Abstract
A 47-year-old woman presented with ascites. There was history of large volume paracentesis and the drained ascitic fluid was found to be positive for malignant cells. Clinical examination revealed a soft tissue nodule over the skin of right iliac fossa and a small umbilical nodule besides presence of ascites. In addition, the patient had a breast lump of 2×2 cm in upper quadrant of right breast. Biopsy from the abdominal wall nodule revealed a malignant tumour with dense desmoplastic response. The tumour cells were pancytokeratin, CA125, WT1 positive and CDX2, CD10, villin, calretinin negative; thus immunohistochemically suggesting a primary tumor arising from ovary. Biopsy from breast lump showed malignant epithelial cells present in sheets with stromal dysplasia. Immunohistochemically tumour cells were positive for CK7. CA125, WT1, thus favouring a metastatic carcinoma to breast with possible primary arising from ovary.
Background
Ovarian cancer spread along the peritoneal surfaces intrabdominally is the rule rather than exception. Even the disease metastasizing to other organ systems including the breast and skin is not unknown. However, sometimes one comes across a clinical situation which baffles even the trained mind as far as frequency of its occurrence is concerned. We report, to the best of our knowledge, the first case of ovarian carcinoma presenting synchronously with skin and breast metastases, both of which are uncommon metastatic sites of ovarian carcinoma.
Case presentation
A 47-year-old woman presented with distension abdomen of 4-month duration. It was associated with loss of weight and appetite. There was history of large volume paracentesis and the drained ascitic fluid was found to be positive for malignant cells. Clinical examination revealed a soft tissue nodule over the skin of right iliac fossa and a small umbilical nodule besides presence of ascites. In addition, the patient had a breast lump of 2×2 cm in upper quadrant of right breast. There was a 4-year history of hysterectomy for a benign pathology.
Investigations
A contrast enhanced CT abdomen revealed a pelvic mass with peritoneal, epiploic, lymph nodal and subcutaneous deposits along with ascites.
Bilateral mammogram revealed a 20×20 mm rounded partly well-defined density in the upper outer quadrant of right breast. An ultrasound correlation of mammographic finding revealed a lobulated 17×13 mm complex solid cystic lesion with extensive hypervascularity with suspicious node, with loss of fatty hilum and central necrosis in the right axilla.
Biopsy from the abdominal wall nodule revealed a malignant tumour with dense desmoplastic response (figure 1). The tumour cells were pancytokeratin, CA125, WT1 positive (figure 2) and CDX2, CD10, villin, , Calretinin negative and thus immunohistochemically suggesting a primary from ovary. Biopsy from breast lump showed malignant epithelial cells present in sheets with stromal dysplasia (figure 3). Immunohistochemically tumour cells were positive for CK7. CA125, WT1, thus favouring a metastatic carcinoma in breast with possible primary arising from ovary.
Figure 1.
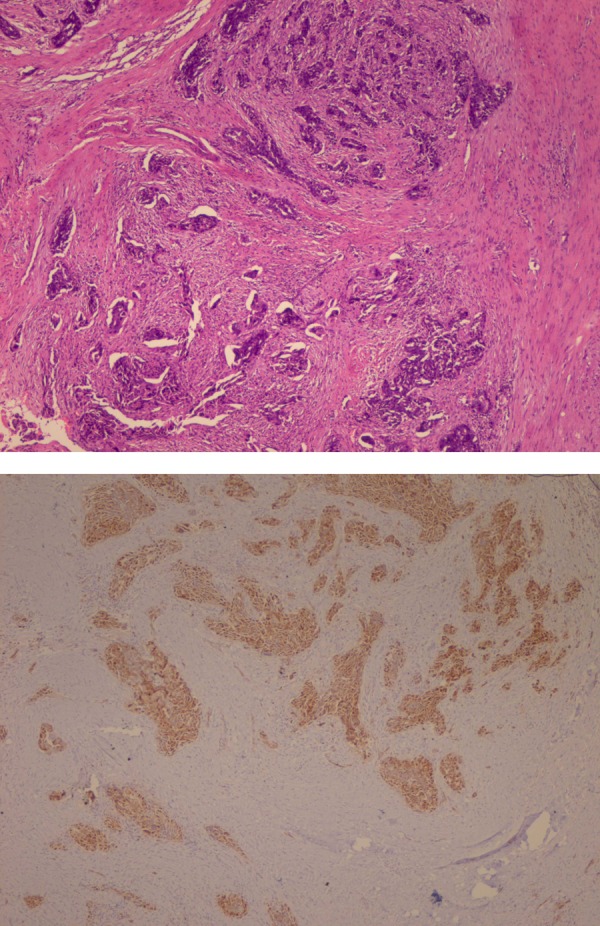
Histopathology of skin nodule.
Figure 2.
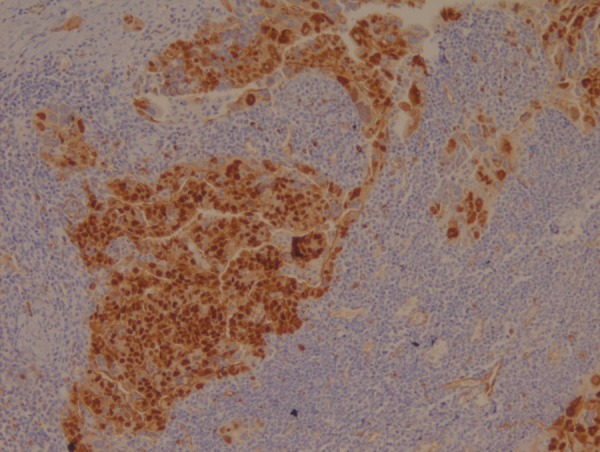
Tumour cells positive for WT1 on immunohistochemistry.
Figure 3.
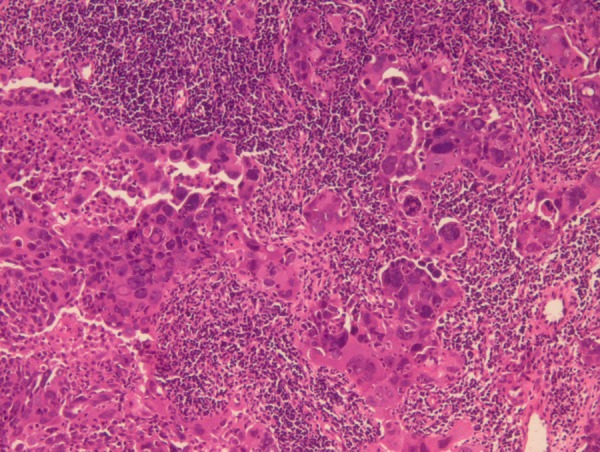
Histopathology of breast lump.
Differential diagnosis
Ovarian cancer metastatic to skin and breast
Breast cancer metastatic to ovaries and skin
Treatment
The patient was managed with taxane and platinum-based palliative chemotherapy. Her skin nodule resolved within few days of starting the treatment. Her ascites and breast lump had resolved by the time she was due for her second chemotherapy cycle. She encountered frequent treatment breaks due to infrequent complications including lung abscess.
Outcome and follow-up
The patient completed five cycles of chemotherapy with prolonged treatment breaks secondary to myelosuppressive toxicities of chemotherapy. She is presently on best supportive care1.5 years from the day of diagnosis.
Discussion
Breast cancer is one of the most common primary malignancies in women, yet metastatic tumours to the breast are infrequent, accounting for only 0.5– 1.3% of breast cancer cases.1 Epithelial ovarian cancer is the second most common gynaecological malignancy.2 The breast is an uncommon site for tumour metastasis. The most common source of metastasis to the breast is a contralateral primary breast tumour, frequently from transthoracic or lymphatic spread. However, haematogenous metastasis from extramammary malignancies has been reported. Frequently seen primary tumours metastasising to breast are usually of haematological origin and melanoma.3 4 Breast metastasis from primary ovarian cancer is rare but is occasionally found after the diagnosis of the primary tumour. The incidence of breast metastasis from primary ovarian/peritoneal carcinomas accounts for 0.03– 0.6% of all breast malignancies.5 Autopsy studies have reported breast metastasis in 1–3% of patients with ovarian cancer.3 6 7 These ovarian cancers are widespread ones and have poor prognosis with breast metastasis. Most common histological variant of ovarian cancer associated with metastatic disease to the breast is papillary serous adenocarcinoma.8 Extramammary tumours should be distinguished from primary breast tumours to avoid any unnecessary surgical procedures. In contrast to primary breast tumours, metastasis to the breast generally consists of firm, well-circumscribed, multinodular, non-calcific masses. They generally lack spiculation and microcalcifications as well as architectural distortions, and other skin changes are potentially diagnostic as well as palliative. Breast metastasis from a primary ovarian tumour is generally diagnosed on an average of 2 years after the initial diagnosis of ovarian cancer.
Cutaneous involvement is unusual at presentation and during the course of ovarian carcinoma. Ovarian carcinoma is the fourth most common origin of skin metastasis in women after carcinoma of the kidney, lung and breast.9 Prognosis after skin metastasis is poor, the most important prognostic factor associated with survival is the interval time between the diagnosis of ovarian cancer and the documentation of cutaneous involvement. In almost all carcinomas, the cutaneous metastases tend to appear late in the course of disease and usually are associated with poor prognosis. There are several theories that try to explain the pathogenesis of skin metastasis in ovarian cancer: direct invasion from the underlying growth, contiguous extension of the tumour cells through out lymphtics and accidental implantation of the tumour cells during surgical procedures such as laparotomic scars, trocar port site, drainage scar and port and catheter scar.10–13
Synchronous skin and breast metastases at presentation, both of which are uncommon metastatic sites of ovarian carcinoma are hitherto unknown till date.
Learning points.
All organ systems should be thoroughly examined and evaluated for the presence of metastasis, whenever a diagnosis of cancer is made.
No site in the body is immune to occurrence of a metastasis from any malignancy.
Both breasts should be thoroughly evaluated in an ovarian patients with cancer; otherwise a metastasis or a second primary might be missed.
Any skin nodule especially at the site through which either any tumour or malignant fluid has been removed, should be subjected to histopathological examination.
Acknowledgments
We acknowledge Dr Rajan Duggal, consultant pathologist, for sharing with us the histopathological findings and microphototgraphs.
Footnotes
Contributors: SB conceived and wrote the draft of case report. RB and AKV edited the draft. AS helped in scientific content writing of the case report.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wadhwa J, Dawar R, Kumar L. Ovarian carcinoma metastatic to the breast. Clin Oncol 1999;2013:419–21 [DOI] [PubMed] [Google Scholar]
- 2.Karem AK, Stempel M, Barakat RR, et al. Patients with history of epithelial ovarian cancer presenting with a breast and/or axillary mass. Gynecol Oncol 2009;2013:490–5 [DOI] [PubMed] [Google Scholar]
- 3.Hajdu SI, Urban JA. Cancer metastatic to the breast. Cancer 1972;2013:1691–6 [DOI] [PubMed] [Google Scholar]
- 4.Sndison AT. Metastatic tumors in the breast. Br J Surg 1959;2013:54–8 [DOI] [PubMed] [Google Scholar]
- 5.Recine MA, Deavers MT, Middleton LP, et al. Serous carcinoma of the ovary and peritoneum with metastases to the breast and axillary lymph nodes: a potential pitfall. Am J Surg Pathol 2004;2013:1646–51 [DOI] [PubMed] [Google Scholar]
- 6.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma. Analysis of 1000 autopsied cases. Cancer 1950;2013:74–85 [DOI] [PubMed] [Google Scholar]
- 7.Dvoretsky PM, Richards KA, Angel C, et al. Distribution of disease at autopsy in 100 women with ovarian cancer. Human Pathol 1988;2013:57–63 [DOI] [PubMed] [Google Scholar]
- 8.Rebecca L, Brown AR, Christian M, et al. Ovarian cancer metastatic to breast presenting as inflammatory breast cancer: a case report & literature review. J Cancer 2010;2013:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownstein MH, Helwig FB. Patients of cutaneous metastasis. Arch Dermatol 1972;2013:862–8 [PubMed] [Google Scholar]
- 10.White JW, Spencer PS, Helm TN. Skin metastases in cancer patients. Cutis 1987;2013:119–21 [PubMed] [Google Scholar]
- 11.Tharakaram S. Metastases to the skin. Int J Dermatol 1988;2013:240–2 [DOI] [PubMed] [Google Scholar]
- 12.Krumerman M, Garret R. Carcinoma metastatic to skin. NY State J Med 1977;2013:1900–3 [PubMed] [Google Scholar]
- 13.Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol 1972;2013:862–8 [PubMed] [Google Scholar]


