Abstract
Parenchymal blood volume (PBV) mapping with flat panel detectors may provide real-time estimates of tissue perfusion during endovascular ischemic stroke procedures. We present two cases of acute middle cerebral artery (MCA) occlusion to demonstrate how PBV may: (1) be used in acute stroke; (2) influence intraprocedural decision-making; and (3) potentially serve as a predicator of clinical outcome. Both cases were successfully recanalized with endovascular embolectomy. Intraprocedural PBV maps were obtained immediately before and after recanalization. Pre-intervention reductions in PBV were seen throughout the MCA territory in both cases, with significant improvement in PBV in one case with good radiographic and clinical outcome and a lack of improvement in PBV in the second case with a large infarct volume. PBV deficit normalization may occur with recanalization of the parent artery and probably represents successful reperfusion. Baseline PBV maps should therefore be interpreted with caution and not interpreted to represent irreversible core infarct.
Keywords: Stroke, Artery, Angiography, Embolic
Background
CT and MRI perfusion imaging are increasingly used to identify stroke patients who are the best candidates for endovascular stroke intervention. These are patients with small core infarcts and large penumbra.1 2 One of the major limitations of these techniques is the amount of time that elapses between an intervention and when initial perfusion imaging is acquired. It is well-known that penumbra reduction, infarct core expansion and neuronal loss increase with the passage of time.3 Parenchymal blood volume (PBV) imaging allows for the visualization of non-quantitative cerebral tissue perfusion during cerebral angiography using C-arm rotation and Syngo Neuro PBV IR software available on Siemens Artis Zee systems. The technical details of image acquisition have been previously described.4 The utility of this novel technique during acute stroke intervention has not been well established. This case report illustrates how PBV changes after successful recanalization may be an important marker of tissue fate and clinical outcome in acute ischemic stroke endovascular intervention.
Case presentation
Case 1
A 53-year-old right-handed man presented with an acute left middle cerebral artery (MCA) syndrome and an NIH Stroke Scale (NIHSS) score of 21. Non-contrast head CT was normal except for a hyperdense left MCA sign. He received intravenous tissue plasminogen activator (tPA) within 2 h of symptom onset. CT angiography demonstrated a cervical internal carotid artery (ICA) dissection to the level of the skull base and intracranial MCA (M1) occlusion. The patient was then taken directly to the neuroangiography suite. No neurologic improvement was noted prior to the intervention.
Case 2
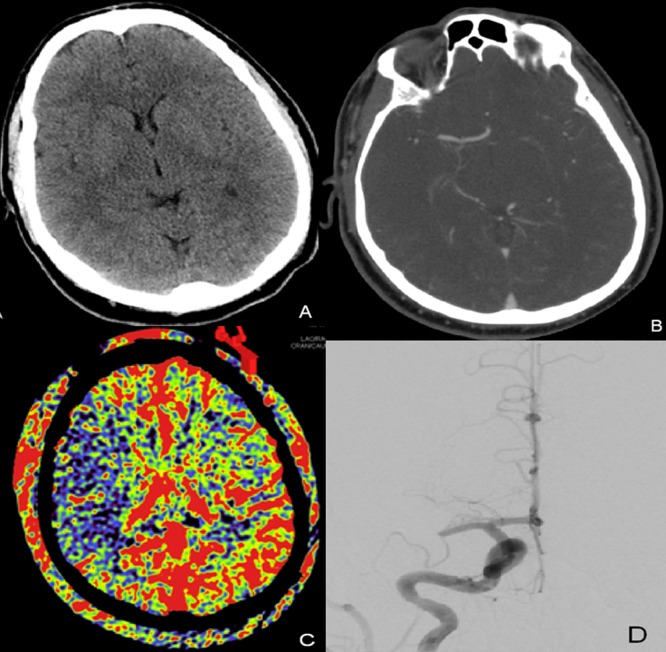
A middle-aged patient without a significant past medical history presented with an acute right MCA syndrome with an NIHSS score of 14. Initial CT and CT angiogram (CTA) demonstrated no large territorial infarction, a hyperdense MCA sign with occlusion of the M1 segment and good leptomeningeal collaterals on CTA. He was treated with intravenous tPA and brought to the angiographic suite without improvement (figure 1).
Figure 1.
(A) Non-contrast head CT on presentation with no signs of early ischemic changes. (B) CT angiogram obtained prior to treatment with right M1 segment occlusion. (C) Pretreatment aortic arch parenchymal blood volume (PBV) acquisition demonstrating decreased PBV throughout the right middle cerebral artery distribution. (D) Right internal carotid artery digital subtraction angiogram (PA) demonstrating right M1 occlusion.
Outcome and follow-up
Case 1
PBV images in case 1, acquired via 4 Fr pigtail aortic arch injections, demonstrated reduced PBV throughout the left MCA distribution. Angiography revealed an occluded cervical left ICA at the origin determined to be an acute dissection and tandem occlusion of the MCA (figure 2). A 054 Penumbra reperfusion catheter was then advanced past the M1 occlusion and hand aspiration with a 60 ml syringe resulted in recanalization of the M1 and superior division of the MCA 4 h from initial symptom onset. Two milligrams of intra-arterial tPA were infused through a Penumbra 041 reperfusion catheter into the inferior division with only partial recanalization.
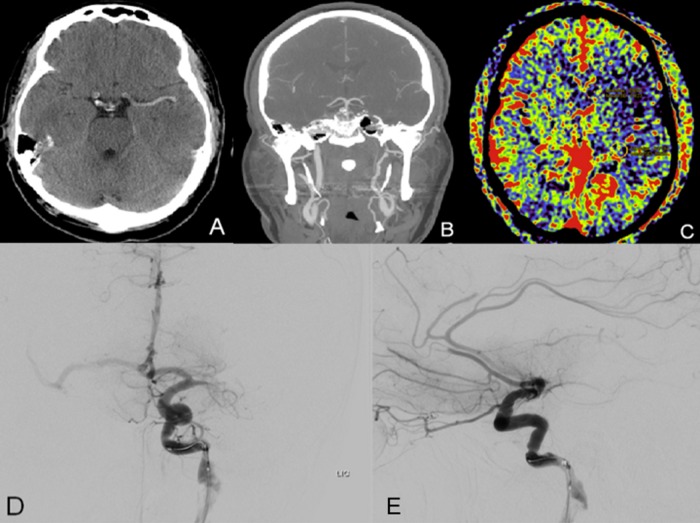
Figure 2.
(A) Non-contrast CT demonstrating a hyperdense middle cerebral artery (MCA) sign on the left without evidence of early ischemic changes. (B) CT angiogram head and neck coronal reconstruction showing dissection in the cervical left internal carotid artery. (C) Pretreatment parenchymal blood volume with decreased blood volume in the left MCA distribution. (D, E) Digital subtraction angiograms of the left internal carotid artery (PA, lateral) demonstrating left M1 segment occlusion and multiple branch occlusions in the bilateral anterior cerebral arteries.
The carotid dissection was then treated with a 7–10 ×40 mm Acculink stent which was deployed from the proximal petrous segment through the high cervical segment of the left ICA. Next, a Protégé EverFlex 8.0×40 mm stent was deployed in telescoping fashion from the proximal aspect of the Acculink into the proximal cervical segment of the left ICA. Additional attempts at intra-arterial thrombolysis and thrombectomy of the inferior MCA division were unsuccessful. A final aortic arch injection PBV acquisition demonstrated no change with reduced PBV in the inferior division and normalized PBV in the superior division (figure 3).
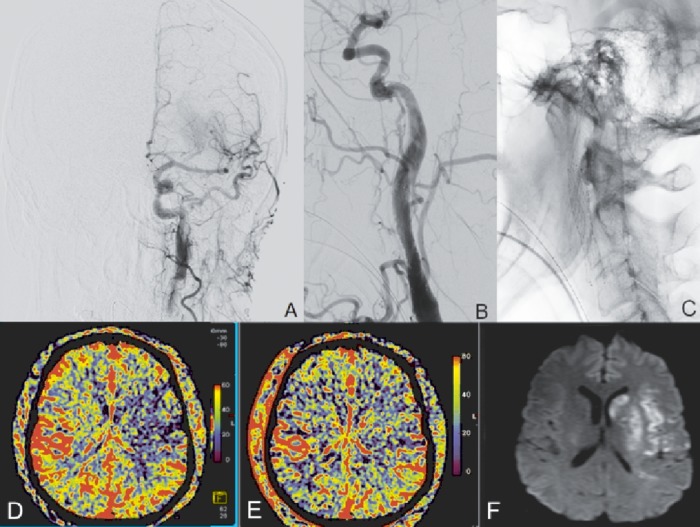
Figure 3.
(A) Post-treatment left internal carotid digital subtraction angiogram (PA) demonstrating recanalization of the left M1 segment. (B) Post-stenting left common carotid digital subtraction (lateral) demonstrating endovascular reconstruction of dissected left internal carotid artery. (C) Native lateral acquisition showing the stent construct in the left common carotid to high cervical internal carotid. (D, E) Pre- and post-recanalization parenchymal blood volume (PBV) aortic arch acquisitions demonstrating normalization of PBV in a significant portion of the middle cerebral artery territory (192 min between pre- and post-PBV acquisitions). (F) Diffusion-weighted image MRI demonstrating final infarct volume.
At discharge he could communicate effectively with mild aphasia and had a moderate hemiparesis with a modified Rankin Scale (mRS) score of 2. He was transferred to inpatient rehabilitation and eventually returned to work full-time with a mRS score of 1 at 1 month.
Case 2
An initial aortic arch injection demonstrated reduced PBV throughout the MCA territory and angiographic images demonstrated MCA (M1 segment) occlusion. TICI IIa recanalization was obtained with 5 mg intra-arterial tPA and angioplasty with a Hyperglide 3 mm×15 mm balloon after failure of Merci, Penumbra,or Solitaire to open the occlusion. Time from onset to recanalization was approximately 8 h. Post-recanalization PBV demonstrated a large persistent PBV deficit in the right MCA territory. The patient went on to have a large infarction of the right MCA territory (figure 4). Clinically, the patient improved significantly with a discharge NIHSS score of 4 with continued dysarthria and mild hemiparesis.
Figure 4.
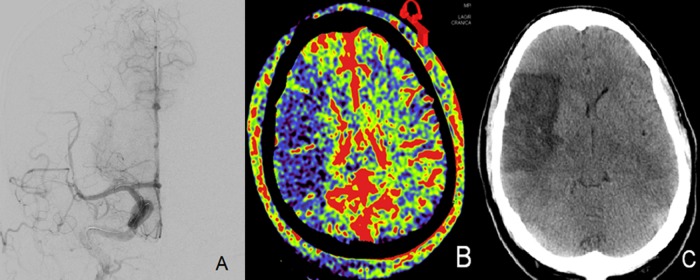
(A) Right internal carotid artery digital subtraction angiogram (PA) demonstrating successful recanalization of the right M1 segment. (B) Post-treatment aortic arch parenchymal blood volume (PBV) acquisition demonstrating persistent decreased PBV in the right middle cerebral artery distribution (199 min elapsed between initial and final PBV acquisitions). (C) Non-contrast head CT obtained 5 days after treatment showing final infarct that closely matches post-treatment aortic arch PBV acquisition.
No complications were encountered in either case as a result of PBV acquisitions.
Discussion
PBV is similar to CT perfusion (CTP) but has the advantage of being obtained in real time during an intervention in the angiography suite. In lieu of cerebral blood volume from CTP, PBV may provide a real-time picture of hypoperfused brain.5 6 The major disadvantage of PBV compared with CTP is that dynamic perfusion imaging cannot be performed due to the limited rotational speed and inability to rotate continuously in the same direction. Mean transit time, the CTP metric known to best correlate with diffusion-weighted images (DWI) infarct in acute stroke without recanalization, cannot therefore be calculated.4 7 In our institution we use a CTA-based protocol for selection of patients for endovascular treatment. We treat patients on the basis of large vessel occlusion and robust CTA collaterals or small diffusion-weighted MRI infarct volume at presentation.8
In our two cases with pre- and post-recanalization PBV, tissue salvage on follow-up imaging was seen only in the case with PBV normalization. This illustrates the concept of successful reperfusion versus futile reperfusion despite recanalization that has been illustrated by CTP.8 9 It remains undetermined what portion of an initial PBV deficit represents core infarct or salvageable penumbral tissue. Case 1 shows that reduced PBV is not always analogous to infarct core, nor is large volume of reduced PBV a malignant radiographic marker similar to a large DWI/perfusion MRI deficit, as reported by the DEFUSE investigators, that predicts symptomatic hemorrhage and poor outcome based on the size of the initial perfusion deficit.2 We therefore feel that the presence of a PBV deficit should not be used to exclude patients from endovascular treatment.
Our second case illustrates futile recanalization without reperfusion/PBV normalization, indicating that there was penumbral loss during the extended time to recanalization. Theoretically, PBV normalization may be useful to the interventionalist in intraprocedural decision-making to stop or forgo attempts at revascularization and ‘settle’ for an imperfect angiographic recanalization but with adequate tissue reperfusion. The favorable clinical outcome of the second patient, despite the large volume of final infarct, is probably due to the sparing of the motor tracks beginning in the primary motor cortex and extending into the internal capsule.
Intraprocedural PBV is a promising new technology that may allow more nuanced decision-making in the interventional suite when treating acute ischemic stroke. Additional work is needed to better define imaging thresholds that will allow reliable characterization of penumbra and infarct core.
Learning points.
Parenchymal blood volume (PBV) measures of cerebral tissue perfusion can be obtained in the angiographic suite with flat panel detectors.
Reperfusion, defined as PBV normalization after recanalization, is a potential marker of good outcome and may serve as a stopping point for acute ischemic stroke embolectomy.
PBV deficits are not analogous with infarct core and should not be used to exclude patients from acute ischemic stroke recanalization therapies.
Footnotes
Contributors: LE contributed to the concept, drafting and editing, research, reviewing and final review of the manuscript; VTD and HT contributed to drafting and editing the manuscript and research; MK contributed to research; ASA contributed to the concept, editing, research, reviewing and final review of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jovin TG, Liebeskind DS, Gupta R, et al. Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: retrospective multicenter analysis of 237 consecutive patients. Stroke 2011;2013:2206–11 [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;2013:508–17 [DOI] [PubMed] [Google Scholar]
- 3.Saver JL. Time is brain–quantified. Stroke 2006;2013:263–6 [DOI] [PubMed] [Google Scholar]
- 4.Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat detector CT in the evaluation of brain parenchyma, intracranial vasculature, and cerebral blood volume: a pilot study in patients with acute symptoms of cerebral ischemia. AJNR Am J Neuroradiol 2010;2013:1462–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittkamp G, Buerke B, Dziewas R, et al. Whole brain perfused blood volume CT: visualization of infarcted tissue compared to quantitative perfusion CT. Acad Radiol 2010;2013:427–32 [DOI] [PubMed] [Google Scholar]
- 6.Zhu HY, Geng DY, Geng CM, et al. An initial study of three-dimensional perfused blood volume computed tomography imaging of patients with anterior circulation hyperacute cerebral infarction. J Neuroimaging 2012;2013:149–54 [DOI] [PubMed] [Google Scholar]
- 7.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;2013:979–85 [DOI] [PubMed] [Google Scholar]
- 8.Souza LC, Yoo AJ, Chaudhry ZA, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol 2012;2013:1331–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soares BP, Tong E, Hom J, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke 2010;2013:e34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]