Abstract
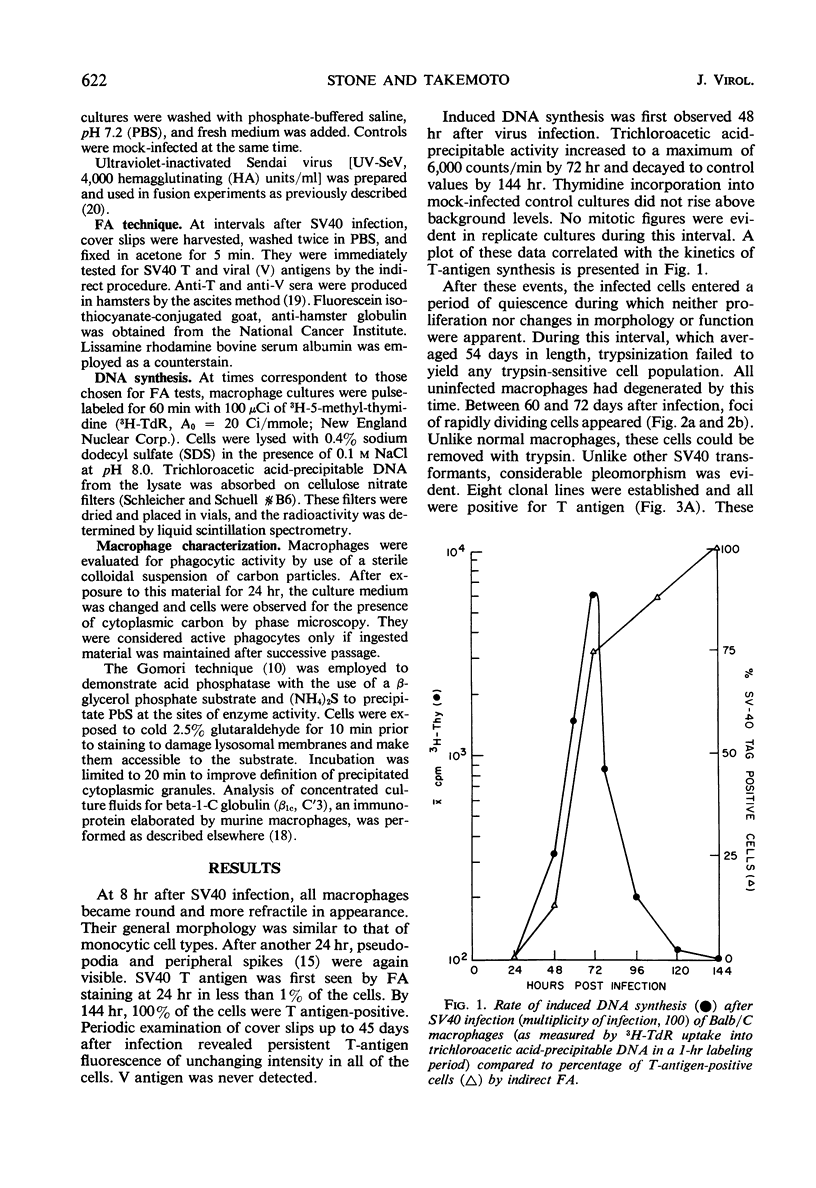
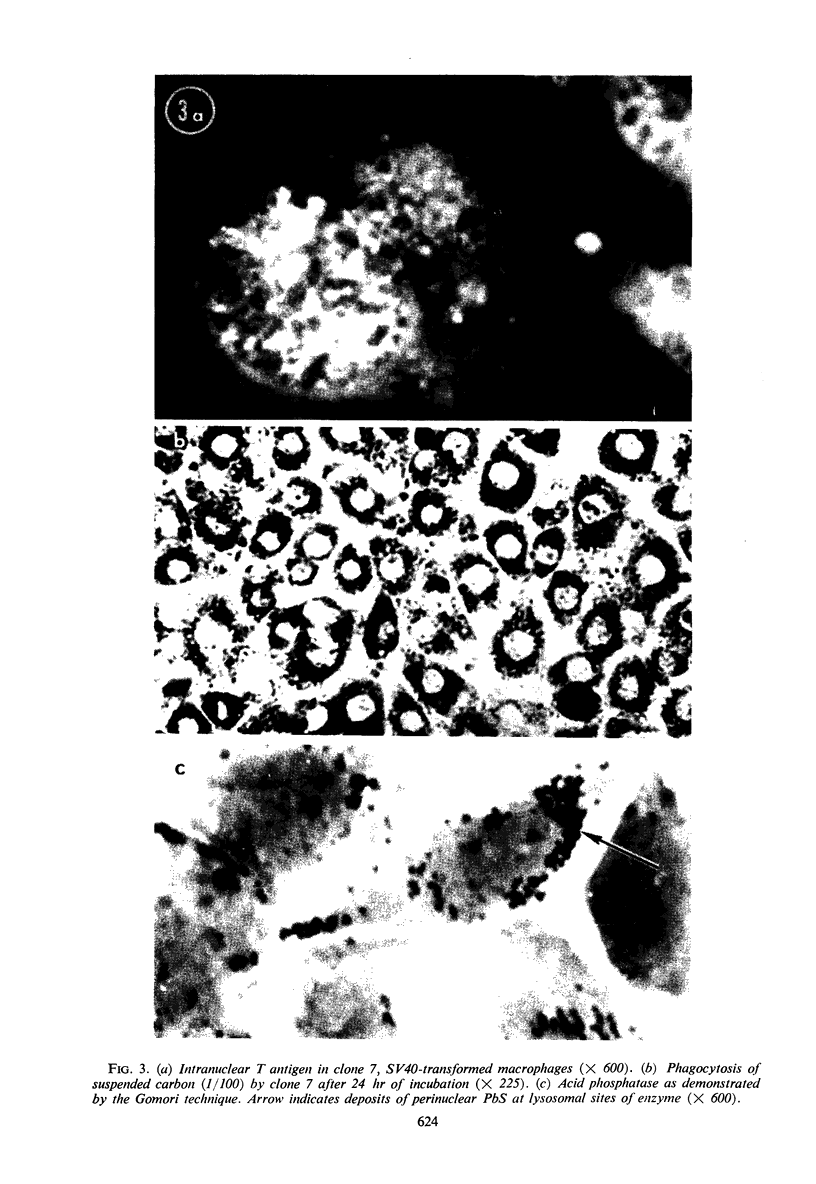
Studies were undertaken to prove that simian virus 40 (SV40) can transform the mouse macrophage, a cell type naturally restricted from deoxyribonucleic acid (DNA) replication. Balb/C macrophages infected with SV40 demonstrated T-antigen production and induced DNA synthesis simultaneously. In the absence of apparent division, these cells remained T antigen-positive for at least 45 days. SV40 could be rescued from nondividing, unaltered macrophages during the T antigen-producing period. Proliferating transformants appeared at an average of 66 days post-SV40 infection. Established cell lines were T antigen-positive and were negative for infectious virus, but yielded SV40 after fusion with African green monkey kidney cells. Their identity as transformed macrophages was substantiated by evaluation of cellular morphology, phagocytosis, acid phosphatase, β1c synthesis, and aminoacridine incorporation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Takahashi K., Okamoto E., Midorikawa O. Establishment of a macrophage cell line derived from a testicular interstitial cell tumor in A-Jax strain mice. Lab Invest. 1969 Feb;20(2):170–177. [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Marin G., di Mayorca G. Requirement for the integrity of the viral genome for the induction of host DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1966 Jul;56(1):208–215. doi: 10.1073/pnas.56.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- Fogel M., Defendi V. Infection of muscle cultures from various species with oncogenic DNA viruses (SV40 and polyoma). Proc Natl Acad Sci U S A. 1967 Sep;58(3):967–973. doi: 10.1073/pnas.58.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes I. J. Mitosis in mouse peritoneal macrophages. J Immunol. 1966 Apr;96(4):734–743. [PubMed] [Google Scholar]
- GLASGOW L. A., HABEL K. Interferon production by mouse leukocytes in vitro and in vivo. J Exp Med. 1963 Jan 1;117:149–160. doi: 10.1084/jem.117.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P., Black P. H., Oxman M. N., Weissman S. M. Stimulation of DNA synthesis in mouse cell line 3T3 by Simian virus 40. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1170–1176. doi: 10.1073/pnas.56.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci L. T-antigen and DNA synthesis in macrophages infected with polyoma virus. Nature. 1969 Aug 9;223(5206):630–632. doi: 10.1038/223630a0. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Black P. H. Inhibition of SV40 T antigen formation by interferon. Proc Natl Acad Sci U S A. 1966 May;55(5):1133–1140. doi: 10.1073/pnas.55.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I., GONATAS N. K. DYNAMICS OF ACRIDINE ORANGE-CELL INTERACTION. II. DYE-INDUCED ULTRASTRUCTURAL CHANGES IN MULTIVESICULAR BODIES (ACRIDINE ORANGE PARTICLES). J Cell Biol. 1964 Apr;21:49–62. doi: 10.1083/jcb.21.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher V. J., Thorbecke G. J. Sites of synthesis of serum proteins. OI. Serum proteins produced by macrophages in vitro. J Immunol. 1967 Oct;99(4):643–652. [PubMed] [Google Scholar]
- Takemoto K. K., Habel K. Hamster ascitic fluids containing complement-fixing antibody against virus-induced tumor antigens. Proc Soc Exp Biol Med. 1965 Oct;120(1):124–127. doi: 10.3181/00379727-120-30464. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Todaro G. J., Habel K. Recovery of SV40 virus with genetic markers of original inducing virus from SV40-transformed mouse cells. Virology. 1968 May;35(1):1–8. doi: 10.1016/0042-6822(68)90299-7. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Green H. Cell growth and the initiation of transformation by SV40. Proc Natl Acad Sci U S A. 1966 Feb;55(2):302–308. doi: 10.1073/pnas.55.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R., Smith B. Induction of cellular DNA synthesis by polyoma virus. 3. Induction in productively infected cells. Proc Natl Acad Sci U S A. 1966 Apr;55(4):956–960. doi: 10.1073/pnas.55.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A. The origin and turnover of mononuclear cells in peritoneal exudates in rats. J Exp Med. 1966 Aug 1;124(2):241–254. doi: 10.1084/jem.124.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTS J. W., HARRIS H. Turnover of nucleic acids in a non-multiplying animal cell. Biochem J. 1959 May;72(1):147–153. doi: 10.1042/bj0720147. [DOI] [PMC free article] [PubMed] [Google Scholar]