Abstract
Inflammation and vascular injury triggered by ischemia/reperfusion (I/R) represent a leading cause of morbidity and mortality in a number of clinical settings. Wnt and its homolog partners R-spondins, in addition to regulating embryonic development have recently been demonstrated to serve as wound-healing agents in inflammation-associated conditions. Here we ask whether R-spondins could prevent inflammation-associated tissue damage in ischemic disorders and thus investigate the role of R-spondin3 (R-spo3) in a mouse model of mesenteric I/R. We demonstrate that R-spo3 ameliorates mesenteric I/R-induced local intestinal as well as remote lung damage by suppressing local and systemic cytokine response and deposition of IgM and complement in intestinal tissues. We also show that decreased inflammatory response is accompanied by tightening of endothelial cell junctions and reduction in vascular leakage. We conclude that R-spo3 stabilizes endothelial junctions and inhibits vascular leakage during I/R and thereby mitigates the inflammatory events and associated tissue damage. Our findings uniquely demonstrate a protective effect of R-spo3 in I/R-related tissue injury and suggest a mechanism by which it may have these effects.
Keywords: permeability, tissue repair, organ damage, junctional integrity
The susceptibility of ischemic tissue to ensuing catastrophic episodes mediated during the reestablishment of blood flow is known as ischemia/reperfusion (I/R) injury. Mesenteric ischemia (ischemia of the small intestine) is a common life-threatening abdominal emergency encountered in a variety of clinical and surgical settings (1–3). Acute mesenteric ischemia induces local cellular changes such as cytoskeletal disorganization, up-regulation of adhesion molecules, and neoantigen expression that provokes an intense inflammatory response and endothelial barrier dysfunction during subsequent reperfusion. Several molecules such as natural antibodies, complement, cytokines, and chemokines together with various cells including neutrophils, endothelial cells, T cells, B cells, NK cells, and platelets, collectively choreograph the inflammation and vascular injury that occur during mesenteric I/R (3–5). A number of pharmacological agents and therapeutic strategies to attenuate the immune response triggered by intestinal I/R have been proposed, including the use of antioxidant agents, leukocyte and lymphocyte depletion, and inhibition of complement activation (4, 5). Despite significant advances in this field, no agent or therapy has yet gained widespread clinical use for the prevention and treatment of ischemic-related conditions.
The human and mouse R-spondins encode a family of proteins that includes four paralogs (R-spo1–4), each of which contains a leading signal peptide, two cysteine-rich furin-like domains, and one thrombospondin type I domain (6). R-spondins are secreted proteins found primarily in the extracellular region and are known to promote β-catenin signaling. Although structurally related, R-spondins reveal a strikingly different expression pattern of transcripts in mouse embryos at different developmental stages (7). The embryonic expression of R-spondins often overlaps with that of known Wnt genes. Wnt signaling is essential for embryonic development, cell differentiation, cell polarity generation, and adult tissue homeostasis (8) and R-spondins activate the canonical Wnt pathway by inducing β-catenin/T-cell factor (TCF)-dependent gene activation (6, 9). R-spo3 is also involved in Wnt/planar cell polarity signaling through clathrin-mediated endocytosis-involving syndecan 4 (10). R-spondins have been claimed to function as mitogens, morphogens, stem cell growth factors, angiogenic factors, and wound healing agents (11, 12). New studies also highlight the role of Wnt and its homolog partners R-spondins in autoimmune and inflammatory disease models including hepatic I/R injury (13, 14). R-spo1 ameliorates inflammation-associated tissue damage in murine models of experimental colitis (14), graft-versus-host disease (15), and arthritis (16) and may have clinical value. Considering the growing number of studies that implicate the beneficial role of R-spondins, we asked if these proteins could also prevent inflammation-associated tissue damage in ischemic disorders.
In this study, we describe the effect of R-spo3 in mesenteric I/R and illustrate a mechanism for its protective role. Our findings reveal that R-spo3 protects tissues against mesenteric I/R by tightening endothelial cell junctions and improving vascular integrity, which consequently dampens vascular leakage and the commencement of inflammatory events triggered during reperfusion.
Results
R-Spondin3 Is Predominantly Expressed in Endothelial Cells.
We first sought to determine the endogenous mRNA expression pattern of R-spondins during mesenteric I/R in the small intestine of C57B6/J mice using qPCR. As shown in Fig. S1, R-spo1, -2, and -4 were detected at negligible amounts, whereas R-spo3 was weakly expressed in the intestinal tissues of mice subjected to sham and I/R. We also assessed the gene expression pattern of R-spondins in different mouse and human cell types including monocytes, granulocytes, dendritic cells, lymphocytes, and epithelial (intestinal) and endothelial cells. R-spo3 was the only family member predominantly expressed in the endothelial cells from mice and humans (Fig. S2 A and B). Following tumor necrosis factor alpha (TNFα) stimulation of endothelial cells, R-spo3 levels increased, suggesting a role in the inflammatory process (Fig. S3). These observations prompted us to determine the ability of R-spo3 to modulate I/R-instigated injury.
R-Spondin3 Attenuates Mesenteric I/R-Induced Tissue Damage.
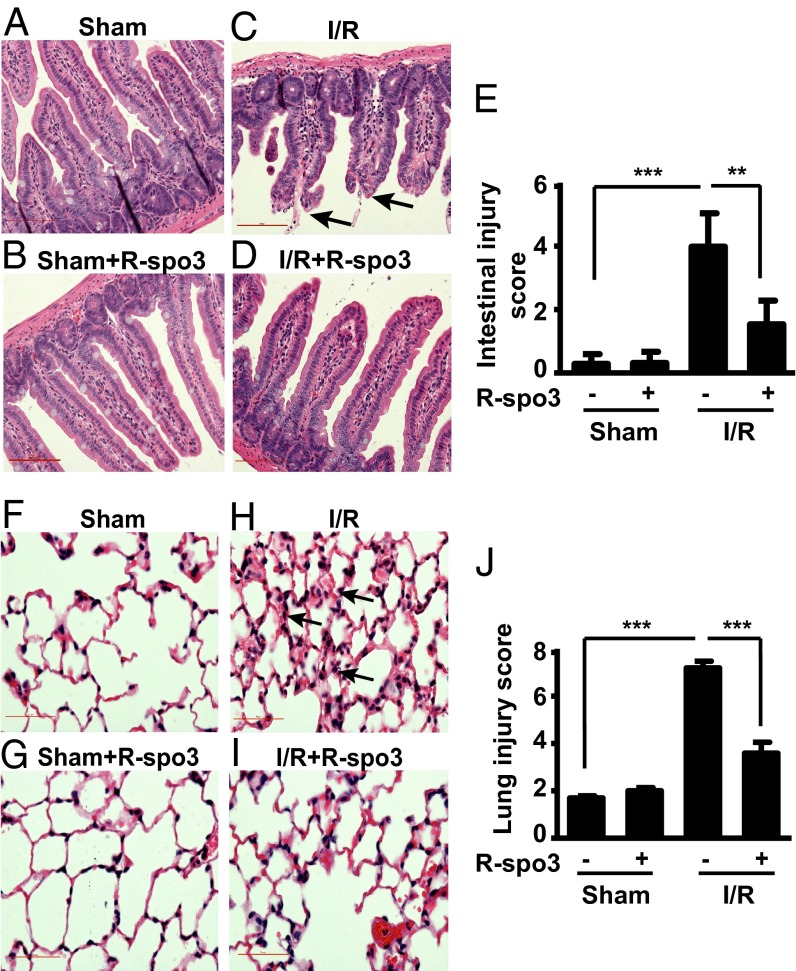
Recombinant mouse R-spo3 was obtained from a commercial source and its purity and functional activity were measured by its ability to stabilize β-catenin (Fig. S4 A and B). After characterization, the mouse R-spo3 was used to evaluate its efficacy in a mouse model of mesenteric I/R-induced tissue injury. As illustrated in Fig. 1C, 30 min of gastrointestinal ischemia followed by 3 h of reperfusion resulted in disruption and sloughing of epithelial cells, exudation of lamina propria, and denudation of villi, which are characteristic features of mesenteric I/R-induced intestinal tissue damage. Administration of R-spo3 30 min before ischemia, however, resulted in a remarkable decrease of the locally induced intestinal damage (Fig. 1D). In sham-operated animals, intestinal tissue integrity was maintained (Fig. 1 A and B). Intestinal mucosal damage was evaluated using a six-parameter scoring system and the cumulative data are shown in Fig. 1E. Administration of R-spo3 significantly decreased the injury score from 3.98 ± 0.6–1.84 ± 0.3 with P < 0.01.
Fig. 1.
R-spo3 attenuates mesenteric I/R-induced local intestinal and remote lung injury. Representative images of hematoxylin and eosin stained intestinal sections of mice from groups (A) sham, (B) sham + R-spo3, (C) I/R, and (D) I/R + R-spo3 (n = 5 per group) are shown. Arrowheads (C) emphasize villi damage characterized by exudation of lamina propria. (E) Average score of intestinal injury for each group was quantified using a scoring system (SI Experimental Procedures). Representative images of hematoxylin and eosin stained lung sections of mice from groups (F) sham, (G) sham + R-spo3, (H) I/R, and (I) I/R + R-spo3 (n = 5 per group) are shown. Arrowheads (H) emphasize lung damage characterized by alveolar/interstitial infiltration. (J) Average score of lung injury for each group was determined based on the criteria described in SI Experimental Procedures. The extent of I/R is 30/180 min. All photomicrographs are 200× magnification. Error bars represent SEM (**P < 0.01, ***P < 0.005). Results are representative of three independent experiments.
The sequence of events that occurs during intestinal I/R injury triggers systemic inflammatory responses, affecting remote organs such as liver, lungs, and kidneys (17). Based on evidence that mesenteric I/R is followed invariably by pulmonary inflammation, we chose to examine lung tissues to evaluate if R-spo3 can also prevent I/R-induced remote lung injury. Lung damage, characterized by pneumonitis (alveolar/interstitial infiltration) shown in Fig. 1H in mice subjected to I/R was reduced from 7.2 ± 0.28 to 3.6 ± 0.46 (Fig. 1J) in mice subjected to I/R + R-spo3 (Fig. 1I), with P < 0.005. Thus, R-spo3 pretreatment attenuates both local intestinal and remote pulmonary damage that occurs following mesenteric I/R.
Administration of R-Spondin3 Mitigates I/R-Instigated Inflammatory Response.
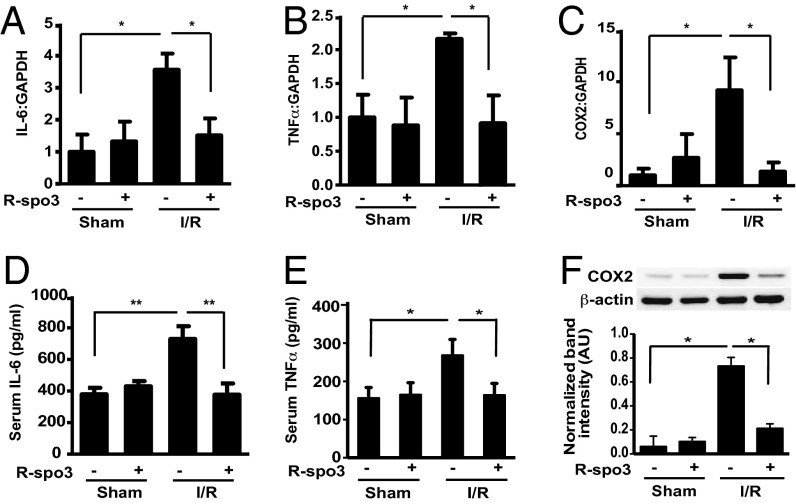
The existing body of evidence overwhelmingly suggests that local inflammation plays an active role in the pathogenesis of I/R injury (4). Proinflammatory cytokines such as TNFα, interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), produced by monocytes, macrophages, granulocytes, lymphocytes, dendritic cells, intestinal epithelial cells, and endothelial cells are crucial players. To determine whether R-spo3 suppresses the cytokine response after mesenteric I/R, we first evaluated mRNA levels of proinflammatory cytokines in the intestine using quantitative PCR. Mesenteric I/R increased the gene expression of IL-6 and TNFα, whereas R-spo3 pretreatment decreased the mRNA levels of these cytokines (Fig. 2 A and B). Similarly, R-spo3 suppressed the production of cyclooxygenase-2 (Cox2), another inflammatory molecule that serves as a specific indicator of tissue damage after mesenteric I/R at both mRNA and protein levels (Fig. 2 C and F). To further investigate the effect of R-spo3 on mesenteric I/R-induced systemic inflammatory response, we measured the levels of IL-6 and TNFα in the serum. As anticipated, I/R-augmented IL-6 and TNFα production were significantly reduced in R-spo3 pretreated mice subjected to I/R (Fig. 2 D and E). However, administration of recombinant R-spo3 failed to significantly suppress lipopolysaccharide-induced mRNA expression of proinflammatory cytokines, such as IL-6 and TNFα in in vitro cultures of monocytes and granulocytes isolated from human peripheral blood (Fig. S5 A and B).
Fig. 2.
R-spo3 mitigates the expression of I/R-enhanced proinflammatory mediators. Relative mRNA expression of proinflammatory mediators such as IL-6 (A), TNFα (B), and COX2 (C) were analyzed using quantitative RT-PCR in the intestinal tissue lysates. These experiments were repeated three times. Each time three mice per group were used. Serum levels of cytokines, IL-6 (D), and TNFα (E) were evaluated using ELISA (n = 9 per group for IL-6 and n = 15 per group for TNFα). (F) Representative Western blot of COX2 and actin in the intestinal tissue lysates. Relative expression of COX2 was measured by densitometric analysis (n = 5 per group, *P < 0.05, **P < 0.005). The extent of I/R is 30/180 min.
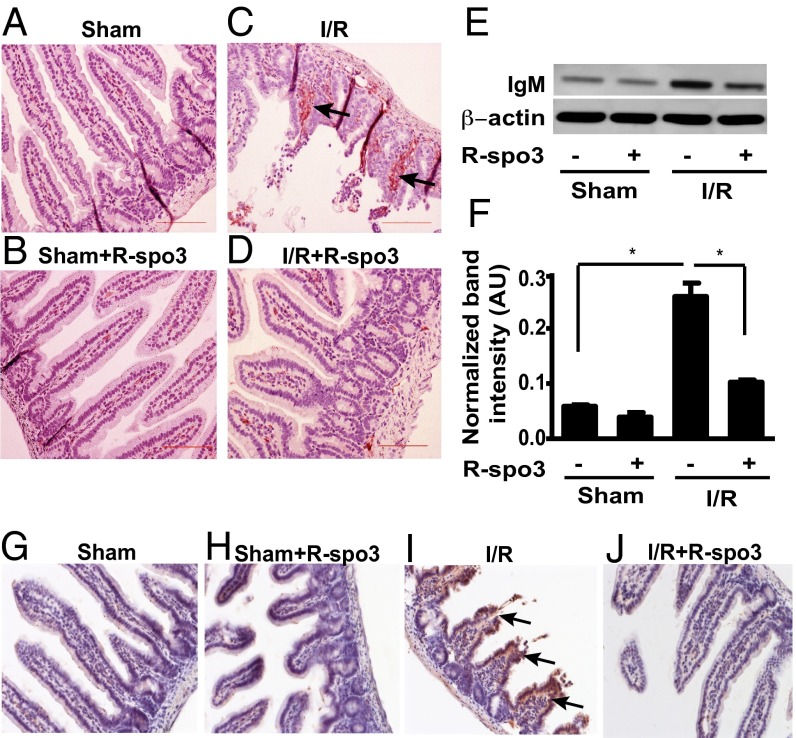
Immunoglobulin M (IgM) and complement (C3) deposition have been recognized as key determinants in the initiation and amplification of dysregulated inflammatory response triggered by mesenteric I/R (18). Interestingly, I/R-enhanced IgM deposition was substantially reduced in R-spo3–treated mice subjected to I/R to levels comparable to those recorded in sham-operated animals (Fig. 3 A–D). We further corroborated this observation by documenting decreased IgM levels in the intestinal tissue lysates using the Western blot method as shown in Fig. 3 E and F. Similarly, I/R-enhanced C3 deposition in intestinal tissues was also remarkably reduced in animals pretreated with R-spo3 (Fig. 3 G–J). Collectively, these results indicate that R-spo3 contribute to the reduction of inflammation in mesenteric I/R.
Fig. 3.
Decreased deposition of IgM and complement C3 in the intestine of R-spo3–pretreated I/R mice. IgM deposition was evaluated in the intestinal tissues by IHC and Western blotting. Representative images of IgM staining from groups (A) sham, (B) sham + R-spo3, (C) I/R, and (D) I/R + R-spo3 are shown (n = 3 per group). Brown staining in the lamina propia indicates IgM deposition (arrows) and blue shows nuclear staining with hematoxylin. (E) Representative Western blots of IgM and actin from each group. (F) Quantitation of IgM signal by densitometry analysis from Western blots normalized to actin (n = 3 per group, *P < 0.05). Complement deposition was evaluated in the intestinal tissues by IHC with anti-C3 antibody. Representative images of C3 staining from groups (G) sham, (H) sham + R-spo3, (I) I/R, and (J) I/R + R-spo3 are shown (n = 3 per group). Brown staining shows C3 deposition (arrows) in the epithelium and lamina propia and blue staining indicates nuclear staining with hematoxylin. The extent of I/R is 30/180 min. All photomicrographs are 200× magnification. Results are representative of three experiments for Western blot and three experiments for immunohistochemistry (IHC).
R-Spondin3 Mitigates Endothelial Dysfunction in Vivo.
Given R-spo3 is produced primarily by endothelial cells (Fig. S2), we surmised that under physiological conditions, it preserves endothelial structure and function in an autocrine fashion and that the noted protective effect against I/R-initiated tissue damage may also involve the sustenance of endothelial cells. Endothelial cell damage is important in various clinical conditions including hypertension, sepsis, atherosclerosis, and I/R (5, 19). Endothelial dysfunction is linked to leukocyte infiltration into surrounding tissues and loss of vascular integrity and permeability (20). Thus, we sought to evaluate the parameters of endothelial dysfunction that occur during I/R.
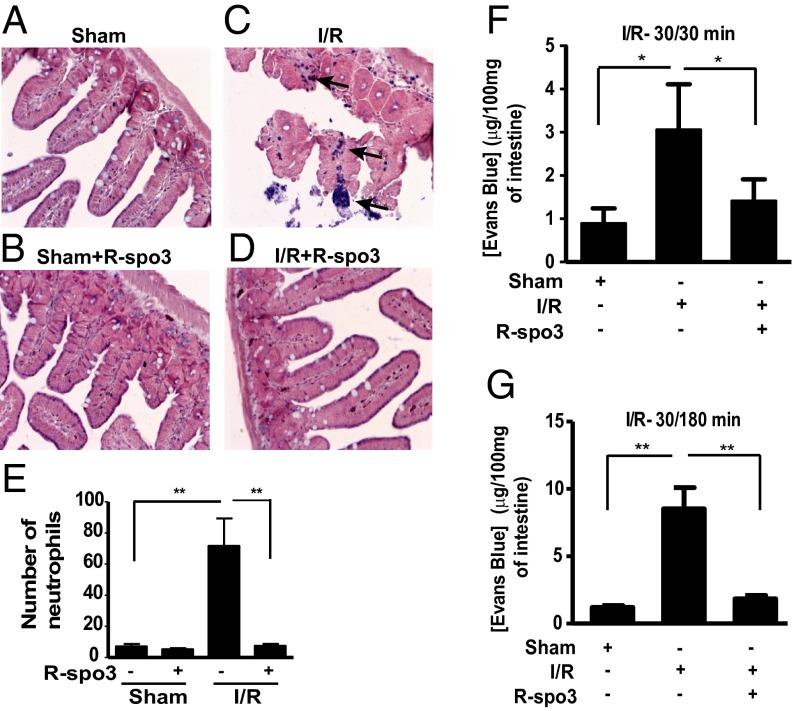
First, we tested the effect of R-spo3 on leukocyte infiltration in intestinal tissues of mice subjected to I/R using the esterase-staining method. Whereas we observed an enhanced influx of neutrophils in the intestine of mice subjected to I/R, we noted a significantly lower number of neutrophils within the intestinal villi of R-spo3–treated I/R mice (Fig. 4 A–D). This lower number of neutrophils was comparable to the levels noted in sham-operated groups. Cumulative data on neutrophil cell number is shown in Fig. 4E. Because the expression of endothelial cell adhesion molecules such as intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) has been implicated in the regulation of leukocyte attachment and migration into surrounding tissues, we measured the mRNA levels of these molecules by qPCR. The general kinetic pattern of transcript accumulation for ICAM1 and VCAM1 in intestinal tissues was first evaluated in a reperfusion time-dependent manner (30 min ischemia followed by 15, 30, 60, 120, and 180 min reperfusion). In the intestinal tissues, the transcripts for ICAM1 and VCAM1 were weakly expressed in all of the groups and displayed a pattern with increase in their expression at 30/15 min and 30/30 min I/R. The message levels gradually dropped at 30/60 min and 30/120 min and then increased at 30/180 min I/R (Fig. S6A). We repeatedly observed this expression pattern for both ICAM1 and VCAM1 in intestinal tissues following mesenteric I/R. Because changes in the influx of neutrophils were noted at 180 min reperfusion time point, we considered it appropriate to determine the effect of R-spo3 on mRNA levels of ICAM1 and VCAM1 at an earlier time point of 30/30 min I/R. Interestingly, it appeared that R-spo3 preconditioning suppressed the transcript expression of ICAM1 and VCAM1 in the intestine of 30/30 min I/R + R-spo3 group compared with the corresponding I/R group (Fig. S6B). We then measured ICAM1 and VCAM1 levels in TNFα-stimulated human umbilical vein endothelial cells (HUVECs) cultures in the presence and absence of R-spo3. Whereas ICAM1 and VCAM1 expressions were induced upon TNFα stimulation, R-spo3 pretreatment substantially inhibited the expression of these molecules in TNFα-stimulated cultures (Fig. S6 C and D).
Fig. 4.
R-spo3 inhibits leukocyte infiltration and vascular leakage in the intestine. Influx of neutrophils to the site of damage was evaluated using naphthol AS-D choloroacetate substrate by esterase staining method. Representative images of intestinal sections, stained for neutrophils from groups (A) sham, (B) sham + R-spo3, (C) I/R, and (D) I/R + R-spo3 are shown. A lot of neutrophils were found in the interstitial spaces of the intestine in the I/R group as shown by the dark blue staining (arrows) and very few neutrophils were found in R-spo3–treated I/R mice. (E) Cumulative data on neutrophil numbers from each group (n = 9 per group, **P < 0.01). The extent of I/R for neutrophil staining is 30/180 min. Vascular leakage in the intestine was measured by extravasation of Evans blue dye at two different reperfusion time periods: 30/30 min I/R (F) and 30/180 min I/R (G) (n = 9 per group, *P < 0.05, **P < 0.01). Mesenteric I/R-induced intestinal vascular leakage was significantly reduced in the intestine of R-spo3–treated I/R mice at both indicated time periods.
To further characterize the role of R-spo3 in endothelial dysfunction, we investigated the loss of barrier integrity and permeability in an in vivo mesenteric I/R model of 30 min ischemia followed by 30 and 180 min reperfusion (Fig. S7). A shorter interval of 30/30 I/R was performed to determine the effect of R-spo3 on vascular integrity at the onset of I/R injury. As anticipated, intestinal vascular leakage measured by extravasation of Evans blue dye was significantly higher in mice subjected to I/R compared with sham-handled mice (Fig. 4 F and G). By contrast, mice treated with R-spo3 showed a significant decrease in the reperfusion-induced vascular permeability at both reperfusion time periods.
Taken together, these data indicate that R-spo3 (i) may regulate the expression of cell adhesion molecules, ICAM1 and VCAM1 and thereby prevent the influx of neutrophils into intestinal tissues during mesenteric I/R; (ii) inhibits vascular leakage and as a result, may prevent the entry of circulating inflammatory molecules such as natural antibodies and complement factors into interstitial spaces of the intestine after I/R; and (iii) exhibits its protective effect on endothelium at the onset of I/R injury, which is evident from decreased intestinal vascular leakage as early as in 30 min reperfusion.
R-Spondin3 Maintains the Integrity of the Endothelial Cell Junctions.
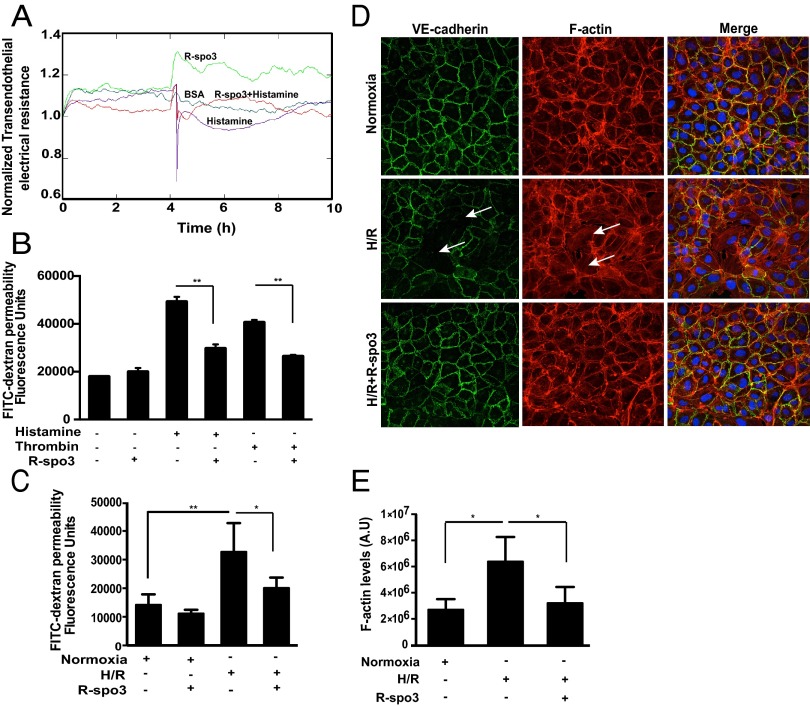
Endothelial barrier function depends on the integrity of intercellular junctions (21). We investigated whether R-spo3 affects endothelial junctional integrity by measurement of transendothelial electrical resistance (TEER). In HUVEC monolayers exposed to recombinant hR-spo3 (1 µg/mL), TEER increased within the first minute and the increase was sustained at a steady level for a continued period (Fig. 5A), indicating that it can tighten and stabilize the endothelial cell junctions. Exposure of monolayers to BSA (1 µg/mL) did not change TEER from the baseline, and histamine (10 µM, negative control) induced a drop in TEER, confirming a decreased barrier function. In cells subjected to R-spo3 + histamine, R-spo3 pretreatment restored TEER and rescued the cells from histamine’s negative effect on endothelial barrier function, thus suggesting its physiological relevance. We also tested the ability of R-spo3 to reduce endothelial permeability following exposure to histamine and thrombin, two inflammatory mediators that play a role in tissue injury. Pretreatment of HUVECs with hR-spo3 (1 µg/mL) reduced the histamine and thrombin-induced permeability as shown in Fig. 5B. Alternatively, barrier integrity in HUVECs under hypoxia/reoxygenation (H/R) conditions, an in vitro surrogate of I/R was examined. We applied fluorescein isothiocyanate (FITC)-dextran of high molecular weight (500 kDa), comparable to the size of IgM molecules, to endothelial monolayers and measured its ability to pass through as a measure of cell permeability. As shown in Fig. 5C, cells treated with R-spo3 showed a significant reduction in the H/R-induced endothelial leakage compared with H/R-subjected cells.
Fig. 5.
R-spo3 tightens endothelial cell junctions and preserves barrier function. (A) Effect of R-spo3 on endothelial junctional integrity, as measured by transendothelial electrical resistance (TEER) in HUVECs exposed to different conditions such as BSA, histamine, R-spo3, and R-spo3 + histamine. (B) R-spo3 pretreatment decreased histamine and thrombin-induced endothelial permeability in HUVECs measured using FITC-dextran passage (n = 4, **P < 0.01). (C) R-spo3 pretreatment decreased H/R-induced endothelial leakage in HUVECs measured using FITC-dextran passage (n = 5, *P < 0.05, **P < 0.01). (D) Effect of R-spo3 on localization of VE-cadherin (adherens junction marker) and F-actin was evaluated by immunofluorescence and phalloidin staining in HUVECs subjected to H/R. H/R resulted in the loss of the VE-cadherin at the cell junctions and increased the number of stress fibers (arrows), whereas R-spo3 preserved VE-cadherin and F-actin at the cell periphery and decreases the formation of stress fibers in cells subjected to H/R. One representative image of three experiments is shown. (E) Quantification of F-actin content in HUVECs under normoxic, H/R, and H/R + R-spo3 conditions. Cumulative data from three independent experiments *P < 0.05.
Vascular endothelial (VE)-cadherin is a marker of endothelial adherens junctions and mediates homotypic cell–cell adhesion and it associates with the cortical actin cytoskeleton to maintain junctional integrity (22). Confluent HUVECs were stained for VE-cadherin and F-actin under H/R conditions. Under normoxic conditions, VE-cadherin is organized at the cell periphery and formed a honeycomb-like structure at places where the peripheral membrane of the neighboring cells overlapped. In these cells, F-actin was also localized around the cell periphery representing junction-bound actin but also showed a certain basal level of stress fiber formation. Upon exposure to H/R VE-cadherin appeared thinner, dispersed, and the junctions were discontinuous with small gaps. In these cells, we also observed numerous stress fibers traversing the cell body from one side to the other. In contrast, R-spo3 restored cortical localization of VE-cadherin and F-actin along the cell periphery and the number of discontinuous adherens junctions and stress fibers in response to H/R were overall reduced as shown in Fig. 5D; quantification is shown in Fig. 5E. Based on these findings, we conclude that R-spo3 reduces endothelial permeability by tightening intercellular junctions and improving barrier integrity.
It is well established that an increase in endothelial permeability induced by vasoactive agents or by hypoxia is regulated by Rho GTPases, well-known regulators of the actin cytoskeleton (22). RhoA appears to primarily induce stress fiber formation and has destabilizing effects on endothelial barrier properties after hypoxia through its effector Rho kinase. We first sought to determine the expression pattern of Rho kinase in HUVECs at various hypoxic time points: 5, 15, 30, 60, and 360 min. Rho kinase levels peaked at 15 and 30 min hypoxia (Fig. S8A). Hypoxia-induced Rho kinase at 30 min was suppressed in the presence of R-spo3 (Fig. S8B), suggesting that R-spo3 may modulate the signaling of RhoA and thereby may regulate the adhesion dynamics and cytoskeletal reorganization.
Discussion
In this study, we provide evidence and a unique mechanism whereby R-spo3 attenuates intestinal injury following mesenteric I/R. In vivo R-spo3 (i) mitigates mesenteric I/R-induced intestinal tissue damage; (ii) attenuates lung injury (a sequential event following mesenteric I/R); (iii) dampens the inflammatory response mediated by proinflammatory cytokines, limited deposition of IgM, and complement C3; and (iv) preserves endothelial barrier function by preventing neutrophil infiltration, inhibiting intestinal vascular leakage. Subsequent in vitro studies reveal that R-spo3 has the ability to tighten endothelial cell junctions and maintain the localization of VE-cadherin and F-actin at the cell periphery and thereby it preserves the endothelial barrier integrity.
Considering the evolving studies implicating R-spo3 in organ development and disease, it is not surprising that mice lacking R-spo3 are embryonically lethal (23). It is noteworthy that R-spo3 expression is crucial for survival because other members of the R-spondin family cannot compensate for its absence. Analysis of R-spo3 by gain- and loss-of-function experiments in both mouse and Xenopus revealed its essential role in vertebrate vasculogenesis and angiogenesis (24). Recent progress in R-spondin research has revealed their expression pattern during mouse embryonic and fetal development as provided by Nam et al. (7). However, the expression data of R-spondin genes in different cell types has not been determined in detail. We screened the mRNA profile of R-spondins in various immune and immune-like cells under basal and proinflammatory conditions and found R-spo3 to be the only member predominantly expressed in endothelial cells. This initial observation suggested a putative role for R-spo3 in the context of I/R-related inflammation and vascular injury.
Uncontrolled local and systemic inflammation is the underlying component of mesenteric I/R pathology (4). Vascular endothelial cells play a pivotal role defining the inflammatory process in I/R by coordinating and regulating immune responses elicited in inflamed tissues. In fact, endothelial dysfunction characterized by enhanced leukocyte- and platelet-endothelial cell adhesion, impaired endothelial integrity and increased permeability, is a prelude to reperfusion injury (25). Consequently, preventing inflammation and vascular injury induced by mesenteric I/R may minimize local and remote organ damage. As demonstrated in this study, R-spo3 significantly decreases mesenteric I/R-induced local intestinal injury. Our data also reveal that R-spo3 suppresses I/R enhanced inflammation, including reduced expression of proinflammatory cytokines, IgM and C3 deposition, and leukocyte infiltration into the lamina propria. Decrease in local inflammatory response relates to reduced lung damage as shown in Fig. 1J. Our finding further supports the observation that R-spo3 treatment markedly reduced IL-6 and TNFα levels in the serum. However, in vitro studies suggest that R-spo3 may not act as a direct anti-inflammatory protein. A possible explanation for the R-spo3–induced anti-inflammatory effect could be attributed to the reduction in endothelial adhesion molecule expression and associated leukocyte accumulation leading to preservation of tissue integrity in the intestine. Two other salient molecules that have been considered as key contributors in initiating intestinal I/R injury are natural antibodies and complement. Natural antibodies such as IgM are large pentameric molecules present in very low quantities in interstitial tissues under normal conditions. In contrast, circulating IgM diffuse into the interstitium resulting in exacerbated deposition on local tissues during reperfusion, subsequently activating the complement system observed by C3 deposition. Based on the observation that R-spo3 limits IgM and C3 deposition in tissues, we considered R-spo3 to exhibit its protective effect at the onset of reperfusion injury. Our finding that R-spo3 treatment dramatically reduced infiltration of leukocytes into intestinal tissues also supports this notion. Previously, others and we have shown that ischemia induces the expression of neoantigens on the cellular surface, to which natural antibodies bind and activate the complement system, leading to tissue damage (18, 26). However, whether suppression of inflammation in mesenteric I/R mice is a direct effect of R-spo3 on the expression of neoantigens on ischemic cell surface needs further experimental investigation.
Several studies have implied that tissue hypoxia affects the integrity of adherens junctions and promotes increased vascular permeability. This breakdown of the barrier has been shown to be associated with increased levels of inflammatory mediators such as thrombin, histamine, and VEGF (27), which further contribute to adverse outcomes of vascular dysfunction. Vascular permeability has thus become a hallmark of numerous autoimmune and inflammatory conditions including I/R (28, 29). Hence there is a need for identification of therapeutic agents and strategies that can prevent and/or reverse vascular leakage. During the last decade, a myriad of vascular growth factors have been discovered, essential for blood vessel formation and normal vascular development including R-spo3 (24). Our study uniquely documents that R-spo3 also acts as an antipermeability agent. Adherens junction proteins such as VE-cadherin and cytoskeletal proteins mediate barrier integrity of endothelium localized at intercellular junctions (22). Numerous reports suggest that ischemia/hypoxia perturbs actin dynamics and induces contractility of actin cytoskeleton in vascular endothelium and thus alters the endothelial barrier functions (30). Results from in vivo and in vitro leakage studies including TEER data clearly present evidence that R-spo3 plays a role in maintaining the endothelial barrier integrity. These findings were further supported by localization of VE-cadherin at membrane junctions and F-actin bound to cell periphery in R-spo3–treated cells, suggesting that R-spo3 signaling may regulate the adhesion and cytoskeleton dynamics in endothelial cells.
Based on our findings and existing studies, we propose a mechanism by which R-spo3 may achieve its protective effect in the endothelium and thereby prevent I/R-induced tissue damage (Fig. S8C). Hypoxia induces activation of RhoA, a small GTPase that acts through its effector Rho kinase. Increased Rho kinase leads to increased myosin light chain (MLC) phosphorylation promoting MLC-dependent contraction with subsequent formation of stress fibers and reduction of VE-cadherin–mediated adhesion (30). Existing literature supports that RhoA activation occurs through syndecan-4–dependent pathway (31). Syndecans are a conserved family of heparan- and chondroitin- sulfate carrying transmembrane proteins that play a central role in maintaining the interactions of extracellular matrix components and soluble ligands with cell surface (32). These interactions are important to maintain the cell polarity. According to a recent report, R-spo3 is involved in Wnt/planar cell polarity signaling through clathrin-mediated endocytosis-involving syndecan4 (10). We speculate that R-spo3 may promote Wnt/planar cell polarity signaling through conventional Wnt receptors/syndecan4 and suppress the activation of RhoA and its downstream effector molecules such as Rho kinase, which appears to primarily have destabilizing effects on endothelial barrier properties after hypoxia. Thereby, R-spo3 reverses RhoA-mediated endothelial barrier dysfunction by decreasing the formation of stress fibers and tightening endothelial cell–cell junctions. Among the Ras family of small GTPases, whose member (RhoA/Rac/Cdc42) plays an active role in R-spo3–mediated signaling needs to be resolved. Future studies should explore the molecular mechanisms of the R-spo3–Wnt system in relation to the Ras family of small GTPases.
Taken together, this study concludes that R-spo3 can limit I/R injury by preserving endothelial cell function and preventing the subsequent inflammatory responses. We propose that R-spo3 is important in the field of vascular biology and may have clinical benefits in I/R-related clinical conditions.
Experimental Procedures
Mice.
Nine-week-old male C57BL/6J mice were obtained from The Jackson Laboratory and mesenteric I/R was performed on 10-wk-old mice (SI Experimental Procedures).
Administration of R-spo3.
Mice were randomly assigned to the following experimental groups: (i) sham procedure + saline (sham), (ii) sham procedure + R-spo3 (sham + R-spo3), (iii) I/R procedure + saline (I/R), and (iv) I/R + R-spo3 procedure (I/R + R-spo3). A half-hour before the induction of ischemia, the mice were preconditioned with recombinant mouse R-spo3 (5 µg/mouse; R&D Systems), or vehicle (normal saline) by i.p. injection.
Mesenteric Model of I/R.
Animals were subjected to mesenteric I/R as previously described (33) and the I/R protocol is elaborated in SI Experimental Procedures.
Histology and Tissue Injury Scoring.
About 15 cm of the small intestinal jejunal segments and lung tissues were harvested for histological analysis and scoring was performed as described previously (33) (SI Experimental Procedures).
mRNA Analysis.
Total RNA from cells and tissues were isolated and standard procedures were used to determine the mRNA expression of different target genes (SI Experimental Procedures).
ELISA.
Serum levels of IL-6 and TNFα were quantified using the ELISA kit specific for mouse IL-6 and TNFα (eBiosciences) (SI Experimental Procedures).
Immunohistochemistry.
Immunohistochemistry (IHC) staining was performed using formalin-fixed paraffin sections of small intestine as described previously (33) (SI Experimental Procedures).
Western Blotting.
Western blotting on cell and tissue lysates was performed via standard procedures (SI Experimental Procedures).
Esterase Staining.
To detect neutrophils in tissue sections, we performed the esterase-staining method using a substrate Naphthol AS-D Chloroacetate (Sigma) (SI Experimental Procedures).
Evaluation of Vascular Leakage in the Gut.
The extravasation of Evans blue dye into the tissue was used as a measure of vascular permeability as described previously (34) (SI Experimental Procedures).
Cell Culture.
HUVECs (Lonza; passages 3–6) were grown in endothelial growth medium (EGM bullet kit; Clonectics) containing 10% (vol/vol) FCS at 37 °C (SI Experimental Procedures).
Measurement of TEER.
HUVECs were seeded onto fibronectin (5 μg/mL) gold-coated microelectrodes for electrical resistance measurements (SI Experimental Procedures).
Measurement of FITC-Dextran Leakage.
HUVECs plated on 0.1% gelatin-coated Transwell inserts (0.4 μm pores size; Costar) were used for FITC-dextran permeability assays (SI Experimental Procedures).
Hypoxia/Reoxygenation Injury Condition.
As an in vitro model of ischemia, hypoxia was achieved using published methods (30) (SI Experimental Procedures).
Immunofluorescence and Localization of F-Actin.
HUVECs cultured on 0.1% gelatin-coated chamber slides were used to stain for VE-cadherin and F-actin (SI Experimental Procedures).
Statistical Analyses.
Numeric data are presented as means ± SD. Statistical significance of differences between the treatment groups for in vivo and in vitro experiments was determined using Student t test. In all experiments, differences were considered statistically significant when P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Deepak Venkatesh for technical help and providing materials, Robin Bosse, Dr. Masayuki Mizui, and Dr. Takashi Muroya for technical help, and Dr. Kamalpreet Nagpal for reviewing the manuscript. The research presented herein was supported by Grants W81XWH-09-1-0530 and W81XWH-09-1-0536 from Medical Research and Material Command of the Department of the Army.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309393110/-/DCSupplemental.
References
- 1.Kong SE, Blennerhassett LR, Heel KA, McCauley RD, Hall JC. Ischaemia-reperfusion injury to the intestine. Aust N Z J Surg. 1998;68(8):554–561. doi: 10.1111/j.1445-2197.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 2.Stoney RJ, Cunningham CG. Acute mesenteric ischemia. Surgery. 1993;114(3):489–490. [PubMed] [Google Scholar]
- 3.Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: A review. Acta Cir Bras. 2005;20(4):336–343. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- 4.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141(1):3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 6.Kim KA, et al. R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle. 2006;5(1):23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- 7.Nam JS, Turcotte TJ, Yoon JK. Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns. 2007;7(3):306–312. doi: 10.1016/j.modgep.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008;50(4):229–243. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim KA, et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008;19(6):2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell. 2011;20(3):303–314. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Yoon JK, Lee JS. Cellular signaling and biological functions of R-spondins. Cell Signal. 2012;24(2):369–377. doi: 10.1016/j.cellsig.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13(3):242. doi: 10.1186/gb-2012-13-3-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehwald N, et al. 2011. Wnt-beta-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology 141(2):707–718, 718 e701–705.
- 14.Zhao J, et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132(4):1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Takashima S, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208(2):285–294. doi: 10.1084/jem.20101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krönke G, et al. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 2010;62(8):2303–2312. doi: 10.1002/art.27496. [DOI] [PubMed] [Google Scholar]
- 17.Diepenhorst GM, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg. 2009;249(6):889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 18.Fleming SD. Natural antibodies, autoantibodies and complement activation in tissue injury. Autoimmunity. 2006;39(5):379–386. doi: 10.1080/08916930600739381. [DOI] [PubMed] [Google Scholar]
- 19.Lefer AM, Tsao PS, Lefer DJ, Ma XL. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- 20.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100(9):2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 22.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 23.Aoki MMM, et al. R-spondin3 is required for mouse placental development. Dev Biol. 2007;301(1):218–226. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Kazanskaya O, et al. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development. 2008;135(22):3655–3664. doi: 10.1242/dev.027284. [DOI] [PubMed] [Google Scholar]
- 25.Pernow J, Gonon AT, Gourine A. The role of the endothelium for reperfusion injury. Eur Heart J Suppl. 2001;3(Supplement C):C22–C27. [Google Scholar]
- 26.Zhang M, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203(1):141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuboi H, et al. Role of the thrombin/protease-activated receptor 1 pathway in intestinal ischemia-reperfusion injury in rats. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G678–G683. doi: 10.1152/ajpgi.00361.2006. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadi-Yazdi C, Williams B, Oakes S, Moore FD., Jr Attenuation of the effects of rat hemorrhagic shock with a reperfusion injury-inhibiting agent specific to mice. Shock. 2009;32(3):295–301. doi: 10.1097/SHK.0b013e3181995e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willoughby L, Dark P, Warhurst G. Investigation of systemic and mesenteric inflammatory signaling and gut-derived endothelial toxicity in patients undergoing high-risk abdominal aortic surgery. Shock. 2011;36(2):121–127. doi: 10.1097/SHK.0b013e3182205bbd. [DOI] [PubMed] [Google Scholar]
- 30.Jin HG, et al. Hypoxia-induced upregulation of endothelial small G protein RhoA and Rho-kinase/ROCK2 inhibits eNOS expression. Neurosci Lett. 2006;408(1):62–67. doi: 10.1016/j.neulet.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Dovas A, Yoneda A, Couchman JR. PKCbeta-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J Cell Sci. 2006;119(Pt 13):2837–2846. doi: 10.1242/jcs.03020. [DOI] [PubMed] [Google Scholar]
- 32.Tkachenko E, Rhodes JM, Simons M. Syndecans: New kids on the signaling block. Circ Res. 2005;96(5):488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 33.Yoshiya K, et al. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;301(6):G1020–G1030. doi: 10.1152/ajpgi.00239.2011. [DOI] [PubMed] [Google Scholar]
- 34.Souza DG, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173(6):4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.