Abstract
Nutrient administration following an exercise bout vastly affects anabolic processes within the human body, irrespective of exercise mode. Of particular importance are protein and carbohydrates whereby these two macronutrients portray distinct functions as anabolic agents. It has been confirmed that protein and/or amino acid ingestion following resistance training is required to reach a positive protein/nitrogen balance, and carbohydrate intake during recovery is the most important consideration to replenish glycogen stores from an exhaustive exercise bout. Several factors play significant roles in determining the effectiveness of protein and carbohydrate supplementation on post-exercise protein and glycogen synthesis. Improper application of these factors can limit the body’s ability to reach an anabolic status. The provided evidence clearly denotes the importance these two macronutrients have in regards to post-exercise nutrition and anabolism. Therefore, the purpose of this review is to discuss the impact of dietary protein and carbohydrate intake during the recovery state on muscle protein synthesis and glycogen synthesis.
Key points.
Post-exercise nutrient intake is essential for promoting protein synthesis and glycogen synthesis.
The timing and amount of protein and/or carbohydrate ingested affects the rate and amount of synthesis.
The type/form of protein and/or carbohydrate ingested after exercise alters anabolic processes during the recovery period.
Key words: Protein supplementation, carbohydrate supplementation, anabolism
Introduction
In recent years, post-exercise nutrition has evolved as an imperative part of training regimens among athletic populations. Athletes of all ages, abilities, and skill levels are adopting some form of post-exercise nutrition to improve performance and enhance the body’s recovery processes following exercise. Athletes in particular are highly susceptible to the detriments of heavy training regimens, because they are constantly depleting their energy substrates and stressing skeletal muscle tissues simultaneously. The macronutrients that have drawn much attention, in reference to the recovery phase of exercise, are protein and carbohydrates. Protein and carbohydrates have their own distinct functions, yet both work to generate an anabolic state within the body when ingested after the completion of an exercise bout. It is necessary for individuals who seek to gain lean muscle mass to induce a positive protein turnover as often as possible. It has been confirmed that protein and/or amino acid ingestion is required to reach a positive protein/nitrogen balance (Borsheim et al., 2004a; Koopman et al., 2006; Tipton et al., 2004), and carbohydrate ingestion alone provides marginal benefits on protein synthesis rates (Roy, 1997). Carbohydrate intake during recovery has been shown to replenish depleted glycogen after intense or exhaustive exercise (Ivy et al., 2002; Ivy et al., 1988b; Reed et al., 1989). The addition of protein can further enhance this process (Ivy, et al., 2002), but only in situations when an inadequate amount of carbohydrate is made available in the diet (van Loon et al., 2000). A lack of glycogen stores in the muscle and liver will limit the performance capacities of the body during prolonged or higher intensity bouts of exercise (Coyle et al., 1986). The provided evidence clearly denotes the importance these two macronutrients have in regards to post-exercise nutrition and anabolism. Therefore, the purpose of this review is to discuss the impact of dietary protein and carbohydrate intake during the recovery state on muscle protein synthesis and glycogen synthesis.
Resistance training and protein turnover
It is of paramount importance to delineate the role that resistance training plays in protein turnover. The work of Biolo and colleagues (Biolo et al., 1995b) examined protein synthesis and degradation rates before and three hours after resistance training in healthy untrained men. Three hours after the exercise bout, protein turnover and amino acid transport increased in addition to increases in protein degradation above baseline levels, resulting in a net negative protein balance. Similar findings were reported by (Phillips et al., 1999), as they measured fractional synthesis rates (FSR) and breakdown rates (FBR) in resistance trained and untrained individuals. FSR values increased when measured immediately following the resistance training bout. However, FBR values were also elevated in response to resistance training, concluding that protein turnover rates remained negative (protein degradation rates remained higher than synthesis rates). One interesting note of this study was that FBR, following the training session, increased higher in the untrained subjects. This demonstrates that trained individuals with adequate resistance training experience will undergo reduced protein turnover rates when compared to untrained counterparts. The duration of elevated protein synthesis rates following resistance training was analyzed by Chesley and colleagues (Chesley et al., 1992). Their results show a 50% and 109% increase in protein synthesis rates at four and 24 hours post- exercise respectively. This elevation seems to diminish and return to near baseline values between 36 hours (MacDougall et al., 1995) and 48 hours (Phillips et al., 1997) after the exercise bout. Several other previous investigations have also supported these findings on the relationship between resistance exercise, void of nutritional intervention, and protein turnover (Biolo et al., 1995a; Durham et al., 2004; Hasten et al., 2000; Phillips et al., 2002; Pitkanen et al., 2003; Tipton et al., 1996).
Protein intake
A number of studies have identified the effects of dietary protein intake, void of any exercise intervention, on markers of protein synthesis. Tipton and colleagues (1999b) supplemented four healthy volunteers with 13.4 g of essential amino acids (EAA) and 35 g of sucrose which elevated arterial EAA concentrations between 100% and 400% between 10 and 30 minutes post-ingestion. This demonstrates that the combination of a small quantity of amino acids coupled with a carbohydrate (an approximate 3:1 ratio) can effectively stimulate protein synthesis at rest. Furthermore, an EAA dosage similar to the Tipton investigation (15 g) increased protein (fractional) synthesis rates in young (10.3%/hr) and older (8.8% hr) adults in a parallel fashion measured by arterial phenylalanine concentrations (Paddon-Jones et al., 2004). Absorption speed of amino acids is a critical factor in regulating post-prandial protein accretion. Whey protein was superior to that of casein in upregulating protein synthesis (Boirie et al., 1997) due to its ability to digest more rapidly than casein protein. Free form amino acid ingestion acts similarly to whey by displaying a rapid and strong increase in aminoacidemia (Dangin et al., 2001). This is especially important in elderly populations, because a quickly digested protein can increase protein synthesis/stores sufficiently and provide some alleviation against protein losses accompanied with aging when compared to a slower digested protein (Dangin et al., 2003). In the larger scope of things, it appears that protein synthesis rapidly increases for up to two hours after amino acid administration (Bohe et al., 2001), and that a dose-dependent response between amino acid availability and protein synthesis exists up to a point of complete saturation and possible inhibitory mechanisms of amino acid uptake by muscles (Bohe, et al., 2001; Monneret et al., 2003). Several other previous investigations have also supported these findings in regards to protein ingestion void of exercise intervention (Kobayashi et al., 2003; Paddon-Jones et al., 2003; Tang et al., 2009; Volpi et al., 2000; 2003).
Protein intake and resistance training
The intervention of dietary protein or amino acid supplementation in conjunction with resistance training has proven to effectively increase protein synthesis rates. An original investigation in this area of research (Biolo et al., 1997) evaluated the effects of intravenous infusion of amino acids (alanine, phenylalanine, leucine, and lysine) at rest and following a lower extremity resistance exercise bout. Their findings revealed a 291% increase in protein synthesis following the exercise bout, while protein degradation remained unchanged from baseline quantities, a response most largely influenced from the 30% - 100% increase in amino acid transport to the active muscle tissue following exercise. Similar protocols in the elderly resulted in augmented rates of protein synthesis accompanied with unchanged rates in muscle protein breakdown which generated a positive protein balance (Volpi et al., 1998). The practicality, however, for these study designs comes into question due to difficulties associated with intravenous infusion of amino acids after resistance training. Therefore, other researchers have assessed the efficiency of oral administration of amino acids and protein following resistance training. Borsheim and colleagues (2002) found that 3g of EAA ingested one and two hours following a resistance training bout increased protein balance in a similar fashion. Furthermore, it has been established that post-exercise EAA supplementation stimulates protein synthesis, in conjunction with a positive protein balance, comparable to that of intravenous infusion of amino acids (Tipton et al., 1999a), and non-EAA are not necessary to achieve post-exercise anabolism (Borsheim et al., 2002; Tipton et al., 1999a). Esmarck and colleagues (2001) investigated the effectiveness of an oral supplement containing 10g of protein, 7g of carbohydrates, and 3g of fat when taken immediately after or two hours following resistance training on muscle hypertrophy and strength in thirteen elderly men. The cross-sectional area of the vastus lateralis following twelve weeks of resistance training increased when subjects ingested the post-workout supplement immediately upon completion of all training sessions, whereas when taken two hours after completion, no changes in muscle cross-sectional area were observed. In this study, it was not necessary to measure protein synthesis levels, since the increase in muscle cross-sectional area (hypertrophy) is indicative of a net positive protein balance. Also, these conclusions give insight on the possible time course for consuming post-exercise protein and/or amino acids, as hypertrophy resulted only when protein was immediately ingested upon cessation of resistance training. A more recent study (Tipton et al., 2004) explored the acute protein balance after exercise when two different proteins were consumed following resistance training. Twenty three non-resistance trained subjects ingested a placebo, 20g of whey, or 20g of casein one hour after completing ten sets of eight repetitions of leg extensions at 80% of their respective one-repetition maximums. Casein and whey protein ingestion yielded similar values of net positive protein balance, and thus an overall increase in protein synthesis (see Figure 1). A later analysis revealed that soy protein increased protein synthesis in rats similar to that of whey after a treadmill exercise protocol (Anthony et al., 2007). A human trial, however, concluded that milk proteins (caseins and whey) in comparison to soy promoted greater muscle protein accretion when they were ingested after regular resistance training (Wilkinson et al., 2007); a response linked closely to their known impacts on splanchnic and peripheral metabolism, respectively (Fouillet et al., 2002). Whey hydrolysate ingested after a resistance exercise bout acutely stimulated mixed muscle protein synthesis 31% greater than soy (Tang, et al., 2009), and post-exercise ingestion of fat-free milk significantly increased lean body mass to a greater extent than soy protein after 12 weeks of resistance training (Hartman et al., 2007). In addition, protein plus amino acid supplementation can up-regulate muscle protein synthesis in conjunction with resistance training (Willoughby et al., 2007), but it seems unnecessary to combine protein and amino acids in an attempt to further stimulate muscle protein synthesis if an adequate amount of protein (20 g) is ingested (Tipton et al., 2009) immediately before or after a resistance exercise bout (Tipton et al., 2007).
Figure 1.
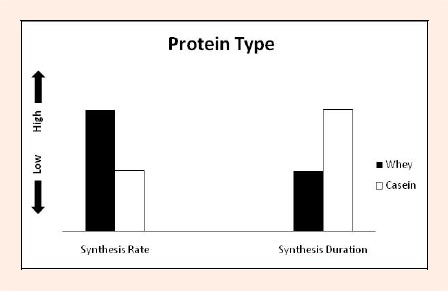
Representation of the two primary supplemented proteins (whey and casein) on protein synthesis rates and protein synthesis duration following resistance training. Whey exhibits a faster digestion rate in comparison with casein. This difference results in whey being responsible for a sharper increase in muscle protein synthesis and minimal effect on protein degradation after administration. Similarly, the slower digestion rate of casein still results in an increase in muscle protein synthesis, albeit it is blunted substantially in direct comparison to administration of whey, but more of a much greater amount of time, while exhibiting a powerful influence over preventing muscle protein breakdown.
Up to this point, several conclusions can be determined from the previous studies: 1) Resistance training increases protein synthesis as well as protein degradation, 2) This increase in protein synthesis is overshadowed by a corresponding elevation in protein degradation, resulting in a net negative protein balance, 3) The intake of dietary protein and/or amino acids after completion of a resistance training bout augments a net positive protein balance, resulting in the potential for skeletal muscle hypertrophy over time (Cribb and Hayes, 2006; Cribb et al., 2007; Hayes and Cribb, 2008; Kerksick et al., 2006; Willoughby et al., 2007), and 4) The intake of dietary protein and/or amino acids immediately following resistance training is more effective in inducing hypertrophy than if nutrient intake is postponed. Figure 2 illustrates the information discussed up to this point regarding the possible nitrogen balance states.
Figure 2.
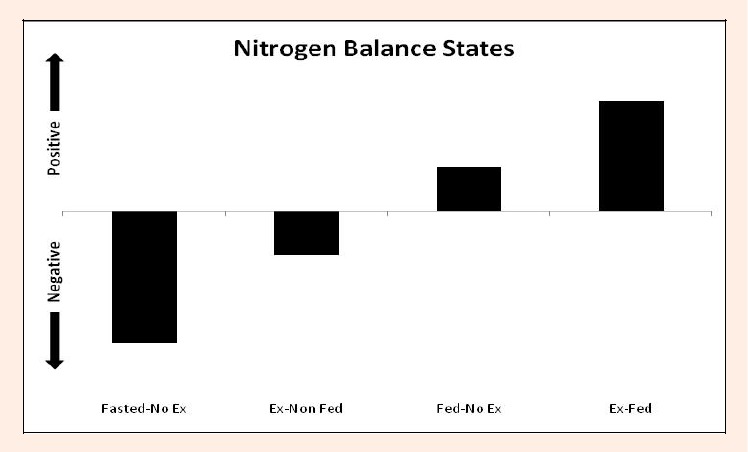
Impact of feeding and exercise status on nitrogen balance. The horizontal axis separates the point at which a positive or negative nitrogen balance occurs. A positive nitrogen balance suggests accumulation of nitrogen; a scenario commonly associated with muscle protein synthesis (e.g., hypertrophy). A negative nitrogen balance suggests an overall loss of nitrogen; a scenario commonly associated with a loss of muscle protein (e.g., atrophy). Fasted-No Ex: State where no resistance training or feeding has occurred. Ex-Non Fed: State where resistance training has occurred without subsequent feeding. Fed-No Ex: State where feeding has occurred but without resistance training bout, a positive nitrogen balance results. Ex-Fed: State where resistance training has occurred with subsequent feeding, a positive nitrogen balance results.
Carbohydrate/protein intake and resistance training
Researchers have additionally defined the functions that carbohydrates carry out in relation to post-exercise nutrition, specifically pertaining to resistance training and protein synthesis. Studies have looked at the effectiveness of carbohydrate consumption alone following exercise, as well as in combination with protein and/or amino acid supplementation. Roy and colleagues (1997) investigated the effect of a glucose supplement administered immediately and one hour after resistance training on anabolic and catabolic markers in resistance trained men. Subjects completed four sets of approximately 8-10 repetitions each of unilateral leg press and knee extension exercises at 85% of 1RM, and either received a glucose supplement (1g·kg-1) or placebo following the exercise bout. Fractional muscle protein synthesis rates in the exercised leg muscle increased 36%, where only a 6% increase was observed in the placebo condition. Urinary urea excretion and 3-methlyhistidine (markers of muscle protein degradation) were lower in the treatment group (urea excretion: 8.6 g·g-1 creatinine, 3-methlyhistidine: 110.4 μmol·g-1 creatinine) compared to placebo (urea excretion: 12.3 g·g-1 creatinine, 3-methlyhistidine: 120.1 μmol·g-1 creatinine), signifying a reduction in protein degradation. The overall effect of the glucose supplement caused a suppressed protein degradation rate compared to the placebo group, resulting in a more positive protein balance. These results were supported by a later analysis that concluded 100 g of carbohydrates improves overall protein balance when ingested one hour following a resistance exercise bout (Borsheim et al., 2004b).
Borsheim and colleagues (2004b) determined if an amino acid, protein, and carbohydrate solution elicited a greater anabolic response following resistance training than carbohydrates alone. Eight recreationally active subjects completed two trials of 10 sets X 8 repetitions of leg extensions, and ingested either a solution containing 77.4g carbohydrates, 17.5g of whey protein, and 4.9g of amino acids or 100g of carbohydrates for each trial, one hour upon cessation of exercise. Arterial phenylalanine concentration increased rapidly in the protein/amino acid/carbohydrate group and remained elevated until 210 minutes after the completion of exercise, causing a net positive muscle phenylalanine balance for a short period. Phenylalanine concentrations remained close to baseline levels in the carbohydrate group, inhibiting net muscle phenylalanine balance from reaching a positive state. Therefore, the addition of protein and amino acids to a carbohydrate solution increases net muscle protein synthesis to a higher degree than carbohydrates alone and shifted the overall balance of muscle protein metabolism to a positive state. A more recent study (Tang et al., 2007) elicited similar results to the Borsheim investigation after eight resistance trained males completed two unilateral trials in random order and ingested either a whey mixture (10g of whey and 21g of fructose) or carbohydrate mixture (21g of fructose and 10g of maltodextrin) following the completion of each trial. In both nutritional conditions, muscle protein synthesis after the exercise bout was elevated in the exercised leg when compared to their respective resting legs. Moreover, fractional synthesis rates were significantly higher when whey protein was ingested when contrasted with only carbohydrate ingestion. These studies provide solid evidence that carbohydrates only have a minimal effect on protein synthesis in the absence of protein ingestion, but can be depended upon as a nutrition source to minimize protein breakdown when ingested alone. With this being said, a small amount of whey protein in addition to carbohydrate consumption in the recovery phase of exercise is a more sufficient means of increasing protein synthesis. Koopman and colleagues (2007) found supporting evidence, as they examined the effects of ingesting differing amount of carbohydrates with adequate protein intake on post-exercise protein synthesis. Healthy volunteers completed three resistance training bouts, separated by one week of rest, and consumed protein (3 g·kg-1·hour-1) with 0, 0.15, or 0.6 grams of carbohydrate/kg/hour respectively for each trial during a six hour time period following exercise. Protein synthesis, protein degradation, and net muscle protein synthesis values were constant across all groups. This suggests that carbohydrates, when supplemented with adequate quantities of dietary protein, do not heighten the anabolic response when consumed during the post-exercise period. The interested reader is encouraged to read these additional studies pertaining to the effects of combining protein with carbohydrates following resistance training on muscle protein synthesis (Koopman et al., 2005; Rasmussen et al., 2000; Tipton et al., 2001).
This group of studies has given insight on several important components related to anabolism in the post-exercise state. Carbohydrates alone seem to have a minimal effect on the net protein balance following exercise. Whether they marginally reduce protein degradation or slightly increase protein synthesis, carbohydrates unaccompanied by protein are unable to generate a positive protein balance and stimulate skeletal muscle hypertrophy. Different forms, sources and/or quantities of protein supplemented with carbohydrates can interact to create a greater anabolic environment in the post-exercise state by elevating protein synthesis levels far greater than carbohydrates alone could initiate. If a positive protein balance and subsequently muscle hypertrophy is desired, protein must be added to carbohydrate supplementation in order to fuel these processes. The combined effects of carbohydrate and amino acid/protein supplementation on protein synthesis are equivalent to their independent effects (Miller et al., 2003).
Glycogen synthesis
Glycogen is a vital fuel source for high intensity and prolonged exercise, and the dependence of this energy substrate increases as exercise intensity rises (Bergstrom and Hultman, 1966; 1967). Consequently, glycogen synthesis during the post-exercise time period is essential for replenishing energy stores and aiding the body in the recovery process. It has been determined that glycogen synthesis following exercise occurs in two distinct phases. The rapid phase lasts approximately 30-60 minutes and does not require the presence of insulin (Maehlum et al., 1977). This phase likely transpires when glycogen reserves have been depleted to extremely low levels (Maehlum et al., 1977), or if carbohydrates are ingested immediately following the exercise bout (Ivy et al., 1988a). The other phase of glycogen synthesis is the slow phase, which can last up to several hours if carbohydrate availability is high and insulin levels remain elevated (Ivy, 1991).
Timing of nutrient intake
The timing of post-exercise nutrition is an important factor to consider when attempting to replenish glycogen stores that may have been depleted from exercise. Ivy and colleagues (1988a) demonstrated this phenomenon by evaluating the effectiveness of a 25% carbohydrate solution given to cyclists immediately or two hours after 70 minutes of nonstop exercise on a cycle ergometer. During the initial two hours of recovery, glycogen synthesis was much higher in the individuals who consumed the solution immediately after exercise. The cyclists who ingested the solution two hours after the exercise bout saw an increase in glycogen synthesis during hours three and four, but this elevation still remained below those who ingested carbohydrate at the earlier time point. Ivy concluded that delaying nutrient (carbohydrate) intake by two hours after a prolonged exercise bout decreases muscle glycogen synthesis by 45% when measured four hours after the completion of exercise. Parkin and colleagues (1997) evaluated the effects of delaying nutrient intake on muscle glycogen synthesis following a strenuous exercise bout in endurance trained men. A total of five high glycemic meals were fed to the subjects over a 24 hour period in a manner that allowed one treatment group to receive nutrients roughly two hours after the other at all time points. At eight and 24 hours after exercise, both treatment groups displayed similar muscle glycogen stores. These findings imply that delaying nutrient intake by two hours after a prolonged exercise regimen has no effect on the rate of muscle glycogen synthesis. This is an area in the literature where some conflict and gray areas have presented themselves. Ivy et al.’s work measured glycogen synthesis rates up to four hours post-exercise, where the work by Parkin and colleagues determined glycogen synthesis rates over an eight hour period but changed the amount of carbohydrates ingested in the immediate feeding group from 0.8 g·kg-1·hour-1 in the first four hours to none thereafter. It is not unreasonable to believe that if the feedings remained constant, glycogen stores might have been higher at the eight hour time point. Other investigations have demonstrated mixed results as well. Additional work by Ivy and colleagues (1988b) showed delayed glycogen synthesis rates of 22% and 24% from hours two to four during exercise recovery in contrast with the immediate two hour window following exercise and carbohydrate ingestion. On the contrary and in agreement with the Parkin et al. study, no differences in glycogen synthesis rates have been reported between the first 60-120 minutes after exercise and the 60-120 minutes thereafter (Jentjens et al., 2001; Reed, et al., 1989). The dissimilarities observed in these studies are possibly attributable to the amount and composition of the carbohydrates ingested along with the degree to which the individuals participating in these investigations were glycogen depleted. Also, a participant’s fitness level may play a factor, as endurance trained individuals have shown an ability to replenish glycogen stores more rapidly than untrained counterparts (Hickner et al., 1997). Due to the inconsistency in the Parkin study and possible limitations of others, it can be assumed that athletes should intake nutrients immediately or soon after the completion of a prolonged or high intensity exercise bout, especially if quick replenishment of glycogen stores is required. If fast glycogen recovery is unnecessary and the goal is long-term maintenance of carbohydrate stores, a daily carbohydrate intake greater than 8 g·kg-1·day-1 is recommended to effectively maintain glycogen stores during repeated days of endurance training (Kirwan et al., 1988; Sherman et al., 1993).
Type and form of nutrient intake
Different types of carbohydrates initiate different outcomes on glycogen synthesis and ultimately muscle and liver glycogen storage. The Glycemic Index (GI) was created to distinguish the blood glucose response, and corresponding insulin response, of a specific food after its digestion in comparison with the glucose response of a standard amount of glucose/white bread; the GI is intended to resemble the rate at which a particular food is digested and absorbed into circulation (Wolever et al., 1991). Researchers have scrutinized different GI foods in relation to their ability to accelerate glycogen synthesis. Blom and colleagues (1987) evaluated muscle glycogen synthesis rates when glucose, sucrose, and fructose were ingested at zero, two, and four hours after an exhaustive cycling bout. Glucose and sucrose supplementation initiated a greater increase in glycogen synthesis when compared to fructose ingestion. Fructose must be catabolized in the liver before it can enter circulation through the blood and contribute to glycogen synthesis within skeletal muscle. It would appear that fructose reduces the availability of circulating glucose compared to other sugars even though contradicting evidence exists (Wallis et al., 2008), and in one case, sucrose replenished glycogen stores to a lesser extent when contrasted with a glucose polymer solution (Bowtell et al., 2000). Kiens and colleagues (1990) explored the effects of ingesting a high or low GI meal, containing 70% of calories from carbohydrates, following exercise on glycogen synthesis rates. Subjects who consumed the high GI meal experienced a 61% larger increase in muscle glycogen synthesis rates. These studies conclude that high GI foods/carbohydrates are more promising in replenishing glycogen stores in the early hours following exercise, and in addition, the mode of nutrient application (oral or IV) does not seem to matter (Blom, 1989).
Other research (Keizer et al., 1987; Reed, et al., 1989) has focused on the effects of ingesting a solid or liquid meal following exercise on the rate of glycogen synthesis. Both of these inquires reached similar conclusions by determining that carbohydrates in liquid and solid form are equally effective in replenishing glycogen stores after exhaustive bouts on a cycle ergometer and that gastric emptying does not impede the process of glycogen synthesis following exercise.
Amount of nutrient ingestion
Another factor that is of upmost importance in determining the rate of glycogen synthesis after exercise is the amount of carbohydrates (determined by body weight) ingested. The typical rate of muscle glycogen storage after carbohydrate supplementation immediately upon cessation of exercise is 20-50 mmol/kg dw/h (Blom, 1989; Blom et al., 1987; Ivy et al., 1988a; Jentjens and Jeukendrup, 2003; Maehlum et al., 1977; 1978; Piehl Aulin et al., 2000; Reed, et al., 1989; Tarnopolsky et al., 1997; Zachwieja et al., 1991). Little research has focused on the direct rates of carbohydrate supplementation and its effects on muscle glycogen synthesis. Blom and colleagues (Blom, et al., 1987) first demonstrated this phenomenon by increasing the rate of muscle glycogen storage approximately 150% when increasing the amount of carbohydrate intake from 0.18 to 0.35 g·kg-1·hour-1. More recent studies have analyzed the effects of higher quantities of carbohydrate consumption on glycogen storage rates. Ivy and associates (Ivy et al., 1988b) utilized dosages of 0.75 and 1.5 g·kg-1·hour-1 over a four hour period following a 120 minute cycling bout. The results indicate similar rates of glycogen synthesis for both treatment dosages which alludes to a possible cap or maximum rate of nutrient consumption that can effectively increase glycogen storage. Nonetheless, it has been found that increasing post-exercise carbohydrate intake to 0.8 to 1.2 g·kg-1·hour-1 results in higher rates of glycogen synthesis (van Loon, et al., 2000), and it seems that a carbohydrate dosage of 1.2 g·kg-1·hour-1 is optimal for reaching maximal post-exercise muscle glycogen synthesis rates (Jentjens and Jeukendrup, 2003; Jentjens, et al., 2001; van Loon, et al., 2000). Many studies display similar findings and support the notion that increasing the amount of carbohydrate intake above 0.35 g·kg-1·hour-1 can further stimulate glycogen synthesis (Casey et al., 1995; McCoy et al., 1996; Piehl Aulin, et al., 2000; Tarnopolsky, et al., 1997; van Hall et al., 2000).
Intervention of protein
The addition of protein to carbohydrate consumption in the post-exercise period has led to mixed results. Zawadzki and colleagues (1992) investigated the effects of carbohydrate, protein, and carbohydrate plus protein supplements on muscle glycogen synthesis after two hours of cycling. Participants ingested either 112g of carbohydrates, 41 grams of protein, or 112 grams of carbohydrate and 41 grams of protein immediately, and two hours after three separate exercise bouts. Supplementing carbohydrates and protein together resulted in higher glycogen stores than the carbohydrate and protein groups. Original research in this area concluded that the increase in glycogen synthesis is directly related to the upregulatory effect that certain amino acids have on insulin (Floyd et al., 1966; Knopf et al., 1966). Therefore, it is reasonable to believe that carbohydrate plus protein intake following exhaustive exercise will further enhance glycogen synthesis over carbohydrates alone. On the contrary, the study design of Zawadzki limits the generalization of the findings due to the variation in caloric values provided to the treatment groups. Glycogen stores may have been further replenished in the carbohydrate + protein group, because additional calories were consumed. A more recent study took a second look at the possible impact of protein + carbohydrate supplement on glycogen synthesis when compared to a carbohydrate solutions of equal caloric value and equal carbohydrate content (Ivy, et al., 2002). Cyclists completed two hours of exercise on three different occasions to analyze all treatments. Supplements were ingested immediately and two hours following exercise. Four hours after the completion of the exercise bout, 47% of glycogen depleted during exercise bout was restored in the carbohydrate + protein group. The equal caloric value and carbohydrate content groups experienced 31% and 28% glycogen restorations respectively. The conclusions of the Ivy study help to support the inferences made earlier by Zawadzki and colleagues. It is apparent that protein can further augment glycogen synthesis when ingested with an adequate amount of carbohydrates. However, conflicting evidence does exist. van Loon and colleagues (2000) concluded that the ingestion of ample carbohydrates is the limiting factor in determining the magnitude of glycogen synthesis after exercise. When protein was added to a sufficient carbohydrate solution, glycogen synthesis was not further stimulated. Other investigations have seen enhanced rates of glycogen synthesis during the post-exercise period (Berardi et al., 2006; Bowtell et al., 1999; Tarnopolsky, et al., 1997) whereas others disagree (Carrithers et al., 2000; Jentjens, et al., 2001; Yaspelkis and Ivy, 1999). In conclusion, it looks as if glycogen synthesis can increase with the addition of protein under certain circumstances, although some evidence lacks in supporting this claim. A summary of factors affecting glycogen synthesis immediately after exercise are displayed in Figure 3.
Figure 3.
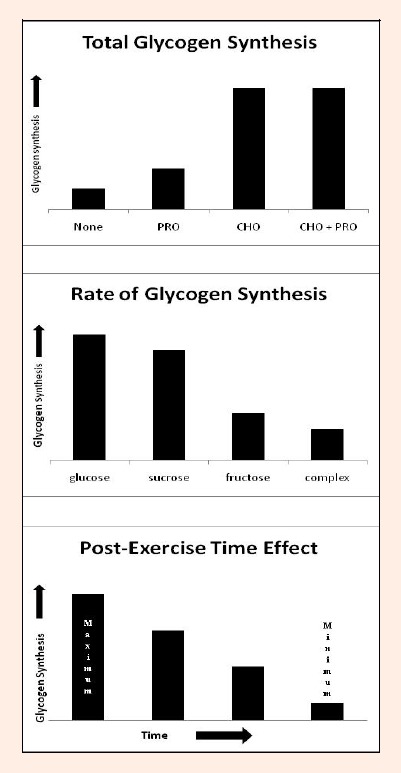
Hypothetical models of factors affecting post-exercise glycogen synthesis. Top: The type of nutrient administered affects total glycogen synthesis post-endurance exercise. Protein (PRO) promotes the least amount of glycogen synthesis where carbohydrate with protein (CHO + PRO) and carbohydrates alone stimulate maximal rates. Middle: The form/complexity of carbohydrate ingested after exercise determines the rate of glycogen synthesis, simple carbohydrates being the most effective. Bottom: A window of time exists following exercise that allows for maximal glycogen synthesis rates. Immediate nutrient administration is most effective, and as time elapses, the ability to replenish glycogen reduces.
Conclusion
The purpose of this review was to discuss the impact of dietary protein and carbohydrate intake during the recovery state on anabolic markers such as muscle protein synthesis and glycogen synthesis. The anabolic processes of muscle protein synthesis and glycogen synthesis are affected by many different variables. Resistance training alone is not potent enough to stimulate a positive protein balance where protein synthesis exceeds protein degradation. The supplementation of protein and/or amino acids following a resistance training bout results in a net positive protein balance that enables skeletal muscle hypertrophy to take place. Carbohydrates play a limited role in protein synthesis, and thus are probably not necessary to prompt hypertrophy training effects. However, carbohydrates are vital to replenish glycogen stores diminished from prolonged or high intensity exercise. Past research has clearly defined that timing of ingestion, GI value of the food, amount ingested, and nutrient composition of the food are all important factors in determining the effectiveness of glycogen synthesis rates. Future research is needed to elucidate the equivocal findings surrounding the combination of protein and carbohydrate supplementation in reference to glycogen synthesis after exercise.
Biographies

Chris Poole
Employment
A PhD student currently working in the Applied Biochemistry and Molecular Physiology Laboratory in the Health and Exercise Department at the University of Oklahoma.
Degree
MSc.
Research interests
Cellular, molecular, and endocrine responses to various forms of exercise in young and elderly populations and sport nutrition and supplementation.
E-mail: cpoole@ou.edu

Colin Wilborn
Employment
Assistant Professor of Exercise Science & Director of the Human Performance Lab, at the University of Mary Hardin-Baylor.
Degree
PhD.
Research interests
The effects of sport supplements and exercise on body composition, metabolism, and performance.
E-mail: cwilborn@umhb.edu

Lem Taylor
Employment
Assistant Professor of Exercise Physiology and the Director of the Exercise Biochemistry Lab at the University of Mary Hardin-Baylor.
Degree
PhD.
Research interests
Skeletal muscle adaptation following various forms of exercise and nutritional intervention from both a basic and applied scientific approach.
E-mail: LTalyor@umhb.edu

Chad Kerksick
Employment
Assistant Professor of Exercise Physiology in the Health & Exercise Science department at the University of Oklahoma.
Degree
PhD.
Research interests
Sport nutrition as well as the biochemical, cellular and molecular adaptations relative to various forms of exercise and nutrition interventions.
E-mail: chad_kerksick@ou.edu
References
- Anthony T.G., McDaniel B.J., Knoll P., Bunpo P., Paul G. L., McNurlan M.A. (2007) Feeding meals containing soy or whey protein after exercise stimulates protein synthesis and translation initiation in the skeletal muscle of male rats. Journal of Nutrition 137, 357-362 [DOI] [PubMed] [Google Scholar]
- Berardi J.M., Price T.B., Noreen E.E., Lemon P.W. (2006) Postexercise muscle glycogen recovery enhanced with a carbohydrate-protein supplement. Medicine and Science in Sports and Exercise 38, 1106-1113 [DOI] [PubMed] [Google Scholar]
- Bergstrom J., Hultman E. (1966) Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature 210, 309-310 [DOI] [PubMed] [Google Scholar]
- Bergstrom J., Hultman E. (1967) A study of the glycogen metabolism during exercise in man. Scandinavian Journal of Clinical and Laboratory Investigation 19, 218-228 [DOI] [PubMed] [Google Scholar]
- Biolo G., Fleming R.Y., Maggi S.P., Wolfe R.R. (1995a) Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. American Journal of Physiology 268, E75-84 [DOI] [PubMed] [Google Scholar]
- Biolo G., Maggi S.P., Williams B.D., Tipton K.D., Wolfe R.R. (1995b) Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology 268, E514-520 [DOI] [PubMed] [Google Scholar]
- Biolo G., Tipton K.D., Klein S., Wolfe R.R. (1997) An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. American Journal of Physiology 273, E122-129 [DOI] [PubMed] [Google Scholar]
- Blom C.S. (1989) Post-exercise glucose uptake and glycogen synthesis in human muscle during oral or i.v. glucose intake. European Journal of Applied Physiology and Occupational Physiology 59, 327-333 [DOI] [PubMed] [Google Scholar]
- Blom P.C., Hostmark A.T., Vaage O., Kardel K.R., Maehlum S. (1987) Effect of different post-exercise sugar diets on the rate of muscle glycogen synthesis. Medicine and Science in Sports and Exercise 19, 491-496 [PubMed] [Google Scholar]
- Bohe J., Low J.F., Wolfe R.R., Rennie M.J. (2001) Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. Journal of Physiology 532, 575-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y., Dangin M., Gachon P., Vasson M.P., Maubois J.L., Beaufrere B. (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proceedings of the National Academy of Sciences U S A 94, 14930-14935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsheim E., Aarsland A., Wolfe R.R. (2004a) Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. International Journal of Sport Nutrition and Exercise Metabolism 14, 255-271 [DOI] [PubMed] [Google Scholar]
- Borsheim E., Cree M.G., Tipton K.D., Elliott T.A., Aarsland A., Wolfe R.R. (2004b) Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. Journal of Applied Physiology 96, 674-678 [DOI] [PubMed] [Google Scholar]
- Borsheim E., Tipton K.D., Wolf S.E., Wolfe R.R. (2002) Essential amino acids and muscle protein recovery from resistance exercise. American Journal of Physiology Endocrinology and Metabolism 283, E648-657 [DOI] [PubMed] [Google Scholar]
- Bowtell J.L., Gelly K., Jackman M.L., Patel A., Simeoni M., Rennie M.J. (1999) Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. Journal of Applied Physiology 86, 1770-1777 [DOI] [PubMed] [Google Scholar]
- Bowtell J.L., Gelly K., Jackman M.L., Patel A., Simeoni M., Rennie M.J. (2000) Effect of different carbohydrate drinks on whole body carbohydrate storage after exhaustive exercise. Journal of Applied Physiology 88, 1529-1536 [DOI] [PubMed] [Google Scholar]
- Carrithers J.A., Williamson D.L., Gallagher P.M., Godard M.P., Schulze K.E., Trappe S.W. (2000) Effects of postexercise carbohydrate-protein feedings on muscle glycogen restoration. Journal of Applied Physiology 88, 1976-1982 [DOI] [PubMed] [Google Scholar]
- Casey A., Short A.H., Hultman E., Greenhaff P. L. (1995) Glycogen resynthesis in human muscle fibre types following exercise-induced glycogen depletion. Journal of Physiology 483 (Pt 1), 265-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesley A., MacDougall J.D., Tarnopolsky M.A., Atkinson S.A., Smith K. (1992) Changes in human muscle protein synthesis after resistance exercise. Journal of Applied Physiology 73, 1383-1388 [DOI] [PubMed] [Google Scholar]
- Coyle E.F., Coggan A.R., Hemmert M.K., Ivy J.L. (1986) Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. Journal of Applied Physiology 61, 165-172 [DOI] [PubMed] [Google Scholar]
- Cribb P.J., Hayes A. (2006) Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Medicine and Science in Sports and Exercise 38, 1918-1925 [DOI] [PubMed] [Google Scholar]
- Cribb P.J., Williams A.D., Stathis C.G., Carey M.F., Hayes A. (2007) Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Medicine and Science in Sports and Exercise 39, 298-307 [DOI] [PubMed] [Google Scholar]
- Dangin M., Boirie Y., Garcia-Rodenas C., Gachon P., Fauquant J., Callier P., Ballevre O., Beaufrere B. (2001) The digestion rate of protein is an independent regulating factor of postprandial protein retention. American Journal of Physiology Endocrinology and Metabolism 280, E340-348 [DOI] [PubMed] [Google Scholar]
- Dangin M., Guillet C., Garcia-Rodenas C., Gachon P., Bouteloup-Demange C., Reiffers-Magnani K., Fauquant J., Ballevre O., Beaufrere B. (2003) The rate of protein digestion affects protein gain differently during aging in humans. Journal of Physiology 549, 635-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham W.J., Miller S.L., Yeckel C.W., Chinkes D.L., Tipton K.D., Rasmussen B.B., Wolfe R. R. (2004) Leg glucose and protein metabolism during an acute bout of resistance exercise in humans. Journal of Applied Physiology 97, 1379-1386 [DOI] [PubMed] [Google Scholar]
- Esmarck B., Andersen J.L., Olsen S., Richter E.A., Mizuno M., Kjaer M. (2001) Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. Journal of Physiology 535, 301-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd J.C., Jr, Fajans S.S., Conn J.W., Knopf R.F., Rull J. (1966) Insulin secretion in response to protein ingestion. Journal of Clinical Investigation 45, 1479-1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillet H., Mariotti F., Gaudichon C., Bos C., Tome D. (2002) Peripheral and splanchnic metabolism of dietary nitrogen are differently affected by the protein source in humans as assessed by compartmental modeling. Journal of Nutrition 132, 125-133 [DOI] [PubMed] [Google Scholar]
- Hartman J.W., Tang J.E., Wilkinson S.B., Tarnopolsky M.A., Lawrence R.L., Fullerton A.V., Phippips S. M. (2007) Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. American Journal of Clinical Nutrition 86, 373-381 [DOI] [PubMed] [Google Scholar]
- Hasten D.L., Pak-Loduca J., Obert K.A., Yarasheski K.E. (2000) Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 yr olds. American Journal of Physiology Endocrinology and Metabolism 278, E620-626 [DOI] [PubMed] [Google Scholar]
- Hayes A., Cribb P. J. (2008) Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Current Opinion in Clinical Nutrition and Metabolic Care 11, 40-44 [DOI] [PubMed] [Google Scholar]
- Hickner R.C., Fisher J.S., Hansen P.A., Racette S.B., Mier C.M., Turner M.J., Holloszy J. O. (1997) Muscle glycogen accumulation after endurance exercise in trained and untrained individuals. Journal of Applied Physiology 83, 897-903 [DOI] [PubMed] [Google Scholar]
- Ivy J.L. (1991) Muscle glycogen synthesis before and after exercise. Sports Medicine 11, 6-19 [DOI] [PubMed] [Google Scholar]
- Ivy J.L., Goforth H.W., Jr, Damon B.M., McCauley T.R., Parsons E.C., Price T.B. (2002) Early postexercise muscle glycogen recovery is enhanced with a carbohydrate-protein supplement. Journal of Applied Physiology 93, 1337-1344 [DOI] [PubMed] [Google Scholar]
- Ivy J.L., Katz A.L., Cutler C.L., Sherman W.M., Coyle E.F. (1988a) Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. Journal of Applied Physiology 64, 1480-1485 [DOI] [PubMed] [Google Scholar]
- Ivy J.L., Lee M.C., Brozinick J.T., Jr, Reed M.J. (1988b) Muscle glycogen storage after different amounts of carbohydrate ingestion. Journal of Applied Physiology 65, 2018-2023 [DOI] [PubMed] [Google Scholar]
- Jentjens R.L., Jeukendrup A. (2003) Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Medicine 33, 117-144 [DOI] [PubMed] [Google Scholar]
- Jentjens R.L., van Loon L.J., Mann C.H., Wagenmakers A.J., Jeukendrup A.E. (2001) Addition of protein and amino acids to carbohydrates does not enhance postexercise muscle glycogen synthesis. Journal of Applied Physiology 91, 839-846 [DOI] [PubMed] [Google Scholar]
- Keizer H.A., Kuipers H., van Kranenburg G., Geurten P. (1987) Influence of liquid and solid meals on muscle glycogen resynthesis, plasma fuel hormone response, and maximal physical working capacity. International Journal of Sports Medicine 8, 99-104 [DOI] [PubMed] [Google Scholar]
- Kerksick C.M., Rasmussen C.J., Lancaster S.L., Magu B., Smith P., Melton C., Greenwood M., Almada A., Earnest C., Kreider R. (2006) The effects of protein and amino acid supplementation on performance and training adaptations during ten weeks of resistance training. Journal of Strength and Conditioning Research 20, 643-653 [PubMed] [Google Scholar]
- Kiens B., Rabern B., Valeur A.K., Richter E.A. (1990) Benefit of dietary simple carbohydrates on the early postexercise muscle glycogen repletion in male athletes [abstract 524]. Medicine and Science in Sports and Exercise 22, S88 [Google Scholar]
- Kirwan J.P., Costill D.L., Mitchell J.B., Houmard J.A., Flynn M.G., Fink W.J., Beltz T.Z. (1988) Carbohydrate balance in competitive runners during successive days of intense training. Journal of Applied Physiology 65, 2601-2606 [DOI] [PubMed] [Google Scholar]
- Knopf R.F., Conn J.W., Floyd J.C., Jr, Fajans S.S., Rull J.A., Guntsche E.M., Thiffault C.A. (1966) The normal endocrine response to ingestion of protein and infusions of amino acids. Sequential secretion of insulin and growth hormone. Transactions of the Association of American Physicians 79, 312-321 [PubMed] [Google Scholar]
- Kobayashi H., Borsheim E., Anthony T. G., Traber D. L., Badalamenti J., Kimball S. R., Jefferson L. S., Wolfe R. W. (2003) Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B. American Journal of Physiology Endocrinology and Metabolism 284, E488-498 [DOI] [PubMed] [Google Scholar]
- Koopman R., Beelen M., Stellingwerff T., Pennings B., Saris W.H., Kies A.K., Kuipers H., van Loon L.J.C. (2007) Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. American Journal of Physiology Endocrinology and Metabolism 293, E833-842 [DOI] [PubMed] [Google Scholar]
- Koopman R., Verdijk L., Manders R.J., Gijsen A.P., Gorselink M., Pijpers E., Wagenmakers A.J.M., van Loon L.J.C. (2006) Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. American Journal of Clinical Nutrition 84, 623-632 [DOI] [PubMed] [Google Scholar]
- Koopman R., Wagenmakers A.J., Manders R.J., Zorenc A.H., Senden J.M., Gorselink M., Keizer H.A., van Loon L.J.C. (2005) Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. American Journal of Physiology Endocrinology and Metabolism 288, E645-653 [DOI] [PubMed] [Google Scholar]
- MacDougall J.D., Gibala M.J., Tarnopolsky M.A., MacDonald J.R., Interisano S.A., Yarasheski K.E. (1995) The time course for elevated muscle protein synthesis following heavy resistance exercise. Canadian Journal of Applied Physiology 20, 480-486 [DOI] [PubMed] [Google Scholar]
- Maehlum S., Felig P., Wahren J. (1978) Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. American Journal of Physiology 235, E255-260 [DOI] [PubMed] [Google Scholar]
- Maehlum S., Hostmark A.T., Hermansen L. (1977) Synthesis of muscle glycogen during recovery after prolonged severe exercise in diabetic and non-diabetic subjects. Scandinavian Journal of Clinical and Laboratory Investigation 37, 309-316 [DOI] [PubMed] [Google Scholar]
- McCoy M., Proietto J., Hargreaves M. (1996) Skeletal muscle GLUT-4 and postexercise muscle glycogen storage in humans. Journal of Applied Physiology 80, 411-415 [DOI] [PubMed] [Google Scholar]
- Miller S.L., Tipton K.D., Chinkes D.L., Wolf S.E., Wolfe R.R. (2003) Independent and combined effects of amino acids and glucose after resistance exercise. Medicine and Science in Sports and Exercise 35, 449-455 [DOI] [PubMed] [Google Scholar]
- Monneret G., Debard A.L., Venet F., Bohe J., Hequet O., Bienvenu J., Lepape A. (2003) Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Critical Care Medicine 31, 2068-2071 [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D., Sheffield-Moore M., Creson D.L., Sanford A.P., Wolf S.E., Wolfe R.R., Ferrando A.A. (2003) Hypercortisolemia alters muscle protein anabolism following ingestion of essential amino acids. American Journal of Physiology Endocrinology and Metabolism 284, E946-953 [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D., Sheffield-Moore M., Zhang X.J., Volpi E., Wolf S. E., Aarsland A., Ferrando A.A., Wolfe R.R. (2004) Amino acid ingestion improves muscle protein synthesis in the young and elderly. American Journal of Physiology Endocrinology and Metabolism 286, E321-328 [DOI] [PubMed] [Google Scholar]
- Parkin J.A., Carey M.F., Martin I.K., Stojanovska L., Febbraio M.A. (1997) Muscle glycogen storage following prolonged exercise: effect of timing of ingestion of high glycemic index food. Medicine and Science in Sports and Exercise 29, 220-224 [DOI] [PubMed] [Google Scholar]
- Phillips S.M., Parise G., Roy B.D., Tipton K.D., Wolfe R.R., Tamopolsky M.A. (2002) Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Canadian Journal of Physiology and Pharmacology 80, 1045-1053 [DOI] [PubMed] [Google Scholar]
- Phillips S.M., Tipton K.D., Aarsland A., Wolf S.E., Wolfe R.R. (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. American Journal of Physiology 273, E99-107 [DOI] [PubMed] [Google Scholar]
- Phillips S.M., Tipton K.D., Ferrando A.A., Wolfe R.R. (1999) Resistance training reduces the acute exercise-induced increase in muscle protein turnover. American Jouurnal of Physiology 276, E118-124 [DOI] [PubMed] [Google Scholar]
- Piehl Aulin K., Soderlund K., Hultman E. (2000) Muscle glycogen resynthesis rate in humans after supplementation of drinks containing carbohydrates with low and high molecular masses. European Journal of Applied Physiology 81, 346-351 [DOI] [PubMed] [Google Scholar]
- Pitkanen H.T., Nykanen T., Knuutinen J., Lahti K., Keinanen O., Alen M., Komi P.V., Mero A.A. (2003) Free amino acid pool and muscle protein balance after resistance exercise. Medicine and Science in Sports and Exercise 35, 784-792 [DOI] [PubMed] [Google Scholar]
- Rasmussen B.B., Tipton K.D., Miller S.L., Wolf S.E., Wolfe R.R. (2000) An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Journal of Applied Physiology 88, 386-392 [DOI] [PubMed] [Google Scholar]
- Reed M.J., Brozinick J.T., Jr, Lee M.C., Ivy J.L. (1989) Muscle glycogen storage postexercise: effect of mode of carbohydrate administration. Journal of Applied Physiology 66, 720-726 [DOI] [PubMed] [Google Scholar]
- Roy B.D., Tarnopolsky M.A., MacDougall J.D., Fowels J., Yarasheski E. (1997) Effect of glucose supplement timing on protein metabolism after resistance training. Journal of Applied Physiology 82, 1882-1888 [DOI] [PubMed] [Google Scholar]
- Sherman W.M., Doyle J.A., Lamb D.R., Strauss R.H. (1993) Dietary carbohydrate, muscle glycogen, and exercise performance during 7 d of training. American Journal of Clinical Nutrition 57, 27-31 [DOI] [PubMed] [Google Scholar]
- Tang J.E., Manolakos J.J., Kujbida G.W., Lysecki P.J., Moore D.R., Phillips S.M. (2007) Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Applied Physiology Nutrition and Metabolism 32, 1132-1138 [DOI] [PubMed] [Google Scholar]
- Tang J. E., Moore D. R., Kujbida G. W., Tarnopolsky M. A., Phillips S. M. (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology 107, 987-992 [DOI] [PubMed] [Google Scholar]
- Tarnopolsky M.A., Bosman M., Macdonald J.R., Vandeputte D., Martin J., Roy B.D. (1997) Postexercise protein-carbohydrate and carbohydrate supplements increase muscle glycogen in men and women. Journal of Applied Physiology 83, 1877-1883 [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Elliott T.A., Cree M.G., Aarsland A.A., Sanford A.P., Wolfe R.R. (2007) Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. American Journal of Physiology Endocrinology and Metabolism 292, E71-76 [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Elliott T.A., Cree M.G., Wolf S.E., Sanford A.P., Wolfe R.R. (2004) Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Medicine and Science in Sports and Exercise 36, 2073-2081 [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Elliott T.A., Ferrando A.A., Aarsland A.A., Wolfe R.R. (2009) Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Applied Physiology Nutrition and Metabolism 34, 151-161 [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Ferrando A.A., Phillips S.M., Doyle D., Jr, Wolfe R.R. (1999a) Postexercise net protein synthesis in human muscle from orally administered amino acids. American Journal of Physiology 276, E628-634 [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Ferrando A.A., Williams B.D., Wolfe R.R. (1996) Muscle protein metabolism in female swimmers after a combination of resistance and endurance exercise. Journal of Applied Physiology 81, 2034-2038 [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Gurkin B.E., Matin S., Wolfe R.R. (1999b) Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. Journal of Nutritional Biochemistry 10, 89-95 [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Rasmussen B.B., Miller S.L., Wolf S.E., Owens-Stovall S.K., Petrini B.E., Wolfe R.R. (2001) Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. American Journal of Physiology Endocrinology and Metabolism 281, E197-206 [DOI] [PubMed] [Google Scholar]
- van Hall G., Shirreffs S.M., Calbet J.A. (2000) Muscle glycogen resynthesis during recovery from cycle exercise: no effect of additional protein ingestion. Journal of Applied Physiology 88, 1631-1636 [DOI] [PubMed] [Google Scholar]
- van Loon L.J., Saris W.H., Kruijshoop M., Wagenmakers A.J. (2000) Maximizing postexercise muscle glycogen synthesis: carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. American Journal of Clinical Nutrition 772, 106-111 [DOI] [PubMed] [Google Scholar]
- Volpi E., Ferrando A.A., Yeckel C.W., Tipton K.D., Wolfe R.R. (1998) Exogenous amino acids stimulate net muscle protein synthesis in the elderly. Journal of Clinical Investigation 101, 2000-2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E., Kobayashi H., Sheffield-Moore M., Mittendorfer B., Wolfe R.R. (2003) Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. American Journal of Clinical Nutrition 78, 250-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E., Mittendorfer B., Rasmussen B.B., Wolfe R.R. (2000) The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. Journal of Clinical Endocrinology and Metabolism 85, 4481-4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis G.A., Hulston C.J., Mann C.H., Roper H.P., Tipton K.D., Jeukendrup A.E. (2008) Postexercise muscle glycogen synthesis with combined glucose and fructose ingestion. Medicine and Science in Sports and Exercise 40, 1789-1794 [DOI] [PubMed] [Google Scholar]
- Wilkinson S.B., Tarnopolsky M.A., Macdonald M.J., Macdonald J.R., Armstrong D., Phillips S.M. (2007) Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. American Journal of Clinical Nutrition 85, 1031-1040 [DOI] [PubMed] [Google Scholar]
- Willoughby D.S., Stout J.R., Wilborn C.D. (2007) Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 32, 467-477 [DOI] [PubMed] [Google Scholar]
- Wolever T.M., Jenkins D.J., Jenkins A.L., Josse R.G. (1991) The glycemic index: methodology and clinical implications. American Journal of Clinical Nutrition 54, 846-854 [DOI] [PubMed] [Google Scholar]
- Yaspelkis B.B., 3rd, Ivy J.L. (1999) The effect of a carbohydrate--arginine supplement on postexercise carbohydrate metabolism. International Journal of Sports Nutrition 9, 241-250 [DOI] [PubMed] [Google Scholar]
- Zachwieja J.J., Costill D.L., Pascoe D.D., Robergs R.A., Fink W.J. (1991) Influence of muscle glycogen depletion on the rate of resynthesis. Medicine and Science in Sports and Exercise 23, 44-48 [PubMed] [Google Scholar]
- Zawadzki K.M., Yaspelkis B.B., 3rd., Ivy J.L. (1992) Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. Journal of Applied Physiology 72, 1854-1859 [DOI] [PubMed] [Google Scholar]


