Abstract
Intensive land use reduces the diversity and abundance of many soil biota, with consequences for the processes that they govern and the ecosystem services that these processes underpin. Relationships between soil biota and ecosystem processes have mostly been found in laboratory experiments and rarely are found in the field. Here, we quantified, across four countries of contrasting climatic and soil conditions in Europe, how differences in soil food web composition resulting from land use systems (intensive wheat rotation, extensive rotation, and permanent grassland) influence the functioning of soils and the ecosystem services that they deliver. Intensive wheat rotation consistently reduced the biomass of all components of the soil food web across all countries. Soil food web properties strongly and consistently predicted processes of C and N cycling across land use systems and geographic locations, and they were a better predictor of these processes than land use. Processes of carbon loss increased with soil food web properties that correlated with soil C content, such as earthworm biomass and fungal/bacterial energy channel ratio, and were greatest in permanent grassland. In contrast, processes of N cycling were explained by soil food web properties independent of land use, such as arbuscular mycorrhizal fungi and bacterial channel biomass. Our quantification of the contribution of soil organisms to processes of C and N cycling across land use systems and geographic locations shows that soil biota need to be included in C and N cycling models and highlights the need to map and conserve soil biodiversity across the world.
Keywords: soil fauna, modeling, soil microbes, nitrogen
Soils are of central importance for delivering ecosystem services, such as food production and climate mitigation. These services strongly depend on carbon (C) sequestration and nutrient cycling, processes that are governed by soil biota. Increasing demand for the production of food, fiber, and biofuel has resulted in intensification of agricultural production, which reduces soil organic matter content (1) and the biomass and diversity of most soil biota (2), with consequent impacts on processes of C and nutrient cycling. Specifically, land use-induced shifts to more bacterial-dominated microbial communities have been linked to increased nitrogen (N) losses (3–5) and reduced C sequestration (6). Conversely, fungal-dominated microbial communities, which are common in less intensively managed land use systems, are linked to more conservative nutrient cycling and greater storage of C (5, 7, 8). Although soil microbes are the primary actors in C and N cycling, their biomass and activity are greatly influenced by higher trophic levels of the soil food web. For instance, animals that consume microorganisms can stimulate rates of nutrient mineralization (9) and plant productivity (10), whereas bioturbators, such as earthworms, can further increase nutrient availability for plants (11), although they can also increase N2O emissions from soil (12).
Although there is evidence from field studies that soil microbial communities are linked to ecosystem functioning (13, 14), most studies on relationships between soil fauna and ecosystem function have been done in controlled (microcosm) experiments (15). As a result, our understanding of the functional importance of different groups of soil biota and the connections between them (the soil food web) in the field is limited, and it is not known how changes in soil food web structure across contrasting locations and land use systems impact on ecosystem functioning. There is some evidence to suggest that the role of the soil food webs relative to abiotic factors in regulating ecosystem functions will vary across geographical locations and environmental gradients (16). Moreover, differences in land use have been shown to affect the resistance and the resilience of soil food webs to simulated drought, with consequences for processes of C and N cycling (17). Therefore, quantifying general relationships between soil biota and processes of C and N cycling is of pivotal importance for predicting how these processes will be affected by global change.
Our aim was to quantify, across geographically contrasting locations in Europe, how changes in soil food web composition resulting from land use systems influence the ecosystem services that they deliver. We hypothesized that, across European land use systems, processes of C and N cycling are explained by soil food web properties on top of variation explained by other factors, such as land use and soil physical and chemical properties. Specifically, we hypothesized that (i) more intensive land use consistently reduces the biomass of soil fungi and their consumers and increases the dominance of bacteria and their consumers [i.e., decrease the fungal/bacterial (F/B) channel ratio] and (ii) a shift to greater dominance of bacteria and their consumers (i.e., decrease F/B channel ratio) increases rates of C and N cycling and loss.
To test these hypotheses, we measured C and N fluxes at 60 sites in four European countries (Sweden, United Kingdom, Czech Republic, and Greece) distributed across five locations in each country representing intensive annual crop rotation [high intensity (H)], extensive rotation, including legumes or ley [medium intensity (M)], or permanent grassland [low intensity (L)]. Measurements included potential N mineralization, which is a measure of the release of N for plant uptake, and losses of N and C from soil both as gases and in drainage waters. Gaseous emissions from agricultural soils, as N2O, CO2, and CH4, contribute significantly to global warming and atmospheric pollution (18), and leaching of C and N in drainage waters contributes to eutrophication of ground and surface water (19). We also quantified the biomass of key functional groups in the soil food web, including fungi and bacteria, protozoa, nematodes, earthworms, Enchytraeids, mites, and collembolans. To relate the structure of the soil food web to C and N fluxes, we calculated traditional soil food web properties, such as the number of feeding groups in the food web. In addition, to test our hypothesis that land use will alter the relative importance of the fungal and bacterial energy channels, we calculated measures based on the fungal, bacterial, and root energy channels (SI Appendix, SI Methods).
Soil food webs and their corresponding C and N fluxes are likely to be affected by factors, such as land use, soil properties, and spatial structure of sampling sites. To determine whether soil food web characteristics explained a unique proportion of variation in ecosystem services and deduce meaningful relationships between soil food web properties and C and N fluxes, we also accounted for variation caused by the spatial structure of the sites (which can be caused by autocorrelation between values of the response variable or underlying factors, such as climate and geology), land use, and soil properties. We accounted for spatial autocorrelation in the measured variables by calculating spatial filters using principle coordinates of neighbor matrices (20), and we used a hierarchical modeling approach that has previously been used to explain landscape-scale variation in soil microbial communities on the basis of climatic factors, soil properties, and plant traits (21) (Materials and Methods and SI Appendix).
Results and Discussion
Across all four countries, soil food web structure was strongly influenced by land use (SI Appendix, Tables S1 and S2). The number of feeding groups, total biomass of the soil food web, and biomass of the fungal, bacterial, and root energy channel [which consists of arbuscular mycorrhizal fungi (AMF), root-feeding fauna, and their predators] were all lower under the M and H land use categories relative to the L category (SI Appendix, Table S1). The biomass of many individual feeding groups of soil biota was lower under these more-intensive land uses (SI Appendix, Table S2). These results indicate that, across contrasting sites in Europe, land use intensification consistently reduces the biomass of all components of the soil food web. However, in contrast to our hypothesis, land use intensification did not influence the ratio of fungal to bacterial biomass or the ratio of fungal energy channel to bacterial energy channel biomass in any of the countries sampled. Instead, land use intensification equally reduced the biomass of most feeding groups in the soil food web. However, the biomass of the groups that are part of the root energy channel was reduced more than the biomass of the organisms of the fungal and bacterial energy channel together (the detritus energy channel) (SI Appendix, Table S1). This difference can be explained by the effect of tillage, which was included in the M and H land use forms, because it disrupts root-associated organisms and their consumers (8, 22).
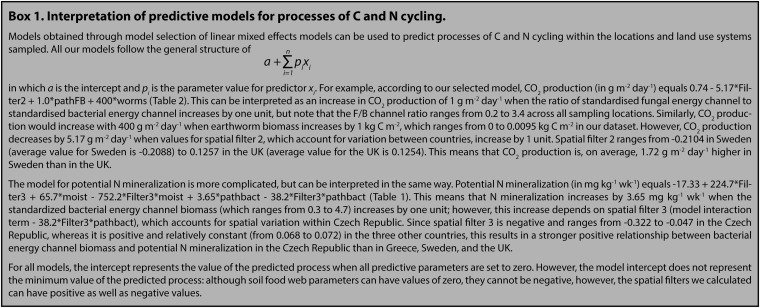
All our models explaining processes of C and N cycling included one or more soil food web measures, indicating that relationships between soil biota and ecosystem functioning are surprisingly consistent across contrasting sites in Europe (Tables 1 and 2). In four of six models, land use was not included as an explanatory variable, which indicates that soil food web properties are better predictors of processes of C and N cycling than the three land use systems. Land use might not be included in our models because within our three broad land use categories, differences in management might have impacted on soil food web structure, which in turn, affected ecosystem processes (SI Appendix, Tables S4–S7 shows management details of all sites). The inclusion of spatial filters in all final models illustrates the importance of accounting for the spatial structure of sampling sites across this scale (Tables 1 and 2) and shows that C and N fluxes varied both among and within European regions. Still, only the model for potential N mineralization included an interaction term between a soil food web property (namely bacterial energy channel biomass) and a spatial filter (filter 3), indicating that the relationship between bacterial energy channel biomass and N mineralization was dependent on geographical location (Box 1 and Table 1). For all other models, the relationship between soil food web properties and the process of C or N cycling was independent of location.
Table 1.
Selected models for potential N mineralization, total N leached, and N2O production
| Potential N mineralization |
Total N leached |
N2O |
||||
| Parameter value | P | Parameter value | P | Parameter value | P | |
| Intercept | −17.33 | 0.0096 | 774 | <0.0001 | 0.606 | 0.0009 |
| Spatial filters | +224.7*Filter3 | <0.0001 | −1,932*Filter2 | 0.0004 | −2.445*filter5 | 0.0054 |
| Soil physical properties | +65.7*moist; −752.2*Filter3*moist | <0.0001; <0.0001 | ||||
| Land use | ||||||
| N and C stocks | ||||||
| Soil food web structure | +3.64*pathbact; −38.2*Filter3*pathbact | 0.0074; 0.0027 | ||||
| Biomass of individual functional groups | −60,114*AM fungi; +16,357,441* bacnem | 0.004; 0.024 | −4,678*flagellates | 0.0196 | ||
| Model R2 | 0.45 | 0.34 | 0.17 | |||
For each N cycling process, the best explaining model is shown, with intercept, parameters, their parameter value (within each category of parameters), and P value as obtained by an L-ratio deletion test (SI Appendix, SI Methods). Interpretation of the models is in Box 1. Bacnem, biomass of bacterial-feeding nematodes; moist, moisture content; pathbact, standardized biomass of the bacterial energy channel.
Table 2.
Selected models for CO2 production, CH4 production, and DOC leached
| CO2 |
CH4 |
DOC leached |
||||
| Parameter value | P | Parameter value | P | Parameter value | P | |
| Intercept | 0.74 | 0.033 | −0.27 | 0.044 | 296 | <0.0001 |
| Spatial filters | −5.17*Filter2 | 0.0003 | −658*Filter2; −230*Filter4 | 0.001; 0.28 | ||
| Soil physical properties | ||||||
| Land use | −0.08*L; +0.17*M | 0.0078 | +326*L; −1,317*Filter4*L | <0.0001; 0.0001 | ||
| N and C stocks | ||||||
| Soil food web structure | +1.0*pathFB | 0.0003 | −0.08*F/B ratio | 0.046 | ||
| Biomass of individual functional groups | +400*worms | <0.0001 | +6.65*bacteria | 0.049 | +8,106,164*fungcoll; +5,798,305*bacnem | <0.0001; 0.017 |
| Model R2 | 0.53 | 0.24 | 0.77 | |||
For each C cycling process, the best explaining model is shown, with intercept, parameters, their parameter value (within each category of parameters), and P value as obtained by an L-ratio deletion test (SI Appendix, SI Methods). Interpretation of the models is in Box 1. Bacnem, biomass of bacterial-feeding nematodes; fungcoll, biomass of fungal-feeding Collembola; pathFB, fungal-to-bacterial energy channel biomass ratio; worms, earthworm biomass.
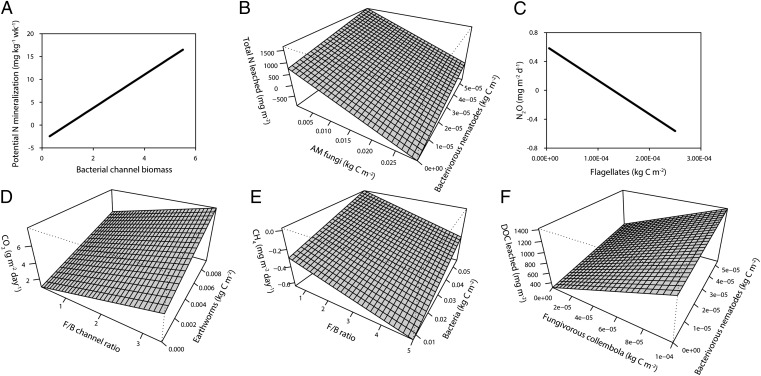
In line with our second hypothesis, across all 60 European farmland sites, the biomass of the bacterial energy channel was positively related to rates of N mineralization (Fig. 1 and Table 1). Interestingly, although the bacterial energy channel was reduced by intensive land use, N mineralization was not affected by land use (SI Appendix, Fig. S1), suggesting that the relationship between the bacterial energy channel and N mineralization was independent of land use. Field studies have shown that fungal-based soil food webs have lower N leaching losses from soil (5, 23) and lower rates of N mineralization (24). In laboratory studies, greater bacterial abundance has been linked to increased rates of N mineralization, and the presence of bacterial feeders in soil has often been shown to increase rates of N mineralization both indirectly through stimulating bacterial activity and directly through excreting N compounds (9, 25, 26). However, our study shows that N mineralization rates increase with greater biomass of the entire bacterial decomposition channel. This observation suggests that the intensification-induced reduction in bacterial channel biomass might increase the dependency on mineral fertilizer.
Fig. 1.
Fitted relationships between ecosystem services and soil food web properties. Variables that were included in the models but not shown in the graphs (Tables 1 and 2) were kept constant at their mean value in the dataset. (A) Potential N mineralization explained by standardized biomass of the bacterial energy channel. (B) Total N leached explained by AMF biomass and biomass of bacterivorous nematodes. (C) N2O production explained by biomass of flagellates. (D) CO2 production explained by F/B channel ratio and earthworm biomass. (E) CH4 production explained by F/B ratio and bacterial biomass (relationship shown is for intensive wheat rotation and permanent grassland) (extensive rotation CH4 production increases with 0.17 mg m−2 d−1 are shown in Table 2). (F) DOC leached from soil explained by fungivorous collembolans and bacterivorous nematodes (relationship shown is for intensive wheat rotation and extensive rotation) (permanent grassland DOC leaching increases with 1,317 mg m−2 as shown in Table 2).
Mineralization of N can turn into a disservice when N supply is too high for crop uptake and excess N is washed away in drainage waters or lost to the atmosphere through denitrification (27). Across all sites, leaching of N was strongly explained by the biomass of two functional groups, which together accounted for more than one-half of the variation explained by the full model (SI Appendix, Table S3). N leaching increased with greater biomass of bacterial-feeding nematodes (Fig. 1 and Table 1), which is in line with our hypothesis and the stimulating effect of bacterial grazers on N mineralization. In addition, we found that N leaching decreased with increasing biomass of AMF across all sites. Laboratory studies have shown that AMF reduce leaching of N and phosphorus (P) (28), but we are not aware of such a relationship being detected in the field, which we show here. Surprisingly, N leaching was not affected by land use across sites (SI Appendix, Fig. S1), which shows that its relationship with AMF is independent of the impact of land use on AMF.
Production of N2O—a product of the denitrification process in soil—decreased across all locations with increasing biomass of flagellates, a group of protozoa that are part of the bacterial energy channel (Table 1). A mechanistic link between protozoa and N2O production has never been reported before. Because protozoa are aquatic organisms, this correlation probably reflects that denitrification predominantly occurs in anoxic zones in the soil (12). Although N2O emission is generally strongly affected by agricultural management (29), we did not find a link with land use here (SI Appendix, Fig. S1).
Across all sites, we found that the three land use types were all methane sinks, and the intensive rotation and permanent grassland were stronger methane sinks than the extensive rotation. Legumes were included in the extensive rotation in three of four countries (SI Appendix, Fig. S1 and Tables S4–S7) and have been shown to reduce the strength of the methane sink in grasslands (30). Methane consumption also decreased with decreasing F/B biomass ratio and increasing biomass of bacteria (Table 2), which suggests that the decrease in bacterial biomass as a result of land use intensification, such as was found here, might affect the abundance of methanotrophs (for example, through an increase in nitrifiers at the expense of methanotrophs) (31).
Production of CO2 measured in situ is a measure of soil heterotrophic activity and root respiration, and it forms a pathway of C loss from soil. Production of CO2 was greatest in the permanent grassland (SI Appendix, Fig. S1), which is consistent with these soils having the greatest C content (SI Appendix, Tables S4–S7). Production of CO2 was also positively related to the biomass of earthworms, which were most abundant in the permanent grassland (SI Appendix, Table S2). Several field-based experiments have shown significant impacts of earthworms on C and N cycling (12), but evidence for impacts of earthworms on respiration in the field is scarce. In addition and in contrast to our hypothesis, CO2 production increased with greater importance of the fungal energy channel (greater F/B channel ratio) (Fig. 1 and Table 2), a relationship that was independent of land use. Fungal-dominated soil food webs are thought to be more efficient in their C use, although evidence is limited (6). The positive relationship found here between the fungal decomposition pathway and CO2 production might be a consequence of the fact that C-rich soils are generally fungal-dominated (5); consistent with this explanation, we found a positive relationship between biomass of the fungal energy channel and soil organic C (SI Appendix, Table S1). However, a greater CO2 production does not necessarily mean a greater loss of soil C given that soil C content is determined by the balance between C loss by respiration and C gain by photosynthesis.
Similar to CO2 production, leaching of dissolved organic carbon (DOC) was greatest from permanent grassland across all sites (Table 2). In addition, DOC leaching increased with the biomass of fungal-feeding collembolans and bacterial-feeding nematodes. This increase might be a consequence of the greater biomass of the fungal energy channel with greater soil C stocks (SI Appendix, Table S1), although the biomass of fungal-feeding collembolans itself was not related to soil organic C (SI Appendix, Table S2). The link between DOC leaching and fungal-feeding collembolans suggests that this functional group might be a sensitive indicator for changes in labile C availability. In addition, labile C constitutes an easily decomposable food source for microbes, which might stimulate microbial growth and increase the biomass of bacterial and fungal grazers through bottom-up effects (32).
In sum, we found strong and consistent impacts of land use on the structure of soil food webs across land use systems in four climatically different regions in Europe; land use intensification reduced the abundance of most functional groups of soil organisms. In turn, soil food web properties strongly influenced processes of C and N cycling, and these relationships were consistent across land use types and sampling locations. The predictive power of soil food web structure or functional groups varied between the processes measured but was of equal importance as abiotic factors (SI Appendix, Table S3). Although relationships between soil food web properties and processes of C cycling were mostly related to land use intensity, relationships with N cycling processes were not. In all cases, soil food web properties were better predictors of processes of C and N cycling than the tree land use systems. Although ultimately correlative, the relationships that we found between bacterial-feeding animals, AMF, and earthworms and C and N cycling are in line with results from mechanistic experiments (9, 12, 28). Therefore, our results strongly suggest that including soil food web parameters will enhance the predictive capacity of C and N cycling models.
Process-based C and N cycling models require detailed input information that is often not available on regional scales (33), and general relationships between soil food web properties and processes of C and N cycling have the potential to simplify these models. Although more validation is needed (for example, within the countries and soil types sampled), the simple relationships between earthworms and CO2 production or between AMF abundance and N leaching might help parameterize C cycling (34) and ecosystem service models (35). Moreover, explicitly incorporating soil food web properties and their response to land use and climate change (17) in dynamic global vegetation models might improve predictions of climate change impacts on terrestrial ecosystem functions and their feedbacks to climate change (36). Finally, there is an urgent need to identify and evaluate indicators for soil-based ecosystem services (37). The quantitative relationships between relatively simple soil food web measures and ecosystem services shown in our analysis could be used to assess soil-based ecosystem services and disservices, such as N leaching from soil. Although the relationships revealed by our analysis require additional validation, they are an important first step to quantifying general relationships between soil food web properties and ecosystem processes in the field. Soil biodiversity is under threat by a range of pressures but remains severely understudied (38); our results explicitly quantify the contribution of soil organisms to processes of C and N cycling across a range of management and environmental conditions and thus, warrant efforts to map and conserve soil biodiversity across the world.
Materials and Methods
Field Sites and Sampling.
We selected four countries across Europe: Sweden, United Kingdom, Czech Republic, and Greece. In each country, sampling was done at five locations, and each location had three managements: intensive rotation (H), extensive rotation (M), and permanent grassland (L). This nested design resulted in 60 sampling sites (4 countries × 5 farms × 3 managements). Between May and July of 2009, in each site, two 1-m2 plots were randomly selected, and for each soil nutrient, microbial and faunal analyses of separate replicate soil cores (5-cm diameter and 10-cm depth) were taken from each plot and kept cool (4 °C) until analysis (see below). Gas samples were taken in situ: in each plot, a 10-cm inner diameter collar consisting of a PVC cylinder was pushed 5 cm into the soil. Then, a 5-cm-high PVC lid was fitted into a butyl rubber-lined groove in each collar. An 8-mL gas sample was taken immediately and 30 min after attaching the lids. SI Appendix, Tables S4–S7 has climate data of sampling regions and details on soil properties and management.
Soil Analyses.
Total soil C and N were analyzed on air-dried soil with a Leco CNS-2000 analyzer, and total organic C was measured in a PrimacsSLC TOC Analyzer on dried (100 °C) soil. Soil pH and gravimetric moisture content were determined using standard methods. Water-holding capacity was determined by placing saturated undisturbed soil cores on a suction pressure plate, and after drying at 105 °C, bulk density was calculated. All soil, food web, and nutrient flux measures were expressed per meter squared, except potential N mineralization.
C and N Fluxes.
Gas samples were analyzed for CO2, N2O, and methane as described in the work by Priemé and Christensen (39). Soil leachates were obtained and analyzed for concentrations of inorganic N and DOC and total N as described in the work by de Vries et al. (5). Potential N mineralization was assessed by incubating a 5-g soil sample at 60% water holding capacity for 1 and 3 wk at 25 °C, extracting with KCl, and analyzing inorganic N. The net amount of inorganic N mineralized in 2 wk was calculated as the difference in inorganic N between weeks 3 and 1.
Food Web Analyses.
Phospholipid fatty acids (PLFAs) were extracted from 3 g soil according to the work by Frostegård and Bååth (40). The PLFAs 15:0, i15:0, a15:0, i16:0, 16:0ω9, i17:0, a17:0, cy17:0, 18:1ω7, and cy19:0 were used as markers of bacterial biomass (40). The amount of PLFA 18:2ω6 was used as a marker of nonmycorrhizal fungal biomass, and the neutral lipid fatty acid 16:1ω5 was used as a marker for AMF (41). Fatty acids were converted into biomass C using the following factors: bacterial biomass, 363.6 nmol PLFA = 1 mg carbon (40); fungal biomass, 11.8 nmol PLFA = 1 mg carbon (42); and AMF biomass, 1.047 nmol neutral lipid fatty acid = 1 µg carbon (41). Protozoa numbers were estimated using a modified most probable number method, and enchytraeid worms were extracted from intact soil core samples using wet funnels. Nematodes were extracted from a 150-mL sample with the modified Cobb sieving and decanting method (43), and soil mesofauna were extracted from undisturbed samples using Tullgren funnels. Nematodes were identified to the genus level and allocated to trophic groups; Collembola, Acari, and Oribatida were determined to species level. More information on food web analyses and biomass calculations is in SI Appendix.
Statistical Analysis.
We generated statistical models for each ecosystem service using spatial filters, soil properties, land use, and soil food web characteristics. We used linear mixed effects models with a farm-level random effect term to account for the clustering of fields in sampling locations. Analysis was conducted using the lme function of R version 2.11.1 (R Development Core Team 2009). Model selection followed the hierarchical procedure used in the work by de Vries et al. (21). In short, the order in which variables were added to linear mixed effects models followed a hypothesized sequence of controls, being such that variables added later in the modeling process are unlikely to affect those variables added earlier. The first terms added to the models were spatial filters, after which we sequentially added soil properties, land use, soil C and N contents, and finally, soil food web properties. Models were selected based on Akaike Information Criterion, and true significance of retained terms was assessed by a χ2 likelihood ratio deletion test. Detailed information on the modeling procedure is in SI Appendix.
Supplementary Material
Acknowledgments
We thank all landowners for kindly letting us use their fields, and George Boutsis, Maria Karmezi, Sofia Nikolaou, Evangelia Boulaki, Charisis Argiropoulos, Annette Spangenberg, and Helen Quirk for help in the field and laboratory. We also thank two anonymous referees for their helpful comments on the manuscript. This project was part of the European Union Seventh Framework funded SOILSERVICE Project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305198110/-/DCSupplemental.
References
- 1.Stoate C, et al. Ecological impacts of arable intensification in Europe. J Environ Manage. 2001;63(4):337–365. doi: 10.1006/jema.2001.0473. [DOI] [PubMed] [Google Scholar]
- 2.Postma-Blaauw MB, de Goede RGM, Bloem J, Faber JH, Brussaard L. Soil biota community structure and abundance under agricultural intensification and extensification. Ecology. 2010;91(2):460–473. doi: 10.1890/09-0666.1. [DOI] [PubMed] [Google Scholar]
- 3.de Vries FT, Van Groenigen JW, Hoffland E, Bloem J. Nitrogen losses from two grassland soils with different fungal biomass. Soil Biol Biochem. 2011;43(5):997–1005. [Google Scholar]
- 4.Hunt HW, et al. The detrital food web in a shortgrass prairie. Biol Fertil Soils. 1987;3(1):57–68. [Google Scholar]
- 5.de Vries FT, et al. Extensive management promotes plant and microbial nitrogen retention in temperate grassland. PLoS One. 2012;7(12):e51201. doi: 10.1371/journal.pone.0051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Six J, Frey SD, Thiet RK, Batten KM. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J. 2006;70(2):555–569. [Google Scholar]
- 7.Bardgett RD, Wardle DA. Aboveground–Belowground Linkages. Biotic Interactions, Ecosystem Processes, and Global Change. New York: Oxford Univ Press; 2010. [Google Scholar]
- 8.de Vries FT, Bardgett RD. Plant-microbial linkages and ecosystem N retention: Lessons for sustainable agriculture. Front Ecol Environ. 2012;10(8):425–432. [Google Scholar]
- 9.Postma-Blaauw MB, et al. Within-trophic group interactions of bacterivorous nematode species and their effects on the bacterial community and nitrogen mineralization. Oecologia. 2005;142(3):428–439. doi: 10.1007/s00442-004-1741-x. [DOI] [PubMed] [Google Scholar]
- 10.Setälä H, Huhta V. Soil fauna increase Betula pendula growth: Laboratory experiments with coniferous forest floor. Ecology. 1991;72(2):665–671. [Google Scholar]
- 11.Postma-Blaauw MB, et al. Earthworm species composition affects the soil bacterial community and net nitrogen mineralization. Pedobiologia. 2006;50(3):243–256. [Google Scholar]
- 12. Lubbers IM, et al. (2013) Greenhouse-gas emissions from soils increased by earthworms. Nat Clim Chang 3(3):187–194.
- 13.Hallin S, Jones CM, Schloter M, Philippot L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009;3(5):597–605. doi: 10.1038/ismej.2008.128. [DOI] [PubMed] [Google Scholar]
- 14.Allison SD, et al. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology. 2013;94(3):714–725. doi: 10.1890/12-1243.1. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen UN, Ayres E, Wall DH, Bardgett RD. Soil biodiversity and carbon cycling: A review and synthesis of studies examining diversity-function relationships. Eur J Soil Sci. 2011;62(1):105–116. [Google Scholar]
- 16.Wall DH, et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Chang Biol. 2008;14(11):2661–2677. [Google Scholar]
- 17.de Vries FT, et al. Land use alters the resistance and resilience of soil food webs to drought. Nat Clim Chang. 2012;2(4):276–280. [Google Scholar]
- 18.Smith P. Agricultural greenhouse gas mitigation potential globally, in Europe and in the UK: What have we learnt in the last 20 years? Glob Chang Biol. 2012;18(1):35–43. [Google Scholar]
- 19.Schlesinger WH. On the fate of anthropogenic nitrogen. Proc Natl Acad Sci USA. 2009;106(1):203–208. doi: 10.1073/pnas.0810193105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borcard D, Legendre P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Modell. 2002;153(1-2):51–68. [Google Scholar]
- 21.de Vries FT, et al. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol Lett. 2012;15(11):1230–1239. doi: 10.1111/j.1461-0248.2012.01844.x. [DOI] [PubMed] [Google Scholar]
- 22.Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW. Ploughing up the wood-wide web? Nature. 1998;394(6692):431. doi: 10.1038/28764. [DOI] [PubMed] [Google Scholar]
- 23.de Vries FT, Hoffland E, van Eekeren N, Brussaard L, Bloem J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol Biochem. 2006;38(8):2092–2103. [Google Scholar]
- 24.Högberg MN, Chen Y, Högberg P. Gross nitrogen mineralisation and fungi-to-bacteria ratios are negatively correlated in boreal forests. Biol Fertil Soils. 2007;44(2):363–366. [Google Scholar]
- 25.Schröter D, Wolters V, De Ruiter PC. C and N mineralisation in the decomposer food webs of a European forest transect. Oikos. 2003;102(2):294–308. [Google Scholar]
- 26.Fraterrigo JM, Balser TC, Turner MG. Microbial community variation and its relationship with nitrogen mineralization in historically altered forests. Ecology. 2006;87(3):570–579. doi: 10.1890/05-0638. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Ricketts TH, Kremen C, Carney K, Swinton SM. Ecosystem services and dis-services to agriculture. Ecol Econ. 2007;64(2):253–260. [Google Scholar]
- 28.van der Heijden MGA. Mycorrhizal fungi reduce nutrient loss from model grassland ecosystems. Ecology. 2010;91(4):1163–1171. doi: 10.1890/09-0336.1. [DOI] [PubMed] [Google Scholar]
- 29.Van Groenigen JW, Velthof GL, Oenema O, Van Groenigen KJ, Van Kessel C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur J Soil Sci. 2010;61(6):903–913. [Google Scholar]
- 30.Niklaus PA, Wardle DA, Tate KR. Effects of plant species diversity and composition on nitrogen cycling and the trace gas balance of soils. Plant Soil. 2006;282(1-2):83–98. [Google Scholar]
- 31.Willison TW, Oflaherty MS, Tlustos P, Goulding KWT, Powlson DS. Variations in microbial populations in soils with different methane uptake rates. Nutr Cycl Agroecosyst. 1997;49(1-3):85–90. [Google Scholar]
- 32.de Vries FT, et al. Legacy effects of drought on plant growth and the soil food web. Oecologia. 2012;170(3):821–833. doi: 10.1007/s00442-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 33.Luo ZK, et al. Meta-modeling soil organic carbon sequestration potential and its application at regional scale. Ecol Appl. 2013;23(2):408–420. doi: 10.1890/12-0672.1. [DOI] [PubMed] [Google Scholar]
- 34.Ciais P, Gervois S, Vuichard N, Piao SL, Viovy N. Effects of land use change and management on the European cropland carbon balance. Glob Chang Biol. 2011;17(1):320–338. [Google Scholar]
- 35.Aitkenhead MJ, Albanito F, Jones MB, Black HIJ. Development and testing of a process-based model (MOSES) for simulating soil processes, functions and ecosystem services. Ecol Modell. 2011;222(20-22):3795–3810. [Google Scholar]
- 36.Ostle NJ, et al. Integrating plant-soil interactions into global carbon cycle models. J Ecol. 2009;97(5):851–863. [Google Scholar]
- 37.Pulleman M, et al. Soil biodiversity, biological indicators and soil ecosystem services-an overview of European approaches. Curr Opin Environ Sustain. 2012;4(5):529–538. [Google Scholar]
- 38.Gardi C, Jeffery S, Saltelli A. An estimate of potential threats levels to soil biodiversity in EU. Glob Chang Biol. 2013;19(5):1538–1548. doi: 10.1111/gcb.12159. [DOI] [PubMed] [Google Scholar]
- 39.Priemé A, Christensen S. Natural perturbations, drying-wetting and freezing-thawing cycles, and the emission of nitrous oxide, carbon dioxide and methane from farmed organic soils. Soil Biol Biochem. 2001;33(15):2083–2091. [Google Scholar]
- 40.Frostegård Å, Bååth E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils. 1996;22(1-2):59–65. [Google Scholar]
- 41.Olsson PA, Bååth E, Jakobsen I, Söderström B. The use of phospholipid and neutral lipid fatty-acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol Res. 1995;99(5):623–629. [Google Scholar]
- 42.Klamer M, Bååth E. Estimation of conversion factors for fungal biomass determination in compost using ergosterol and PLFA 18: 2 omega 6,9. Soil Biol Biochem. 2004;36(1):57–65. [Google Scholar]
- 43.S'jacob JJ, Van Bezooijen J. A Manual for Practical Work in Nematology. Wageningen, The Netherlands: Wageningen University; 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.