Abstract
Base excision repair (BER) removes at least 20,000 DNA lesions per human cell per day and is critical for the maintenance of genomic stability. We hypothesize that aberrant BER, resulting from mutations in BER genes, can lead to genomic instability and cancer. The first step in BER is catalyzed by DNA N-glycosylases. One of these, nth endonuclease III-like (NTH1), removes oxidized pyrimidines from DNA, including thymine glycol. The rs3087468 single nucleotide polymorphism of the NTH1 gene is a G-to-T base substitution that results in the NTH1 D239Y variant protein that occurs in ∼6.2% of the global population and is found in Europeans, Asians, and sub-Saharan Africans. In this study, we functionally characterize the effect of the D239Y variant expressed in immortal but nontransformed human and mouse mammary epithelial cells. We demonstrate that expression of the D239Y variant in cells also expressing wild-type NTH1 leads to genomic instability and cellular transformation as assessed by anchorage-independent growth, focus formation, invasion, and chromosomal aberrations. We also show that cells expressing the D239Y variant are sensitive to ionizing radiation and hydrogen peroxide and accumulate double strand breaks after treatment with these agents. The DNA damage response is also activated in D239Y-expressing cells. In combination, our data suggest that individuals possessing the D239Y variant are at risk for genomic instability and cancer.
Keywords: DNA repair, mutagenesis, MCF10A breast epithelial cells
The base excision repair (BER) pathway is responsible for the removal of at least 20,000 DNA lesions per cell per day (1), making it critical for the maintenance of genomic stability. The simplest and most common form of BER is short patch BER, which can be initiated by one of several different DNA glycosylases, each having preferences for specific types of lesions (2). Monofunctional DNA glycosylases recognize DNA lesions and catalyze the hydrolysis of the N-glycosylic bond to generate an abasic site. The abasic site is nicked at its 5′ side by the APE1 endonuclease, leaving a 3′OH and a 5′deoxyribose phosphate (dRP). DNA polymerase beta (pol β) fills in the single nucleotide gap and catalyzes removal of the dRP group. Bifunctional glycosylases, which usually recognize oxidative lesions, generate an abasic site and then catalyze its removal via β-elimination to generate a 3′dRP and 5′phosphate. APE1 then catalyzes removal of the 3′dRP, leaving a 3′OH, to which pol β can bind and fill in the resulting single nucleotide gap. In both cases, the XRCC1/Ligase IIIα or XRCC1/Ligase I complex catalyzes ligation of the resulting ends. An alternative BER pathway, that does not depend on APE1, is used when the NEIL glycosylases initiate repair (3). NEIL 1, 2, and perhaps 3 catalyze excision of the damaged base via β,δ elimination, leaving a 3′phosphate and a 5′phosphate. The 3′phosphate is removed by polynucleotide kinase, leaving a gap that is most often filled by pol β, followed by ligation.
NTH1 is one of four human bifunctional DNA glycosylases that recognize and remove oxidized pyrimidines and formamidopyrimidines (4). NTH1 is constitutively expressed and recognizes a fairly broad spectrum of oxidized pyrimidines, including 5-hydroxycytosine and thymine glycol (5–8). The rs3087468 single nucleotide polymorphism (SNP) of the NTH1 gene is a G-to-T base substitution that occurs in ∼6.2% of the global population and is found in Europeans, Asians, and sub-Saharan Africans (www.ncbi.nlm.nih.gov/SNP/). This nonsynonymous alteration results in substitution of a Tyr codon for an Asp codon (D239Y) within the active site of the protein. Approximately 0.6% of the global population is homozygous for this SNP.
In this study we tested the hypothesis that the D239Y human NTH1 (hNTH) variant has a functional phenotype that could drive carcinogenesis. We found that the D239Y variant is able to bind to oxidized pyrimidines, but is unable to excise damaged bases from DNA. Expression of the D239Y variant in both human and mouse cells induces genomic instability and cellular transformation, likely as a result of the accumulation of DNA double-strand breaks (DSBs). Our results are consistent with the interpretation that individuals who carry the rs3087468 SNP are at increased risk for cancer.
Results
hNTH1 D239Y Is an Inactive DNA Glycosylase.
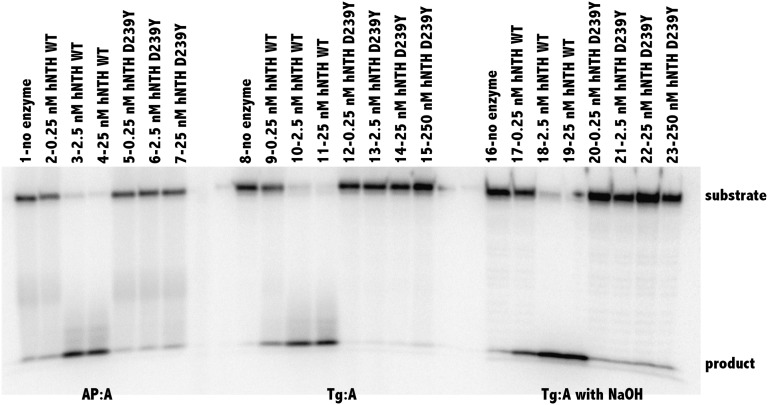
Both the crystal structures of Escherichia coli (9) and Bacillus stereothermophilus (10) Nth and site-directed mutagenesis of a highly conserved aspartate (9), Asp239 in hNTH1, suggested that Asp239 is the protein’s active site nucleophile. Therefore, we reasoned that alteration of Asp239 to Tyr would result in a significantly less active hNTH1 DNA glycosylase than wild type (WT). To test this hypothesis, we subcloned the WT hNTH1 cDNA into pGEX6P3 and used site-directed mutagenesis to generate the alteration of Asp239 to Tyr. We then overexpressed either WT or D239Y hNTH1 in the E. coli nth nei double mutant that exhibits a high spontaneous mutation frequency due to its inability to remove oxidized bases (11). As shown in Fig. S1, expression of WT hNTH1 in this strain significantly reduces the mutation frequency, whereas expression of the D239Y variant does not. These results suggest that, unlike the WT human protein, the D239Y variant is not able to repair oxidized pyrimidines. To confirm this observation, we purified both WT and D239Y hNTH1 and characterized their activities with DNA glycosylase assays. As shown in Fig. 1, D239Y hNTH1 is unable to excise thymine glycol (Tg) opposite template A and has little, if any, lyase activity, in comparison with the WT protein. We also show that D239Y is unable to excise dihydrouracil (DHU) from DNA (Fig. S2). Both D239Y and WT bind with similar affinities to DNA containing Tg (Fig. S3). Interestingly, addition of hD239Y to WT hNTH1 results in decreased overall glycosylase activity, suggesting that D239Y and WT compete for binding to damaged bases, in this case Tg (Fig. S4). In combination, these results indicate that D239Y hNTH1 is an inactive DNA glycosylase that has the ability to recognize and bind to Tg and DHU lesions and likely other oxidized pyrimidines.
Fig. 1.
D239Y hNTH1 is unable to excise Tg from oligodeoxyribonucleotides. In vitro glycosylase/lyase assays with hNTH1 WT and the hNTH1 D239Y variant were performed as described in Experimental Procedures and SI Text. Double-stranded substrate apurinic/apyrimidinic (AP):A (25 nM) was incubated without enzyme (lane 1) or with hNTH WT (0.25, 2.5, and 25 nM; lanes 2, 3, and 4, respectively) or hNTH1 D239Y (0.25, 2.5, and 25 nM; lanes 5, 6, and 7, respectively). Double-stranded Tg:A (25 nM) was incubated without enzyme (lane 8) or with hNTH WT (0.25, 2.5 and 25 nM; lanes 9, 10, and 11, respectively) or hNTH1 D239Y (0.25, 2.5, 25, and 250 nM; lanes 12, 13, 14, and 15, respectively). To determine glycosylase only, reactions were terminated with NaOH, heated, and followed by the addition of formamide loading dye. Double-stranded substrate, Tg:A (25 nM), was incubated without enzyme (lane 16) or with hNTH WT (0.25, 2.5, and 25 nM; lanes 17, 18, and 19, respectively) or hNTH1 D239Y (0.25, 2.5, 25, and 250 nM; lanes 20, 21, 22, and 23, respectively).
Expression of D239Y hNTH1 in Human Cells Results in Cellular Transformation.
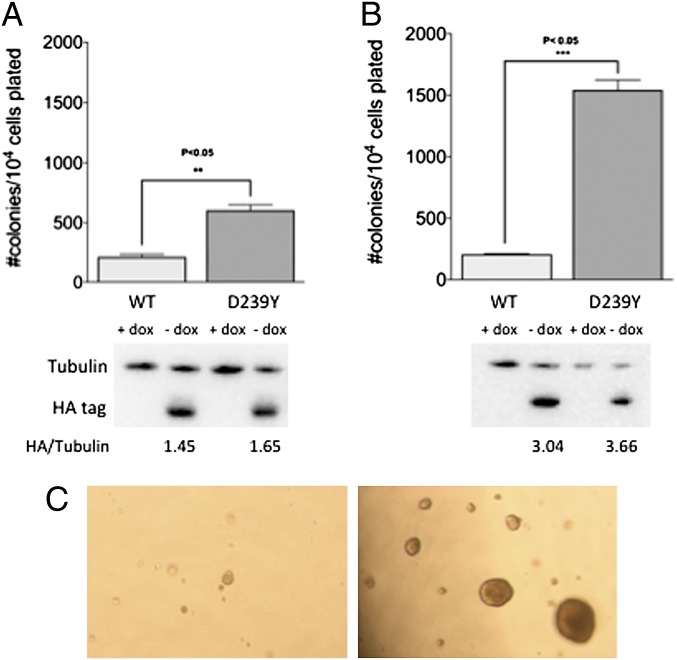
Cells that are not able to remove oxidized bases are likely to exhibit genomic instability that leads to a cancerous phenotype. To test this hypothesis, we subcloned either hemagglutinin (HA)-tagged WT or D239Y hNTH1 cDNA into the pRVYTET vector, prepared retrovirus, and infected MCF10A human breast epithelial cells. Tagging the proteins with the HA epitope enables us to distinguish endogenous from exogenous protein. We selected two independent pools of MCF10A cells expressing either WT or D239Y hNTH1 as described (12, 13). The MCF10A cells are an immortalized but nontransformed cell line that also expresses endogenous WT hNTH1 (Fig. S5). Therefore, cells that express D239Y also express endogenous WT hNTH1. Expression of the protein is under control of doxycyline (Dox); when Dox is present in the growth medium, expression is off and, when absent, the exogenous protein is expressed (13). As shown in the Western blot in Fig. 2 A and B, in the absence of Dox, the WT-HA and D239Y-HA NTH1 proteins are expressed in their respective pools at equivalent levels, relative to tubulin. Passage 12 cells grown in the absence of Dox were plated in soft agar as described (12), and colonies were counted after 5 wk. As shown in Fig. 2 A and B, cells from two independent pools expressing D239Y hNTH1 formed significantly greater numbers of colonies in soft agar that those expressing WT. Moreover, a significant fraction of the colonies expressing D239Y were substantially larger than those expressing WT, as shown in Fig. 2C.
Fig. 2.
Expression of D239Y hNTH1 in MCF10A cells induces cellular transformation. Pools of MCF10A cells expressing either WT or D239Y hNTH1 were passaged 12 times and plated in soft agar as described in Experimental Procedures and SI Text. (A) Anchorage-independent growth for pool 1. (B) Anchorage-independent growth for pool 2. Western blots below each graph show equivalent expression of WT and D239Y in their respective pools. (C) Photographs of anchorage-independent growth of colonies expressing WT (Left) and D239Y (Right). Note that colonies expressing D239Y are significantly larger than those expressing WT hNTH1.
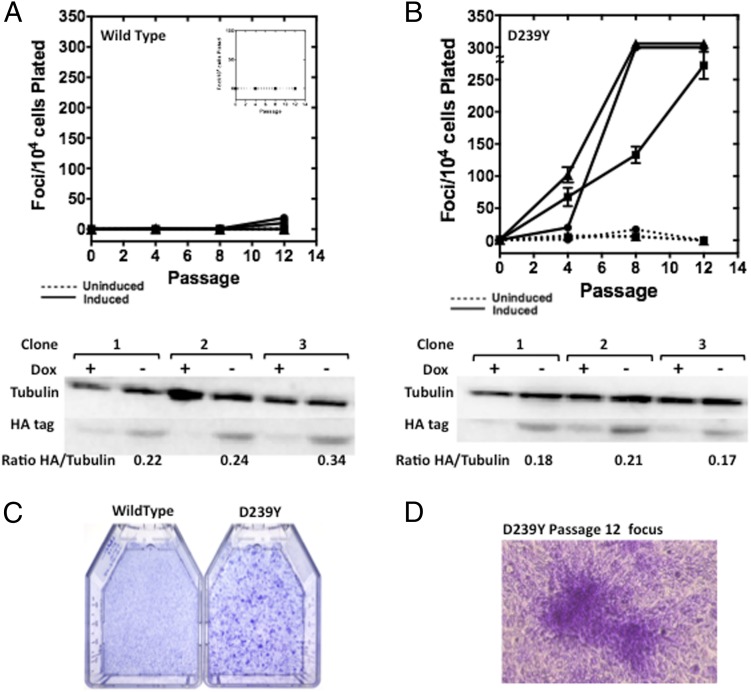
We also expressed either WT-HA or D239Y-HA in C127 mouse mammary epithelial cells, isolated individual stable clones, and characterized focus formation as described (13). Focus formation results from cells losing contact inhibition and growing on top of each other as pictured in Fig. 3D. As shown in Fig. 3 A and C, expression of WT hNTH1 in each of three individual clones does not induce focus formation. However, we observed focus formation in each of the three clones of C127 cells that express D239Y (Fig. 3 B and C). We then characterized the invasive index (14) of clones expressing either WT or D239Y. As shown in Fig. S6, expression of D239Y in the C127 cells results in invasion through the basement membrane, whereas cells expressing WT hNTH1 exhibit a low invasive index. In combination, our results suggest that expression of D239Y hNTH1 results in cellular transformation. This finding is consistent with the interpretation that individuals who carry the D239Y hNTH1 variant are at increased risk for cancer.
Fig. 3.
Expression of D239Y induces cellular transformation in mouse C127 epithelial cells. Either WT (A) or D239Y (B) proteins were expressed in mouse C127 cells, and focus formation was monitored as an endpoint. (A) Three individual WT clones, 1 (●), 2 (■), and 3 (▲), were induced (solid lines) or not induced (dashed lines, Inset) to express WT hNTH1. No significant levels of focus formation were observed when Dox was removed (inducing conditions) from the medium to allow expression of the exogenous protein. Western blot under graph shows levels of expression normalized to tubulin. Membranes were probed with antibodies to the hemagglutinin tag (HA tag), and alpha tubulin as a loading control. The ratio of HA tag to tubulin shows a similar expression level among all clones. (B) Three individual D239Y clones, 1 (●), 2 (■), and 3 (▲) were induced (solid lines) or not induced (dashed lines) to express D239Y hNTH1. All three clones induce significant levels of focus formation at various passages when Dox was removed from the medium allowing expression of the exogenous protein, as shown in the Western blot under the graph. (C) Photograph of flasks of C127 cells stained for focus formation and expressing either WT (Left) or D239Y (Right) hNTH1. (D) Photograph of a focus of cells expressing D239Y at passage 12.
Expression of D239Y hNTH1 Results in Genomic Instability.
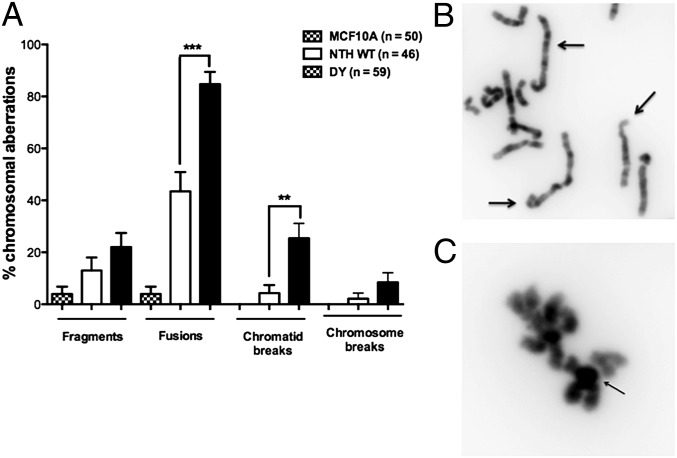
Deficient excision of oxidized bases could result in an increased mutation frequency that eventually leads to cellular transformation. To determine whether this was the case, we used the λcII mutation assay that we previously described (15). Point mutations and small insertions and deletions are observed in this forward mutation assay. The mutation frequencies of cells expressing D239Y and WT were 1.7 × 10−4 and 9.1 × 10−5, respectively, and therefore not significantly different from each other. These data suggest that it is unlikely that point mutations, which accumulate in the cells expressing D239Y, lead to cellular transformation. As shown in Fig. 1, WT but not D239Y hNTH1 excises Tg, a replication-blocking lesion (16–19). We speculated that cells expressing D239Y hNTH1 may not remove Tg as efficiently as cells exclusively expressing WT protein, leading to replication blocks that result in chromosomal aberrations. To test this hypothesis, we scored metaphase spreads, prepared as described (12), from MCF10A cells expressing either D239Y or WT hNTH1. As shown in Fig. 4, MCF10A cells expressing D239Y exhibit significantly increased levels of chromatid breaks and fusions compared with cells expressing only WT hNTH1. These results suggest that expression of exogenous D239Y in MCF10A cells results in genomic instability that could lead to cellular transformation.
Fig. 4.
Expression of D239Y hNTH1 induces chromosomal aberrations. Metaphase spreads were prepared from early passage MCF10A pools expressing either WT or D239Y as described in Experimental Procedures. (A) Column graph showing that cells expressing D239Y had significantly increased levels of fusions and chromosome breaks. (B) Arrows point to examples of fusions observed in cells expressing D239Y. (C) Arrows point to examples of chromosome breaks in cells expressing D239Y hNTH1.
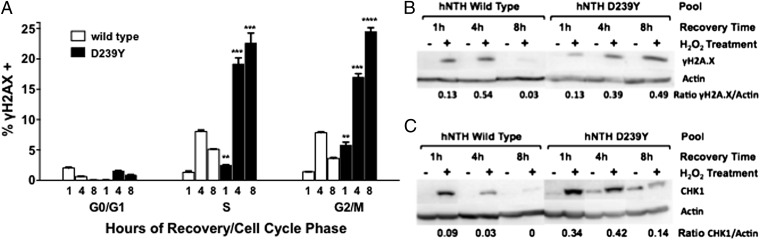
Cells Expressing D239Y hNTH1 Accumulate DSBs.
Chromatid breaks and fusions are likely to arise from a DSB intermediate. Our results showing increased levels of these types of aberrations in cells expressing D239Y suggest that these cells have higher levels of DSBs or that repair of DSBs is slower than in cells expressing WT hNTH1. To determine whether DSBs accumulate in the cells expressing D239Y, we monitored γH2AX levels after treating the cells with H2O2. We surmised that treatment with H2O2 would induce the presence of oxidized bases, including Tg, which would not be excised efficiently in cells expressing the inactive D239Y NTH1 compared with WT cells. Deficient excision of Tg or another unknown replication-blocking lesion could result in collapsed replication forks and DSBs. Pools of MCF10A cells expressing WT or D239Y were synchronized in G1 as described in Experimental Procedures and released into the medium. After 18 h, when cells moved into S phase (Fig. S7), we treated them with H2O2 and monitored γH2AX as a marker for the presence of DSBs; we also stained with propidium iodide (PI) to identify the cell cycle stage. Cells were also allowed to recover for various times, then stained and analyzed to monitor repair of DSBs. As shown in Fig. 5, DSBs accumulate in cells expressing D239Y that are in S and G2/M phases of the cell cycle and continue to be present even after 8 h of recovery, whereas DSBs are more rapidly repaired in cells expressing WT hNTH1. These results suggest that the presence of D239Y induces the accumulation of DSBs, perhaps as a consequence of replication fork collapse upon collision with an unexcised Tg or other replication-blocking lesions. This suggestion is supported by results showing that cells expressing D239Y have increased levels of γH2AX and phosphorylated CHK1 compared with cells expressing WT hNTH1 (Fig. 5 B and C). Importantly, cells expressing D239Y that were not treated with H2O2, also express increased levels of Chk1 as they traverse S phase (Fig. 5C, lanes 7, 9, and 11). These results show that the DNA damage response is activated even in D239Y-expressing cells that are not treated with a DNA-damaging agent, indicating that the cells expressing D239Y do not rapidly repair certain types of endogenous oxidative DNA damage. This damage could lead to genomic instability and cellular transformation.
Fig. 5.
MCF10A cells expressing D239Y accumulate double strand breaks in S and G2/M. (A) MCF10a pools expressing WT or D239Y NTH1 were treated with 30 mM H2O2 for 30 min on ice and allowed to recover for 1, 4 and 8 h. Cells were stained with γH2AX/FITC antibody to assess the levels of double-strand breaks and propidium iodide for cell cycle phase, and analyzed by flow cytometry. Data are plotted as the mean ± SEM (n = 3). Within each cell line, + denotes P < 0.01 and ++ denotes P < 0.0001 comparing 1-h to 4-h and 4-h to 8-h recoveries in each cell-cycle phase. D239Y has a significantly higher percentage of γH2AX-positive cells (P < 0.01) than WT at 1-h, 4-h, and 8-h recovery time and in every phase of the cell cycle, except for the 1-h G0/G1 point where WT is higher (P < 0.01). (B and C) γH2AX (B) and phosphorylated CHK1 (C) Western blots of cells synchronized and treated with 30 mM H2O2 and allowed to recover for 1, 4, and 8 h before lysis, as in A. Lanes 1–6 are WT, and lanes 7–12 are D239Y. Lanes 1, 2, 8, and 9 are 1-h recovery time; lanes 3, 4, 9, and 10 are 4-h recovery time; lanes 5, 6, 11, and 12 are 8-h recovery time. Lanes 1, 3, 5, 7, 9, and 11 are untreated controls; lanes 2, 4, 6, 8, 10, and 12 are H2O2 treated.
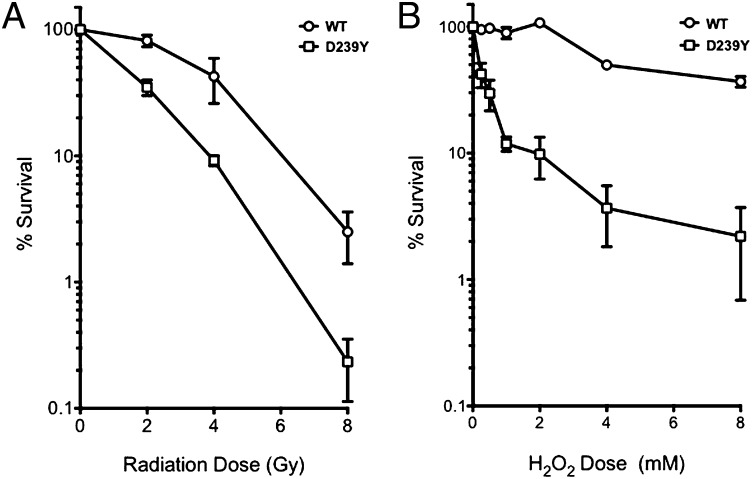
Expression of D239Y Confers Sensitivity to Ionizing Radiation and Hydrogen Peroxide.
The accumulation of DSBs in H2O2-treated cells expressing D239Y suggest that these cells could be sensitive to agents that induce oxidative base damage, namely, H2O2 and ionizing radiation (IR). To determine whether this was the case, we performed clonogenic survival assays with C127 clones expressing either WT or D239Y hNTH1. As shown in Fig. 6, cells expressing D239Y hNTH1 were more sensitive than WT to both H2O2 (Fig. 6A) and IR (Fig. 6B). In combination with our other results, the finding that expression of D239Y confers sensitivity to H2O2 and IR agents suggests that cells expressing this variant do not repair DNA damage as efficiently as WT cells.
Fig. 6.
Expression of D239Y confers sensitivity to H2O2 and IR. A total of 2 × 106 C127 WT or D239Y expressing cells were suspended in serum-free medium and either X-irradiated at 0, 2, 4, and 8 Gy (A) or treated with 0, 2, 4, 6, or 8 mM H2O2 on ice for 30 min (B). Cells were then serially diluted in triplicate 100-mm plates, grown for 12 d, then washed and stained with crystal violet; colonies were then scored.
Summary.
We found that expression of the D239Y hNTH1 germ-line variant in the presence of WT NTH1 induces genomic instability and cellular transformation. These observations, together with our biochemical data demonstrating that D239Y lacks glycosylase activity yet binds to lesion-containing DNA as well if not better than WT NTH1, strongly suggest that a lesion recognized and bound by WT will be repaired but one recognized and bound by D239Y hNTH1 will not. The genomic instability induced by D239Y likely results from its inability to repair oxidative DNA lesions, such as Tg, that can otherwise block replicative DNA polymerases. If the block is in the lagging strand, replication can reinitiate downstream, resulting in formation of a single-strand gap. The remaining lesion could then be processed by homologous recombination (HR)-mediated repair to restore the genetic information. However, should a functionally redundant glycosylase excise the Tg or other fork-blocking lesion within the context of a single-strand gap, a DSB would result that could be processed by nonhomologous end-joining (NHEJ) leading to cell death or genomic instability. Fork-blocking lesions in the leading strand would lead to replication fork collapse. The collapsed fork could be rescued by regression catalyzed by helicases such as BLM, and restart could occur by HR-mediated repair. Alternatively, excision of the lesion by a functionally redundant glycosylase could again result in the formation of a DSB that could be repaired by HR in a error-free manner, lead to cell death, or lead to genomic instability. Our observation that DSBs accumulate in cells expressing D239Y, even after 8 h of recovery, suggests that fork-blocking lesions accumulate over time and that some of these are likely acted upon by redundant glycosylases, leading to DSBs and replication fork collapse. Should the DSB be repaired in an error-prone manner via NHEJ, genomic instability will result leading to cellular transformation. Taken together, our results suggest that individuals who carry D239Y likely accumulate chromosomal changes over time, as BER acts on endogenous oxidative lesions. Because genomic instability leads to cancer, our results suggest that individuals who carry the D239Y germ-line variant are at increased risk for cancer. Should these individuals be exposed to DNA-damaging agents, chromosomal abnormalities may accumulate even more rapidly, leading to an earlier onset of cancer.
Experimental Procedures
Cells and Growth Media.
MCF10A cells are immortalized, nontransformed epithelial cells derived from human mammary tissue (ATCC). These cells were maintained in DMEM/F12 medium (Corning Cellgro) supplemented with 5% (vol/vol) horse serum, 1% (wt/vol) penicillin-streptomycin, insulin (10 mg/mL; Invitrogen), epidermal growth factor (20 ng/mL; Peprotech), hydrocortisone (0.5 mg/mL), and cholera toxin (100 ng/mL; Sigma-Aldrich). C127 is a nontransformed clonal line derived from a mammary tumor of an R III mouse (20). These cells and the GP2-293 virus packaging cell line (Clontech) used for retrovirus preparation were maintained in DMEM (Corning Cellgro) supplemented with 10% (vol/vol) FBS and 1% (wt/vol) penicillin-streptomycin (Invitrogen). All cell lines were grown at 37 °C in a 5% CO2 humidified incubator.
Cloning and Expression of hNTH1.
For protein purification, the hNTH sequence was amplified by the PCR and subcloned into the NdeI, XhoI restriction sites in pET30a. Standard methods of site-directed mutagenesis were used to mutate the WT hNTH1 sequence to generate a cDNA encoding the D239Y variant. The proteins were purified essentially as described (see below) as detailed in SI Text. For expression in tissue culture cells, the WT and D239Y sequences were PCR amplified using a downstream primer that contained the HA tag sequence (YPYDVPDYA) on its 5′ end and cloned into the Not1, BamH1 restriction sites in the pRVY-Tet retroviral vector. Virus preparation, infection, and selection are described in SI Text.
DNA Glycosylase Assays.
Glycosylase assays were carried out as described in ref. 21 and in SI Text.
Western Blotting.
Expression of exogenous HA-tagged NTH1 and characterization of the DNA damage response were analyzed by Western blot as described in detail in SI Text.
Cellular Transformation Assays.
Focus formation assays with the C127 cells were carried out as described (12). Anchorage-independent growth assays with the MCF10A cells were performed as described (12). See SI Text for additional details. Cell invasion assays were carried out using Biocoat Control Inserts (BD Pharmagen no. 354578) and GFR Matrigel Basement Membrane Matrix Invasion Chambers (BD Pharmagen no. 354483) essentially as described (14).
Chromosomal Aberrations.
Metaphase spreads were prepared and chromosomal aberrations were analyzed as described (12). See SI Text for additional details.
Flow Cytometry.
MCF10A cells expressing WT or NTH1 D239Y were seeded at 8 × 105 cells per 10-cm dish, allowed to attach overnight, and synchronized by 24 h of serum and growth factor deprivation. Complete medium was added back and cells incubated at 37 °C/5% CO2 for a further 18 h to reach S phase. Plates were treated with or without 30 mM H2O2 in serum-free medium in the cold for 30 min, then changed back to warm complete medium and allowed to recover for 1 h, 4 h, and 8 h after treatment. Cells were then trypsinized and rinsed with PBS, pelleted and fixed in 2% formalin on ice for 20 min, pelleted again and resuspended by adding 70% ice-cold ethanol dropwise while vortexing. Cells were fixed overnight at −20 °C. The cells were incubated with primary phospho-H2A.X antibody (Millipore 05–636) 1:500 overnight at 4 °C. Following the incubation, cells were washed twice with PBS and incubated with anti-mouse secondary antibody conjugated to FITC 1:500 for 1 h at room temperature. Cells were washed twice with PBS and resuspended in 500 μL of PI/RNase staining buffer (BD Pharmingen). Fluorescence was measured by flow cytometry using the BD LSRII analytical cytometer and analyzed using FlowJo 8.8.7 software.
Clonogenic Survival.
Cells were trypsinized and resuspended in serum-free medium on ice at 2 × 106 cells per 2 mL for H2O2 treatment or 5 mL for X-irradiation. Cells were then treated with 0, 2 mM, 4 mM, or 8 mM H2O2 for 30 min on ice, or with 0, 2 Gy, 4 Gy, or 8 Gy X-rays, followed by serial dilution in 10-cm dishes with DMEM/10% FBS. Cells were grown for 12 d before staining with 0.25% crystal violet in 80% methanol. Colonies were scored by eye at 10× magnification. Only colonies with more than 50 cells were counted, and all experiments were repeated at least twice.
Supplementary Material
Acknowledgments
We thank the Vermont Cancer Center DNA Analysis Core for sequencing all of the clones and Megan Hess for technical assistance. This work was supported by National Institutes of Health Grant P01 CA098993 awarded by the National Cancer Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306752110/-/DCSupplemental.
References
- 1.Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 2.Freidberg EC, Wood RD, Walker GC, Siede W. DNA Repair and Mutagenesis. 2nd Ed. Washington, DC: ASM Press; 2006. [Google Scholar]
- 3.Wiederhold L, et al. AP endonuclease-independent DNA base excision repair in human cells. Mol Cell. 2004;15(2):209–220. doi: 10.1016/j.molcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Wallace SS, Murphy DL, Sweasy JB. Base excision repair and cancer. Cancer Lett. 2012;327(1-2):73–89. doi: 10.1016/j.canlet.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asagoshi K, et al. Recognition of formamidopyrimidine by Escherichia coli and mammalian thymine glycol glycosylases. Distinctive paired base effects and biological and mechanistic implications. J Biol Chem. 2000;275(32):24781–24786. doi: 10.1074/jbc.M000576200. [DOI] [PubMed] [Google Scholar]
- 6.Aspinwall R, et al. Cloning and characterization of a functional human homolog of Escherichia coli endonuclease III. Proc Natl Acad Sci USA. 1997;94(1):109–114. doi: 10.1073/pnas.94.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda S, et al. Purification and characterization of human NTH1, a homolog of Escherichia coli endonuclease III. Direct identification of Lys-212 as the active nucleophilic residue. J Biol Chem. 1998;273(34):21585–21593. doi: 10.1074/jbc.273.34.21585. [DOI] [PubMed] [Google Scholar]
- 8.Eide L, et al. Human endonuclease III acts preferentially on DNA damage opposite guanine residues in DNA. Biochemistry. 2001;40(22):6653–6659. doi: 10.1021/bi0028901. [DOI] [PubMed] [Google Scholar]
- 9.Thayer MM, Ahern H, Xing D, Cunningham RP, Tainer JA. Novel DNA binding motifs in the DNA repair enzyme endonuclease III crystal structure. EMBO J. 1995;14(16):4108–4120. doi: 10.1002/j.1460-2075.1995.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fromme JC, Verdine GL. Structure of a trapped endonuclease III-DNA covalent intermediate. EMBO J. 2003;22(13):3461–3471. doi: 10.1093/emboj/cdg311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang D, Hatahet Z, Blaisdell JO, Melamede RJ, Wallace SS. Escherichia coli endonuclease VIII: Cloning, sequencing, and overexpression of the nei structural gene and characterization of nei and nei nth mutants. J Bacteriol. 1997;179(11):3773–3782. doi: 10.1128/jb.179.11.3773-3782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamtich J, Nemec AA, Keh A, Sweasy JB. A germline polymorphism of DNA polymerase beta induces genomic instability and cellular transformation. PLoS Genet. 2012;8(11):e1003052. doi: 10.1371/journal.pgen.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweasy JB, et al. Expression of DNA Polymerase Beta Mutants in Mouse Cells Results in Cellular Transformation. Proc Natl Acad Sci USA. 2005;102(40):14350–14355. doi: 10.1073/pnas.0505166102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Librach CL, et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113(2):437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalal S, et al. Prostate-cancer-associated I260M variant of DNA polymerase beta is a sequence-specific mutator. Biochemistry. 2005;44(48):15664–15673. doi: 10.1021/bi051179z. [DOI] [PubMed] [Google Scholar]
- 16.Aller P, Rould MA, Hogg M, Wallace SS, Doublié S. A structural rationale for stalling of a replicative DNA polymerase at the most common oxidative thymine lesion, thymine glycol. Proc Natl Acad Sci USA. 2007;104(3):814–818. doi: 10.1073/pnas.0606648104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JM, Beardsley GP. Thymine glycol lesions terminate chain elongation by DNA polymerase I in vitro. Nucleic Acids Res. 1986;14(2):737–749. doi: 10.1093/nar/14.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouet P, Essigmann JM. Possible role for thymine glycol in the selective inhibition of DNA synthesis on oxidized DNA templates. Cancer Res. 1985;45(12 Pt 1):6113–6118. [PubMed] [Google Scholar]
- 19.Ide H, Kow YW, Wallace SS. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985;13(22):8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai CC, Edwards AP, DiMaio D. Productive interaction between transmembrane mutants of the bovine papillomavirus E5 protein and the platelet-derived growth factor beta receptor. J Virol. 2005;79(3):1924–1929. doi: 10.1128/JVI.79.3.1924-1929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandaru V, Blaisdell JO, Wallace SS. Oxidative DNA glycosylases: Recipes from cloning to characterization. Methods Enzymol. 2006;408:15–33. doi: 10.1016/S0076-6879(06)08002-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.