Abstract
The effects of magnesium supplementation on blood pressure (BP) have been studied for over 25 years and results have been inconsistent. Blood pressure reductions in randomized studies have varied from 12 mmHg reductions to no reduction. The objective of this pilot intervention was to investigate the effect of magnesium supplementation on systolic blood pressure whilst resting and during recovery from aerobic and resistance exercise and on performance. A further objective was to see whether the effect of a high vs low habitual dietary magnesium intake affected these results. Sixteen male volunteers were randomly assigned to either a 300 mg·d-1 magnesium oxide supplementation (MO) or a control group (CG) for 14 days. Resting blood pressure (BP) and heart rate (HR) were measured before subjects performed a maximal 30 minute cycle, immediately followed by three x 5 second isometric bench press, both at baseline and after the intervention. Blood pressure and heart rate were recorded immediately post exercise and after five minutes recovery. A 3 day food diary was recorded for all subjects to measure dietary magnesium intake. At the end of the intervention, the supplemented group, had a reduction in mean resting systolic BP by 8.9 mmHg (115.125 ± 9.46 mmHg, p = 0.01) and post exercise by 13 mmHg (122.625 ± 9. 88 mmHg, p = 0.01). Recovery BP was 11.9 mmHg lower in the intervention group compared to control (p = 0.006) and HR decreased by 7 beats per minute in the experimental group (69.0 ± 11.6 bpm, p = 0. 02). Performance indicators did not change within and between the groups. Habitual dietary magnesium intake affected both resting and post exercise systolic BP and the subsequent effect of the magnesium supplementation. These results have an implication in a health setting and for health and exercise but not performance.
Key points.
Magnesium supplementation will have an effect on resting and recovery systolic blood pressure with aerobic exercise.
Magnesium supplementation will have an effect on resting and recovery systolic blood pressure with resistance exercise.
Magnesium supplementation did not have an effect on performance indicators.
A low habitual dietary magnesium intake will negatively affect blood pressure.
A high habitual dietary magnesium intake will impact on the effect of magnesium supplementation.
Key words: Blood pressure, magnesium supplementation, aerobic performance, isometric contraction, recovery, dietary magnesium.
Introduction
Magnesium, one of the most abundant minerals in the body, is essential for over 300 biochemical processes and plays important roles in activating cellular enzymatic activity, such as those needed to synthesize DNA and RNA, and also in metabolism (Musso, 2009). For athletes it is important because of its involvement in glycolysis, the citric acid cycle and creatine phosphate production. It has also been suggested that free magnesium levels can affect excitation contraction coupling of the myocardium (Michailova et al., 2004). Magnesium has been consistently linked with a reduction in blood pressure within both a clinically hypertensive population and a normotensive population (Itoh et al., 1997). Meta-analyses of randomized studies by Jee et al., 2002 and Kass et al., 2012 on the effects of magnesium on BP shows dose dependent reductions. A study by Itoh et al., 1997 on 33 normotensive patients showed that magnesium lowered BP after 4 weeks. The authors attributed the reduction to the excretion of sodium in urine which acts as a relaxant to the blood vessels. However, Doyle et al., 1999 performed a study on healthy females and found no reduction in BP with increased magnesium intake after 4 weeks.
Exercise causes magnesium levels in the body to be depleted through losses in sweat, urine and alterations in the blood magnesium levels (Rayssiguier et al., 1990). An athlete will therefore have a higher requirement for daily magnesium intake than the sedentary population. Athletes on calorie restricted diets may also be at risk of deficiency.
Evidence shows that exercise causes a redistribution of magnesium to the active sites in the body (Nielsen and Lukaski, 2006), and that deficiency of the mineral negatively affects performance (Newhouse and Finstad, 2000). However, there has been little evidence to show that magnesium supplementation in adults with adequate magnesium intake will increase performance (Laires and Monteiro, 2007).
There are two suggested mechanisms for the effect of magnesium on systolic BP at rest. One mechanism is that magnesium acts as a driving force of the sodium potassium pump in the cell membrane and mobilizes more sodium to be excreted (Bara et al., 1993). Reduction of intracellular sodium may cause the smooth muscle cells in the vascular walls to relax and BP to reduce. Another suggested mechanism is that magnesium acts as a calcium channel blocker (Touyz, 2004), working as a relaxant of the smooth muscle in blood vessels, increasing arterial compliance and reducing BP.
There have been suggestions of an inverse relationship between daily dietary magnesium intake and BP (Kawano et al., 1998; Ma et al., 1995), however, dietary magnesium is rarely assessed in the studies reviewed and no large studies have looked at the impact of dietary magnesium on BP in hypertensive patients. Common foods which contain magnesium are grains such as buckwheat flour and oat bran, vegetables such as artichoke, spinach and black and white beans and nuts for example almonds and brazils. It should be considered however that the daily dietary intake of magnesium in Western society has been declining from about 500 mg·day-1 in the 1900’s to a value closer to 175 mg·day-1 (Altura, 1994), increasing the likelihood of an individual being deficient in magnesium. This figure falls someway short of the current UK RNI outlined by the UK Food Standards Agency, (2003) of 300 mg·day-1 for men and 270 mg· day-1 for women (12.35 mmol and 11.10 mmol).
To the best of the authors’ knowledge, no studies have addressed how magnesium impacts maximal aerobic exercise and performance in healthy individuals. Further there are no studies which look at the effect of magnesium supplementation in those with a high vs low habitual dietary magnesium intake.
Although reviews have shown a reduction in blood pressure with magnesium supplementation, (Jee et al., 2002; Kass et al., 2012) there is limited research on the effect of supplementation on BP during exercise or on performance. It is important for magnesium levels to stay high during recovery to affect cellular metabolism and protein synthesis (Groff and Gropper, 2000). Therefore low magnesium levels may impair muscle recovery and BP during and post exercise. Normal BP response post exercise is rapid hypotension (Orri et al., 2004). However, this may differ in response to magnesium supplementation and in the extent of hypotension.
The aims of this pilot investigation were to determine 1) the relationship between magnesium supplementation and systolic BP at rest and post exercise and its effect on performance, 2) to determine whether a high or low dietary magnesium intake impacts on these results. Further objectives were to investigate any changes in heart rate throughout.
Methods
Subjects
Sixteen male subjects were recruited. They were all undertaking aerobic exercise (>4 hours per week), apparently healthy and with no physical impairments. Exercise was defined as any physical activity perceived to be at 13 or above on The Borg Scaling of Perceived Exertion. Fifteen subjects were white Caucasian and 1 was Asian. Subjects volunteered to take part in the project and completed a consent form and health screen. Ethical approval was received from The University of Hertfordshire, Life Science Ethics Committee. Full inclusion and exclusion criteria can be seen in Table 1.
Table 1.
Inclusion and exclusion criteria for subjects.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Male | Female |
| Between 19-24 years old | Under 19 and over 24 years of age |
| Normotensive blood pressure (110/70 mmHg - 135/85 mmHg) | Hypertensive (>135/85) |
| Exercising for minimum of 4 hours per week | Hypotensive (<110/70 mmHg) |
| Exercising less than 4 hours per week | |
| On any medication | |
| Ingested any supplement 72 hours before baseline test | |
| Suffering from any ailment or injury that affects performance | |
| Failed Health Screen |
Design
Subjects were randomized to either the supplement or the control group. Subjects in both groups were asked to come in for familiarization and then twice for testing over a 4 week period, with only the experimental group taking the magnesium supplementation and the control group undertaking the same tests but with no supplementation. A total of 3 visits to the laboratory were required from each of the subjects.
Protocol : Familiarization and Test 1
Diet: Subjects were asked to complete a 3 day dietary recall to assess habitual magnesium dietary intake using dedicated software (Dietplan 6.50 Forestfield Software Ltd., West Sussex, UK). Dietary intake was categorized into high and low categories based on the Committee on Medical Aspects of Food and Nutrition Policy (COMA, 1991) calculated a Reference Nutrient Intake (RNI) of 300 mg/day for adult males (<300 mg·d-1 and > 300 mg·d-1).
Supplementation: Supplements consisted of a vegetarian capsules filled with 150mg of medical grade Magnesium Oxide Light 13138 (Sigma-Aldrich, Missouri, USA). Each capsule was individually weighed (Ohaus adventurer balance AR1530 New Jersey, USA), Subjects were instructed to take two tablets per day resulting in a total of 300mg per day per subject, one in the morning on waking, and at the end of the day for a total of 14 days.
Exercise familiarization: On the first visit to the laboratory subjects were shown the equipment and the full protocol was carried out as described below in order that the subjects were able to familiarize themselves with the process. They were then invited back to the laboratory in a time frame of 3-7 days after familiarization had taken place for the protocol to be repeated and baseline measurements recorded. Supplementation was distributed after baseline measurements were recorded. The protocol was repeated 14 days after baseline measurements had been taken.
Exercise protocol: Following 10 minutes of silent rest, a 3 minute warm up at self-selected pace was undertaken on the cycle ergometer (Monark Ergomedic 874 E Cycle Ergometer, Monark Sports and Medical, Sweden), with 1kg load, with heart rate kept below 140bpm. A Polar T31 transmitter (Polar Electro Oy, Kempele, Finland), measured heart rate throughout all exercise. Subjects then cycled on the cycle ergometer with a 1kg load for 30 minutes at their maximal capacity in order to achieve the greatest distance in the allocated time. After completion of the 30 minute maximal cycle a two minutes rest was given with subjects being advised to continue to pedal for at least 60 seconds of the two minute interval at self-selected intensity that was lower than that undertaken during the 30 minute trial. After the two minute interval, 3 x 5 second maximal isometric bench press contractions were completed, each separated by a 30 seconds rest. The isometric contraction was undertaken with the subject supine on a bench and pushing maximally against a static metal bar on the Marcy Smith Machine Plus, (Marcy Fitness Products, Ontario, Canada) and measured by a Digital Analyser Isometric Transducer (MIE Medical Research Ltd., Yorkshire, UK). The 3 isometric bench press weights were averaged and percentage change calculated between baseline and week 2. As subjects differed in size, bench press output results could be skewed due to the different size of the levers. Therefore, the joint angle of the upper arm and shoulder was standardized to 40°.
Measurements pre and post-exercise from rest to recovery: After 10 minutes of silent rest, BP was measured from the right arm (Omron MX3, Omron Healthcare, Kyoto, Japan). At timepoint 15 minutes, during the 30 minute maximal cycle, gas was analyzed for O2 and CO2 using Cortex Biophysik Metalyser 3B (Leipzig, Germany) with Metasoft 3.9.2 software. Gas was collected again at the 30 minute time mark and respiratory exchange ratio (RER) calculated. After completing all three of the isometric contractions, BP was measured again. A 5 minute seated recovery period then took place with subjects having a final BP and heart rate measurement taken at this time.
Heart rate (HR) was measured pre and post- exercise and recorded 4 times throughout the protocol. Total HR recordings were at Pre-exercise at rest (RHR), after 15 minutes cycling (HR15), at the end of Exercise (HR30), and post-exercise, after 5 minutes recovery (RecHR).
Test 2
After 14 days both groups repeated the exercise protocol, with the same measurements being taken. Subjects came in on the same time of day, and the same tester was present during both tests for all subjects.
Data analysis
Food diaries were input into Dietplan 6 (Dietplan 6.50 Forestfield Software Ltd., West Sussex, UK) and subjects were stratified into high and low habitual dietary magnesium intake based on these results. Data were then analyzed for the cohort as a whole and again stratified for low and high habitual dietary intake. All data were analyzed using Excel and SPSS7 (PASW Statistics v17.0.2 IBM, New York, USA) and tested for normality using a Shapiro-Wilk test. As the data were normally distributed, independent t-tests were performed between groups, and dependent paired t- tests between tests to assess significance. The alpha value was set at 0.5.
Results
Sixteen male subjects were recruited (age 20.88 ± 1.82 yrs, height 1.80 ± 0.07 m, weight 73.91 ± 12.49 kg, resting systolic blood pressure 124.63 ± 6.05mmHg). Baseline characteristics were further broken down into the control and experimental group (Table 2). No statistical significance difference was found between the groups.
Table 2.
Subject characteristics (n=16). Values are means (± Standard Deviation).
| Control (n = 8) | Experimental (n = 8) | All (n = 16) | |
|---|---|---|---|
| Age (years) | 21.38 (1.60) | 20.38 (2.00) | 20.88 (1.82) |
| Height (m) | 1.83 (.04) | 1.78 (.08) | 1.80 (.07) |
| Weight (kg) | 74.44 (10.30) | 73.39 (15.01) | 73.91 (12.49) |
| RHR (bpm) | 74 (12) | 76 (12) | 75 (12) |
| RSBP (mmHg) | 125.25 (5.06) | 122.75 (7.09) | 124.63 (6.05) |
| RDBP (mmHg) | 70.75 (7.09) | 71.13 (10.06) | 71.00 (8.42) |
RHR = Resting heart rate, RSBP = Resting systolic blood pressure, RDBP = Resting diastolic blood pressure.
In the supplemental group three subjects were above the RDA (300 mg·d-1) and 5 were below. The control group had an even amount of subjects above and below the RDA.
Blood pressure
At baseline there were no significant differences between the groups in blood pressure at any of the time points (Table 3). Post intervention there was as a significant difference between the control and the supplemented group for resting pre- exercise, post and recovery blood pressure. A significant difference from baseline was also seen in the supplemented group for resting pre-exercise and post exercise blood pressure after 2 weeks supplementation.
Table 3.
Summary of mean blood pressure values, at baseline and week 2 for both subject groups. Values are means (± Standard Deviation).
| Baseline | Follow-up | |||
|---|---|---|---|---|
| Measurements | Control | Supplement | Control | Supplement |
| RSBP (mmHg) | 125.3 (5.1) | 124.0 (7.2) | 124.5 (3.9) | 115.1 (9.5) *† |
| Post SBP (mmHg) | 136.3 (4.3) | 135.6 (15.3) | 139.1 (4.5) | 122.6 (9.9) *† |
| Rec SBP (mmHg) | 117.5 (8.5) | 118.1 (16.2) | 121.6 (6.1) | 110.4 (9.2) † |
| RHR (bpm) | 74 (12) | 76 (15) | 68 (12) | 69 (12) |
| HR15 (bpm) | 155 (20) | 152 (24) | 163 (16) | 171 (11) |
| HR30 (bpm) | 180 (12) | 187 (13) | 186 (13) | 187 (12) |
| RecHR (bpm) | 102 (15) | 101 (16) | 100 (16) | 106 (11) |
* indicates significance at week 2 compared to baseline (p < 0.05).
† indicates signifincance between control and experimental groups at week 2 (p < 0.05). RSBP - Resting systolic blood pressure. PostSBP - Post exercise systolic blood pressure. RecSBP - Systolic blood pressure after 5 minutes recovery. RHR - Resting heart rate. HR15 - Heart rate at 15 minutes cycling. HR30 - Heart rate at 30 minutes cycling. HR30 - Heart rate at 30 minutes cycling. RecHR - Heart rate after 5 minutes recovery
RSBP decreased by 7.7% post intervention in the experimental group compared to baseline (p = 0.017) and inter-group between experimental and control post intervention (p = 0.014). Post SBP decreased by 10.6% at follow-up in the experimental group compared to baseline (p = 0.017) and between experimental and control groups at follow up (p = 0.000) (Table 3).
Rec SBP decreased by 7.0% at follow-up in the experimental group compared to baseline (p = 0.006) although no significance was found for Rec SBP at follow-up between the baseline and experimental group (Table 3).
Habitual dietary magnesium intake
There was no significant difference found for habitual magnesium intake between control and experimental subjects (p = 0.18). When stratified by dietary magnesium intake, the supplemented group showed significance in resting systolic BP at week 2 compared to baseline for the low habitual dietary magensium intake (114.0 ± 11.6 mmHg, p = 0.02) (Figure 1) and in the high magnesium intake group post exercise (120.7 ± 1.5 mmHg, p = 0.01) (Figure 2). The control group showed no significant change in either resting or post exercise SBP at week 2 compared to baseline (Figures 1 and 2).
Figure 1.
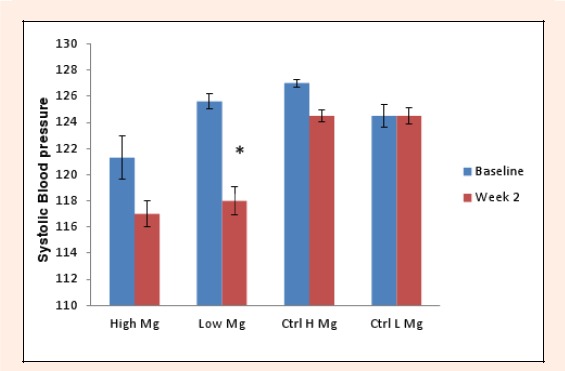
Mean resting systolic blood pressure change in the experimental and control groups split into high (≥300mg) and low (<300mg) magnesium intake subgroups between baseline and week 2.
Figure 2.
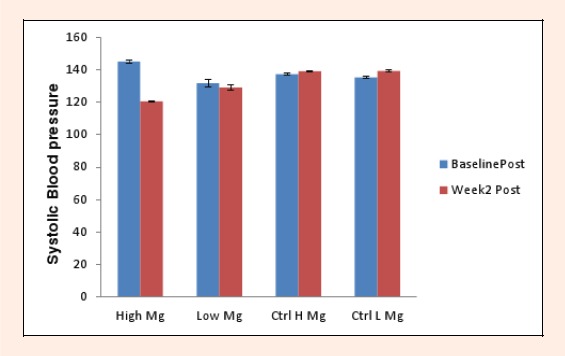
Mean post exercise systolic blood pressure change in the experimental and control groups split into high (≥300mg) and low (<300mg) magnesium intake subgroups between baseline and week 2.
However, significance was found in resting systolic BP at week 2 compared to baseline in the low magensium intake group (114.00 ± 11.60 mmHg, p = 0.02) within the intervention group.
Performance
Cycling time trial distance increased from baseline to week 2 significantly in both groups, however this increased distance was lower at week 2 in the supplemented group compared to the control group. In the experimental group baseline distance was 20.5 ± 2.5 Km and after the intervention increased to 22.7 ± 2.4Km an increase of 10.6%. However, in the control group baseline distance was 19.4 ± 1.8 Km and at the end of the trial was 22.1 ± 2.3 Km, an increase of 14.1%.
The 3 isometric bench press weights were averaged and percentage change calculated between baseline and week 2. Average strength increased by 11.4% in the control group, from 24.2 ± 8.67 kg to 26.9 ± 8. 08 kg and decreased by 18.0% in the experimental group from 35.50 ± 16.72 kg to 29.0 ± 10.88 kg. No significant changes were shown.
Heart rate and RER
No significant difference was found between the control group and the experimental group at any time point. For the RER at the 30 minute time point the intervention group changed from 1.14 ± 0.13 to 1.10 ± 0.07 from baseline to week 2 and the control group from 1.06 ± 0.03 to 1.11 ± 0.02 from baseline to week 2. No significance change was shown. For heart rate, as expected, significance was found timewise in the change from resting to recovery heart rate and throughout the 30 minute cycle in both the control and supplemented group (Table 3).
Discussion
In this pilot study resting, post exercise and recovery systolic BP significantly decreased from baseline to week 2 for the magnesium supplemented group when compared to the control group. This is consistent with other studies that have reported significant reductions of 4.1 mmHg and 5 mmHg respectively for systolic BP (Itoh et al., 1997; Kawano et al., 1998).
Different to most supplementation studies, this pilot investigation looked at whether habitual magnesium intake influenced the BP results at both resting and post exercise conditions. The data were used to stratify results for both the supplemented and control group into high and low magnesium intake. Although not the main focus for the study and the study is not powered to examine this, there is an indication that allowed consideration to be given as to whether supplementation showed the same changes in those who have a naturally high dietary intake of magnesium as to those who have a low dietary intake. In the control group for both the high and low habitual magnesium intake, resting systolic BP did not change at week 2 compared to baseline. However, a significant lowering was found in resting systolic BP at week 2 compared to baseline in the low magensium intake group within the intervention group. This suggests that magnesium supplementation may have a greater effect on resting BP in subjects that have a low habitual dietary magnesium intake than those who have a naturally higher intake above the RNI.
Sanjuliani et al., 1996 found over 10 mmHg reduction in mean BP with 600 mg·d-1 oral magnesium oxide supplement in hypertensive patients. They attributed this significant reduction to decreased intracellular sodium levels and increased magnesium levels in the blood.
Conversely, two studies found no evidence for the role of increased dietary magnesium intake in the reduction of high BP. One study was in women only (Doyle et al., 1999) and magnesium was increased through diet alone but was still below the US RDA.
Mean post exercise BP showed the opposite. The results showed mean post exercise systolic BP significantly decreased by 24.3 mmHg at week 2 when compared to baseline, in the high magnesium intake subgroup of the experimental group (20.2%, p = 0.017). This shows that magnesium supplementation has a bigger effect on post exercise BP in subjects that have a high magnesium intake. The mechanism for this is not explained in the literature. It may be due to the increased demands of magnesium for a physically active person during exercise as it is bound to ATP or that a higher plasma concentration may result in more magnesium being used as a relaxant of the smooth muscle cells in the vascular walls. This has implications in health. Patients suffering from hypertension could gain larger BP reductions from a combined dietary change and oral supplementation program.
Secondary to the main aim, this investigation set out to determine whether supplementation had an effect on aerobic and resistance performance. For the 30 minute cycling time trial the results showed a significant increase of 2.74 kilometers and 2.19 kilometers for mean cycling time trial distance in both control and experimental groups. However, there was no significance found between groups. A review by Newhouse and Finstad, 2000 supports this. They reviewed 33 years of research and concluded that most evidence shows no enhancement of performance. However, a study by Cinar et al., 2007 found that magnesium supplementation did improve performance of subjects who were exercising for 90- 120 minutes per day for 5 days a week, rather than 2 separate bouts of exercise 14 days apart. The suggested mechanism for the increase is that magnesium increases the red blood cell count and hemoglobin levels, allowing greater oxygen distribution.
For the isometric bench presses the results showed the average strength increased in the control group by 11.4% and decreased by 18. 0% in the experimental group, showing a 29.4% difference between the groups at week 2, although baseline and week 2 data were higher in the experimental group compared to control group throughout. None of the data were significant at either baseline or week 2 although a non-significant difference could be seen partly due to the fact that the experimental group had a higher baseline value than the control group. Much of the literature shows the contrary (Brilla and Haley, 1992; Domiguez et al., 2006). Reasons for this may be due to higher dosages of supplements (8 mg·kg-1) over a longer period of supplementation (7 weeks) and untrained individuals rather than active subjects being used. For resistance exercise and isometric contractions magnesium supplementation has shown to produce mixed results on performance. A study by Brilla and Haley, 1992 found a 20% strength increase of peak knee extension torque which they attributed to magnesium’s role at ribosomal level. However, a review of supplemental effects by Clarkson, 1991 identified no relationship between magnesium supplementation and performance at any dosage. Magnesium also activates amino acids and aids the attachment of mRNA to the ribosomes in protein biosynthesis (Groff and Gropper, 2000). This is important in increasing strength and protein synthesis during resistance exercise and recovery. Magnesium has been shown to enhance insulin-like growth factor 1 (IGF-1) which may elevate testosterone (Dominguez et al., 1998). Both being anabolic hormones, supplementation of magnesium may increase strength when given alongside an exercise training program.
Limitations to this pilot study included the short supplementation period of only 2 weeks and also small sample size. Although the cohort were matched for weekly physical activity levels a more robust test of fitness, such as VO2max testing was not carried out. Dietary intake was recorded for 3 days to assess habitual dietary magnesium intake. A cross-over design would also enhance the effect of the supplementation and allow for the trial to be double blinded.
Conclusion
In this pilot study, oral magnesium supplementation significantly reduces resting and post exercise BP. Furthermore, in those with a low background dietary magnesium intake there was a greater effect of supplementation on resting BP than those with a high dietary magnesium intake. Conversely, post exercise BP was reduced more in those with a high dietary magnesium intake. This pilot suggests that the effect of magnesium supplementation is relevant mainly in a health context rather than performance as no enhancements in athletic performance were found. Further research could investigate the effects of higher and longer dosages of magnesium supplementation on both aerobic and resistance exercise performance. An analysis of dietary intake looking at habitual magnesium intake in those showing resting hypertension would also aid in the understanding of magnesium and blood pressure
Acknowledgements
The authors declare that they have no conflict of interest.
Biographies

Lindsy S. Kass
Employment
Senior lecturer at University of Hertfordshire
Degree
MSc
Research interests
Exercise and health, exercise and performance, sport nutrition, magnesium
E-mail: L.s.kass@herts.ac.uk
Philip Skinner
Employment
Referrals Coordinator at The Doctors Laboratory
Degree
BSc
Research interests
Nutritional and physiological improvement on health with exercise
E-mail: phil.skinner1@gmail.com
Filipe Poeira
Employment
MSc by Research candidate employed by the University of Hertfordshire.
Degree
BSc (Hons)
Research interests
Sport performance; nutrition; magnesium and performance; health and fitness training.
E-mail: j.f.poeira2@herts.ac.uk
References
- Altura B.M. (1994) Introduction: importance of Mg in physiology and medicine and the need for ion selective electrodes. Scandinavian Journal of Clinical and Laboratory Investigation. Supplement 217, 5-9 [PubMed] [Google Scholar]
- Bara M., Guiet-Bara A, Durlach J. (1993) Regulation of sodium and potassium pathways by magnesium in cell membranes. Magnesium Research 6(2), 167-177 [PubMed] [Google Scholar]
- Bohl C.H, Volpe S.L. (2002) Magnesium and Exercise. Critical Reviews in Food Science and Nutrition 42(6), 533-563 [DOI] [PubMed] [Google Scholar]
- Brilla L.R., Haley T.F. (1992) Effect of magnesium supplementation on strength training in humans. Journal of the American College of Nutrition 11(3), 326-329 [DOI] [PubMed] [Google Scholar]
- Cinar V., Nizamlioglu M., Mogulkoc R., Baltaci A.K. (2007) Effects of magnesium supplementation on blood parameters of athletes at rest and after exercise. Biological Trace Element Research 115(3), 205-212 [DOI] [PubMed] [Google Scholar]
- Clarkson P.M. (1991) Minerals: exercise performance and supplementation in athletes. Journal of Sports Science 9, 91-116 [DOI] [PubMed] [Google Scholar]
- COMA (1991) Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values, Committee on Medical Aspects of Food and Nutrition Policy. HMSO: London: [PubMed] [Google Scholar]
- Cappuccio F.P., Markandu N.D., Beynon G.W., Shore A.C., Sampson B., MacGregor G.A. (1985) Lack of effect of oral magnesium on high blood pressure: a double blind study. British Medical Journal, 291, 235-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez L.J., Barbagallo M., Sowers J.R., Resnick L.M. (1998) Magnesium Responsiveness to Insulin and Insulin-Like Growth Factor I in Erythrocytes from Normotensive and Hypertensive Subjects. Journal of Clinical Endocrinology and Metabolism 83, 4402-4407 [DOI] [PubMed] [Google Scholar]
- Dominguez L.J., Barbagallo M., Lauretani F., Bandinelli S., Bos A., Corsi A.M., Simonsick E.M, Ferrucci L. (2006) Magnesium and muscle performance in older persons: the InCHIANTI study. American Journal of Clinical Nutrition 84(2), 419-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L., Flynn A., Cashman K. (1999) The effect of magnesium supplementation on biochemical markers of bone metabolism or blood pressure in healthy young adult females. Euopean Journal of Clinical Nutrition 53(4), 255-261 [DOI] [PubMed] [Google Scholar]
- Elin R.J. (1988) Magnesium metabolism in health and disease. Disease-a-month 34(4), 161-218 [DOI] [PubMed] [Google Scholar]
- Finstad E.W., Newhouse I.J., Lukaski H.C., McAuliffe J.E., Stewart C.R. (2001) The effect of magnesium supplementation on exercise performance. Medicine Science in Sport and Exercise 33, 493-498 [DOI] [PubMed] [Google Scholar]
- Golf S.W., Happel O., Graef V., Seim K.E. (1984) Plasma aldosterone, cortisol and electrolyte concentrations in physical exercise after magnesium supplementation. Journal of Clinical Chemistry and Clinical Biochemistry 22(11), 717-721 [DOI] [PubMed] [Google Scholar]
- Groff J.L., Gropper S.S. (2000) Advanced Nutrition and Human Metabolism. 3rd edition California: Wadsworth [Google Scholar]
- Itoh K., Kawasaki T., Nakamura M. (1997) The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Bitish Journal of Nutrition 78(5), 737-750 [DOI] [PubMed] [Google Scholar]
- Jee S.H., Miller III E.R., Guallar E., Singh V.K., Appel L.J., Klag M.J. (2002) The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. American Journal of Hypertension 15, 691-696 [DOI] [PubMed] [Google Scholar]
- Kass L., Weekes J., Carpenter L. (2012) Effect of magnesium supplementation on blood pressure: a meta-analysis. European Journal of Clinical Nutrition 66(4), 411-418 [DOI] [PubMed] [Google Scholar]
- Kawano Y., Matsuoka H., Takishita S., Omae T. (1998) Effects of magnesium supplementation in hypertensive patients. Hypertension 32(2), 260-265 [DOI] [PubMed] [Google Scholar]
- Laires M.J., Monteiro C. (2007) Exercise and magnesium. New Perspectives in Magnesium Research 14, 173-185 [Google Scholar]
- Loosli A.R., Benson J., Gillien D.M., Bourdet K. (1986) Nutrition habits and knowledge in competitive adolescent female gymnasts. Physician and Sportsmedicine 14, 118-130 [DOI] [PubMed] [Google Scholar]
- Ma J., Folsom A.R., Melnick S.L., Eckfeldt J.H., Sharrett A.R., Nabulsi A.A., Hutchinson R.G., Metcalf P.M. (1995) Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes and carotid arterial wall thickness: The ARIC study. Journal of Clinical Epidemiology 48(7), 927-940 [DOI] [PubMed] [Google Scholar]
- McDonald R., Keen C. (1988) Iron, zinc, and magnesium nutrition and athletic performance. Sports Medicine 5, 171-184 [DOI] [PubMed] [Google Scholar]
- Michailova A.P., Belik M.E., McCulloch A.D. (2004) Effects of magnesium on cardiac excitation-contraction coupling. Journal of American College of Nutrition 23(5), 514S-517S [DOI] [PubMed] [Google Scholar]
- Musso C, G. (2009) Magnesium metabolism in health and disease. International Journal of Urology and Nephrology. 41(2), 357-362 [DOI] [PubMed] [Google Scholar]
- Newhouse I.J., Finstad E.W. (2000) The Effects of Magnesium Supplementation on Exercise Performance. Clinical Journal of Sports Medicine 10(3), 195-200 [DOI] [PubMed] [Google Scholar]
- Nielsen F.H., Lukaski H.C. (2006). Update on the relationship between magnesium and exercise. Magnesium Research 19(3), 180-189 [PubMed] [Google Scholar]
- Orri J.C., Griffin S.E., Robergs R.A., James D.S., Wagner D.R., Quintana R.(2004). Intra-arterial blood pressure characteristics during submaximal cycling and recovery. Journal of Exercise Physiology 7(2), 45-53 [Google Scholar]
- Rayssiguier Y., Guezennec C.Y., Durlach J. (1990). New experimental and clinical data on the relationship between magnesium and sport. Magnesium Research 3, 93-102 [PubMed] [Google Scholar]
- Sanjuliani A.F., de Abreu Fagundes V.G., Francischetti E.A. (1996) Effects of magnesium on blood pressure and intracellular ion levels of Brazilian hypertensive patients. International Journal of Cardiology 56(2), 177-183 [DOI] [PubMed] [Google Scholar]
- Terblanche S., Noakes T.D., Dennis S.C., Marais D., Eckert M. (1992) Failure of magnesium supplementation to influence marathon running performance or recovery in magnesium-replete subjects. International Jounal of Sports Nutition 2, 154-164 [DOI] [PubMed] [Google Scholar]
- Touyz R.M. (2004). Magnesium in clinical medicine. Frontiers in Bioscience 9, 1278-1293 [DOI] [PubMed] [Google Scholar]


