Abstract
Mesenchymal stromal cells (MSCs) have been isolated from different tumors and it has been suggested that they support tumor growth through immunosuppression processes that favor tumor cell evasion from the immune system. To date, however, the presence of MSCs in cervical cancer (CeCa) and their possible role in tumor growth remains unknown. Herein we report on the presence of MSCs in cervical tissue, both in normal conditions (NCx-MSCs) and in CeCa (CeCa-MSCs), and described several biological properties of such cells. Our study showed similar patterns of cell surface antigen expression, but distinct differentiation potentials, when we compared both cervical MSC populations to MSCs from normal bone marrow (BM-MSCs, the gold standard). Interestingly, CeCa-MSCs were negative for the presence of human papiloma virus, indicating that these cells are not infected by such a viral agent. Also, interestingly, and in contrast to NCx-MSCs, CeCa-MSCs induced significant downregulation of surface HLA class I molecules (HLA-A*0201) on CaSki cells and other CeCa cell lines. We further observed that CeCa-MSCs inhibited antigen-specific T cell recognition of CaSki cells by cytotoxic T lymphocytes (CTLs). HLA class I downregulation on CeCa cells correlated with the production of IL-10 in cell cocultures. Importantly, this cytokine strongly suppressed recognition of CeCa cells by CTLs. In summary, this study demonstrates the presence of MSCs in CeCa and suggests that tumor-derived MSCs may provide immune protection to tumor cells by inducing downregulation of HLA class I molecules. This mechanism may have important implications in tumor growth.
Introduction
Mesenchymal stromal cells (MSCs) are a heterogeneous subset of stem cells that can be isolated from many adult tissues. They can differentiate into cells of the mesodermal lineage, such as adipocytes, osteocytes, and chondrocytes, as well as cells of other embryonic lineages [1]. MSCs can interact with cells of both the innate and adaptive immune systems and exert profound effects in immune responses, primarily through the production of immunosuppressive molecules, including prostaglandin E2, nitric oxide, indoleamine 2,3-dioxygenase, soluble (s) major histocompatibility complex (MHC), class I, G5 (sHLA-G5), transforming growth factor alpha (TGF-α), and interleukin-10 (IL-10) [1,2], that affect several functions of immunocompetent cells, such as the lymphocyte cytotoxic activity [3].
Some studies suggest that MSCs contribute to the formation of tumor stroma and provide a permissive niche for tumor development through immunosuppression processes that favor evasion from the immune system [4,5]. Such processes have been implicated in several aspects of epithelial tumor biology, such as tumor growth, neoplastic progression, angiogenesis, and metastasis [6,7]. MSCs have been isolated from different tumor types such as ovarian carcinomas [8], giant cell tumors of bone [9], neuroblastomas [10], osteosarcomas [11], lipomas [12], and gastric cancer [13]; however, the presence of MSCs in cervical cancer (CeCa) and their possible role in such tumor growth have not been documented.
It has been shown that tumors have multiple mechanisms to evade the immune response. Among them, they possess the ability to block the maturation and function of antigen-presenting cells (APCs) and cause alterations in T cell signal transduction and function [14]. In this context, the lack or suppression of MHC class I surface expression in cancer cells is accompanied by a reduction in the recognition and lysis of tumor cells by CD8+ CTLs, which is further associated with disease progression [15]. Abnormalities in the surface expression of MHC class I molecules are common in CeCa cells and such abnormalities are often associated with defects in elements of the antigen-processing machinery and are usually influenced by the tumor environment [16,17]. Interestingly, MSCs have been shown to induce changes in the maturation and function of normal APCs, including reduced expression of MHC class I and II antigens and costimulatory molecules, resulting in APCs unable to support T cell response [18].
On the other hand, it is known that MSCs produce and secrete IL-10 [19], a pleiotropic cytokine that displays immunoregulatory effects and that is associated to MHC class I downregulation [20,21]. Indeed, in CeCa patients, a higher expression of IL-10 in cervical tissue has been correlated with a reduced immune response against tumors and with development of high-grade lesions [22,23].
Based on all of these notions, and in trying to contribute to our understanding of the role of MSCs in tumor biology, in the present study, we have looked for the presence of MSCs in the normal cervix (NCx) and in CeCa, and characterized them in terms of their immunophenotype and differentiation potentials. We have further assessed their capacity to modulate the expression of MHC class I molecules on cervical tumor cells. We have also determined the participation of IL-10 in such an expression, and the ability of MSCs to alter immune recognition by T cells. Throughout this study, we have compared cervix MSCs—both normal and neoplastic—with MSCs derived from normal bone marrow (BM), which are considered as the MSC gold standard.
Materials and Methods
Isolation and culture of BM-derived MSCs
BM cells, collected according to institutional guidelines, were obtained from five hematologically normal BM transplant donors. MSCs were obtained by a negative selection procedure (RosetteSep™ System; StemCell Technologies, Inc. [STI]) as previously described by our group [24]. Briefly, mononucleated cells were isolated from BM aspirates and were resuspended in low-glucose-DMEM (Lg-DMEM, [Gibco]) supplemented with 10% fetal bovine serum (FBS; Gibco BRL), and seeded at a density of 0.2×106 cells per cm2 into T25 cell culture flasks (Corning, Inc.). After 4 days, the nonadherent cells were removed and a fresh medium was added. When cultures reached 80% confluence, they were digested with trypsin-EDTA (0.05% Trypsin-0.53 mM EDTA; [Gibco]) and subcultured at a density of 1×103 cells/cm2 into T75 flasks (Corning). At the second passage, cells were harvested and analyzed.
Isolation and culture of NCx and CeCa-derived MSCs
NCx samples were obtained from five normal subjects who had hysterectomy surgery. CeCa samples were obtained from biopsies from two patients in stage IIIB and three patients in stage IIB. These procedures have been approved by the local ethics committee.
In the case of CeCa samples, biopsies were sent to the Pathology Department for routine diagnosis. Another part of the specimen biopsy was immediately frozen at −20°C for human papiloma virus (HPV) typing and the remaining was intended to culture.
NCx and CeCa-derived MSCs were obtained by the enzymatic digestion procedure. Cervical biopsy was dissected into small pieces. The chopped tissues were digested with trypsin-EDTA and single-cell suspension was collected by flushing the tissue parts through a 100-μm nylon filter and centrifuged to obtain the cell pellet. Total numbers of mononucleated and viable cells were determined, seeded, and manipulated as described for BM.
Cell surface antigen analysis of MSCs
Flow cytometry analysis of cultured MSCs was performed as described previously [24]. Directly conjugated antibodies used included one or two of the following mAbs: anti-CD13-PE, anti-CD14-PE, anti-CD29-FITC, anti-CD31-FITC, anti-CD34-FITC, anti-CD44-PE, anti-CD45-FITC, anti-CD54-PE, anti-CD62L-FITC, anti-CD105-PE, and anti-HLA-DR-PE (all: Caltag Laboratories); anti-CD49b-PE, anti-CD58-PE, anti-CD73-PE, anti-CD166-PE, and anti-HLA-ABC-FITC (all: Beckton Dickinson/PharMingen), anti-CD90-FITC (Immunotech) and anti-CD133-PE (Miltenyi Biotec GmbH). Cells incubated with their corresponding isotype control (Caltag Laboratories) were also included. The labeled cells were analyzed on a Coulter Epics Altra Flow Cytometer (Beckman Coulter) by collecting a minimum of 10,000 events. The data were analyzed with CellQuest software (BD Biosciences).
MSCs differentiation
Differentiation analysis of MSCs was performed as described previously [24]. To induce adipogenic differentiation, cells were incubated for 2 weeks in the adipogenic medium consisting of MesenCult™ (STI) supplemented with MSC adipogenic stimulatory supplements (STI), according to the manufacturer's instructions. Cell morphology was examined under a phase-contrast microscope to confirm the formation of neutral lipid vacuoles. The presence of neutral lipids was visualized by staining with Oil Red O (Sigma- Aldrich).
To induce osteogenic differentiation, a Stem Cell Kit™ (SCK; STI), consisting of MesenCult™ supplemented with 15% osteogenic stimulatory supplements, 10−8 M dexamethasone, 0.2 mM ascorbic acid, and 10 mM b-glycerol phosphate, was used. Cells were incubated in the presence of SCK for 3 weeks and osteogenic differentiation was evaluated by detection of calcium deposition stained using the von Kossa technique.
For chondrogenic differentiation, 2.5×105 cells were centrifuged at 150 g for 5 min to form a pelleted micromass in the bottom of the tube. This was incubated for up to 28 days with the chondrogenic induction medium (Cambrex Bio Science Walkersville, Inc.), consisting of the chondrogenic differentiation basal medium supplemented with SingleQuots of dexamethasone, ascorbate, insulin-transferrin-selenium (ITS)+supplement, penicillin/streptavidin, sodium pyruvate, proline, and L-glutamine and 10 ng/mL TGF-β (Cambrex) was added. Every 3–4 days, the medium was changed; after 28 days, the micromass was fixed, embedded, microtome cut and stained with Alcian blue or Mason's trichromic dye (Sigma-Aldrich).
HLA class I expression of CeCa cell lines
Flow cytometry analysis to determine HLA class I expression on cell surface of cultured CaSki (HPV16+, HLA-A*0201+), HeLa (HPV18+), and C33A (HPV−) human CeCa cell lines, was performed as described for MSCs. Monoclonal antibodies were obtained from hybridomas supernatants: PA2.1 (anti-HLA-A2, -A28) mAb was purchased from American Type Culture Collection and the W6/32 mAb, which recognizes a conformational epitope on the intact heavy chain/β2microglobulin complex, was generously supplied by Dr. Gerd Moldenhauer of the German Cancer Research Center, Heilderberg, Germany. Cell samples were analyzed in a FACSCalibur flow cytometer (Becton Dickinson & Co.). After gating out cell debris, 10,000 events were analyzed for their fluorescence intensity. In all experiments, the fluorescence intensity was determined at least three times, where each of the 10,000 events were gated and showed as the mean fluorescence intensity (MFI)±SD. The staining with the FITC-labeled secondary antibody alone was considered as a negative control.
HPV typing
The MY09 and MY11 L1 consensus primers, which recognize a conserved region in the L1 open reading frame, producing a fragment of 450 bp and HPV16 E7-specific primers, which amplify a fragment of 100 bp [25], were used to examine the presence of HPV DNA in the genomic DNA of each β-actin (238 pb)-positive tumor samples and BM-, NCx-, and CeCa-derived MSCs. The positive control consisted of DNA from the CaSki line, which contains the HPV16+. The negative control consisted of DNA from the HeLa line, HPV18+.
The conditions of amplification were as follows: denaturing at 94°C for 15 s, primer annealing at 58°C for 30 s, and extension at 72°C for 1 min, for a total of 35 cycles, the final cycle included an incubation at 72°C for 10 min. Seven microliters of the amplification product was electrophoresed in 1.5% agarose containing 0.5 μg/mL of ethidium bromide and visualized by UV light. The consensus primers used for L1 amplification were MY09: 5′cgtccmarrggawactgatc3′ and MY11: 5′gcmcagggwcataayaatgg3′, while oligonucleotide sequences used in the HPV16+ type-specific PCR were sense 5′gatgaaatagatggtccagc3′ and antisense 5′gctttgtacgcacaaccgaagc3′ [25].
MSC/CeCa cell line cocultures
CeCa cell lines were cultured alone or cocultured for 24, 48, 72, and 96 h with BM-, NCx-, or CeCa-derived MSCs at different ratios 1:100, 1:10, and 1:1. In these experiments, cells were separated from MSCs by a transwell chamber (Millicell chamber). After coculture, CeCa cell lines were analyzed for HLA class I expression by using PA2.1 and W6/32 monoclonal antibodies.
Conditioned media activity and IL-10 quantification and neutralization activity
To analyze the activity of conditioned media from either MSC cultures or MSC/CaSki cocultures, CaSki cells were cultured in the presence of 40% of the corresponding conditioned media. After 96 h of cell culture, expression of the total HLA class I molecules and HLA-A2 alleles was determined by flow cytometry as previously described.
To quantify IL-10 contained in the conditioned media of MSCs and MSC/CaSki cell cocultures, we used a Human IL-10 ELISA Development Kit (Peprotech). The assay was performed according to the manufacturer's protocol.
To determine the effect of rhIL-10 on the expression of HLA class molecules, CeCa cells were cultured in the presence of 40% of the conditioned media (derived from either MSC cultures or MSC-CaSki cocultures) or increasing amounts of hrIL-10 (Peprotech), from 0.125 ng/mL to 4 ng/mL. To neutralize the biological activity of rhIL-10 or IL-10 contained in conditioned media of the MSCs and MSC/CaSki cell cocultures, the rabbit anti-human IL-10 neutralizing specific antibody (Peprotech) was added according to the Peprotech's protocol. After 96 h of culture, the expression of HLA class I molecules was determined on the cells by flow cytometry.
In vitro induction of CTL response
To stimulate CTLs, we used a method previously reported [26]. Briefly, PBLs derived from patients with CeCa and positives for HPV16 and for the HLA-A*0201 allele, were incubated with the antigenic peptides TLGIVCPIC (86–94 sequence) and YMLDLQPETT (11–20 sequence) derived from the E7 HPV16 protein, which specifically binds to the HLA-A2 allele [27]. The cells were restimulated with the T2 cell line previously loaded with the peptides and in the presence of β2-microglobulin plus rIL-2 and rIL-15. Cytotoxicity assays were performed on day 21 after purification of CD8+ T lymphocytes by using the EasySep Negative Human CD8+ kit (StemCell Tech).
Cytotoxicity assays
CaSki cells, previously cultured in the presence of rhIL-10 or cocultured with MSCs, in the presence or absence of the human IL-10 neutralizing specific antibody (1.8 μg/mL) at different ratios (1:100, 1:50, and 1:25), were used as target cells after labeled with [51Cr] (Amersham). Different numbers of effector cells in 50 μL of a complete medium were incubated, and then 104 [51Cr]-labeled target cells were added to triplicate wells of 96-well plates. After 4 h at 37°C, 100 μL of the supernatant was harvested and transferred to counting vials and measured on a γ-counter (Cobra Becton Dickinson). For each pretreated cell group [51Cr], labeled cells incubated with 5% SDS or medium alone were used to determine maximum and spontaneous releases. Spontaneous release was usually less than 10% and never exceeded 15%. The percentage of specific lysis of each well was calculated as (experimental release–spontaneous release)/(maximal release–spontaneous release)×100.
Statistical analysis
All numerical data were expressed as average of values obtained±SD of experiments made by triplicate. Comparisons were evaluated by multivariate statistical analysis using Statistical software, version 9.0.0 (SPSS, Inc.). A P-value <0.05 was considered significant.
Results
MSCs from BM, NCx, and CeCa showed similar patterns of cell surface antigen expression
The presence of MSCs had not been demonstrated in NCx or in CeCa tissue. To test this, MSC cultures were established from NCx and CeCa samples and evaluated for expression of cell surface antigens described for BM-MSCs [28]. As expected, MSCs derived from all sources showed a null or dim expression of hematopoietic markers (i.e., CD14, CD34, and CD45), as well as CD133, CD62L, and CD31 (Table 1). In contrast, they all expressed the adhesion molecules CD29, CD44, CD49b, CD58, CD166, and several other cell surface molecules that have been previously reported for BM-MSCs (i.e., CD105, CD73, CD90, and CD13; Table 1) [28]. In addition, MSCs from these sources were positive for HLA-ABC (class I) and negative for HLA-DR (class II). Interestingly, major differences in the expression of certain antigens were observed. Indeed, 50% of BM-MSCs expressed CD49b, whereas in NCx- and CeCa-MSCs, the proportion was significantly higher (99% and 99%, respectively; Table 1). Furthermore, we observed a significantly higher expression of CD54 in NCx-MSCs (80%) and CeCa-MSCs (64%), as compared to BM-MSCs (25%).
Table 1.
Antigen Expression Profiles on MSCs from BM, NCx, and CeCa
| Ag | BM | NCx | CeCa |
|---|---|---|---|
| CD105 | 89±12 | 99±0.2 | 97±1 |
| CD73 | 97±3 | 99 | 98±0.8 |
| CD90 | 85±14 | 99±0.3 | 94±5 |
| CD13 | 98±0.9 | 99 | 99±0.1 |
| HLA-ABC | 94±9 | 99±0.4 | 97±1 |
| CD29 | 95±7 | 99±0.6 | 96±1 |
| CD44 | 89±13 | 99±0.5 | 99±0.2 |
| CD49b | 50±14 | 99a | 99±0.1a |
| CD58 | 75±32 | 98±1 | 87±12 |
| CD166 | 92±11 | 99±0.1 | 95±6 |
| CD14 | 2±2 | 1±0.5 | 3±1 |
| CD34 | 0.1±0.2 | 4±3 | 1±0.6 |
| CD45 | 0.5±0.5 | 2±0.5 | 1±0.1 |
| CD31 | 1±0.7 | 1±0.6 | 1±0.1 |
| CD133 | 1.4±0.4 | 1.7±1 | 0.8±0.1 |
| CD62L | 0.3±0.3 | 1±0.2 | 1 |
| HLA-DR | 2±3 | 1±1 | 1±0.1 |
| CD54 | 25±17 | 80±16a | 64±6a |
Expression of cell markers was determined by flow cytometry. Results represent mean±SD and correspond to the proportion (%) of cells positive for each particular antigen.
Significantly different (P<0.05) from values shown for BM (BM, n=5; NCx, n=5; CeCa, n=5).
MSCs, mesenchymal stromal cells; BM, bone marrow; NCx, normal cervix; CeCa, cervical cancer.
Distinct differentiation potentials of MSCs from BM, NCx, and CeCa
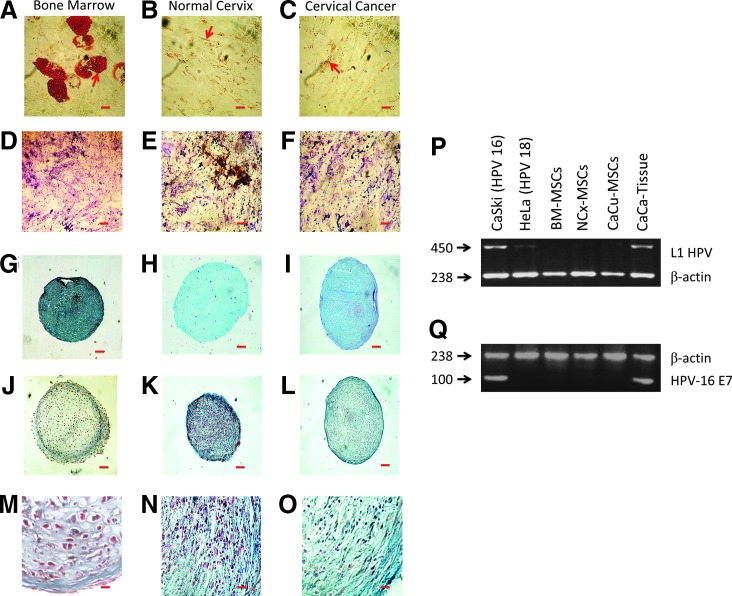
MSCs have the ability to differentiate into adipocytes, osteoblasts, and chondrocytes [28]. Adipogenic induction was apparent in MSC preparations from BM by intracellular accumulation of lipid-rich vacuoles that stained with Oil Red O (Fig. 1A). In contrast, in NCx-MSCs and CeCa-MSCs, we did not observe cells with adipocitic morphology (Fig. 1B, C); only fibroblastoid cells with Oil Red O-positive spots in their cytoplasm were detected. Osteogenic differentiation was observed in all MSC samples, as determined by calcium deposition and defined by von Kossa staining (Fig. 1D–F).
FIG. 1.
Functional characterization of mesenchymal stromal cells (MSCs) from bone marrow (BM), normal cervix (NCx), and cervical cancer (CeCa). MSCs from the three sources (BM, n=5; NCx, n=5; CeCa, n=5) were cultured in the adipogenic, osteogenic, and chondrogenic induction medium for 14, 21, and 28 days, respectively. Adipogenic differentiation was indicated by accumulation of neutral lipid vacuoles that stained with Oil Red O. Arrows indicate cytoplasmic granules positive to staining. Scale bar=20 μm, (A−C). Osteogenic differentiation was indicated by calcium deposition, which stained with von Kossa dye. Scale bar=20 μm, (D−F). Chondrogenic differentiation was indicated by the chondrogenic matrix colored by Alcian blue in cryosections from pelleted micromass. Scale bar=200 μm, (G−I). Chondrogenic differentiation was analyzed by morphology and collagen content in cryosections from pelleted micromass. Scale bar=200 μm and 20 μm, (J−L) and (M−O panels are inset from J−L), respectively. In samples from BM, the presence of small clusters of cells, surrounded by a condensed matrix located in the same area, is evident [hyaline cartilage; (J) and (M, inset from J)]. In samples from NCx and CeCa, numerous collagenous fibers, colored by Mason's trichromic dye (blue), are visible as large irregular bundles between groups of chondrocytes [fibrocartilage; (K), (L), (N, inset from K), and (O, inset from L)]. One representative experiment is showed. In (P, Q), detection and typing of HPV were performed by PCR assay. Amplification pattern shows a conserved region of 450 bp in the L1 HPV open reading frame obtained after amplification on CeCa tissue. No bands were detected in BM-, NCx-, and CeCa-derived MSCs (BM, n=5; NCx, n=5; CeCa, n=5) (P). The CaSki cell line positive for HPV16+ infection was used as a positive control and the Hela cell line HPV18+ was used as a negative control. β-actin (230 bp) was used as an internal control (Q). One representative experiment is showed.
Differentiation toward the chondrogenic lineage was assessed by formation of a matrix-rich, multilayered mass accompanied by accumulation of sulfated proteoglycans, as evidenced by Alcian blue staining (Fig. 1G–I). Interestingly, a distinct type of cartilage formation was observed in pelleted micromasses from NCx and CeCa compared to BM (Fig. 1J–O). Indeed, we observed that in pelleted micromasses from BM samples, cells grouped into small clusters located in the same area, a feature typical of hyaline cartilage [29]; (Fig. 1J, M). In contrast, pelleted micromasses from NCx and CeCa showed minimal matrix formation and predominance of fibrous collagen deposition (as indicated by blue color when using Mason's trichromic stain; Fig. 1K, L, N, O), which indicates the presence of fibrocartilage formation [30].
MSCs from cervix were negative for HPV infection
MSCs derived from the three sources were analyzed to determine the presence of HPV virus by PCR. The CaSki cell line and CeCa tissue samples from patients, both positive for HPV16+ infection, were used as positive controls and the HeLa cell line was used as a negative control. As shown in Fig. 1P and Q, there were no positive MSCs for HPV infection in contrast to the primary tumor sample from which MSCs were obtained.
CeCa-MSCs downregulate the expression of HLA-class I molecules on CeCa cells
It has been clearly demonstrated that MSCs participate in the formation of tumor stroma and support the growth of neoplastic cells [31]; however, little is known about the way MSCs favor tumor growth. Our hypothesis was that since antigen presentation through HLA class I molecules on the cell membrane of tumor cells is essential for recognition by CTLs, MSCs may favor tumor growth by inducing alterations in the expression of HLA class I molecules on tumor cells.
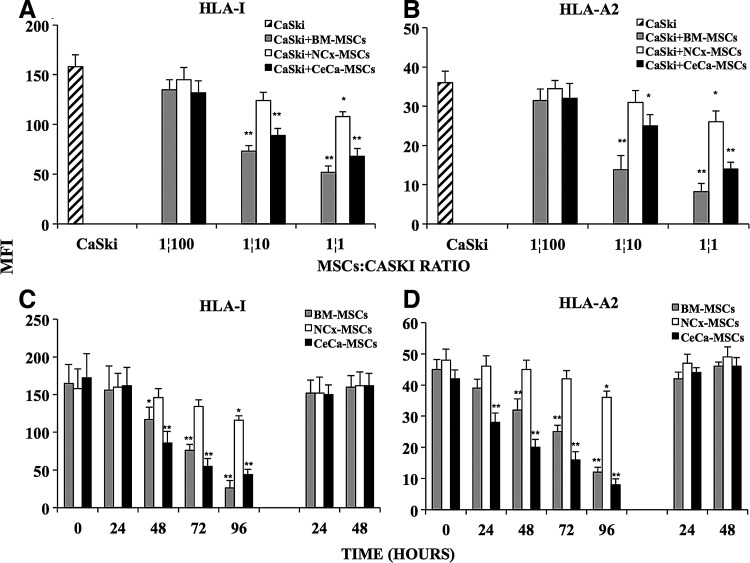
To test this, we performed cocultures of CaSki cells with MSCs derived from BM, NCx, and CeCa, in transwell chambers and analyzed the effect of MSCs on the constitutive expression of HLA class I molecules on CaSki cells. We observed that, as the number of MSCs was increased in culture, HLA class I molecule expression on CaSki cells diminished in a ratio-dependent manner (Fig. 2A). After 96 h, the lowest expression was present at a 1:1 MSC:CaSki cells ratio (Fig. 2C) diminished by 65%, 30%, and 58% in the presence of BM-, NCx-, and CeCa-derived MSCs, respectively (Fig. 2A), while the HLA-A2 allele was decreased by 77%, 30%, and 62% under the same culture conditions (Fig. 2B). It is noteworthy that HLA class I antigen expression on CaSki cells gradually decreased throughout time (Fig. 2C, D). Interestingly, when the transwells containing MSCs were removed and the media culture was changed by a fresh one, after 96 h of cell coculture, CaSki cells recovered the basal expression of both total HLA class I molecules and the HLA-A2 allele (Fig. 2C, D, respectively). These results suggested that soluble factors, secreted into the culture media when MSCs were present, induced downregulation of HLA class I molecules on the tumor cell line, and this effect was strongly increased in the presence of CeCa-MSCs, although in all the cases, it was reversible after removal of MSCs.
FIG. 2.
CeCa- and BM-derived MSCs strongly diminished the expression of HLA class I molecules on CeCa cells. BM-, NCx-, and CeCa-derived MSCs (BM, n=5; NCx, n=5; CeCa, n=5) were seeded into the upper wells in transwell chambers and cocultured at different ratios 1:100, 1:10, and 1:1 with CaSki cells added to the bottom wells. After 96 h, the expression of total HLA class I molecules (A) or HLA-A2 allele (B) was determined by flow cytometry on CaSki cells cultured alone ( ) or cocultured with MSCs as indicated in Materials and Methods. The expression of total HLA class I molecules (C) and HLA-A2 allele (D) on CaSki cells was also monitored after 24, 48, 72, and 96 h of coculture with MSCs (at ratio 1:1). Thereafter, the transwell chamber containing MSCs was removed and the medium of the CaSki cells was changed for a fresh one. Expression of HLA molecules on CaSki cells was determined after 24 and 48 h of cell culture. The mean fluorescence intensity (MFI)±SD from 10,000 events is depicted in each cell treatment. *Indicates significant differences (P<0.05%) and ** (P<0.005%) in comparison to CaSki cells cultured alone. The data are representative of three independent experiments.
) or cocultured with MSCs as indicated in Materials and Methods. The expression of total HLA class I molecules (C) and HLA-A2 allele (D) on CaSki cells was also monitored after 24, 48, 72, and 96 h of coculture with MSCs (at ratio 1:1). Thereafter, the transwell chamber containing MSCs was removed and the medium of the CaSki cells was changed for a fresh one. Expression of HLA molecules on CaSki cells was determined after 24 and 48 h of cell culture. The mean fluorescence intensity (MFI)±SD from 10,000 events is depicted in each cell treatment. *Indicates significant differences (P<0.05%) and ** (P<0.005%) in comparison to CaSki cells cultured alone. The data are representative of three independent experiments.
To determine if MSC cocultures have the same effect on different cell types, C33A and HeLa, CeCa cell lines were cocultured during 96 h with MSCs by using transwell chambers, and then the total expression of the HLA class I molecules was determined. Similar to the CaSki cell line, HLA class I downregulation on these different cell types was strongly favored in the presence of CeCa-MSCs and BM-MSCs, but not in the presence of NCx-MSCs (data not shown). These results indicate that downregulation of HLA-class I molecules by CeCa-MSCs also occurs in different CeCa cell lines via soluble factors.
IL-10 contained in conditioned media from CeCa-MSC/CaSki cell cocultures induced HLA class I downregulation on CeCa cells
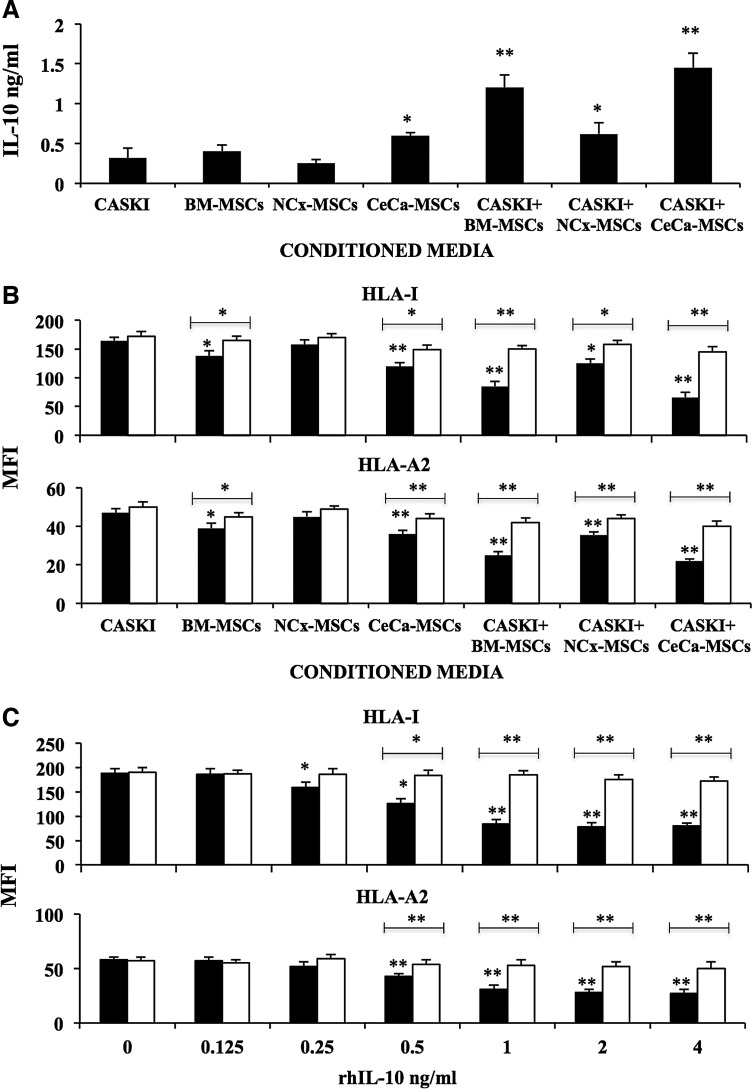
Conditioned media from cultures of BM-, NCx-, CeCa-derived MSCs, as well as from MSC/CaSki cell cocultures, were obtained and evaluated to determine the possible presence of soluble cytokines capable of inducing downregulation of HLA class I on tumor cells. Some authors have reported the capacity of IL-10 to induce HLA class I downregulation on tumor cells [32,33]. To determine the presence of IL-10 in the conditioned media from the different culture conditions, we evaluated the presence of IL-10 by ELISA. In conditioned media from BM-MSC/CaSki and CeCa-MSC/CaSki cocultures, we detected higher IL-10 concentrations (>1.2 ng/mL), as compared to those from CaSki (0.32 ng/mL), BM-MSCs (0.4 ng/mL), NCx-MSCs (0.25 ng/mL), and CeCa-MSCs (0.6 ng/mL) and also NCx-MSCs/CaSki (0.62 ng/mL). Indeed, we detected the highest level of IL-10 in the conditioned media from CeCa-MSC/CaSki cocultures (Fig. 3A).
FIG. 3.
IL-10 produced in the MSC/CaSki cell cocultures diminished the expression of HLA class I molecules on CeCa cells. (A) ELISA assay was performed to determine the content of IL-10 in conditioned media derived either from MSCs cultured alone or MSC/CaSki cell cocultures at a 1:1 ratio (BM, n=5; NCx, n=5; CeCa, n=5). (B) CaSki cells were cultured in the presence of 40% (■) of the conditioned media derived from either MSC cultures or MSC/CaSki cocultures at a 1:1; or with 40% of these conditioned media plus 1.8 μg/mL of rabbit anti-human IL-10 neutralizing specific antibody (□). (C) CaSki cells were also cultured in the presence of increasing amounts of hrIL-10-from 0.125 ng/mL to 4 ng/mL (■), or with these amounts of hrIL-10 plus 1.8 μg/mL of rabbit anti-human IL-10 neutralizing specific antibody (□). After 96 h, the total HLA class I molecules (HLA-I) and HLA-A2 allele expression on CaSki cells was determined by flow cytometry (B, C). The mean fluorescence intensity (MFI)±SD from 10,000 events is depicted in each cell treatment. Significant differences, *(P<0.05) and **(P<0.005) were obtained in comparison to CaSki cells treated with their own conditioned media. The data are representative of three independent experiments.
To determine in a more precise manner, the possible participation of IL-10 in HLA downregulation, a specific neutralizing anti-IL-10 antibody was added to CaSki cell cultures established in the presence of 40% conditioned media from MSCs and MSC/CaSki cocultures. As shown in Fig. 3B, the addition of anti-IL-10 significantly reestablished the expression of HLA class I and HLA-A2 on CaSki cells. On the other hand, total HLA class I as well as HLA-A2 downregulation was also observed when CaSki cells were cultured in the presence of increasing amounts of rhIL-10 (0.125–4 ng/mL), and in the same manner, the addition of anti-IL-10 significantly reestablished the expression of HLA molecules on CaSki cells (Fig. 3C). These data indicated that when interacting in coculture, CeCa tumor cells and/or MSCs—particularly those from CeCa or normal BM—increased their capacity to secrete IL-10, and that this cytokine is one of the responsible ones in inducing HLA downregulation on CeCa cells.
CeCa-MSCs favor protection of CeCa cells from cytotoxic T cell activity via IL-10
To analyze whether the reduced expression of HLA class I molecules on CaSki cells—induced by MSCs in the cell coculture system—affect their recognition by immune cells, T lymphocytes derived from HPV16+ CeCa patients, positive for the HLA-A2 allele, were stimulated with antigenic peptides that specifically bind to the HLA-A*0201 allele [27,34], and then challenged against CaSki cells, previously cultured in the presence of rhIL-10 or cocultured with MSCs, in the presence or absence of the human IL-10 neutralizing specific antibody. In all the experiments, T lymphocytes stimulated with E7 epitopes were always capable to lyse the T2 cell line loaded with the proper antigenic peptide and no reactivity on CaSki cells was observed when T lymphocytes were previously stimulated with an irrelevant peptide GILGFVFTL derived from the protein matrix of the influenza-A (data not shown).
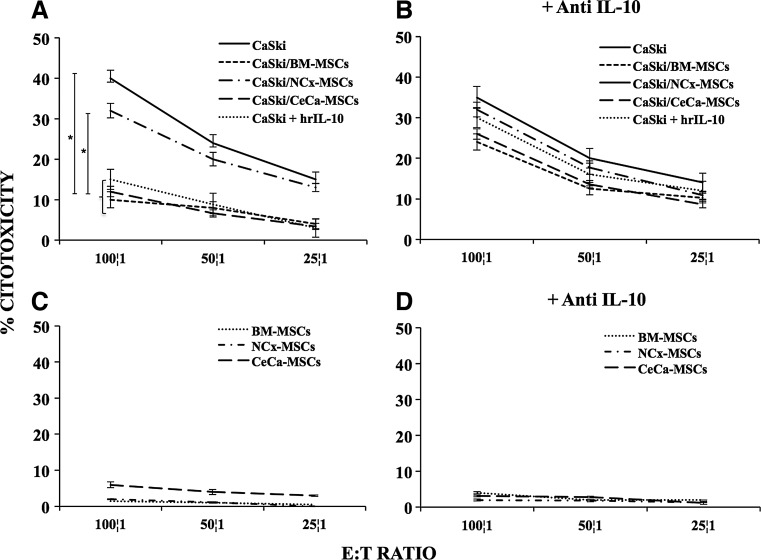
As expected, after T cell stimulation, we observed that T lymphocytes were able to lyse CaSki cells in a dose-dependent manner (Fig. 4A). However, when T stimulated lymphocytes were challenged with CaSki cells previously cocultured with CeCa-MSCs and BM-MSCs at a 1:1 ratio, the cytotoxic activity was strongly decreased by 65%–75% in relation to that observed on CaSki cells or CaSki cells previously cocultured with NCx-MSCs (Fig. 4A). The cytotoxic activity of T stimulated lymphocytes was also decreased by 60% on CaSki cells, previously cultured in the presence of rhIL-10 (1 ng/mL; Fig. 4A). In contrast, when T lymphocytes were challenged with CaSki cells previously cocultured with BM-MSCs, CeCa-MSCs, or pretreated with hrIL-10 in the presence of neutralizing anti-IL-10 antibodies, the immune recognition of tumor cells was reestablished (Fig. 4B). On the other hand, T lymphocytes showed no cytotoxic activity on the three types of MSCs in the absence or presence of neutralizing anti-IL-10 antibodies (Fig. 4C, D). These results support the notion that MSCs have a negative effect on the immune recognition of tumor cells by T cells by downregulating expression of HLA class I molecules and that IL-10 plays a key role in this process.
FIG. 4.
IL-10 produced in the MSC/CaSki cell cocultures diminishes the recognition of CeCa cells by CTLs. CaSki cells were cultured in transwell chambers alone or in the presence of 1 ng/mL of hrIL-10, as well as with BM-, NCx-, or CeCa-MSCs at a ratio of 1:1 (BM, n=5; NCx, n=5; CeCa, n=5), in the absence (A) or presence of neutralizing anti-IL-10 antibody (B). After 96 h, CaSki cells were harvested and challenged against CTLs (CD8+ T lymphocytes) specific for peptides TLGIVCPIC and YMLDLQPETT derived from the E7 HPV16+ protein, which is expressed in CaSki cells by HLA-A2. CTL cytolytic activity was tested by a standard 4 h [51Cr] release assay at different ratios 100:1, 50:1, and 25:1 effector:target cells (E:T). The CTL cytolytic activity was also performed on BM-, NCx-, or CeCa-MSCs obtained from the cell cocultures with CaSki either in the absence (C) or presence (D) of neutralizing anti-IL-10. *Indicates significant differences (P<0.05%) in comparison to CaSki cells cultured alone. The data are representative of three independent experiments and shown as mean values±SD.
Discussion
MSCs have been shown to possess an immunosuppressive activity [35] and contribute to create a specific tumor microenvironment that promotes tumor survival through immunosuppression conditions that favor the evasion of tumor from the immune system [4,5]. Previous reports have shown the isolation of MSCs from several tumors [8–13]; however, the presence of MSCs has not been demonstrated in NCx or in CeCa tissue. Following this, and taking into consideration the immunossupressive properties of MSCs [35], we reasoned that the presence of these cells in cervical tumors may represent an important immunoselective advantage for tumor cells to evade the immune recognition.
To test this notion, in the present study, we evaluated the presence of MSCs in NCx and CeCa and analyzed their in vitro capacity to modulate the immune recognition of tumor cells by T cells. We characterized, in a comparative manner, MSCs from the cervix with those from BM in terms of their immunophenotype and differentiation potential. The present study is, to our knowledge, the first study describing the presence of MSCs in the cervix.
As a first approach, we determined the characteristics of BM-, NCx-, and CeCa-derived MSCs, according to the minimal criteria proposed by the ISCT [28]. It is important to indicate that throughout this study, MSCs were used at the second passage, to avoid cellular heterogeneity in primary cultures. In terms of the immunophenotype, the major antigens described for BM-MSCs, such as CD73, CD105, and CD90 [24,36,37], were also expressed on NCx- and CeCa-derived MSCs. CD13 expression was high and homogeneous in all samples from the three sources, as we previously reported for cord blood- and placenta-derived MSCs [24]; thus, CD13 could represent a specific marker for MSCs from several sources, including the cervix. Expression of CD49b and CD54 was much higher in NCx and CeCa than in BM and these results may reflect that MSCs derived from the cervical tissue are a subpopulation of MSCs—as discussed by others [38]—since they share many MSC properties, but differ in their adipogenic differentiation potential and in the expression of some adhesion molecules. It is also noteworthy that the higher expression of integrins in cervical MSCs may be of importance in homing to cervical tissue [39].
In terms of differentiation potential, our results show that, in contrast to those derived from BM, NCx- and CeCa-derived MSCs were not able to differentiate toward the adipogenic lineage; similar results were reported by our own group for cord blood-derived MSCs [24]. A loss of adipogenic differentiation capacity might be caused by tumor cells through attenuation of TP53 expression that promotes osteoblast differentiation [40], thus possibly inhibiting differentiation into adipocytes. MSCs from all three sources showed osteogenic and chondrogenic potential, which demonstrates the multipontentiality of MSCs derived from NCx and CeCa; however, as in BM, these results may suggest that cultures of MSCs could represent an admixture of morphologically, phenotypically, and functionally different cells, as some authors have proposed [41,42]. Interestingly, fibrocartilage was observed in assays from NCx and CeCa, whereas hyaline cartilage was predominant in pelleted micromass from BM, which suggests distinct functional capacities between MSCs from NCx and CeCa compared with BM. Together, these results shown some differences in functional capacities of MSCs from three sources, however, studies on gene expression are necessary for a better description of them. Finally, we determined that MSCs derived from NCx and CeCa were negative for HPV infection; thus, HPV does not seem to directly affect the biologic properties of MSCs from the cervix.
We have previously shown the presence of immunologic mechanisms that favor evasion of tumor cells from the cytotoxic activity by CTLs in CeCa, in which, HLA class I expression is involved [26]; in this context, it is possible that those MSCs we detected in CeCa tumor samples participate in such a mechanism. To test this, we analyzed the effect of MSCs on the expression of HLA class I molecules on CeCa cell lines. We observed that in the absence of cell–cell contact, BM- and CeCa-, but not NCx-derived MSCs induced a strong decrease in HLA class I and HLA-A2 expression on different CeCa cell lines, which was dependent on the ratio and time of coculture. This effect was clearly dependent on the presence of MSCs in the coculture, since after MSC removal, CeCa cells recovered their HLA class I and HLA-A2 basal expression in the first 24 h, suggesting that soluble factors produced in MSC/CeCa cell cocultures were responsible for this effect. This observation was corroborated when conditioned media obtained from these cocultures produced a significant diminution in HLA class I and HLA-A2 expression on CaSki cells.
The decrease observed in the expression of HLA antigens on CaSki cells may be due to the production of immunosuppressive molecules by MSCs. Indeed, a cytokine-mediated cross talk between MSCs and cancer cells has also been reported [1]. Furthermore, it has been reported that molecules produced by MSCs induce reduced expression of MHC class I on APCs, resulting in APCs unable to support T cell response and pushing APCs away from a proinflammatory (TNF-α, IL-12) phenotype toward an anti-inflammatory (IL-10 production) phenotype [18,19,43]. In this context, it is known that IL-10 is a pleiotropic cytokine produced by cancer cells [44] and MSCs [19] and that downregulates HLA class I expression and protects tumor cells from allo-specific CTLs [20].
Taking into consideration that IL-10 could influence the decrease of HLA class I expression on CaSki cells cocultured with MSCs, we determined the presence of this cytokine in conditioned media derived from MSC/CaSki cocultures and evaluated the effect of rhIL-10 on such antigen expression. We found that, as compared with the levels observed in conditioned media from cultures of CaSki cells, IL-10 was increased 3.7-fold in conditioned media from CaSki cells cocultured with BM-MSCs, 1.9-fold in those cocultured with NCx-MSCs, and 4.5-fold in those cocultured with CeCa-MSCs. Such IL-10 levels may simply be the sum of the individual production of IL-10 by MSCs and tumor cells in cocultures with NCx-MSCs, but in those with BM-MSCs or CeCa-MSCs, where the IL-10 production was higher than the proper sum of the individual cell types, these differences could be the result of the cross talk between CaSki and MSCs during cell coculture through transwell plates, as recently was reported by Liu and cols. [45], who found that rat-derived BM-MSCs increased the IL-10 intracellular expression after transwell cell coculture with dendritic cells, suggesting MSC activation in such cell cocultures. In this regard and in trying to determine which is the source of the IL-10 in our cocultures, we have observed, in preliminary assays, that BM-MSCs and CeCa-MSCs in coculture with CaSki cells increased the IL-10 intracellular expression in comparison with their respective monocultures, however, it is possible also that CaSki cells increase IL-10 expression; these two aspects are under investigation. We cannot exclude the possibility that the increase in IL-10 secretion observed in CeCa-MSC-conditioned media compared with that of NCx-MSCs, could be due to an increase in the number of tumor-associated fibroblasts (TAFs) in tumor, since it has been reported that TAFs secrete cytokines with direct immunosuppressive effects such as IL-10 [38].
On the other hand, conditioned media from BM- and CeCa-derived MSCs and rhIL-10 decreased the HLA class I expression on CaSki cells, suggesting an important participation of this cytokine in such a decrease. Addition of anti-IL-10 neutralizing antibodies to the cocultures or cultures in the presence of rhIL-10, significantly inhibited the effect of IL-10 in HLA-I and HLA-A2 expression on CaSki cells. However, other factors different than IL-10 and also produced in these cocultures, could contribute to downregulate the HLA class I expression on CaSki cells and gene expression profiling of the three different MSC populations, might also be helpful in this respect.
Since loss or downregulation of HLA class I molecules seems to be an important mechanism of cancer cells to evade immune recognition by CTLs [16], we analyzed if CaSki cells previously cocultured with MSCs or rhIL-10 fail to be recognized by CTLs. We found that CaSki cells previously cocultured with BM-MSCs, CeCa-MSCs, and rhIL-10 were weakly recognized by peptide-specific CTLs; addition of neutralizing anti-IL-10 Ab in such previous cultures strongly recovered the recognition of CaSki cells by CTLs. No changes in the cytotoxicity percentage was observed in CaSki cells when they were cocultured with NCx-MSCs, which suggests distinct functional capacities between MSCs from NCx compared with BM and CeCa, which may be related with the lowest concentration of IL-10 we detected in the conditioned media of the former. Taken together, these results indicate that IL-10 plays an important role in the downregulation of HLA class I molecules on tumor cells by MSCs, and that this seems to be a key mechanism through which, MSCs protect to cancer cervical cells of cellular immune response by CTLs.
In fact, in CeCa patients, higher expression of IL-10 in cervical tissue correlates with higher grade lesions [46], thus, the presence of MSCs in the tumor microenviroment may strongly contribute to increase the production of IL-10, and so, to downmodulate HLA class I expression on tumor cells. In this regard, the effects of IL-10 on the expression of molecules associated with the antigen processing machinery are also being analyzed and also if BM-MSCs and CeCa-MSCs are capable of MHC class I downregulation on non-CeCa cells.
On the other hand, it is known that a complete loss of human HLA class I molecules on tumor cells results in evasion to cytotoxic T lymphocyte-mediated lysis, but eventually, tumor cells may result susceptible to natural killer (NK) cell-mediated killing, since NK cells are an important arm of the innate immune system directly involved in the spontaneous recognition and lysis of virus-infected and tumor cells [47]. However, other associated mechanisms can also contribute to an impaired innate, as well as adaptative, antitumor immune response. For example, IL-10 can contribute by increasing HLA-G expression on HLA class I-deficient cells to escape from NK-mediated lysis through interactions with killer inhibitory receptors on NK cells [48,49]. In CeCa lesions, the expression of both HLA-G and IL-10 associated with HPV infection might play an important role in CeCa progression [50,51]. In fact, it has been recently reported that in tissue samples from biopsies derived from patients with CeCa and with loss or downregulation of HLA class I expression, most of the HLA-G-positive cases (87.5%) exhibited upregulation of the IL-10 [52]. According to our results, CeCa-MSCs can contribute to impairing the antitumor immune response by downregulating HLA class I expression on CeCa cells through IL-10 secretion, however, further studies are necessary to resolve whether HLA-G expression is also induced in CeCa cells in our experimental model.
In summary, we have demonstrated the presence of MSCs in NCx and CeCa. Some differences were observed in terms of their differentiation capacity, as compared to their normal marrow counterpart, but immunophenotype analysis showed great similarities. Our results suggest that the presence of MSCs in CeCa tumors could contribute to generate an immunoselective event that provides advantage for tumor cells to escape host immune surveillance and nullify adoptive cancer immunotherapy, and in this manner, MSCs may play a crucial role in supporting tumor cell growth. Further studies are warranted to elucidate if MSCs participate in some other biologic mechanisms involved in the evasion of immune response by tumor cells.
Acknowledgments
We thankfully acknowledge the excellent technical assistance of Teresa Apresa García, Ernesto Javier Rivera Rosales, Juan de Dios Moreno Alvarez, and Héctor Sánchez Peña. We are indebted for CONACYT support to A.M.G. (grant no. 106591) and J.J.M.M. (grant no. 87183); DGAPA-PAPIIT support to MLMG (grant no IN223010, IN217013); and Mexican Institute of Social Security, IMSS, support to A.M.G. (grants no. 762, 800, and 1014) and J.J.M.M. (grant no. 062) are gratefully acknowledged.
Author Disclosure Statement
The authors declare that they have no competing or financial interests.
References
- 1.Uccelli A. Moretta L. Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 2.Spaggiari GM. Capobianco A. Abdelrazik H. Becchetti F. Mingari MC. Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 3.Rasmusson I. Ringdén O. Sundberg B. Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 4.Djouad F. Plence P. Bony C. Tropel P. Apparailly F. Sany J. Noël D. Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 5.Spaeth E. Klopp A. Dembinski J. Andreeff M. Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 6.Yazhou C. Wenlv S. Weidong Z. Licun W. Clinicopathological significance of stromal myofibroblasts in invasive ductal carcinoma of the breast. Tumour Biol. 2004;25:290–295. doi: 10.1159/000081394. [DOI] [PubMed] [Google Scholar]
- 7.Tsujino T. Seshimo I. Yamamoto H. Ngan CY. Ezumi K. Takemasa I. Ikeda M. Sekimoto M. Matsuura N. Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–2090. doi: 10.1158/1078-0432.CCR-06-2191. [DOI] [PubMed] [Google Scholar]
- 8.McLean K. Gong Y. Choi Y. Deng N. Yang K. Bai S. Cabrera L. Keller E. McCauley L. Cho KR. Buckanovich RJ. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–3219. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wulling M. Delling G. Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003;34:983–993. doi: 10.1053/s0046-8177(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 10.Johann PD. Vaegler M. Gieseke F. Mang P. Armeanu-Ebinger S. Kluba T. Handgretinger R. Müller I. Tumour stromal cells derived from paediatric malignancies display MSC-like properties and impair NK cell cytotoxicity. BMC Cancer. 2010;10:501. doi: 10.1186/1471-2407-10-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs CP. Kukekov VG. Reith JD. Tchigrinova O. Suslov ON. Scott EW. Ghivizzani SC. Ignatova TN. Steindler DA. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin TM. Chang HW. Wang KH. Kao AP. Chang CC. Wen CH. Lai CS. Lin SD. Isolation and identification of mesenchymal stem cells from human lipoma tissue. Biochemi Biophys Res Commun. 2007;361:883–889. doi: 10.1016/j.bbrc.2007.07.116. [DOI] [PubMed] [Google Scholar]
- 13.Cao H. Xu W. Qian H. Zhu W. Yan Y. Zhou H. Zhang X. Xu X. Li J. Chen Z. Xu X. Mesenchymal stem cell-like cells derived from human gastric cancer tissues. Cancer Lett. 2009;274:61–71. doi: 10.1016/j.canlet.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 14.Campoli M. Ferrone S. Zea AH. Rodriguez PC. Ochoa AC. Mechanisms of tumor evasion. Cancer Treat Res. 2005:61–88. doi: 10.1007/0-387-27545-2_3. [DOI] [PubMed] [Google Scholar]
- 15.Garrido F. Algarra I. Garcia-Lora AM. The escape of cancer from T lymphocytes: immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol Immunother. 2010;59:1601–1606. doi: 10.1007/s00262-010-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta AM. Jordanova ES. Kenter GG. Ferrone S. Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady CS. Bartholomew JS. Burt DJ. Duggan-Keen MF. Glenville S. Telford N. Little AM. Davidson JA. Jimenez P, et al. Multiple mechanisms underlie HLA dysregulation in cervical cancer. Tissue Antigens. 2000;55:401–411. doi: 10.1034/j.1399-0039.2000.550502.x. [DOI] [PubMed] [Google Scholar]
- 18.Jiang XX. Zhang Y. Liu B. Zhang SX. Wu Y. Yu XD. Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 19.Beyth S. Borovsky Z. Mevorach D. Liebergall M. Gazit Z. Aslan H. Galun E. Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M. Salazar F. Petersson M. Masucci G. Hansson J. Pisa P. Zhang QJ. Masucci MG. Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersson M. Charo J. Salazar-Onfray F. Noffz G. Mohaupt M. Qin Z. Klein G. Blankenstein T. Kiessling R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol. 1998;161:2099–2105. [PubMed] [Google Scholar]
- 22.Bermudez-Morales VH. Gutierrez LX. Alcocer-Gonzalez JM. Burguete A. Madrid-Marina V. Correlation between IL-10 gene expression and HPV infection in cervical cancer: a mechanism for immune response escape. Cancer Invest. 2008;26:1037–1043. doi: 10.1080/07357900802112693. [DOI] [PubMed] [Google Scholar]
- 23.Mindiola R. Caulejas D. Núñez-Troconis J. Araujo M. Delgado M. Mosquera J. Increased number of IL-2, IL-2 receptor and IL-10 positive cells in premalignant lesions of the cervix. Invest Clin. 2008;49:533–545. [PubMed] [Google Scholar]
- 24.Montesinos JJ. Flores-Figueroa E. Castillo-Medina S. Flores-Guzmán P. Hernández-Estévez E. Fajardo-Orduña G. Orozco S. Mayani H. Human mesenchymal stromal cells from adult and neonatal sources: comparative analysis of their morphology, immunophenotype, differentiation patterns and neural protein expression. Cytotherapy. 2009;11:163–176. doi: 10.1080/14653240802582075. [DOI] [PubMed] [Google Scholar]
- 25.Walboomers JM. Jacobs MV. Manos MM. Bosch FX. Kummer JA. Shah KV. Snijders PJ. Peto J. Meijer CJ. Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Mora-García Mde L. Duenas-González A. Hernández-Montes J. De la Cruz-Hernández E. Pérez-Cárdenas E. Weiss-Steider B. Santiago-Osorio E. Ortíz-Navarrete VF. Rosales VH, et al. Up-regulation of HLA class-I antigen expression and antigen-specific CTL response in cervical cancer cells by the demethylating agent hydralazine and the histone deacetylase inhibitor valproic acid. J Transl Med. 2006;4:55. doi: 10.1186/1479-5876-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ressing ME. Sette A. Brandt RM. Ruppert J. Wentworth PA. Hartman M. Oseroff C. Grey HM. Melief CJ. Kast WM. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 28.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop Dj. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey DR. Watt I. Imaging hyaline cartilage. Br J Radiol. 2003;76:777–787. doi: 10.1259/bjr/51504520. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin M. Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- 31.Studeny M. Marini FC. Dembinski JL. Zompetta C. Cabreira-Hansen M. Bekele BN. Champlin RE. Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Nat Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 32.Tsuruma T. Yagihashi A. Torigoe T. Sato N. Kikuchi K. Watanabe N. Hirata K. Interleukin-10 reduces natural killer sensitivity and downregulates MHC class I expression on H-ras-transformed cells. Cell Immunol. 1998;184:121–128. doi: 10.1006/cimm.1998.1266. [DOI] [PubMed] [Google Scholar]
- 33.Yue FY. Dummer R. Geertsen R. Hofbauer G. Laine E. Manolio S. Burg G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer. 1997;71:630–637. doi: 10.1002/(sici)1097-0215(19970516)71:4<630::aid-ijc20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Kast WM. Brandt RM. Sidney J. Drijfhout JW. Kubo RT. Grey HM. Melief CJ. Sette A. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. J Immunol. 1994;152:3904–3912. [PubMed] [Google Scholar]
- 35.Uccelli A. Moretta L. Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 36.Flores-Figueroa E. Montesinos JJ. Flores-Guzmán P. Gutiérrez-Espíndola G. Arana-Trejo RM. Castillo-Medina S. Pérez-Cabrera A. Hernández-Estévez E. Arriaga L. Mayani H. Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leuk Res. 2008;32:1407–1416. doi: 10.1016/j.leukres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Flores-Guzmán P. Flores-Figueroa E. Montesinos JJ. Martínez-Jaramillo G. Fernández-Sánchez V. Valencia-Plata I. Alarcón-Santos G. Mayani H. Individual and combined effects of mesenchymal stromal cells and recombinant stimulatory cytokines on the in vitro growth of primitive hematopoietic cells from human umbilical cord blood. Cytotherapy. 2009;11:886–896. doi: 10.3109/14653240903180076. [DOI] [PubMed] [Google Scholar]
- 38.Paunescu V. Bojin FM. Tatu CA. Gavriliuc OI. Rosca A. Gruia AT. Tanasie G. Bunu C. Crisnic D, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. 2011;15:635–646. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson H. Erkers T. Nava S. Ruhm S. Westgren M. Ringdén O. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol. 2012;167:543–555. doi: 10.1111/j.1365-2249.2011.04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tataria M. Quarto N. Longaker MT. Sylvester KG. Absence of the p53 tumor suppressor gene promotes osteogenesis in mesenchymal stem cells. J Pediatr Surg. 2006;41:624–632. doi: 10.1016/j.jpedsurg.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Vogel W. Grünebach F. Messam CA. Kanz L. Brugger W. Bühring HJ. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88:126–133. [PubMed] [Google Scholar]
- 42.Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007:2884–2889. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- 43.Nauta AJ. Kruisselbrink AB. Lurvink E. Willemze R. Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 44.Todaro M. Zerilli M. Ricci-Vitiani L. Bini M. Perez Alea M. Maria Florena A. Miceli L. Condorelli G. Bonventre S, et al. Autocrine production of interleukin-4 and interleukin-10 is required for survival and growth of thyroid cancer cells. Cancer Res. 2006;66:1491–1499. doi: 10.1158/0008-5472.CAN-05-2514. [DOI] [PubMed] [Google Scholar]
- 45.Liu WH. Liu JJ. Wu J. Zhang LL. Liu F. Yin L. Zhang MM. Yu B. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK/STAT3 signaling pathway. PLoS One. 2013;8:e55487. doi: 10.1371/journal.pone.0055487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Clerici M. Merola M. Ferrario E. Trabattoni D. Villa ML. Stefanon B. Venzon DJ. Shearer GM. De Palo G. Clerici E. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J Natl Cancer Inst. 1997;89:245–250. doi: 10.1093/jnci/89.3.245. [DOI] [PubMed] [Google Scholar]
- 47.Waldhauer I. Steinle A. NK cells and cancer immunosurveillance. Oncogene. 1998;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 48.Riteau B. Menier C. Khalil-Daher I. Martinozzi S. Pla M. Dausset J. Carosella ED. Rouas-Freiss N. HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol. 2001;13:193–201. doi: 10.1093/intimm/13.2.193. [DOI] [PubMed] [Google Scholar]
- 49.Urosevic M. Kurrer MO. Kamarashev J. Mueller B. Weder W. Burg G. Stahel RA. Dummer R. Trojan A. Human leukocyte antigen-G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol. 2001;159:817–824. doi: 10.1016/S0002-9440(10)61756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon BS. Kim YT. Kim JW. Kim SH. Kim JH. Kim SW. Expression of human leukocyte antigen-G and its correlation with interleukin-10 expression in cervical carcinoma. Int J Gynaecol Obstet. 2007;98:48–53. doi: 10.1016/j.ijgo.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 51.Dong DD. Yang H. Li K. Xu G. Song LH. Fan XL. Jiang XL. Yie SM. Human leukocyte antigen-G expression in cervical lesions: association with cancer progression, HPV 16/18 infection, and host immune response. Reprod Sci. 2010;17:718–723. doi: 10.1177/1933719110369183. [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez JA. Galeano L. Palacios DM. Gómez C. Serrano ML. Bravo MM. Combita AL. Altered HLA class I and HLA-G expression is associated with IL-10 expression in patients with cervical cancer. Pathobiology. 2012;79:72–83. doi: 10.1159/000334089. [DOI] [PubMed] [Google Scholar]