Abstract
Dendritic spines are the primary sites of excitatory synaptic transmission in the vertebrate brain, and the morphology of these actin-rich structures correlates with synaptic function. Here we demonstrate a unique method for inducing spine enlargement and synaptic potentiation in dispersed hippocampal neurons, and use this technique to identify a coordinator of these processes; Ras-specific guanine nucleotide releasing factor 2 (RasGRF2). RasGRF2 is a dual Ras/Rac guanine nucleotide exchange factor (GEF) that is known to be necessary for long-term potentiation in situ. Contrary to the prevailing assumption, we find RasGRF2’s Rac-GEF activity to be essential for synaptic potentiation by using a molecular replacement strategy designed to dissociate Rac- from Ras-GEF activities. Furthermore, we demonstrate that Rac1 activity itself is sufficient to rapidly modulate postsynaptic strength by using a photoactivatable derivative of this small GTPase. Because Rac1 is a major actin regulator, our results support a model where the initial phase of long-term potentiation is driven by the cytoskeleton.
Keywords: Rho GTPase, LTP, synaptic plasticity, AMPA receptor
The human brain is estimated to contain over 100 trillion synapses, constituting the major mode of communication between neurons. Postsynaptic depolarization is predominantly initiated at excitatory glutamatergic synapses, which are preferentially localized to actin-rich protrusions called dendritic spines. Transmission of high-frequency stimulation (HFS) to these synapses rapidly increases the size of both excitatory postsynaptic currents (EPSCs) and spines (1). These alterations are believed to form the cellular basis of learning and memory, and are commonly referred to as long-term potentiation (LTP).
LTP can be divided into at least two phases: initial (I-LTP) and maintenance. I-LTP refers of the rising phase of potentiation, when synaptic transmission is enhanced by AMPA receptor (AMPAR) accumulation at the synapse (2, 3). The maintenance phase is considered mechanistically distinct, and converts I-LTP into a stable form (4). The temporal correlation between I-LTP and spine enlargement (1) suggests that both processes potentially share a common regulatory mechanism. Because the Rho family of small GTPases (Rho GTPases) control the actin dynamics that underlie spine morphology (5), the proteins that control Rho GTPase activity may in turn be prime organizers of I-LTP.
Rho GTPases (Rac1, RhoA, Cdc42, and at least 17 others) exist in GTP- and GDP-bound states, corresponding with active and inactive conformations, respectively. When active, Rho GTPases effect actin remodeling through actin-binding proteins, such as cofilin and the Arp2/3 complex (6). Rho GTPases are turned on by guanine nucleotide exchange factors (GEFs), which stimulate GDP for GTP exchange (7). Over 70 GEFs exist for Rho GTPases, highlighting the diversity of GEF function. Because myriad data suggest that Rac1 activity is necessary for both spine formation and enlargement (8, 9), we screened for Rac-GEFs that gain activity in response to HFS-like stimulation. Here we describe the detection of RasGRF2, a dual Ras/Rac-GEF, and subsequently demonstrate the necessity of this protein’s Rac-GEF activity for synaptic potentiation. The duality of this protein suggests that RasGRF2 is a multifunction signaling node for the conversion of calcium transients into altered AMPAR trafficking.
Results
Induction of Synaptic Potentiation in Dispersed Hippocampal Neurons.
Because synapses potentiate and spines enlarge within minutes of HFS, we reasoned that any biochemical pathway coordinating these phenomena must be even faster. With this in mind, we chose chemical stimulation of dispersed hippocampal neurons as our experimental system for two major benefits relative to traditional in situ LTP induction methods: (i) rapid protein extraction following stimulation, and (ii) completeness of synaptic sites stimulated. Neurons cultured to 19 d in vitro (DIV) display spines similar to in situ preparations.
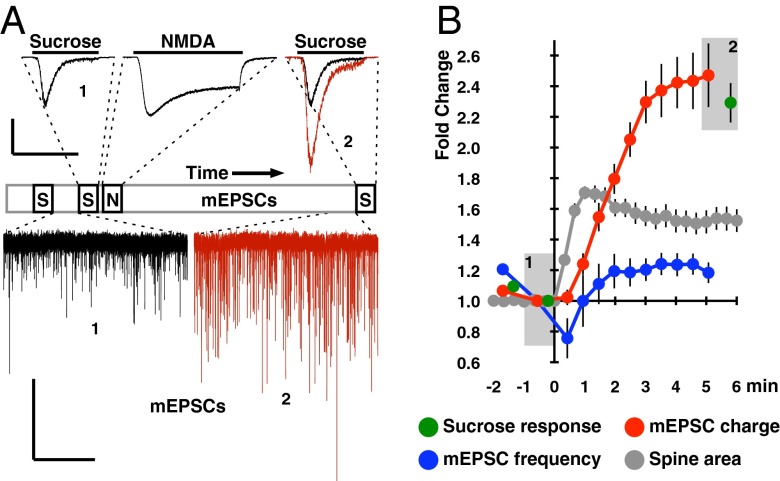
During our investigation of a protocol that uses spontaneous presynaptic glutamate release to drive NMDA receptor (NMDAR)-dependent synaptic potentiation in vitro (10), we unexpectedly observed that transient NMDA stimulation itself is sufficient to rapidly enhance both mEPSC (miniature EPSC) charge and spine area (Fig. 1) [mEPSC size is quantified as integrated charge and not amplitude because of the former being less affected by dendritic filtering (11), although similar results were obtained using either metric]. To provide a higher signal-to-noise correlate to mEPSC charge measurements, we perfused neurons with hyperosmotic external solution (+500 mOsm from sucrose) to accelerate the spontaneous glutamate release rate (12). The sucrose response increased by the same magnitude as mEPSC charge, indicating that we were accurately quantifying synaptic strength by mEPSC charge measurements (Fig. 1). We could not compare the time course of NMDA-LTP to HFS-LTP as HFS causes presynaptic effects, such as posttetanic potentiation, that obscure the rising phase of potentiation. However, the kinetically faster spine enlargement effect we observed compares favorably with spine enlargement time courses reported in situ (1, 3, 13–16). We also note that the synaptic response to transient NMDA stimulation has previously been described in situ (17–19), where it was found that NMDA stimulation specifically induces I-LTP. In agreement with this finding, we observed NMDA-LTP to be severely decremented 2 h after stimulation (Fig. S1).
Fig. 1.
NMDA stimulation induces synaptic potentiation in dispersed hippocampal neurons. (A) Schematic of the timeline for NMDA and sucrose stimulations during the 8-min experiment duration; mEPSCs were otherwise continuously recorded. Representative traces corresponding to the timeline demonstrate mEPSC and sucrose response enhancement induced by NMDA stimulation and the NMDA current itself. Black traces and the number 1 represent responses before NMDA stimulation, whereas red traces and the number 2 correspond to responses after NMDA stimulation. This color and number convention is used repeatedly throughout this article. (B) Quantification of mEPSC charge, mEPSC frequency, and sucrose response magnitudes over time in response to NMDA stimulation (0 min). mEPSC charge potentiation is saturated by 3–5 min after NMDA stimulation. The transient drop in mEPSC frequency observed following sucrose stimulations is because of the resultant vesicle depletion. This figure and Fig. S1 were the only experiments where hypertonic solutions were perfused. Data were normalized per cell to the last timepoint before NMDA application and expressed as mean ± SEM (n = 14 neurons). Spine area was quantified from independent experiments and is normalized per spine (n = 40 spines from 6 neurons). Spine area increase is saturated by 90 s after NMDA stimulation. [Scale bars: 1 nA and 3 s (sucrose and NMDA), 40 pA and 10 s (mEPSCs).]
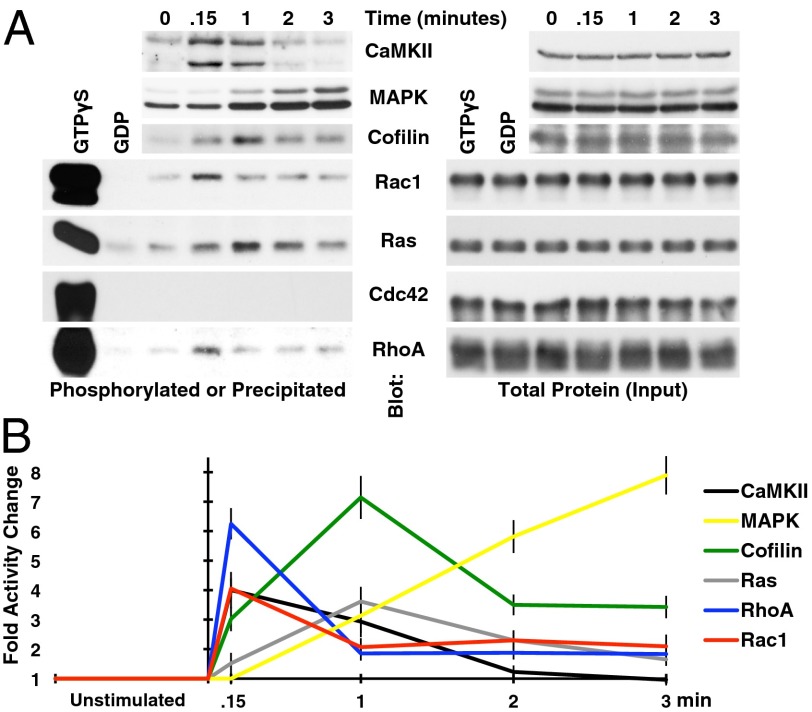
To determine signaling pathways that could credibly be playing a role in NMDA-LTP, we measured the activation time course of proteins canonically associated with LTP or the cytoskeleton. Protein activity was either indirectly probed with phospho-specific antibodies for CaMKII (Ca2+/calmodulin-dependent protein kinase II), cofilin, and MAPK (Mitogen-activated protein kinase), or directly assayed by precipitating the active form of GTPases Ras, RhoA, and Rac1/Cdc42 with the GTPase-binding domain of their effector proteins Raf, Rhotekin, and PAK, respectively (Fig. 2). It was evident that CaMKII and the Rho GTPases Rac1 and RhoA were activated with the fastest time course, having maximal activation within seconds of NMDA stimulation. Our observed time courses for CaMKII and RhoA activation are similar to those reported using optical probes in situ (13, 14). There is currently no equivalent literature describing the time course of Rac1 activation, and we did not detect any endogenous Cdc42 activity by this assay. Not surprisingly, the actin severing protein cofilin was maximally phosphorylated (inactivated) immediately following the wave of Rac1 and RhoA activation, with the Ras/MAPK pathway being significantly slower (Fig. 2). However, based on time courses alone, neither the cytoskeletal nor Ras/MAPK pathways can be excluded from playing a role in actual synaptic potentiation.
Fig. 2.
Biochemical properties of NMDA-LTP. (A) The phosphorylation or precipitation of proteins associated with LTP or the cytoskeleton was assayed by immunoblot to gauge chronological activity following NMDA stimulation. The time after NMDA stimulation is indicated above the blots, with “0 min” corresponding to the unstimulated condition, and “.15 min” corresponding with 9 s after NMDA stimulation. CaMKII, cofilin, and MAPK were detected using both phospho- and nonphospho-specific antibodies, the latter being used to normalize for protein level present in samples (input). The doublet in the phospho-CaMKII blot is a result of this antibody recognizing both α and β isoforms. Rac1, Ras, Cdc42, and RhoA activities were assayed by precipitation with agarose-immobilized substrates; see main text for details. Only nonphsopho antibodies were used in this case, and GTPγS and GDP controls were included to detect the maximum and minimum possible signals, respectively. The protein present in the lysates used for precipitation was quantified for the same reasons as with phosphoproteins. (B) The time courses were then quantified from these blots and normalized to the unstimulated condition (n = 3 repeats), revealing distinct waves of activation.
Identification of RasGRF2.
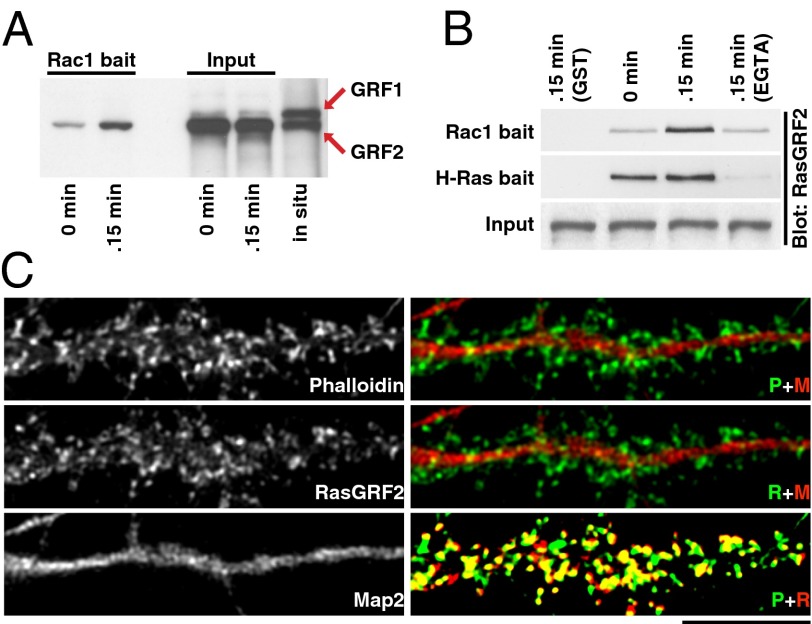
Our finding that Rac1 is rapidly activated following NMDA stimulation lead us to hypothesize that a Rac-GEF had gained activity. Because active Rac1 can cause spine enlargement (9), we further hypothesized that this GEF might play a role in NMDA-LTP. To identify this hypothetical Rac-GEF, we performed a precipitation assay similar to those used to identify active GTPases, only now the “bait” protein was the GTPase itself, mutated to tightly bind activated GEFs [Rac1-G15A; GTPase is permanently nucleotide-free (20)]. This bait was used to “fish” for Rac-GEFs, with the central premise that there should be more precipitation of a Rac-GEF immediately following NMDA stimulation (compared with the unstimulated condition) if it had gained activity. We immunoblotted the precipitates for different neuronally expressed Rac-GEFs: Kalirin-7, RasGRF1/2, SOS1, and Tiam1. Of these Rac-GEFs, we only detected an increase in the precipitation of RasGRF2 (Fig. 3A and Fig. S2), suggesting that the Rac-GEF activity of RasGRF2 is activated immediately following NMDA stimulation. Interestingly, RasGRF2 is a dual Ras/Rac-GEF that also precipitated with H-Ras bait (H-Ras-G15A) (Fig. 3B). This GEF has previously been demonstrated to be necessary for HFS-LTP in situ (21, 22), although this necessity was assumed to be a function of its Ras-GEF activity. RasGRF2 was also present in spines as detected by immunocytochemistry (Fig. 3C), reinforcing the plausibility of RasGRF2 being involved in NMDA-LTP.
Fig. 3.
Identification of RasGRF2, a NMDA-responsive Rac-GEF. (A) RasGRF2’s precipitation with agarose-immobilized Rac1-G15A (bait) was enhanced by NMDA stimulation; compare 0.15 min (9 s after NMDA stimulation) to 0 min (unstimulated). The G15A mutation tightly binds activated GEFs by creating a nucleotide-free GTPase (20). Because the RasGRF2 antibody we used cross-reacts with both isoforms of RasGRF (38), it was evident that RasGRF1 was not present in the cultured hippocampal neurons used for precipitation (input), although it was present in adult rat whole-brain lysate (in situ). (B) We precipitated RasGRF2 under four conditions: unstimulated, NMDA stimulated, NMDA stimulated with EGTA added to the lysate, and precipitation without bait (GST). These results demonstrate that Ca2+/calmodulin-binding is likely necessary for both RasGRF2’s Rac- and Ras-GEF activities; see main text for details. The blots are representative of n ≥ 3 repeats. (C) RasGRF2 is present in spines. Hippocampal neurons were triple-labeled with antibodies against RasGRF2 and microtubule-associated protein 2 (Map2), and with phalloidin to visualize F-actin (abbreviated R, M, and P, and color-coded in merged images). Map2 and phalloidin primarily labels dendrites and spines, respectively. RasGRF2 staining is present in phalloidin-intense areas, indicating RasGRF2’s presence in spines. Contrast was enhanced in the phalloidin/RasGRF2 merge. (Scale bar, 10 µm.)
Because RasGRF2 is known to bind Ca2+/calmodulin through its IQ (Isoleucine/Glutamine motif) domain (23), we examined the function of this interaction using lysis buffer supplemented with 100 µM CaCl2 to activate all calmodulin available in the lysates (Fig. 3B). The addition of EGTA (2 mM) during precipitation from NMDA stimulated lysates reduced RasGRF2’s affinity for both the Rac1 and H-Ras bait. This finding supports the notion that Ca2+/calmodulin binding is necessary for activating both GEF functions. However, there was a relative lack of difference in precipitation between NMDA stimulated and unstimulated lysates specifically with the H-Ras bait. This finding, in turn, suggests that Ca2+/calmodulin binding is sufficient to induce the Ras-GEF activity of RasGRF2, but not the Rac-GEF activity.
Interestingly, when we compared lysates from our in vitro system to adult rat whole-brain lysates (i.e., in situ), it was evident that the higher molecular weight RasGRF1 isoform was absent in vitro (Fig. 3A). RasGRF1 is highly homologous to RasGRF2, but has been shown to specifically play a role in long-term depression rather than LTP (21). This role may be related to RasGRF1 and -2 functioning downstream of NMDAR subunits NR2B and -A, respectively (24, 25). We were not able to significantly improve RasGRF1 expression by culturing neurons up to 48 DIV (Fig. S3). Because RasGRF1 (but not RasGRF2) is an imprinted gene, we speculate that the unsilencing of the paternal allele by DNA methyltransferase that typically occurs in vivo (23) does not occur when neurons are grown in isolation.
The Rac-GEF Activity of RasGRF2 Is Necessary for NMDA-LTP.
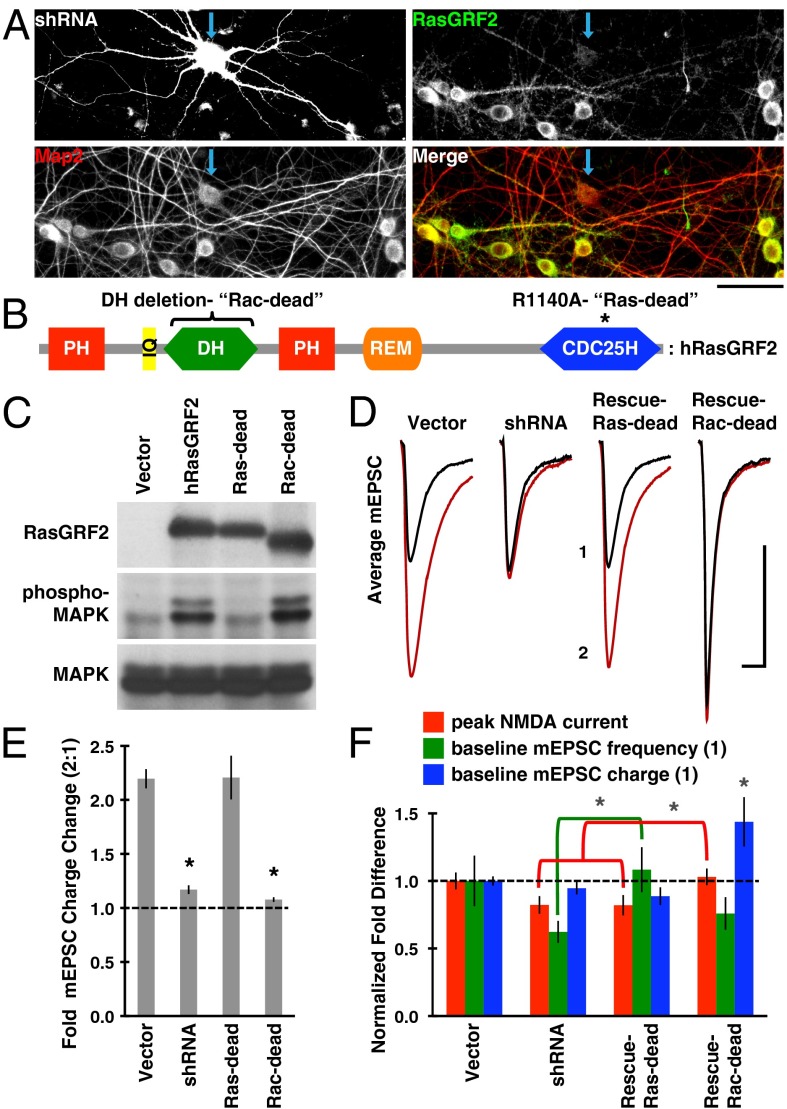
To determine the necessity of RasGRF2’s Rac-GEF activity for NMDA-LTP, we performed knockdown and rescue experiments with mutants of RasGRF2 designed to dissociate Rac- from Ras-GEF activity. A short hairpin RNA (shRNA) was created against the rat form (r) of RasGRF2 (Fig. 4A) (knockdown efficiency averaged greater than 80%), and a human (h) RasGRF2 construct resistant to this shRNA was used as the basis for mutagenesis. To isolate Rac-GEF activity, we used the point mutation R1140A (Fig. 4B), which is known to inhibit Ras binding to the catalytic CDC25H domain (26), and thus prevent Ras activation. When this Ras-GEF-dead (Ras-dead) mutant was expressed in HEK-293T cells (which natively lack RasGRF1/2), it failed to activate the Ras/MAPK pathway relative to wild-type RasGRF2 (Fig. 4C). We also observed that prolonged Ras activity itself activates the Rac pathway (Fig. S4), complicating analysis of RasGRF2’s Rac-GEF activity in these cells. To generate an unambiguous Rac-dead mutant, we therefore deleted the entire Rac-GEF Dbl homology (DH) domain of RasGRF2 (Fig. 4B). A known side effect of this deletion is the enhancement of RasGRF2’s Ras-GEF activity in the unstimulated (i.e., Ca2+/calmodulin-free) state (27, 28).
Fig. 4.
Mutations testing the necessity of RasGRF2’s Rac-GEF activity for NMDA-LTP. (A) To replace native RasGRF2 with mutant forms, we created a shRNA that effectively knocks down rat RasGRF2 in our system. As evidenced in the RasGRF2/MAP2 merge, transfected neurons (blue arrow) had significantly reduced levels of RasGRF2 (green), but not Map2 (red), than surrounding untransfected neurons. Knockdown efficiency averaged greater than 80%. (Scale bar, 50 µm.) (B) Domain structure of RasGRF2, with indicated mutations used to dissociate Rac- from Ras-GEF activities. PH, Pleckstrin homology domain; IQ, Isoleucine/Glutamine motif; DH, Dbl homology (Rac-GEF) domain; REM, Ras exchanger motif (noncatalytic); CDC25H, CDC25 homology (catalytic Ras-GEF) domain. (C) The Ras-GEF activity of RasGRF2 constructs was assayed in HEK-293T cells by blotting for phospho-MAPK. The Ras-dead construct lacks Ras/MAPK activity, whereas the Rac-dead construct is known to have enhanced (i.e., leaky) Ras-GEF activity in the Ca2+/calmodulin-free state (27, 28). Blot is representative of n ≥ 3 repeats. (D) shRNA transfected neurons poorly expressed NMDA-LTP, but this defect was rescued with a form of RasGRF2 that lacks Ras-GEF activity (Ras-dead); see main text for details. The mEPSCs shown are pooled (n > 4,000 mEPSCs from n = 12 neurons per condition), representing the minute before NMDA stimulation (denoted 1), and the fifth minute after NMDA stimulation (denoted 2). (Scale bar, 10 ms and 10 pA.) (E) Quantification of data shown in D, but averaged by neuron. (F) Quantification of the baseline (i.e., unstimulated) synaptic properties of the four conditions used, normalized to vector. Baseline mEPSC charge was significantly enhanced in the Rac-dead rescue condition (P = 0.019). These data also suggest that RasGRF2’s Rac-GEF activity affects baseline mEPSC frequency (green comparison; P = 0.030) and RasGRF2’s Ras-GEF activity affects the peak NMDA current (red comparison; P = 0.032 vs. shRNA and P = 0.031 vs. Rac-dead rescue). Normal mEPSC frequency, peak NMDA current, and mEPSC charge were 11.20 Hz, 1.83 nA, and 68.83 pA·ms, respectively. Black asterisks denote a significant difference from vector condition with P < 0.01.
Knockdown of RasGRF2 strongly suppressed NMDA-LTP, and this defect was rescued with the Ras-dead mutant (Fig. 4 D and E). We believe the most parsimonious interpretation of these data is that RasGRF2’s Rac-GEF rather than Ras-GEF activity is necessary for NMDA-LTP. In agreement with this, the Rac-dead mutant did not rescue NMDA-LTP; however, baseline mEPSC charge was significantly larger to begin with (Fig. 4 D–F). This finding suggests that elevated Ras activity (the side effect of the Rac-dead mutant) does lead to stronger synapses on longer timescales. A similar enhancement of baseline EPSC size has been reported in situ from neurons expressing constitutively active H-Ras-G12V, yet synaptic strength was not saturated despite this altered state (29). We obtained similar results when H-Ras-G12V was expressed in our system (Fig. S5), arguing that NMDA-LTP is not defective in the Rac-dead rescue condition because of saturation of synaptic strength.
Upon further examination of the baseline synaptic properties of these neurons (i.e., before NMDA stimulation), it was evident that RasGRF2’s Rac-GEF activity affects mEPSC frequency and its Ras-GEF activity modulates the peak NMDA current (Fig. 4F). The former conclusion is supported by the significant difference in mEPSC frequency between the RasGRF2 knockdown and Ras-dead rescue conditions, whereas the latter conclusion is supported by the reduction in peak NMDA current in both of these Ras-deficient conditions. Paralleling this mEPSC frequency effect, RasGRF2 knockdown reduced spine density and synaptic postsynaptic density protein 95 (PSD-95) content, whereas overexpression of Ras-dead RasGRF2 or constitutively active Rac1 had the opposite effect (Fig. S6). In summary, RasGRF2’s Rac-GEF activity appears to regulate NMDA-LTP and synapse number, but its Ras-GEF activity influences NMDAR expression or function and synaptic strength on longer timescales.
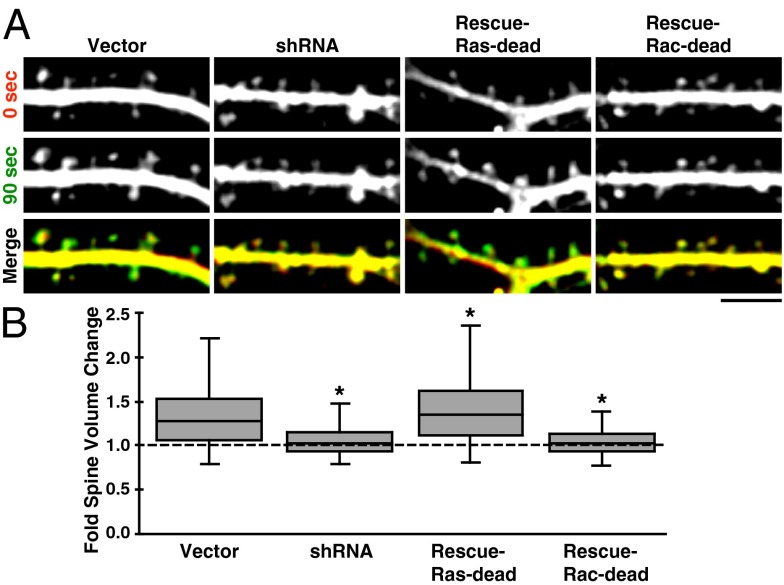
Subsequently, we repeated RasGRF2 knockdown and rescue experiments to determine RasGRF2’s contribution to spine enlargement. To accurately quantify the distribution of spine morphology effects in these conditions, we measured the change in spine volume at 90 s after NMDA stimulation (when enlargement is maximal) (Fig. 1) for all spines. shRNA transfected neurons had significantly reduced enlargement compared with vector, which was rescued by the Ras-dead, but not Rac-dead, RasGRF2 mutant (Fig. 5). In fact, spine enlargement in Ras-dead rescued neurons was greater than in vector-transfected neurons by a small but significant margin. We conclude that RasGRF2’s Rac-GEF activity plays a similarly important role in both NMDA-LTP and the correlated rapid spine enlargement. Because the average magnitude of spine enlargement we observed is smaller than magnitudes reported using single-spine stimulation techniques [such as glutamate uncaging (1, 3, 13, 14, 16)], we conjecture that spine enlargement may be hindered when many spines are enlarging simultaneously because of finite membrane and cytosol.
Fig. 5.
RasGRF2’s Rac-GEF activity is necessary for spine enlargement. (A) Stretches of dendrite and spines are shown from the indicated conditions demonstrating the degree of NMDA-induced spine enlargement. Images were assigned the same color as their time-label [i.e., before or 90 s after NMDA stimulation, when spine enlargement is maximal (Fig. 1B)] and merged in the bottom row; green-shifted spines are larger. Contrast enhancement as used for these images was not used for data analysis. (Scale bar, 5 µm.) (B) Box-and-whisker plot of spine volume changes between the 0- and 90-s timepoints (n > 500 spines pooled from n = 9 neurons per condition); see main text for details. Spine volume changes were diminished in the RasGRF2 knockdown and Rac-dead rescue conditions relative to vector. Ras-dead rescued spines enlarged slightly more than those in the vector condition, although the difference is significant because of the large number of spines analyzed. Whiskers represent data falling within 1.5 × IQR. Asterisks denote a significant difference from vector condition with P < 0.01.
Rac1 Rapidly Modulates Postsynaptic Strength.
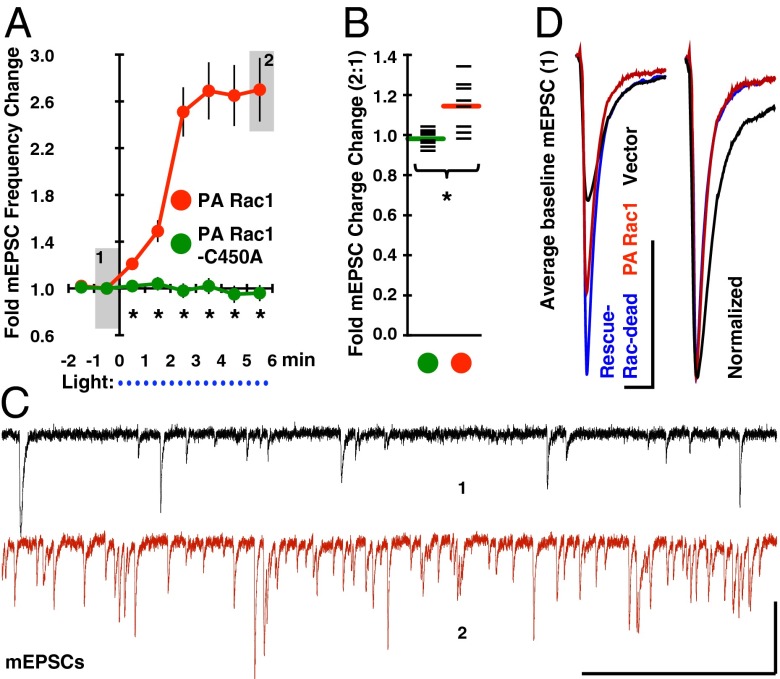
Another way to examine the synaptic action of Rac1 is to test how transient Rac1 activation affects synaptic function. We transfected neurons with a constitutively active form of Rac1 that is sterically hindered from interacting with effectors until photoactivated with blue light [PA Rac1 (30, 31)]. Photoactivation resulted in a rapid increase in mEPSC frequency, and a small but significant increase in mEPSC charge (Fig. 6 A–C). Neurons expressing a light-insensitive mutant of this construct (PA Rac1-C450A) failed to show a response to light (Fig. 6 A and B). As in earlier experiments, transfection efficiency was low, and the axons of transfected neurons infrequently intersect their own dendrites, which indicates the effect of Rac1 on mEPSC frequency is of postsynaptic origin. Lending support to this interpretation, neurons transiently expressing the lit-state mutant of PA Rac1 (PA Rac1-I539E; essentially constitutively active Rac1) had excess dendritic spines and synaptic PSD-95 content (Fig. S6). Presumably, PA Rac1 affects synaptic function by effecting synapse formation, splitting, or unsilencing.
Fig. 6.
Rac1 rapidly modulates postsynaptic strength. (A) PA Rac1 or its light-insensitive mutant (PA Rac1-C450A) were transfected into 19 DIV neurons 12 h before experimentation. After establishing a stable baseline recording under red light (>600 nm), blue light (450–490 nm) was pulsed through the patching objective for 2 s every 20 s, as indicated by blue dots below the time axis. mEPSC frequency rapidly increased specifically with the nonmutated version of PA Rac1, and (B) mEPSC charge was also significantly increased by the 5–6 min timepoint (black bars denote individual data points). (C) Representative traces are shown from the light-sensitive PA Rac1 condition representing the timepoint immediately before illumination (denoted 1), and the final time point (denoted 2). (Scale bar, 500 ms and 40 pA.) (D) Average baseline mEPSCs from this and earlier experiments (Fig. 4D) are overlaid to demonstrate the altered mEPSC kinetics observed in PA Rac1 and Rac-dead RasGRF2-expressing neurons relative to vector expressing neurons. These effects are likely initiated by the elevated GTPase-tone induced by both constructs’ leakiness (27, 28, 30). (Scale bar, 10 ms and 10 pA.) Asterisks denote a significant difference between conditions with P < 0.01.
The time course of the PA Rac1 mEPSC frequency effect closely matches that of mEPSC charge increase during NMDA-LTP (Fig. 1). Potentially, the discrepancy in primary effect (mEPSC frequency vs. charge) is related to PA Rac1 being activated everywhere in the neuron rather than being controlled by NMDAR activation. PA Rac1 expressing neurons were also in an altered state, as evidenced by their mEPSC kinetics being similar to conditions with enhanced Ras-tone (Fig. 6D) [PA Rac1 is not completely inert in the dark-state (30)]. Finally, PA Rac1 activation is not supplemented with the numerous biochemical pathways transduced by NMDAR activation. With these caveats in mind, the difference in effect of PA Rac1 compared with NMDA-LTP is not surprising. Because neurons expressing dominant-negative PA Rac1-T17N display stunted NMDA-LTP (Fig. S5), we conclude that Rac1 can positively regulate synaptic function on a rapid timescale.
Discussion
Here we provide evidence encompassing three primary points: (i) a pulse of NMDA induces synaptic potentiation in dispersed hippocampal neurons; (ii) RasGRF2’s Rac-GEF activity is necessary for this potentiation and its associated spine enlargement; and (iii) Rac1 activity rapidly modulates postsynaptic strength.
Strong evidence was provided over 25 y ago that transient NMDA stimulation induces authentic I-LTP in situ (17), yet this technique has rarely been used since. Among the evidence supporting this authenticity was the finding that HFS-LTP completely occludes NMDA-LTP specifically during the initial phase, which would be the expected result if both stimuli induce the same process. Here we have demonstrated that combining NMDA stimulation with a system optimized for rapid protein extraction allows for a biochemical examination of the initial seconds of LTP, which to our knowledge is a unique capability. This temporal resolution is critical because the morphological and electrophysiological traits of LTP begin expression within seconds of induction.
No causal relationship between spine enlargement and synaptic potentiation has yet been proven, but the temporal correlation between these phenomena is tantalizing. Spine enlargement is controlled by the underlying actin cytoskeleton (5, 32), and actin dynamics control AMPAR diffusion into synapses (33). This latter notion is supported by our observation that exogenous Rac1 activation rapidly modulates synaptic strength, and evidence from others that AMPARs are not exocytosed directly into synapses (3, 15). Indeed, evidence supports a model where the preexisting reservoir of extrasynaptic AMPARs (i.e., already on the membrane surface) is the predominant source of AMPARs destined for the synapse during I-LTP (2, 3, 15). Because Rac1 is the leading candidate of the Rho GTPases to effect spine enlargement (9), we screened for proteins that turn this GTPase on and identified RasGRF2, a dual Ras/Rac-GEF. RasGRF2 has previously been demonstrated to be necessary for in situ LTP, contextual fear-conditioning, and function of the dopaminergic reward system (21, 22, 34). We verified that this protein was also necessary for NMDA-LTP in our system, but our finding of the necessity of its Rac-GEF activity is unique. This core result was also reiterated when we examined RasGRF2’s Rac-GEF function in spine enlargement. There is some precedence for Rac1 playing a role in LTP; acute pharmacological disruption of Rac1 activation specifically attenuates I-LTP in a dose-dependent manner in situ (35). Nonetheless, RasGRF2’s function in LTP was previously assumed to be wholly because of its Ras-GEF activity, and as its name implies, RasGRF2 is generally thought of as a Ras-GEF first. Our data does support a role for RasGRF2’s Ras-GEF activity in synaptic strengthening, but not rapid (i.e., initial) strengthening. It is interesting to speculate that RasGRF2 could not only initiate but bridge the initial and maintenance phases of LTP. Ras activity is likely more strongly associated with the latter of these phases because of its ability to effect new protein synthesis and resupply surface AMPARs by exocytosis (16).
Over 20 Rac-GEFs are coded for in the human genome (7), many of which are expressed in the brain. Before this report, only one had been shown to be involved with LTP: Kalirin-7 (36, 37). We did not observe significant Kalirin-7 activation by precipitation following NMDA stimulation (Fig. S2), likely indicating that RasGRF2 and Kalirin-7 differentially regulate Rac1 activation. GEFs can also effect GTPase activation by cellular relocalization toward the GTPase; this mode of action would be undetectable by precipitation or kinetic assays and may pertain to Kalirin-7. We also believe it is likely that Rac1 is not the only upstream regulator of actin dynamics in spines during synaptic potentiation. For example, it is evident that RhoA is activated in our system and in situ (14) with kinetics similar to Rac1, potentially indicating some form of cooperation between these GTPases. Furthermore, we cannot rule out Rac1 regulators other than RasGRF2 from contributing to its activation. Multiple Rac-GEFs, GTPase-activating proteins, and guanine nucleotide dissociation inhibitors could all hypothetically contribute to the Rac1 activation we observed. Coupling precipitation assays analogous to those used here with mass spectrometry could shed light on these possibilities in a completely unbiased manner.
Materials and Methods
All materials were purchased from Sigma, unless otherwise noted.
DNA Constructs.
hRasGRF2 cDNA (MHS4426-99239507; ThermoFisher) was partially digested and cloned into the pcDNA3.1 expression vector (Invitrogen) with EcoRI. The DH-domain deletion and R1140A mutants were generated using Stratagene’s QuikChange site-directed mutagenesis kit with forward primers 5′-GTTCTCCTCATGCTGAAAGTAGCGACACTGAAAACATAAG-3′ and 5′-CTGAAGGAAGATTTAAAAATCTTGCAGAAACCCTTAAAAATTGTAAC-3′, respectively. rRasGRF2 was knocked down using shRNA complementary to target sequence 5′-TGGATTGATGACTATAGTC-3′, generated as previously described (8). Constructs encoding PA Rac1, PA Rac1-T17N, PA Rac1-C450A, PA Rac1-I539E, mCherry transfection marker, GFP-tagged Tiam1 N-terminal deletion (DH-PH domain construct), GFP-tagged H-Ras-S17N, and GFP-tagged H-Ras-G12V were acquired from Addgene (22024, 22029, 22025, 22026, 26823, 20154, 18665, and 18666, respectively). GFP (enhanced) transfection marker and FLAG-Tiam1 have been previously described (8). Constructs were verified by DNA sequencing.
GST-Substrates, Antibodies, and Drugs.
GST-Raf1 was acquired from Addgene (13338), GST-Rhotekin from Cytoskeleton (RT02), and GST-PAK has been previously described (8). GST-Rac1-G15A was a gift from Channing Der (University of North Carolina at Chapel Hill, Chapel Hill, NC), and GST-H-Ras was a gift from Larry Feig (Tufts University, Medford, MA), which we mutated to G15A. Antibodies were acquired as follows: α/β-CaMKII (phospho-Thr286/287; 06–881), Map2 (AB5622), Kalirin (07-122), actin (MAB1501), Rac1 (05-389; Millipore), α-CaMKII (3357), p44/42 MAPK (4695), p44/42 MAPK (phospho-Thr202/Tyr204; 4370), cofilin (3312), cofilin (phospho-Ser3; 3311), RhoA (2117), Cdc42 (2466), pan-Ras (3339; Cell Signaling), Tiam1 (872), SOS1 (256), RasGRF2 (7591; Santa Cruz), PSD-95 (MA1-046; ThermoFisher), VGLUT1 (135-303; Synaptic Systems), phospho-PAK [previously described (8)], fluorophore-conjugated secondary antibodies (Jackson ImmunoResearch), and HRP-conjugated secondary antibodies (Calbiochem). Alexa Fluor 488 phalloidin was purchased from Invitrogen, and all pharmacological compounds were purchased from Tocris.
Electrophysiology.
Whole-cell voltage-clamp recordings were performed with an Axopatch 200B amplifier controlled by Clampex 10 (Molecular Devices) at 24 °C. Data were acquired at 10 kHz and low-pass filtered at 2 kHz with the membrane potential clamped at −60 mV. Series resistance was only compensated for large currents (i.e., sucrose and NMDA perfusion), and only cells with series resistance <14 MΩ were recorded. Whole-cell parameters were monitored at least once per minute to ensure stability, and only data from cells with a steady holding current in the range of 0 to −80 pA were analyzed. The patch pipette solution contained: 115 mM Cs methanesulfonate, 20 mM CsCl, 10 mM Hepes, 4 mM ATP-Mg, 0.4 mM GTP-Na, 2 mM MgCl2, 1 mM EGTA, 10 mM phosphocreatine, 50 U/mL phosphocreatine kinase (300 mOsm, pH 7.4). The external perfusion solution contained 140 mM NaCl, 2.4 mM KCl, 10 mM Hepes, 10 mM glucose, 2 mM CaCl2, 2 mM MgCl2, 0.0005 mM TTX, 0.1 mM picrotoxin (300 mOsm, pH 7.4). NMDA stimulation was achieved by a 5-s pulse of this solution modified to have 0 MgCl2, 0.1 mM NMDA, 0.01 mM glycine, 0.001 mM strychnine, and NaCl was replaced with NMDG or sucrose. Perfusion (1 mL/min) and solution switching was achieved with valves feeding a multichannel flow-pipe. mEPSCs were detected off-line using Axograph X software (AxoGraph Scientific).
Imaging.
Widefield epifluroesence microscopy was performed using a Zeiss Axio Observer Z1 inverted microscope and Photometrics CoolSNAP HQ2 camera (6.45 × 6.45 μm pixel size) at 32× or 63× magnification. For spine-enlargement experiments, cells were treated identically as in electrophysiology experiments, although not patched or sucrose stimulated. Photomicrographs were analyzed using ImageJ (National Institutes of Health). Spine volume was calculated as follows: background fluorescence was subtracted from cropped images featuring the same stretch of dendrite, and a multiplier was then applied to equalize the total remaining fluorescence (i.e., from the dendrite and spines) between images. This method corrects for bleaching because GFP protein levels should not change over the stretch of dendrite and spines in this time course. Spine-specific fluorescence was then measured for individual spines, and the “fold volume change” was calculated per-spine as the ratio of spine fluorescence 90 s after NMDA stimulation to spine fluorescence immediately before stimulation. All useable stretches of dendrite and spines from the original images were processed as such. Care was taken to avoid pixel saturation during image acquisition. Spine area was measured as follows: Images acquired from GFP-expressing neurons were deconvolved and converted to binary. The pixels constituting spines were quantified as a measurement of area. Pixel counts were expressed as a ratio to the last time point before stimulation like in volume measurements. Only spines that clearly showed area increases before image manipulation were analyzed. For immunocytochemical experiments, three-channel images were acquired using spectrally distinct fluorophores and filter-set combinations: Alexa Fluor 488 or GFP (Zeiss 38 HE), Cy3 (Zeiss 20), and Cy5 (Zeiss 50). Cells were paraformaldehyde fixed and stained using standard procedures (8). For PA Rac1 experiments, photostimulation was accomplished using the Zeiss 38 HE filter-set and X-Cite 120XL light source at full power. Time-lapse imaging was precluded in this case because of insufficient accumulation of the transfection marker (mCherry).
Cell Culture and Transfection.
E18 dissociated hippocampal neuron cultures were prepared from rats as previously described (8), and plated at 40,000–50,000 cells per cm2 in Neurobasal/B27 (Invitrogen) on glass coverslips or plastic coated with PDL/Laminin (BD biosciences). 100 μM d-AP5 [D(-)-2-amino-5-phosphonovaleric acid] was added to media at 8 and 15 DIV, except where noted. Neurons were sparsely transfected by the calcium phosphate technique (8) at 4–5 DIV or 12 h before experimentation for PA Rac1 experiments. GFP was used as transfection marker for all but PA Rac1 electrophysiology experiments (mCherry). All experiments involving neurons were preformed at 19–20 DIV, or older where indicated. HEK-293T cells were maintained and transfected as previously described (8).
Biochemical Experiments.
For biochemical experiments involving NMDA stimulation, TTX was added to cultures (10-cm dish) 20 min before experiment start. Upon commencement, cultures were rinsed with Mg2+/Ca2+ free PBS, stimulated for 5 s with NMDA solution, and then quenched with a bolus of MgCl2 (2 mM) while the NMDA solution was removed. Cells were immediately lysed or rinsed and returned to their original media. Unstimulated cells were treated the same as NMDA stimulated cells except NMDA, glycine, and strychnine were omitted from the solution. For nonprecipitation experiments, cells were lysed in SDS/EGTA sample buffer (8) and immediately boiled, then frozen until further use. For precipitation experiments, the lysis buffer contained: 25 mM Hepes, 150 mM NaCl, 1% Nonidet P-40, 5 mM MgCl2, 1 mM DTT, 1 mM Na3VO4, 10 mM NaF, 10 mM β-glycerol phosphate, and protease inhibitors, pH 7.5; 0.1 mM CaCl2 was added where noted. Lysates were briefly sonicated and then spun at low speed to remove large insolubles. The resulting supernatant was rotated for 20 min with 15 µg of GST-substrate conjugated to glutathione agarose beads, and subsequently pelleted and washed three times with lysis buffer. Lysates were kept at 4 °C throughout. After the final wash, beads were pelleted, boiled in SDS sample buffer, and frozen until used. Proteins were detected by immunoblot using standard techniques (8).
Statistical Analysis.
Student t test or ANOVA with Fisher’s LSD test were used, with P < 0.05 considered significantly different. All electrophysiology, imaging, and biochemical data used for comparisons were derived from sister cultures, and pooled from at least three independent experiments. Data analysis was performed blind when not completely automated. P values were calculated with KaleidaGraph (Synergy Software). Black asterisks in graphs denote a significant difference between conditions with P < 0.01, and gray asterisks denote 0.05 > P > 0.01. Data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Drs. Channing Der, Klaus Hahn (University of North Carolina at Chapel Hill), Karel Svoboda (Howard Hughes Medical Institute), Tobias Meyer (Stanford University), Derrick Rossi (Harvard University), and Larry Feig (Tufts University) for reagents; and Drs. Joseph Duman and Matthew Weston (Baylor College of Medicine) for helpful comments on the manuscript. This work was funded by National Institutes of Health Grants R01 NS062829 (to K.F.T.) and R01 NS051262 (to C.R) and the German Research Council Excellence cluster EXC 257 (to C.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304340110/-/DCSupplemental.
References
- 1.Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493(7433):495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron. 2009;64(3):381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter JD, Klann E. Making synaptic plasticity and memory last: Mechanisms of translational regulation. Genes Dev. 2009;23(1):1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 5.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: Connecting dynamics to function. J Cell Biol. 2010;189(4):619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sit S-T, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124(Pt 5):679–683. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 7.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 8.Tolias KF, et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45(4):525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: Evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26(3):429–440. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Lu W, et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29(1):243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 11.Bekkers JM, Stevens CF. Cable properties of cultured hippocampal neurons determined from sucrose-evoked miniature EPSCs. J Neurophysiol. 1996;75(3):1250–1255. doi: 10.1152/jn.1996.75.3.1250. [DOI] [PubMed] [Google Scholar]
- 12.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16(6):1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee S-JR, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458(7236):299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472(7341):100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26(7):2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci USA. 2010;107(36):15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauer JA, Malenka RC, Nicoll RA. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988;334(6179):250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- 18.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manabe T, Renner P, Nicoll RA. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992;355(6355):50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- 20.García-Mata R, et al. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Tian X, Hartley DM, Feig LA. Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J Neurosci. 2006;26(6):1721–1729. doi: 10.1523/JNEUROSCI.3990-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai JA, Li S, Hartley DM, Feig LA. Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J Neurosci. 2009;29(5):1496–1502. doi: 10.1523/JNEUROSCI.5057-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Medarde A, Santos E. The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim Biophys Acta. 2011;1815(2):170–188. doi: 10.1016/j.bbcan.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Krapivinsky G, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40(4):775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 25.Jin SX, Feig LA. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS ONE. 2010;5(7):e11732. doi: 10.1371/journal.pone.0011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Hoog CL, et al. Ras binding triggers ubiquitination of the Ras exchange factor Ras-GRF2. Mol Cell Biol. 2001;21(6):2107–2117. doi: 10.1128/MCB.21.6.2107-2117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan WT, Koch CA, de Hoog CL, Fam NP, Moran MF. The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr Biol. 1998;8(16):935–938. doi: 10.1016/s0960-9822(07)00376-4. [DOI] [PubMed] [Google Scholar]
- 28.Buchsbaum R, Telliez JB, Goonesekera S, Feig LA. The N-terminal pleckstrin, coiled-coil, and IQ domains of the exchange factor Ras-GRF act cooperatively to facilitate activation by calcium. Mol Cell Biol. 1996;16(9):4888–4896. doi: 10.1128/mcb.16.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110(4):443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- 30.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461(7260):104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz DM, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15(6):891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto K-I, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7(10):1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 33.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58(4):472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stacey D, et al. IMAGEN Consortium RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc Natl Acad Sci USA. 2012;109(51):21128–21133. doi: 10.1073/pnas.1211844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez LA, Tejada-Simon MV. Pharmacological inactivation of the small GTPase Rac1 impairs long-term plasticity in the mouse hippocampus. Neuropharmacology. 2011;61(1-2):305–312. doi: 10.1016/j.neuropharm.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemtiri-Chlieh F, et al. Kalirin-7 is necessary for normal NMDA receptor-dependent synaptic plasticity. BMC Neurosci. 2011;12:126. doi: 10.1186/1471-2202-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Z, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56(4):640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian X, et al. Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk and CREB. EMBO J. 2004;23(7):1567–1575. doi: 10.1038/sj.emboj.7600151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.