–446. doi: 10.1016/j.ceb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414(6864):652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 3.Jarosch E, et al. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol. 2002;4(2):134–139. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- 4.Bays NW, Wilhovsky SK, Goradia A, Hodgkiss-Harlow K, Hampton RY. HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol Biol Cell. 2001;12(12):4114–4128. doi: 10.1091/mbc.12.12.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vashist S, Ng DT. Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J Cell Biol. 2004;165(1):41–52. doi: 10.1083/jcb.200309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho P, Goder V, Rapoport TA. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell. 2006;126(2):361–373. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Cormier JH, Tamura T, Sunryd JC, Hebert DN. EDEM1 recognition and delivery of misfolded proteins to the SEL1L-containing ERAD complex. Mol Cell. 2009;34(5):627–633. doi: 10.1016/j.molcel.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosokawa N, Kamiya Y, Kato K. The role of MRH domain-containing lectins in ERAD. Glycobiology. 2010;20(6):651–660. doi: 10.1093/glycob/cwq013. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, et al. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. J Cell Biol. 2011;192(5):825–838. doi: 10.1083/jcb.201008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burr ML, et al. HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation. Proc Natl Acad Sci USA. 2011;108(5):2034–2039. doi: 10.1073/pnas.1016229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyler RE, et al. Unassembled CD147 is an endogenous endoplasmic reticulum-associated degradation substrate. Mol Biol Cell. 2012;23(24):4668–4678. doi: 10.1091/mbc.E12-06-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes EA, Hammond C, Cresswell P. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci USA. 1997;94(5):1896–1901. doi: 10.1073/pnas.94.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernasconi R, Galli C, Calanca V, Nakajima T, Molinari M. Stringent requirement for HRD1, SEL1L, and OS-9/XTP3-B for disposal of ERAD-LS substrates. J Cell Biol. 2010;188(2):223–235. doi: 10.1083/jcb.200910042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuda-Shimizu Y, Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require Herp. Mol Cell. 2007;28(4):544–554. doi: 10.1016/j.molcel.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt EW, et al. Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation. EMBO J. 2002;21(10):2418–2429. doi: 10.1093/emboj/21.10.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, et al. Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. J Cell Biol. 2007;177(4):613–624. doi: 10.1083/jcb.200611063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho P, Stanley AM, Rapoport TA. Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell. 2010;143(4):579–591. doi: 10.1016/j.cell.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikura S, Weissman AM, Bonifacino JS. Serine residues in the cytosolic tail of the T-cell antigen receptor alpha-chain mediate ubiquitination and endoplasmic reticulum-associated degradation of the unassembled protein. J Biol Chem. 2010;285(31):23916–23924. doi: 10.1074/jbc.M110.127936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu Y, Okuda-Shimizu Y, Hendershot LM. Ubiquitylation of an ERAD substrate occurs on multiple types of amino acids. Mol Cell. 2010;40(6):917–926. doi: 10.1016/j.molcel.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, et al. Ube2j2 ubiquitinates hydroxylated amino acids on ER-associated degradation substrates. J Cell Biol. 2009;187(5):655–668. doi: 10.1083/jcb.200908036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagg HR, et al. The TRC8 E3 ligase ubiquitinates MHC class I molecules before dislocation from the ER. J Cell Biol. 2009;186(5):685–692. doi: 10.1083/jcb.200906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Story CM, Furman MH, Ploegh HL. The cytosolic tail of class I MHC heavy chain is required for its dislocation by the human cytomegalovirus US2 and US11 gene products. Proc Natl Acad Sci USA. 1999;96(15):8516–8521. doi: 10.1073/pnas.96.15.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamu CE, Story CM, Rapoport TA, Ploegh HL. The pathway of US11-dependent degradation of MHC class I heavy chains involves a ubiquitin-conjugated intermediate. J Cell Biol. 1999;147(1):45–58. doi: 10.1083/jcb.147.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassink GC, Barel MT, Van Voorden SB, Kikkert M, Wiertz EJ. Ubiquitination of MHC class I heavy chains is essential for dislocation by human cytomegalovirus-encoded US2 but not US11. J Biol Chem. 2006;281(40):30063–30071. doi: 10.1074/jbc.M602248200. [DOI] [PubMed] [Google Scholar]
- 25.Barel MT, et al. Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation. J Immunol. 2003;171(12):6757–6765. doi: 10.4049/jimmunol.171.12.6757. [DOI] [PubMed] [Google Scholar]
- 26.Furman MH, Loureiro J, Ploegh HL, Tortorella D. Ubiquitinylation of the cytosolic domain of a type I membrane protein is not required to initiate its dislocation from the endoplasmic reticulum. J Biol Chem. 2003;278(37):34804–34811. doi: 10.1074/jbc.M300913200. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, et al. The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site. Proc Natl Acad Sci USA. 2006;103(2):341–346. doi: 10.1073/pnas.0506618103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonifacino JS, Cosson P, Klausner RD. Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell. 1990;63(3):503–513. doi: 10.1016/0092-8674(90)90447-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.