Abstract
A characteristic of memory T (TM) cells is their ability to mount faster and stronger responses to reinfection than naïve T (TN) cells do in response to an initial infection. However, the mechanisms that allow this rapid recall are not completely understood. We found that CD8 TM cells have more mitochondrial mass than CD8 TN cells and, that upon activation, the resulting secondary effector T (TE) cells proliferate more quickly, produce more cytokines, and maintain greater ATP levels than primary effector T cells. We also found that after activation, TM cells increase oxidative phosphorylation and aerobic glycolysis and sustain this increase to a greater extent than TN cells, suggesting that greater mitochondrial mass in TM cells not only promotes oxidative capacity, but also glycolytic capacity. We show that mitochondrial ATP is essential for the rapid induction of glycolysis in response to activation and the initiation of proliferation of both TN and TM cells. We also found that fatty acid oxidation is needed for TM cells to rapidly respond upon restimulation. Finally, we show that dissociation of the glycolysis enzyme hexokinase from mitochondria impairs proliferation and blocks the rapid induction of glycolysis upon T-cell receptor stimulation in TM cells. Our results demonstrate that greater mitochondrial mass endows TM cells with a bioenergetic advantage that underlies their ability to rapidly recall in response to reinfection.
Keywords: metabolism, lymphocytes
Naïve T (TN) and memory T (TM) cells are quiescent, but upon T-cell receptor (TCR)-mediated recognition of antigen (Ag) and costimulation, they become activated, undergo clonal expansion, and acquire effector functions. Although both TN and TM cells acquire effector functions, a noted characteristic of TM cells is their ability to respond more rapidly than TN cells to Ag (1–4). Several factors underlie the accelerated recall response of TM cells. For example, Ag-specific TM cells are present in greater numbers than TN cells. In addition, several intrinsic aspects of TM cells have been suggested to contribute to their ability to respond more efficiently, such as increased activity of proximal TCR signaling components, an “open” chromatin conformation of cytokine genes, and altered transcriptional profiles (1, 2, 5–9). However, whether bioenergetic differences contribute to this process is not clear.
Resting cells like TN and TM cells interchangeably use glucose, amino acids, and lipids to fuel the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) for ATP production (10–12). However, proliferating cells like activated T cells promote aerobic glycolysis, which supports cell growth, proliferation, and effector functions (13–16). Although TN and TM cells both have a similar metabolism, there are metabolic differences between these cells (17). TM cells from Listeria monocytogenes-infected mice have more mitochondria than TN cells, which is consistent with our finding that TM cells but not TN cells have considerable spare respiratory capacity (SRC) (17). Because SRC is important for cellular survival and function (17–19) and TM cells are characterized by their ability to respond vigorously to Ag reencounter (20), we investigated whether bioenergetic differences between TM and TN cells contribute to the ability of TM cells to rapidly recall in response to reinfection. We show here that TM cells have more mitochondria and ATP than TN cells and that, upon activation, secondary TE cells proliferate faster, produce cytokines more quickly, and maintain more ATP than primary effector T (TE) cells. In addition, TM cells use both OXPHOS and glycolysis to a greater and more prolonged degree after activation than TN cells, suggesting that more mitochondria in TM cells not only promote OXPHOS, but also glycolysis. We show that both the rapid proliferation and induction of glycolysis in TM cells depend on mitochondrial ATP. We also found that optimal mitochondrial function is predominantly fueled by fatty acid oxidation (FAO) in TM cells and depends on the association of hexokinase (HK) with mitochondria. Thus, our data demonstrate that greater mitochondrial mass underlies the rapid recall ability of TM cells.
Results
To explore whether bioenergetic differences between TM and TN cells account for the ability of TM cells to rapidly recall upon activation, we generated “memory” T cells in vitro by activating OT-I TN cells with OVA peptide and IL-2 for 3 d and then differentially cultured cells for 3–4 additional days in IL-15 (17, 21). To determine whether IL-15 TM cells have more mitochondria than TN cells, as we have shown for TM cells generated after infection (17), we stained cells with Mitotracker green and found that IL-15 TM cells had more mitochondrial mass (Fig. 1 A and B). Although IL-15 TM cells are slightly bigger than TN cells, greater size does not necessarily correlate with increased mitochondrial content. We have shown that in vitro-generated IL-2–induced TE (IL-2 TE) cells are much larger than IL-15 TM cells and actually have less mitochondrial content (17). IL-15 TM cells also have a significantly higher ratio of mitochondrial DNA/nuclear DNA (mtDNA/nDNA) than TN cells (Fig. 1C). Together these data indicate that in vitro-generated IL-15 TM cells, like TM cells generated after infection (17), have greater mitochondrial mass than TN cells and provide a model from which to study bioenergetic differences between these cells.
Fig. 1.
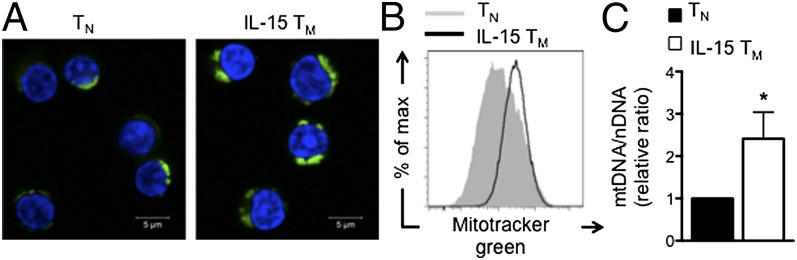
IL-15 TM cells have more mitochondrial mass than TN cells. OT-I cells were activated with OVA peptide for 3 d in IL-2 and cultured in IL-15 for 3–4 d to generate IL-15 TM cells, which were compared with OT-I TN cells. (A) TN and IL-15 TM cells stained with Mitotracker green (green) and DRAQ5 (blue). (B) Mitotracker green staining was quantified by flow cytometry. Data are representative of three experiments. (C) mtDNA/nDNA ratio, mean ± SEM, from six experiments; *P < 0.05.
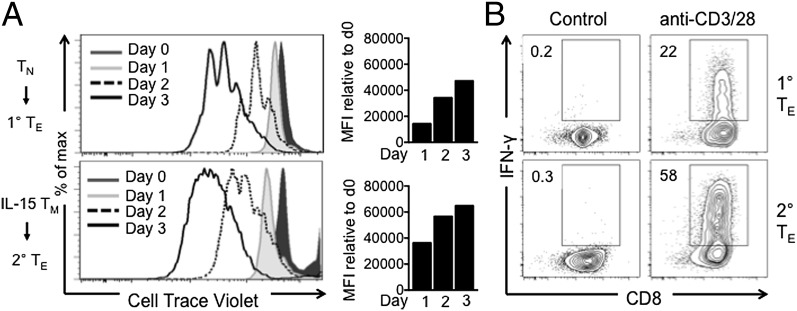
To verify that IL-15 TM cells respond faster to stimulation than TN cells, we measured proliferation of TN and IL-15 TM cells after activation with anti-CD3/28. Secondary TE cells (derived from IL-15 TM cells) proliferated faster than primary TE cells (derived from TN cells; Fig. 2A). We also found that secondary TE cells produced more IFN-γ (Fig. 2B) and IL-2 (Fig. S1). Together, these data confirm that IL-15 TM cells proliferate more quickly and produce more cytokines after activation than TN cells.
Fig. 2.
Secondary TE cells proliferate faster and make more IFN-γ than primary TE cells. Naïve OT-I and IL-15 TM cells were (re)stimulated with anti-CD3/28 for 3 d. (A) Proliferation by Cell Trace Violet dilution at indicated time points. Bar graphs represent difference in mean fluorescence intensity (MFI) at the indicated time point relative to t = 0. (B) IFN-γ production 2 d after (re)stimulation; representative of ≥2 experiments.
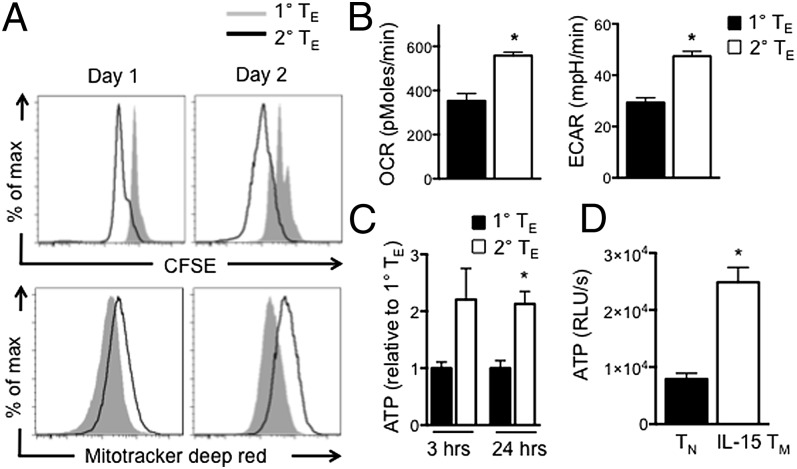
To determine whether differences in mitochondrial content between TN and IL-15 TM cells result in metabolic differences after activation, we stained cells with Mitotracker Deep Red, a dye that localizes to respiring mitochondria. Secondary TE cells proliferated faster and had more respiring mitochondria than primary TE cells (Fig. 3A). We also measured O2 consumption rates (OCR), an indicator of OXPHOS, and extracellular acidification rates (ECAR), an indicator of aerobic glycolysis. Two days after restimulation, secondary TE had higher OCR and ECAR than primary TE cells (Fig. 3B), indicating increased OXPHOS and aerobic glycolysis, respectively, and correlating with the faster proliferation of these cells (Figs. 2A and 3A). We measured ATP and found that secondary TE cells have more ATP than primary TE cells after restimulation and that resting IL-15 TM cells have more ATP than resting TN cells (Fig. 3 C and D). Taken together, these data demonstrate that secondary TE cells have more metabolic activity and ATP than primary TE cells.
Fig. 3.
Enhanced secondary TE cell proliferation is marked by greater metabolic activity and ATP production. Naïve OT-I and IL-15 TM cells were (re)stimulated with anti-CD3/28. (A) Proliferation and Mitotracker deep red staining 1 and 2 d after (re)stimulation. (B) OCR and ECAR in primary and secondary TE cells 2 d after stimulation; mean ± SEM; *P < 0.0001. (C and D) ATP in primary and secondary TE cells 3 and 24 h after (re)stimulation with anti-CD3/28 (C), and in naïve and IL-15 TM cells (D). Data are shown as mean ± SEM; *P < 0.005 (C) and < 0.001 (D); representative of ≥2 experiments.
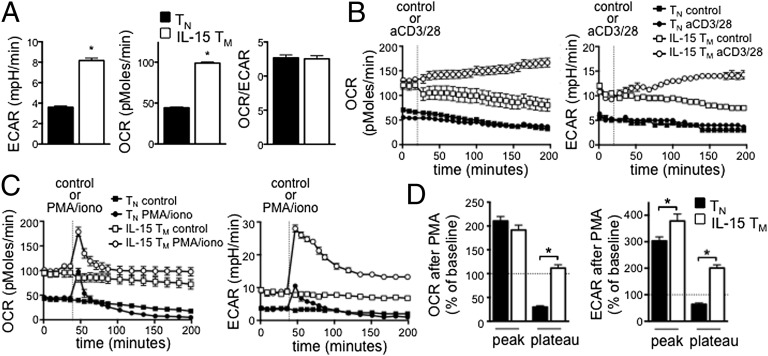
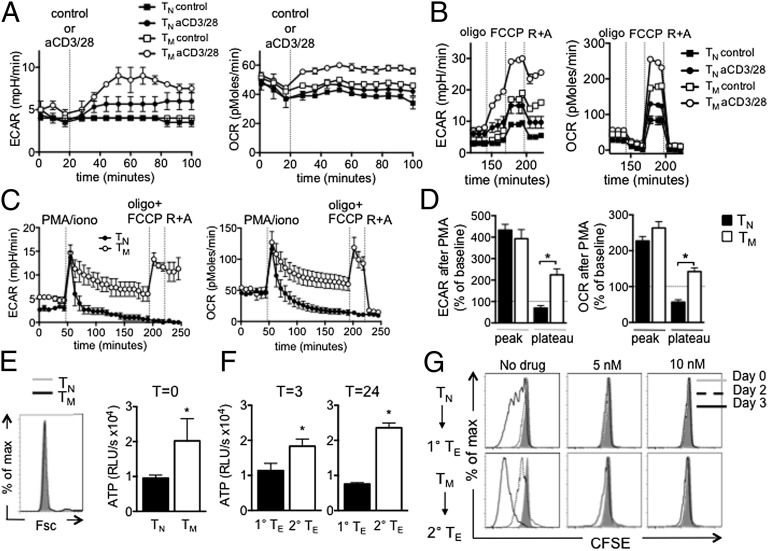
Given our findings that IL-15 TM cells have enhanced mitochondrial content and more ATP than TN cells, we next determined their metabolic phenotype in resting conditions. IL-15 TM cells had higher OCR and ECAR than TN cells (Fig. 4A), indicating that resting IL-15 TM cells are metabolically more active. Relative utilization of glycolysis and OXPHOS, as indicated by OCR/ECAR ratio, is the same in both cells (Fig. 4A), suggesting that the IL-15 TM cells revert to a quiescent metabolic state rather than preferentially using glycolysis over OXPHOS like activated TE cells. Because TM cells respond to reinfection more rapidly than TN cells, we measured the bioenergetics of T cells immediately after activation. We activated TN and IL-15 TM cells with anti-CD3/28 and found that IL-15 TM cells increased OCR and ECAR (Fig. 4B). As TN cells minimally responded in this time frame, we used a stronger stimulus that would also bypass differences in TCR signaling (6). TN and IL-15 TM cells rapidly increased OCR after PMA/ionomycin (iono) (Fig. 4 C and D), which was proportionally similar at the peak, but more sustained in IL-15 TM cells. Both TN and IL-15 TM cells increased ECAR after PMA/iono; however, this increase was higher at the peak and sustained longer in IL-15 TM cells, indicating their greater capacity to promote and sustain glycolysis upon stimulation (Fig. 4 C and D). Furthermore, unlike TN cells, mitochondrial activity in IL-15 TM cells remained intact after PMA/iono, as indicated by increased OCR in response to the uncoupler carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), which reveals maximum respiratory capacity, in the presence of oligomycin (oligo) (blocks ATP synthase), and decreased OCR after etomoxir (FAO inhibitor), and rotenone plus antimycin A (R+A) [electron transport chain (ETC) inhibitors] (Fig. S2). Together these data indicate that IL-15 TM cells are bioenergetically different from TN cells and have a greater capacity to promote and sustain glycolysis and OXPHOS after activation.
Fig. 4.
IL-15 TM cells have enhanced glycolytic capacity compared with TN cells after activation. (A) Basal ECAR and OCR were measured in OT-I TN and IL-15 TM cells; *P < 0.0001. TN and IL-15 TM cells were stimulated with anti-CD3/28 beads (B) or with PMA/iono (C) and OCR and ECAR measured. Data in A–C are from the same experiment and are representative of ≥3 experiments. (D) Compiled and baselined data as shown in C, from two experiments; peak is first measurement after PMA/iono, plateau is at 120 min. *P < 0.0001 (Left), and < 0.05 and < 0.001 (Right); mean ± SEM.
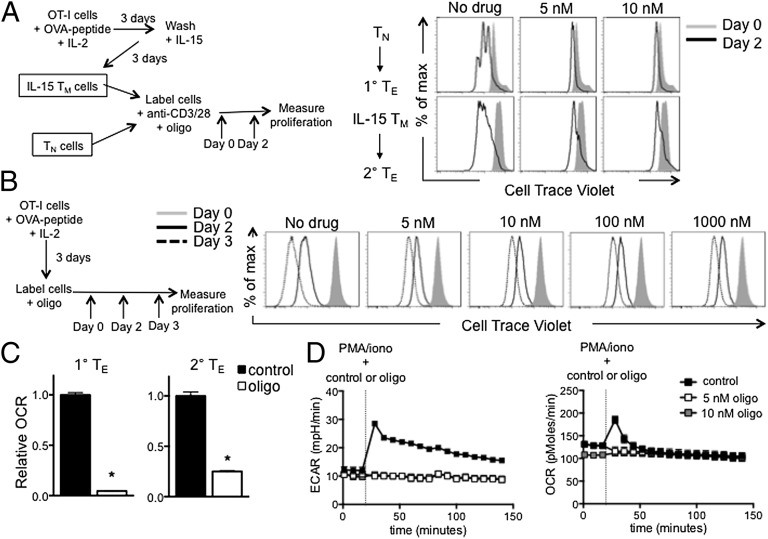
We next questioned how having more mitochondrial mass could contribute to the rapid recall ability and the enhanced glycolytic capacity of IL-15 TM cells. We activated TN and IL-15 TM cells with anti-CD3/28 and followed proliferation in the absence or presence of oligo. Similar to our findings in CD4 TN cells (16), initiation of proliferation of both CD8 TN and IL-15 TM cells was impaired in the presence of low doses of oligo (5–10 nM) (Fig. 5A and Fig. S3). In contrast, even high doses of oligo (1 μM) did not stop proliferation of actively proliferating T cells (Fig. 5B), showing that the initiation of proliferation, rather than the process of active proliferation, requires mitochondrial ATP. The low doses of oligo that inhibited proliferation (Fig. 5A) also reduced OCR (Fig. 5C). Together these data indicate that although IL-15 TM cells were previously activated, they have returned to a resting metabolic state and have the same requirements for activation as TN cells. To determine whether mitochondrial ATP was required for the greater glycolytic capacity of IL-15 TM cells, we added oligo together with PMA/iono. The increase in OCR after activation was diminished in IL-15 TM cells when low doses of oligo were present (Fig. 5D), indicating that OXPHOS is induced after activation and produces mitochondrial ATP. The increase in ECAR in IL-15 TM cells after activation was also inhibited by low doses of oligo (Fig. 5D), suggesting that mitochondrial ATP is needed for the induction of glycolysis and the rapid proliferation of IL-15 TM cells after activation.
Fig. 5.
Mitochondria-derived ATP is required for the initiation of proliferation and facilitates glycolysis in IL-15 TM cells after PMA/iono. (A) OT-I TN and IL-15 TM cells were (re)stimulated with anti-CD3/28 with or without oligo (added at day 0), and proliferation is shown at days 0 and 2; representative of four experiments. (B) OT-I cells were activated with OVA peptide and IL-2 for 3 d, then labeled with Cell Trace Violet and oligo added (day 0). Proliferation was observed at days 0, 2, and 3; representative of two experiments. (C) Relative OCR in primary and secondary TE cells 2 d after anti-CD3/28, ±5 nM oligo; mean ± SEM, representative of two experiments; *P < 0.0001. (D) IL-15 TM cells were restimulated with PMA/iono, with or without oligo, and OCR and ECAR were measured. Data are shown as mean ± SEM, representative of three experiments.
We next measured the bioenergetics of in vivo-generated TM cells isolated from L. monocytogenes (expressing OVA, LmOva) infected mice. Anti-CD3/28 stimulation increased OCR and ECAR in TM cells and, to a lesser extent, ECAR in polyclonal TN cells (Fig. 6A). Two hours after activation, both cell types responded to oligo, FCCP, and R+A (Fig. 6B), indicating intact mitochondrial function. In addition, both TN and TM cells rapidly increased ECAR and OCR after PMA/iono, which was sustained in TM but not TN cells (Fig. 6 C and D). Like IL-15 TM cells (Fig. S2), TM cells increased OCR when exposed to oligo plus FCCP 2 h after activation and reduced OCR after R+A, whereas TN cells did not (Fig. 6C). Together these data indicate that TM cells sustain glycolysis and OXPHOS after activation and that their mitochondrial function remains intact after PMA/iono. TN cells did not lose mitochondrial function when exposed to media (Fig. S4) or anti-CD3/28 (Fig. 6B), suggesting that this loss of mitochondrial function is not an intrinsic defect of TN cells, but rather a result of PMA/iono. We also verified that resting TM cells had more ATP than TN cells, which was not due to greater cell size (Fig. 6E). Likewise, secondary TE cells derived from TM cells had more ATP (Fig. 6F) and proliferated faster than primary TE cells, whereas the initiation of proliferation in both TN and TM cells was impaired by low doses of oligo (Fig. 6G). Together, these data indicate that TM cells, like IL-15 TM cells, have a greater glycolytic capacity and proliferate faster upon stimulation than TN cells, which is facilitated by mitochondrial ATP.
Fig. 6.
TM cells have enhanced glycolysis, more ATP, and proliferate faster than TN cells after activation. CD8 TN (CD44lo CD62Lhi) and TM (CD44hi CD62Lhi) cells were isolated from naïve and LmOVA-infected mice. OCR and ECAR in TN and TM cells after stimulation with aCD3/28 coated beads (A), and after subsequent oligo (1 μM), FCCP (1.5 μM), and rotenone (100 nM) plus antimycin A (1 µM) injections (B) (data in A and B are from the same experiment). (C) TN and TM cells were PMA/iono stimulated, exposed to oligo + FCCP, and R+A, and OCR and ECAR measured. TN is the same as Fig. S4, TM is the same as Fig. 8F and Fig. S7. (D) Compiled and baselined data as shown in C, from two experiments; peak is first measurement after PMA/iono, plateau is at 120 min. *P < 0.0005 (Left), and < 0.0001 (Right). Forward scatter and ATP of resting TN and TM cells (E), and ATP in primary and secondary TE cells 3 and 24 h after aCD3/28 (F). (G) TN and TM cells stimulated with aCD3/28 with or without oligo (day 0) and proliferation is shown. TN control is the same as in Figs. S6B and S12B. TM control is the same as Fig. 7A and 8E; mean ± SEM, representative of two (A, B, and E), 3 (C), or one (F and G) experiment(s).
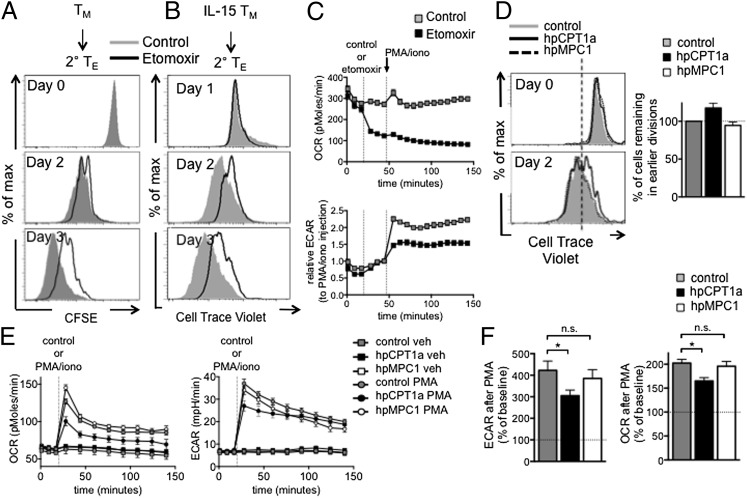
We have shown that TM cells preferentially use FAO for their energy (17). To determine whether FAO contributes to the mitochondrial ATP required for TM cell activation, we activated TM and IL-15 TM cells in the presence of etomoxir and followed proliferation. We found that proliferation was attenuated, but not completely suppressed, when FAO was blocked (Fig. 7 A and B and Fig. S5), suggesting that FAO contributes to, but is not the only pathway fueling OXPHOS in these cells. TN cell proliferation was also reduced when FAO was blocked (Fig. S6). To test whether FAO inhibition impairs the rapid induction of glycolysis after activation, we exposed IL-15 TM cells to etomoxir immediately before activation with PMA/iono. Etomoxir inhibited the increase in OCR and blunted ECAR after activation (Fig. 7C), indicating that FAO fuels OXPHOS and contributes to the rapid activation of IL-15 TM cells. TM cells in the presence of etomoxir failed to increase OCR, but not ECAR, after PMA/iono (Fig. S7), and also failed to increase OCR after oligo plus FCCP, indicating that FAO contributes to the maximum respiratory capacity after activation. We also modulated the expression of carnitine palmitoyltransferase 1a (CPT1a), a protein that is the rate-limiting step to FAO, and the target of etomoxir (22, 23). We transduced T cells with retrovirus expressing shRNA against CPT1a (hpCPT1a) (17). Inhibiting CPT1a expression in IL-15 TM cells slowed initial proliferation after anti-CD3/28 activation (Fig. 7D), although this effect was more subtle and variable than the consistently strong inhibition by etomoxir. To investigate whether pyruvate fuels this process, we used a retrovirus expressing shRNA against the mitochondrial pyruvate carrier (hpMPC1) (24). Although this shRNA resulted in lower MPC1 mRNA expression (Fig. S8), there was no difference in proliferation compared with the control cells (Fig. 7D). Consistent with our data showing that FAO contributes to the proliferation of IL-15 TM cells after activation, we found that hpCPT1a IL-15 TM cells had impaired induction of OCR and ECAR after PMA/iono stimulation, whereas hpMPC1 IL-15 TM cells did not (Fig. 7 E and F). Flux analysis of 13C-glucose comparing IL-2 TE cells, which are highly glycolytic and use substantial amounts of glucose, to IL-15 TM cells showed that the relative abundance of unlabeled carbon was greater than that of labeled carbon for TCA cycle intermediates in IL-15 TM cells (Fig. S9). These data indicate that pyruvate flux into the TCA cycle is lower in IL-15 TM cells, which may suggest that other substrates contribute to OXPHOS. Together these data indicate that FAO fuels the OXPHOS required for the rapid recall response of IL-15 TM cells.
Fig. 7.
FAO fuels the OXPHOS needed for rapid recall of TM cells. (A) Proliferation of TM cells from LmOVA-infected mice after anti-CD3/28 ± etomoxir (day 0); control is same as Fig. 6G and 8E. Data are from one experiment. (B) Proliferation of IL-15 TM cells after anti-CD3/28 ± etomoxir; representative of four experiments. (C) OCR and ECAR of IL-15 TM cells after PMA/iono ± etomoxir; mean ± SEM, representative of four experiments. (D–F) Proliferation of IL-15 TM cells expressing control (shRNA against luciferase), hpCPT1a (shRNA against CPT1a), or hpMPC1 (shRNA against MPC1) retrovirus after anti-CD3/28 (D); graph shows percent of cells normalized to control cells in gate with fewer divisions (separated by line), generated from two experiments. (E) OCR and ECAR of control, hpCPT1a, and hpMPC1 IL-15 TM cells after PMA/iono. (F) Compiled and baselined data as shown in E, from three experiments; peak is at first measurement after PMA/iono, plateau is at 120 min. n.s., not significant. *P < 0.05; mean ± SEM (E and F) and representative of two (D) or four (E) experiments.
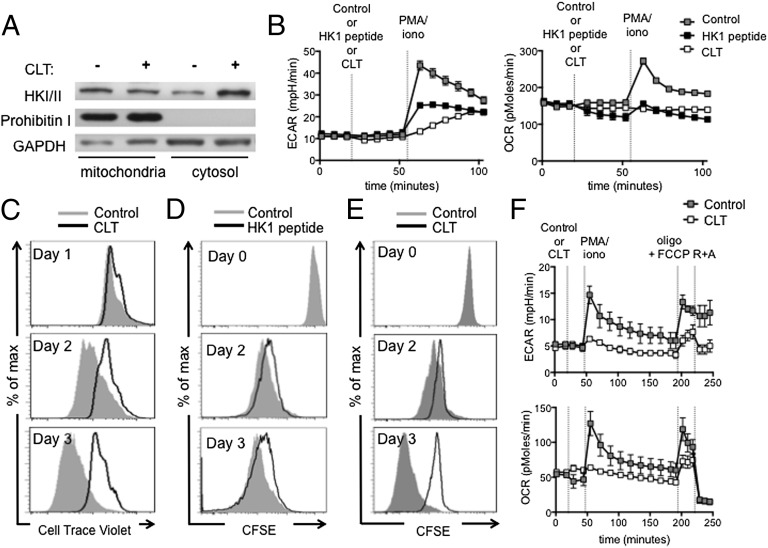
We next wanted to determine how mitochondrial ATP could contribute to the rapid increase in glycolysis in IL-15 TM cells upon activation. HK mediates the ATP-dependent conversion of glucose into glucose-6-phosphate in the first step of glycolysis. Upon T-cell activation, HK is recruited to the mitochondria where ATP is abundant (25). We used clotrimazole (CLT), a drug that dissociates HK from the mitochondria (Fig. 8A) (26), or peptides that consist of the N-terminal sequence of HK and compete with HK for mitochondrial binding (27, 28), to explore whether mitochondrial-associated HK is critical for the rapid induction of glycolysis in (IL-15) TM cells. We found that the ability of IL-15 TM cells to rapidly increase OCR and ECAR after PMA/iono was impaired in the presence of CLT or HK peptides (Fig. 8B and Fig. S10). CLT did not destroy mitochondrial activity, because R+A decreased OCR after CLT (Fig. S11A). Likewise, 10 µM of either HK-1 or HK-2 peptides left OXPHOS intact, whereas the combination of both resulted in the inability to increase OCR after oligo + FCCP (Fig. S11B), indicating a loss of mitochondrial function. In addition, IL-15 TM and TN cell proliferation after anti-CD3/28 stimulation was impaired by CLT (Fig. 8C and Fig. S12) or HK-1 competing peptide (Fig. 8D). CLT also decreased proliferation (Fig. 8E) and impaired OCR and ECAR after activation (Fig. 8F) in TM cells. Mitochondrial function remained intact in TM cells treated with CLT as shown by the increase in OCR after oligo plus FCCP and decrease in OCR after R+A (Fig. 8F). These data indicate that mitochondria-associated HK is critical for the rapid induction of glycolysis and proliferation of (IL-15) TM cells.
Fig. 8.
Dissociation of HK from mitochondria impairs proliferation and the rapid engagement of glycolysis in (IL-15) TM cells after activation. (A) Western blot analysis for HK I and II in IL-15 TM cells incubated ± CLT for 2 h; GAPDH and prohibitin I are loading controls for cytosolic and mitochondrial fractions, respectively; representative of two experiments. (B) OCR and ECAR of IL-15 TM cells stimulated with PMA/iono in the presence of control or HK-1 peptide (10 μM) or CLT (25 μM); mean ± SEM, representative of ≥2 experiments. IL-15 TM cells were stimulated with anti-CD3/28 and proliferation ± CLT; representative of five experiments (C), or with control or HK-1 peptide (10 μM); representative of two experiments, is shown (D). (E and F) TM cells from LmOVA-infected mice. Proliferation after anti-CD3/28 ± CLT; control is same as Fig. 6G and 7A, from one experiment (E); OCR and ECAR after PMA/iono ± CLT and exposed to oligo (1 μM) plus FCCP (1.5 μM), and rotenone (100 nM) plus antimycin A (1 µM); mean ± SEM and representative of two experiments; control is the same as Fig. 6C and Fig. S7 (F).
Discussion
We propose a model where substantial mitochondrial mass and ATP enable TM cells to rapidly recall upon restimulation. The enhanced availability of mitochondrial ATP provides the energy that is required for the rapid engagement of glycolysis observed in T cells after activation. The HK-mediated conversion of glucose into glucose-6-phosphate requires ATP, and we show here that blocking the generation of mitochondrial ATP, and the dissociation of HK from the mitochondria, impair the rapid induction of glycolysis and hamper the fast proliferation of TM cells after activation. The proliferation of TN cells after activation was also impaired when mitochondrial ATP was unavailable, suggesting that both TN and TM cells have similar requirements for activation and that quantitative bioenergetic differences underlie the distinct abilities of these cells to respond to Ag. Qualitative bioenergetic differences may also exist between TN and TM cells. Although both TN and TM cells increased glycolysis after anti-CD3/28, and responded to uncoupling and ETC inhibitors, TN cells lost metabolic activity after PMA/iono, suggesting that after this stimulation, TN cells cannot efficiently fuel their mitochondria, whereas TM cells can. Several factors could contribute to this disparity, such as differences in the strength of activation, the signaling pathways involved, or the acquisition or storage of substrates.
Although quantitative differences in Ag-specific TN and TM cells can contribute to the superior protection conferred by TM cells upon reinfection, studies where equal cell numbers were transferred into mice have shown that these cells respond with distinct kinetics (1, 3, 5, 29, 30). TM cells enter the cell cycle earlier and proliferate faster than TN cells after activation (3–5). Because TM cells are different from TN cells in their ability to migrate to peripheral tissues (30–32), these experiments do not exclude the possibility that TM cells respond more quickly because they encounter Ag before TN cells. Consistent with previous studies (3, 4, 33), we show here that TM cells are intrinsically different as they proliferate faster and make more cytokine after activation in vitro. This more ready-to-respond state of TM cells has been attributed to several intrinsic differences such as an “open” chromatin conformation of cytokine genes, altered transcription profiles, and enhanced activity of proximal TCR signaling components (1, 2). We now show here that rapid proliferation and engagement of glycolysis by TM cells after activation requires mitochondrial ATP to enable optimal HK function. Although primary TE cells have been shown to exhibit prolonged proliferation after infection compared with secondary TE cells (5, 34), and ultimately TN cells have a greater per-cell expansion than TM cells (34), these observations do not contradict that TM cells have a greater capacity to proliferate more rapidly immediately after stimulation.
CD8 TM cells use FAO, which contributes to their substantial SRC that is important for survival and stable TM development (17). We show here that FAO in TM cells also provides the substrates, at least partly, for the OXPHOS that is required for the rapid induction of glycolysis and the fast proliferative response of these cells. When IL-15 TM cells are transduced with the hpCPT1a retrovirus, the effect on the inhibition of OCR and proliferation is more subtle and variable than when fully differentiated IL-15 TM cells are acutely exposed to etomoxir. Our published data show 50% reduction in CPT1a mRNA by using this construct (17), suggesting only partial FAO inhibition. Unlike direct exposure to etomoxir, the hpCPT1a retrovirus does not acutely impair FAO. When transduced cells are cultured for 5 d before proliferation and OCR are measured, it is likely that they adapt and begin to rely less on FAO. Given the importance of CPT1a in in vivo TM cell development, and that IL-15 induces CPT1a expression in vitro (17), we expect that the hpCPT1a-transduced T cells that do survive until day 6 in the IL-15 cultures are selected on their ability to use other substrates, i.e., cells that rely most heavily on FAO while expressing hpCPT1a might not survive. This phenomenon could explain the more striking effect on proliferation and OCR when FAO is acutely inhibited by etomoxir. Our data also suggest that pyruvate flux via MPC does not substantially contribute to OXPHOS in T cells during the recall response. Although we have not ruled out the possibility that pyruvate still enters the mitochondria in hpMPC cells, it is likely that, in addition to long chain fatty acids, glutamine or medium and short chain fatty acids contribute to OXPHOS in TM cells.
Our results indicate that mitochondrial association of HK is important for the rapid recall of TM cells. Recruitment of HK to the mitochondria happens quickly in response to Akt activation (25), and the conversion of glucose into glucose-6-phosphate is a process that requires ATP. Because mitochondrial HK has been shown to exclusively use intramitochondrial ATP to phosphorylate glucose (35–37), we speculate that the direct availability of ATP supports the rapid activation of HK when associated with mitochondria. Higher expression of glycolysis enzymes in TM cells might also account for their capacity to quickly increase glycolysis.
OT-I TN cells did not increase OCR or ECAR after anti-CD3/28, whereas polyclonal TN cells slightly increased ECAR. It is possible that this difference would not occur if given more time or a stronger stimulus, or perhaps polyclonal TN cells exist in a slightly more activated state than transgenic TN cells and that this more activated state contributes to their faster metabolic activity. We also found that etomoxir inhibited the increase in OCR, but not ECAR, in TM cells after PMA/iono, differing from IL-15 TM cells, which show a decrease in both ECAR and OCR. These data suggest that TM cells are able to engage glycolysis briefly after PMA/iono in the presence of etomoxir, but importantly, this engagement is dissipated after oligo and FCCP. This difference may be due to the fact that IL-15 TM cells are a homogenous population exposed to optimized culture conditions, whereas in vivo-generated TM cells have varied substrate and growth factor availability and might not rely solely on FAO to initially engage glycolysis after activation, but need it to maintain mitochondrial and glycolytic activity when maximal respiratory capacity is required.
We show here that bioenergetic differences between CD8 TN and TM cells contribute to the differential responses of CD8 TN and TM cells to activation, i.e., greater mitochondrial mass endows TM cells with the ability to rapidly recall. Agents that induce mitochondrial biogenesis have been of interest for treatment of numerous pathologies (38). Our findings indicate that drugs that induce mitochondrial biogenesis could hold promise as immunotherapeutics to improve vaccination strategies.
Materials and Methods
Full methods are available as SI Materials and Methods. Mice were purchased from Jackson Laboratory. OT-I cells were activated with OVA-peptide and IL-2 for 3 d and, subsequently, cultured in the presence of IL-2 or IL-15 for 3–4 d to generate IL-2 TE and IL-15 TM cells, respectively (17). OCR and ECAR were measured by using the XF-24 or XF-96 Extracellular Flux Analyzers (Seahorse Bioscience). Statistical comparisons for two groups were calculated by using unpaired two-tailed Student’s t tests.
Supplementary Material
Acknowledgments
We thank Yuliya Shakir, Erica Lantelme, and Dorjan Brinja for technical assistance. This work was supported by National Institutes of Health (E.L.P. and E.J.P.) and Netherlands Organisation for Scientific Research (G.J.W.v.d.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221740110/-/DCSupplemental.
References
- 1.DiSpirito JR, Shen H. Quick to remember, slow to forget: Rapid recall responses of memory CD8+ T cells. Cell Res. 2010;20(1):13–23. doi: 10.1038/cr.2009.140. [DOI] [PubMed] [Google Scholar]
- 2.Farber DL. Biochemical signaling pathways for memory T cell recall. Semin Immunol. 2009;21(2):84–91. doi: 10.1016/j.smim.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1(1):47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 4.Cho BK, Wang C, Sugawa S, Eisen HN, Chen J. Functional differences between memory and naive CD8 T cells. Proc Natl Acad Sci USA. 1999;96(6):2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169(7):3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 6.Kersh EN, et al. TCR signal transduction in antigen-specific memory CD8 T cells. J Immunol. 2003;170(11):5455–5463. doi: 10.4049/jimmunol.170.11.5455. [DOI] [PubMed] [Google Scholar]
- 7.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177(2):1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 8.Kersh EN, et al. Rapid demethylation of the IFN-gamma gene occurs in memory but not naive CD8 T cells. J Immunol. 2006;176(7):4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 9.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 10.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: Energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5(11):844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 11.Jones RG, Thompson CB. Revving the engine: Signal transduction fuels T cell activation. Immunity. 2007;27(2):173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249(1):27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krauss S, Brand MD, Buttgereit F. Signaling takes a breath—new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15(4):497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- 14.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6(3):683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 15.Roos D, Loos JA. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp Cell Res. 1973;77(1):127–135. doi: 10.1016/0014-4827(73)90561-2. [DOI] [PubMed] [Google Scholar]
- 16.Chang CH, et al. Post-transcriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37(Pt 6):1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls DG, et al. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. 2010;2010(46):2511. doi: 10.3791/2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19(3):315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172(12):7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 22.Deberardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281(49):37372–37380. doi: 10.1074/jbc.M608372200. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay RR, Zammit VA. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol Aspects Med. 2004;25(5-6):475–493. doi: 10.1016/j.mam.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Bricker DK, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majewski N, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16(5):819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Penso J, Beitner R. Clotrimazole and bifonazole detach hexokinase from mitochondria of melanoma cells. Eur J Pharmacol. 1998;342(1):113–117. doi: 10.1016/s0014-2999(97)01507-0. [DOI] [PubMed] [Google Scholar]
- 27.Gelb BD, et al. Targeting of hexokinase 1 to liver and hepatoma mitochondria. Proc Natl Acad Sci USA. 1992;89(1):202–206. doi: 10.1073/pnas.89.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nederlof R, et al. Pathophysiological consequences of TAT-HKII peptide administration are independent of impaired vascular function and ensuing ischemia. Circ Res. 2013;112(2):e8–e13. doi: 10.1161/CIRCRESAHA.112.274308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Northrop JK, Wells AD, Shen H. Cutting edge: Chromatin remodeling as a molecular basis for the enhanced functionality of memory CD8 T cells. J Immunol. 2008;181(2):865–868. doi: 10.4049/jimmunol.181.2.865. [DOI] [PubMed] [Google Scholar]
- 30.Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: Homing properties rather than initial frequencies are crucial. J Immunol. 1999;163(10):5535–5543. [PubMed] [Google Scholar]
- 31.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 32.Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: Parallels and distinctions. Immunotherapy. 2011;3(10):1223–1233. doi: 10.2217/imt.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206(2):435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin MD, Condotta SA, Harty JT, Badovinac VP. Population dynamics of naive and memory CD8 T cell responses after antigen stimulations in vivo. J Immunol. 2012;188(3):1255–1265. doi: 10.4049/jimmunol.1101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brdiczka D. Interaction of mitochondrial porin with cytosolic proteins. Experientia. 1990;46(2):161–167. doi: 10.1007/BF02027312. [DOI] [PubMed] [Google Scholar]
- 36.BeltrandelRio H, Wilson JE. Interaction of mitochondrially bound rat brain hexokinase with intramitochondrial compartments of ATP generated by oxidative phosphorylation and creatine kinase. Arch Biochem Biophys. 1992;299(1):116–124. doi: 10.1016/0003-9861(92)90252-r. [DOI] [PubMed] [Google Scholar]
- 37.Cesar MdeC, Wilson JE. Further studies on the coupling of mitochondrially bound hexokinase to intramitochondrially compartmented ATP, generated by oxidative phosphorylation. Arch Biochem Biophys. 1998;350(1):109–117. doi: 10.1006/abbi.1997.0497. [DOI] [PubMed] [Google Scholar]
- 38.Beeson CC, Beeson GC, Schnellmann RG. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem. 2010;404(1):75–81. doi: 10.1016/j.ab.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.