Abstract
BACKGROUND
The trends in hospitalization rates and risk factors for severe bronchiolitis have not been recently described, especially after the routine implementation of prophylaxis for respiratory syncytial virus (RSV) infections.
OBJECTIVES
To define the burden of hospitalizations related to RSV and non-RSV bronchiolitis in a tertiary-care children’s hospital from 2002 to 2007 and to identify the risk factors associated with severe disease.
METHODS
Medical records of patients hospitalized for bronchiolitis were reviewed for demographic, clinical, microbiologic, and radiologic characteristics as well as the presence of underlying medical conditions. Differences were evaluated between children with RSV and non-RSV bronchiolitis, and multivariable logistic regression analyses were performed to identify independent risk factors for severe disease.
RESULTS
Bronchiolitis hospitalizations in children younger than 2 years old (n =4800) significantly increased from 536 (3.3%) in 2002 to 1241 (5.5%) in 2007, mainly because of RSV infections. Patients with RSV bronchiolitis (n = 2840 [66%]) were younger at hospitalization and had a lower percentage of underlying medical conditions than children hospitalized with non-RSV bronchiolitis (27 vs 37.5%; P < .001). However, disease severity defined by length of hospitalization and requirement of supplemental oxygen, intensive care, and mechanical ventilation was significantly worse in children with RSV bronchiolitis. RSV infection and prematurity, regardless of the etiology, were identified as independent risk factors for severe bronchiolitis.
CONCLUSIONS
There was a significant increase in hospitalizations for RSV bronchiolitis from 2002 to 2007. A majority of the children with RSV bronchiolitis were previously healthy, but their disease severity was worse compared with those hospitalized with non-RSV bronchiolitis.
Keywords: bronchiolitis, RSV, disease severity, ICD-9
Bronchiolitis is the leading cause of hospitalization of infants and young children in the United States.1 Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis in this age group and accounts for 50% to 80% of cases.1–3 Between 1980 and 2003, and for reasons that are not completely understood, there was an increase in both hospitalization rates and outpatient visits attributed to bronchiolitis.1–3
The majority of studies of bronchiolitis have been focused on the burden of RSV disease and its associated risk factors.4– 6 However, trends in hospitalization rates, epidemiology, or disease severity of bronchiolitis caused by viruses other than RSV during the first 2 years of life remain to be fully defined. Authors of previous studies3,7–10 documented the burden of viral respiratory illnesses in children younger than 5 years. In those studies, trends in hospitalization rates were not addressed. In addition, children with underlying high-risk medical conditions were excluded, which may have diluted the burden of disease in 1 of the target populations for these infections.
The aim of this study was to provide a more current estimate of the number and rate of bronchiolitis-associated hospitalizations at our institution after the implementation of anti-RSV prophylaxis and to define the differences in epidemiologic, clinical, microbiological, and radiologic features between children younger than 2 years with RSV bronchiolitis and those with bronchiolitis caused by other respiratory viruses. In addition, we examined how different risk factors influenced disease severity in children with RSV and non-RSV bronchiolitis.
METHODS
Patient Population
Children younger than 2 years who were hospitalized for bronchiolitis at Children’s Medical Center in Dallas, Texas, from January 1, 2002, to December 31, 2007, were identified via International Classification of Diseases, Ninth Revision codes with a primary diagnosis of RSV bronchiolitis (466.11) and bronchiolitis attributed to other infectious organisms (466.19). The project was approved by the institutional review board of the University of Texas Southwestern Medical Center (institutional review board No. 032008-045).
Data Collection
Medical records were reviewed for the following: (1) viral diagnostic tests performed in respiratory samples, including the rapid RSV test (enzyme immunoassay), the direct fluorescent antibody (DFA) test, and viral culture; (2) demographic and epidemiologic characteristics including age, gender, race/ethnic group, gestational age, weight at hospitalization, year and month of hospitalization, and the presence of underlying medical conditions including prematurity, congenital heart disease (CHD), chronic lung disease (CLD), trisomy 21, congenital or acquired immunodeficiencies, cystic fibrosis, neuromuscular disorders, presence of other congenital abnormalities, and preexisting respiratory tract morbidity; (3) outcomes of care or disease-severity parameters including length of stay, requirement and duration of supplemental oxygen, admission to and length of stay in the PICU, need and length of mechanical ventilation, and mortality11,12; (4) other microbiological diagnostic tests performed including blood, urine, and cerebrospinal fluid bacterial cultures. Serious bacterial infection was defined as bacteremia, bacterial meningitis, or urinary tract infection in children younger than 3 months and as bacteremia or bacterial meningitis in children older than 3 months13; and (5) chest radiographic findings, which were grouped into 7 different categories: (a) no pathologic findings; (b) bronchial wall thickening; (c) interstitial markings and/or atelectasis; (d) focal opacity; (e) pleural effusion; (f) focal opacity with pleural effusion and; (g) other findings.
Statistical Analysis
Descriptive Analysis
Descriptive analyses were performed by using frequency distributions or rates. Means (SD) or medians (25th–75th percentiles) were used to summarize the demographic data and patients’ baseline characteristics. The χ2 test for trends was used to determine significant changes in hospitalization rates over time.
Bivariate Analysis
Associations between categorical and continuous variables were analyzed by using the χ2 tests with Yates correction for continuity or Fisher’s exact tests and the 2-tailed Student’s t or Wilcoxon rank-sum tests as appropriate.
Multivariable Analysis
We performed multivariable analyses to determine which factors independently predicted the risk of severe disease. We selected the following as primary outcomes: supplemental oxygen; PICU and intubation requirement; and length of hospitalization. Statistical models were built by using multivariable logistic regression for binary outcome variables (supplemental oxygen, PICU, and intubation requirement) and linear regression models for the continuous outcome length of hospitalization. Three independent predictors were considered for the models: (a) group (RSV or non-RSV); (b) age at hospitalization (months), gender, race, and weight (kg); and (c) the presence of underlying medical conditions (prematurity, CHD, CLD, trisomy 21, congenital abnormalities, neuromuscular disorders, and preexisting respiratory tract morbidity). Multivariable logistic regression analysis was performed by constructing a full stepwise sequence, and the final model was selected on the basis of the Akaike criteria.14,15 Because the extremely skewed distribution of length of stay, multivariable linear regression was performed after log transformation and restricted to cases with values within 3 SDs of the mean of log-transformed length of stay (only 25 cases of 4285 were excluded).16,17 The final regression model was selected by using the backward-elimination method. Association of predictors with supplemental oxygen, PICU, and intubation requirement is displayed using by odds ratios and 95% CIs. Associations of risk factors with length of stay were displayed as ratios and 95% CIs, which represent the antilog of the regression parameter estimates and confidence limits. Predictor variables with a P value of <.05 and multivariate odds ratios and 95% CIs that did not include 1 were considered significant. Statistical analyses were performed by using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Bronchiolitis Hospitalizations, Viral Etiology, and Seasonality
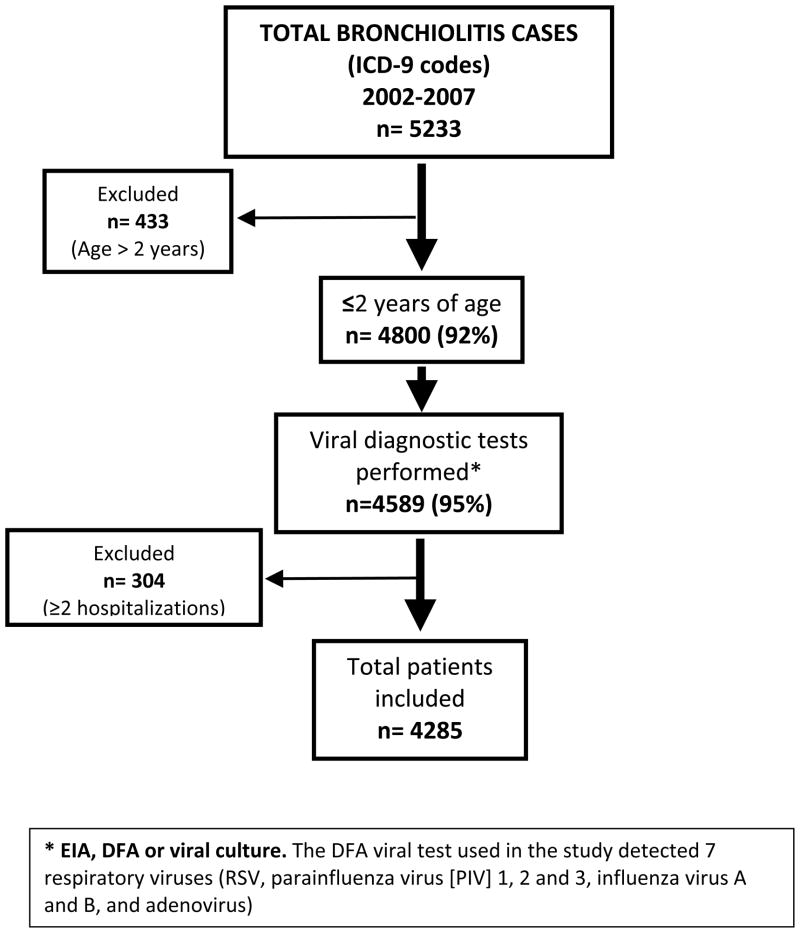
From January 1, 2002, to December 31, 2007, 5233 hospitalizations were attributed to bronchiolitis. Of those hospitalizations, 4800 (92%) occurred in children younger than 2 years and 4589 (95%) had a viral diagnostic test performed, which included rapid antigen tests for 1650 (35%), DFA test for 3559 (77%), and viral culture for 1766 (38%) subjects. Per the hospital’s policy, samples with negative rapid-test results were automatically tested by using a DFA assay, and samples that tested negative by rapid test and/or DFA test underwent viral culture. This policy was applied to 97% of the study subjects.
A total of 255 children had more than 1 hospitalization, accounting for 304 hospitalizations (6%). Only the first hospitalization was considered for subsequent analysis, for a total of 4285 patients younger than 2 years with a viral test performed and a single hospitalization (Fig 1). Overall, a respiratory virus was identified in 73% of the cases. The virus most commonly identified was RSV (66% [n = 2840]), followed by parainfluenza virus (3%), rhinovirus (3%), adenovirus (1%), and influenza virus A and B in less than 1% of the cases. In 1170 (27%) cases, a viral test was performed and no etio-logic agent was identified. More than 1 virus was identified in 24 (0.5%) patients. The most frequent association was RSV and rhinovirus. We referred to the group of patients with bronchiolitis caused by viruses other than RSV and those with negative viral testing results as the non-RSV bronchiolitis group.
FIGURE 1.
Flow diagram of selection of study patients. From 2002 to 2007, 5233 hospitalizations were attributed to bronchiolitis at Children’s Medical Center Dallas. A total of 92% occurred in children younger than 2 years, and 95% had a viral test performed. Only the first hospitalization was considered for subsequent analysis, which left a total of 4285 unique patients. ICD-9 indicates International Classification of Diseases, Ninth Revision. a Enzyme immunoassay, DFA, or viral cultures. The DFA viral test used in the study detected 7 respiratory viruses (RSV, para-influenza virus 1, 2, and 3, influenza virus A and B, and adenovirus).
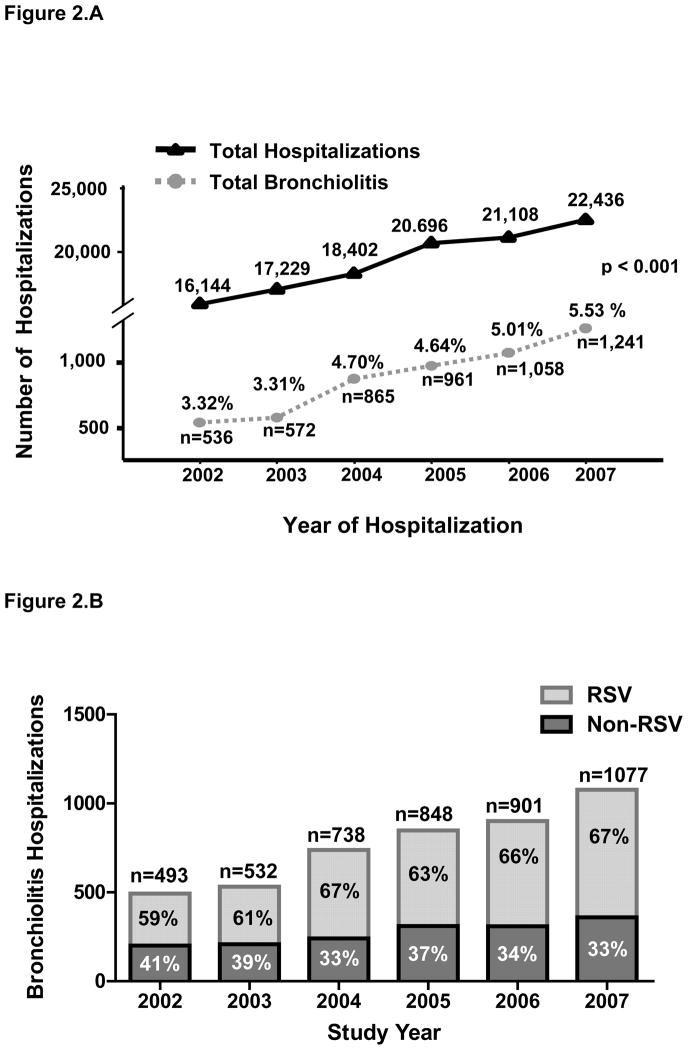
The total number and percentage of yearly hospitalizations for bronchiolitis significantly increased from 536 (3.3%) in 2002 to 1241 (5.5%) in 2007 (Fig 2A). Whereas hospitalizations attributed to RSV bronchiolitis increased from 59% (292 cases) in 2002 to 67% (718 cases) in 2007 (P < .01), the percentage of bronchiolitis hospitalizations in the non-RSV group decreased from 41% in 2002 (201 cases) to 33% in 2007 (359 cases) (P = .003) (Fig 2B).
FIGURE 2.
Bronchiolitis hospitalizations at CMCD. A, Bronchiolitis hospitalizations significantly increased from 2002 to 2007. The x-axis represents each study year. The y-axis reflects the total number of hospital admissions and the total number and percentage of bronchiolitis hospitalizations, which significantly increased from 2002 to 2007 (P < .001; χ2 for trends). B, RSV and non-RSV bronchiolitis hospitalizations per year from 2002 to 2007 at Children’s Medical Center Dallas. Although the proportion of children hospitalized with non-RSV bronchiolitis decreased from 2002 to 2007, hospitalizations attributed to RSV significantly increased from 2002 to 2007 (P < .01).
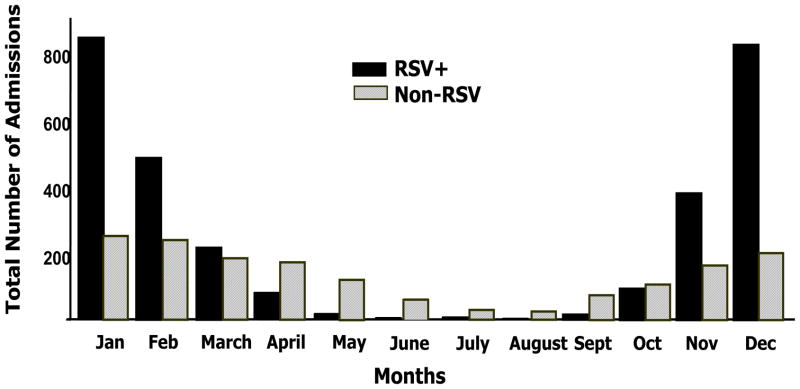
RSV hospitalizations began at the end of October or November, peaked in December and January, and ended in March or April, except for 2003 (which was a late RSV season), in which they peaked in January and February. A small number of RSV cases were diagnosed off-season throughout the study. Cases of non-RSV bronchiolitis were diagnosed all year round and peaked from November to April, but compared with RSV hospitalizations they were proportionally more common from May to October (Fig 3). Overall, the variability in the number of hospitalizations per month was greater in the RSV group (~5% between years) than in non-RSV bronchiolitis (<3%) (Supplemental Fig 5).
FIGURE 3.
Monthly distribution of bronchiolitis hospitalizations. The horizontal (x) axis represents the months of the year for RSV (■) and non-RSV bronchiolitis (gray dashed bars) hospitalizations; the vertical axis displays the aggregates for each month (total number of cases identified per month) during the study period.
Demographic Characteristics and Risk Factors
First, we compared the baseline demographic characteristics and risk factors between patients with RSV and non-RSV bronchiolitis. The mean (±SD) gestational age in children hospitalized for RSV was significantly higher than in children with non-RSV infections (38.3 ±3.0 vs 37.4 ± 4.3 weeks). However, patients with RSV bronchiolitis were younger than patients with non-RSV bronchiolitis (6.3 vs 8.0 months, respectively; P < .001). Eighty-three percent of the children with RSV bronchiolitis were 12 months of age or younger versus 74% of those in the non-RSV group (P < .001). Male was the predominant gender, and Hispanic was the predominant ethnic population in both groups, which likely reflects the geographic location of the study (Table 1).
TABLE 1.
Demographic Characteristics and Risk Factors of Children Younger Than 2 Years of Age Hospitalized With RSV and Non-RSV Bronchiolitis
| RSV Bronchiolitis (N = 2840) | Non-RSV Bronchiolitis (N = 1445) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Gestational age, mean ± SD, wk | 38.3 ±3.4 | 37.4 ±4.3 | <.001 |
| Weight, mean ± SD, kg | 7.0 ±2.6 | 7.6 ±2.8 | <.001 |
| Age, mean ± SD, mo | 6.3 ±5.6 | 8.0 ±6.0 | <.001 |
| Gender, n (%) | .314 | ||
| Male | 1619 (57.01) | 847 (58.62) | |
| Female | 1221 (42.99) | 598 (41.38) | |
| Race or ethnic group, n (%) | .009 | ||
| White | 643 (22.64)a | 278 (19.24) | |
| Black | 540 (19.01) | 319 (22.08)a | |
| Hispanic | 1504 (52.96) | 784 (54.26) | |
| Other | 153 (5.39) | 64 (4.43) | |
| Risk factors, n (%) | |||
| Prematurity, gestational age at birth, wk | 580 (20.42) | 418 (28.93) | <.001 |
| ≤28 | 71 (2.50) | 81 (5.61) | |
| 29–32 | 81 (2.85) | 85 (5.88) | |
| 32–35 | 194 (6.83) | 112 (7.75) | |
| 35–37 | 234 (8.24) | 140 (9.69) | |
| Congenital heart disease | 91 (3.20) | 78 (5.40) | <.001 |
| Chronic lung disease | 58 (2.04) | 77 (5.33) | <.001 |
| Trisomy 21 | 34 (1.20) | 34 (2.35) | .004 |
| Congenital syndromesb | 46 (1.62) | 32 (2.21) | .280c |
| Immunodeficiencies | 7 (0.25) | 5 (0.35) | .761c |
| Cystic fibrosis | 2 (0.007) | 1 (0.07) | .000c |
| Neuromuscular disorders | 34 (1.20) | 23 (1.59) | .287 |
| Respiratory tract morbidityd | 53 (1.87) | 34 (2.35) | .286 |
| No known risk factors | 2074 (73.02) | 903 (62.49) | <.001 |
RSV bronchiolitis was significantly more frequent in white children, and non-RSV bronchiolitis was significantly more frequent in black children.
Congenital syndromes: Prader-Willi syndrome, trisomy 18, VACTERL association, Shwachman-Diamond syndrome, Noonan and Goldenhar syndromes, hemoglobinopathies, congenital lymphedema, congenital hypothyroidism, and Kasabach-Merritt syndrome.
Exact test.
Respiratory tract morbidity included the following: neonatal intubation of 10 or more days for meconium aspiration syndrome, gastroschisis, congenital diaphragmatic hernia, tracheoesophageal fistula, pulmonary hypertension, and spontaneous pneumothorax and upper airway abnormalities including laryngo/tracheomalacia, subglottic stenosis, cleft palate, and Pierre-Robin sequence.
Second, we analyzed the presence of different risk factors that have been associated with severe RSV disease.2 The majority of children with RSV bronchiolitis were previously healthy with no risk factors identified. Indeed, the proportion of children hospitalized with RSV bronchiolitis with previously known risk factors was significantly lower than in children with non-RSV bronchiolitis (27 vs 37.5%; P <.001). Children hospitalized with non-RSV bronchiolitis had a significantly higher prevalence of CHD, CLD, trisomy 21, and prematurity (Table 1). In addition, the proportion of infants aged 32 weeks or younger hospitalized with RSV bronchiolitis was significantly lower than in the non-RSV group (5.4 vs 11.5%; P <.001), which possibly reflects the effect of anti-RSV prophylaxis. On the other hand, the proportion of infants born at 32 to 35 weeks’ gestation was similar between groups (7%), but the age at hospitalization was significantly lower for children with RSV versus non-RSV bronchiolitis (4 vs 8 months; P ≤ .001). Other risk factors such as cystic fibrosis and congenital or acquired immunodeficiencies were identified at low rates and were comparable between groups.
Microbiological and Radiologic Evaluations
Among 4285 patients, equal proportions of RSV and non-RSV bronchiolitis (11% in each group) underwent full sepsis evaluation (blood, urine, and cerebrospinal fluid cultures). A partial sepsis evaluation (blood culture, with or without urine culture) was performed for 1651 patients (RSV, n = 1110 [39%] vs non-RSV bronchiolitis, n = 541 [38%]; P = .56). The overall frequency of serious bacterial infection in children hospitalized with bronchiolitis was low: 32 (1%) cases in the RSV group versus 10 (0.7%) cases in children with non-RSV bronchiolitis (P = .23). Urine cultures were performed for 1423 (33%) patients. Children with RSV bronchiolitis had urine cultures performed more frequently than those with non-RSV bronchiolitis (34 vs 30%, respectively; P =.002), but concomitant urinary tract infections were diagnosed at a similar frequency in both groups (6 vs 4.8% in RSV versus non-RSV bronchiolitis, respectively). Cerebrospinal fluid cultures were negative for all patients. The pathogens identified are displayed in Table 2.
TABLE 2.
Sepsis Evaluation and Pathogens Identified in Hospitalized Children With Bronchiolitis
| Sepsis Evaluation, n Positive Cultures/n Total (%) | RSV Bronchiolitis (N = 2840) | Non-RSV Bronchiolitis (N = 1445) | P |
|---|---|---|---|
| Blood culture | 10/1421 (0.70) | 6/702 (0.85) | .79a |
| Urine culture | 60/985 (6.09) | 25/438 (4.79) | .39a |
| Cerebrospinal fluid culture | 0/321 (0.00) | 0/164 (0.00) | — |
Data represent the number of children with positive culture/total number of cultures performed. Numbers in parenthesis represent the percentage of positive cultures. The pathogens most commonly isolated in blood cultures were S aureus (methicillin-susceptible S aureus, n = 6; methicillin-resistant S aureus, n = 1), followed by S pneumoniae, Klebsiella spp, and group B Streptococcus (2 cases each) and group A Streptococcus, N meningitidis, and Candida spp isolated in a single case each. Other bacterial pathogens identified and classified as contaminants included coagulase-negative Staphylococci (n =54 [1.1%]), α-hemolytic Streptococcus (n =9 [0.2%]), Bacillus spp, and Corynebacterium spp. In urine cultures, E coli was the bacterial pathogen most commonly identified, followed by group D Enterococcus, Klebsiella spp, Group B Streptococcus, Proteus spp, Candida spp, and Enterobacter spp. A total of 28 urine cultures had findings suggestive of polymicrobial contamination.
Fisher exact test.
A chest radiograph was performed on 3807 (88.8%) case subjects. The radiologic pattern most commonly described for both groups was the presence of bronchial wall thickening followed by atelectasis and/or increased interstitial markings. Children with non-RSV bronchiolitis had chest radiographs performed more often and had their chest radiograph reported as normal more commonly than children with RSV bronchiolitis (P =.023). On the other hand, children with RSV bronchiolitis had a significantly higher proportion of focal opacities than those with non-RSV bronchiolitis. The radiologic findings in children with RSV and non-RSV bronchiolitis are summarized in Table 3.
TABLE 3.
Radiologic Evaluation and Patterns in Hospitalized Children With Bronchiolitis
| RSV Bronchiolitis (N = 2840) | Non-RSV Bronchiolitis (N = 1445) | P | |
|---|---|---|---|
| Radiologic evaluation | 2465 (86.79) | 1342 (92.87) | <.001 |
| No pathologic findings | 236 (9.57) | 160 (11.92) | .02 |
| Abnormal chest radiograph | 2229 (90.43)a | 1182 (88.08)a | .02 |
| Bronchial wall thickening | 1066 (43.25) | 609 (45.38) | .21 |
| Interstitial markings/atelectasis | 794 (32.21) | 418 (31.15) | .51 |
| Focal opacity | 357 (14.48) | 150 (11.18) | .002 |
| Pleural effusion | 7 (0.28) | 3 (0.22) | .80 |
| Focal opacity/pleural effusion | 4 (0.16) | 2 (0.15) | .72 |
| Other findings | 1 (0.04) | 0 (0.00) | .74 |
Numbers in parentheses represent the percentage calculated from patients who had a chest radiograph performed.
Differences in Disease Severity Between RSV and Non-RSV Bronchiolitis
To assess differences in disease severity between children with RSV and non-RSV bronchiolitis we compared (1) length of hospitalization, (2) requirement and duration of supplemental oxygen, including continuous positive airway pressure support, (3) requirement and length of stay in the PICU, and (4) need and duration of intubation. Disease severity was worse in children hospitalized with RSV bronchiolitis in all parameters evaluated. The median length of hospitalization, length of stay in the PICU, and length of intubation for children with RSV bronchiolitis were significantly longer than for those in the non-RSV group. The number of children who required supplemental oxygen, PICU care, and ventilatory support was also significantly higher in the RSV group. However, duration of supplemental oxygen was not significantly different between groups (Table 4). Because of the possibility of false-negative assay results, particularly when rapid-antigen RSV tests were used, we also compared disease severity between children with RSV bronchiolitis and those with (1) non-RSV bronchiolitis who tested negative for respiratory viruses and (2) non-RSV bronchiolitis who tested positive for other respiratory virus. These analyses confirmed that RSV bronchiolitis was more severe than non-RSV bronchiolitis, with no viruses identified in any parameters evaluated. On the other hand, when we compared RSV bronchiolitis with bronchiolitis caused by other viruses, there were differences in length of hospitalization and oxygen requirement but not in the other parameters evaluated (Supplemental Table 5).
TABLE 4.
Disease Characteristics in children Hospitalized With RSV and non-RSV Bronchiolitis
| Parameters of Disease Severity | RSV Bronchiolitis (N = 2840) | Non-RSV Bronchiolitis (N = 1445) | P |
|---|---|---|---|
| Length of stay, median (IQR [25th–75th percentile]), d | 3 (2–5) | 2 (2–4) | <.001 |
| Supplemental oxygen | |||
| Requirement, n (%) | 1600 (56.3) | 675 (46.7) | <.001 |
| Duration, median (IQR [25th–75th percentile]), d | 2 (1–4) | 2 (1–4) | .239 |
| ICU | |||
| Requirement, n (%) | 329 (11.6) | 119 (8.2) | <.001 |
| Duration, median (IQR [25th–75th percentile]), d | 4 (2–7) | 2 (1–5) | <.001 |
| Ventilatory support | |||
| Requirement, n (%) | 171 (6) | 46 (3.2) | <.001 |
| Duration, median (IQR [25th–75th percentile]), d | 6 (4–9) | 4 (1–7) | .003 |
Numbers in parentheses represent the percentage of children who required oxygen, ICU, or ventilatory support. IQR indicates interquartile range.
We documented 5 deaths (0.1%). Three fatal cases were attributed to RSV: a 3-month-old child with CHD; a 17-month-old child with Moebius syndrome; and a previously healthy 5-month-old child. Two children with non-RSV bronchiolitis died: a 17-month-old child with CHD and a 3-month-old child born at 33 weeks’ gestation who developed methicillin-resistant Staphylococcus aureus pneumonia.
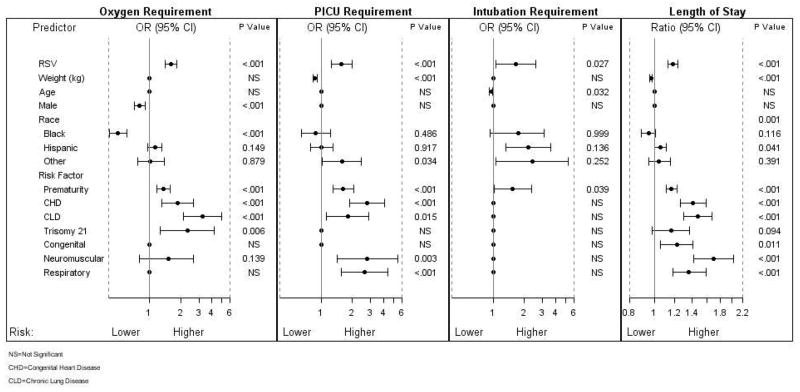
Risk Factors for Disease Severity
Several parameters were independently associated with outcomes of care regardless of the etiology of the bronchiolitis (Fig 4 and Supplemental Table 6). Of those parameters, RSV diagnosis and prematurity were found to be independent predictors for all 4 markers of disease severity.
FIGURE 4.
Odds ratios (ORs) for risk factors associated with disease severity in bronchiolitis-related hospitalizations. According to multiple logistic and multiple linear regression analyses, the independent significant risk factors associated with disease severity of oxygen, PICU, and intubation requirement and length of hospital stay are those with a P value of <.05. The reference for disease group RSV is non-RSV, for male subjects are female subjects, and for race is white race. The odds of PICU admission and intubation were inversely exponentially related to the weight and age of the patients. Given that the other risk factors are constant, for every increase (decrease) in weight by 1 kg, the odds of PICU admission decrease (increase) by 9.2% (10.2%). Similarly, for every increase (decrease) in age by 1 month, the odds of intubation decrease (increase) by 1.8% (17.9%), respectively. NS indicates not significant.
Predictors of Supplemental Oxygen Requirement
Patients with CLD, trisomy 21, CHD, RSV infection, and prematurity were more likely to require oxygen.
Predictors of PICU Admission
Children with RSV bronchiolitis had an increased risk for PICU admission compared with children with non-RSV bronchiolitis. In addition, children with CHD, neuromuscular disorders, preexisting respiratory tract morbidity, CLD, prematurity, and lower weights at hospitalization also had an increased risk for PICU admission.
Predictors of Intubation
Patients with RSV infection, younger age, and prematurity had a higher risk of requiring mechanical ventilation.
Predictors of Length of Hospitalization
Except for age and gender, all other parameters included in the model were found to be independent predictors of longer hospital stay (Fig 4 and Supplemental Table 6).
DISCUSSION
Results of our study of a large hospital-based cohort offer a comprehensive description of the burden of bronchiolitis in the inpatient setting from 2002 to 2007. The fact that 95% of children had a viral diagnostic test performed allowed us to compare the differences in demographic, clinical, microbiological, and radiologic characteristics and the presence of risk factors predictive of severe disease in children younger than 2 years with RSV and non-RSV bronchiolitis.
In agreement with previous studies conducted in the 1990s,1,4 we found that the proportion of hospitalizations for bronchiolitis significantly increased during the study period, from 3.3% in 2002 to 5.5% in 2007. Whereas the percentage of hospitalizations attributed to non-RSV bronchiolitis decreased throughout the study, those caused by RSV significantly increased during the same period and, during the last 4 years of the study, doubled the number of non-RSV bronchiolitis hospitalizations (63%–67% vs 33%–37%, respectively).
Recently, in a population-based surveillance study, Hall et al6 revealed that RSV was not only associated with substantial morbidity in inpatients, but it was also responsible for a high proportion of outpatient visits. In that study, the majority of children with RSV infection had no coexisting illness. In agreement with those observations, we found that 73% of children hospitalized with RSV infection had no underlying medical conditions. In fact, the proportion of children with underlying medical conditions was significantly higher for those with non-RSV bronchiolitis, which possibly reflects the effect of anti-RSV prophylaxis. We found several factors that independently correlated with the severity of illness regardless of the etiology of the bronchiolitis. Although prematurity, CLD, and CHD have been previously associated with more severe disease in patients with RSV infection,18–20 we found that trisomy 21, lower weights on admission, neuromuscular disorders, and RSV infection, per se, were independent predictors for severe bronchiolitis. Altogether, these findings underscore the need for an effective RSV vaccine and, in the mean time, the necessity to develop novel strategies that may allow the implementation of anti-RSV prophylaxis in broader patient populations.
In agreement with previous study results, 21,22 we found low rates of bacterial coinfections in children hospitalized with bronchiolitis. Whereas Purcell et al21 reported 1.6% positive bacterial cultures, mostly urine cultures, Oray-Schrom et al22 reported a rate of 7.2%, which was closer to our rate of 6.1% in children hospitalized with RSV bronchiolitis. These findings confirm that concurrent bacterial infections are uncommon in children with bronchiolitis and emphasize the need for viral testing to accurately diagnose these patients’ conditions. Overall, 88% of children had radiologic studies performed. In contrast to other studies,23 the most common findings identified, regardless of the etiology of the bronchiolitis, were bronchial wall thickening and atelectasis. Children with non-RSV bronchiolitis had a significantly higher frequency of chest radiographs performed than children with RSV bronchiolitis, which suggests that either children with non-RSV bronchiolitis had a more severe disease at presentation or that the diagnosis of RSV influenced the physician’s decision on whether a chest radiograph was needed.
Our study has a number of limitations. Its retrospective design has limited our ability to collect precise information on the use of anti-RSV prophylaxis, which may have provided specific data on the effectiveness of this strategy in patients at high-risk. We limited the identification of patients with bronchiolitis to 2 International Classification of Diseases, Ninth Revision codes, which may have underestimated the actual burden of the disease because patients with RSV infection may have been hospitalized with a diagnosis other than bronchiolitis, such as viral pneumonia or fever without a source. The same type of bias applied to the non-RSV bronchiolitis group, which makes the data set comparable. The data on disease severity should be interpreted with caution, because the diagnosis of RSV may have biased the management of these patients in terms of hospital admissions, lengths of stay, or even admission to the PICU. However, more objective parameters of severe disease, such as the need for supplemental oxygen or intubation, were significantly more common in children with RSV bronchiolitis. The majority of the study patients were of Hispanic background, which could limit the generalizability of the results. Nevertheless, the proportion of Hispanic subjects was comparable in the RSV and non-RSV groups (53 vs 54%, respectively), and yet children with RSV had more severe disease in all parameters evaluated. Finally, the fact that nonmolecular techniques were used for the diagnosis of non-RSV bronchiolitis limited our ability to appropriately classify these patients and warrants future studies to characterize how specific viruses influence bronchiolitis disease severity.
CONCLUSIONS
Bronchiolitis-related hospitalizations were associated with considerable morbidity in children younger than 2 years. The majority of children hospitalized with RSV bronchiolitis was previously healthy and yet had more severe disease than children hospitalized with non-RSV bronchiolitis. These data underscore the need to develop new tools for the identification of previously healthy children whose risk for severe disease cannot be predicted. They also suggest that other patient populations may benefit from novel prophylaxis strategies beyond those currently directed at the traditional high-risk groups.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT
Respiratory syncytial virus (RSV) is the leading cause of hospitalization in infants.
WHAT THIS STUDY ADDS
Hospitalization for respiratory syncytial virus increased significantly from 2002 to 2007. The majority of children with RSV bronchiolitis were previously healthy, but they had more severe disease than did chidren with non-RSV bronchiolitis.
Acknowledgments
Funded by the National Institutes of Health (NIH).
Dr Bhore was supported in part by NIH grant 1 UL1 RR024982-01, and Dr Mejias was supported in part by the RGK Foundation.
ABBREVIATIONS
- RSV
respiratory syncytial virus
- DFA
direct fluorescent antibody
- CHD
congenital heart disease
- CLD
chronic lung disease
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282(15):1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 3.Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58– 64. doi: 10.1542/peds.2007-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21(7):629–632. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 suppl):S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113(6):1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 8.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25(4):320–324. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 10.Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38(3):221–226. doi: 10.1016/j.jcv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Sheeran P, Jafri H, Carubelli C, et al. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18(2):115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Malley R, DeVincenzo J, Ramilo O, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178(6):1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro E. Fever without localizing signs. In: Long SS, editor. Principles and Practice of Pediatric Infectious Diseases. Philadelphia, PA: Churchill Livingstone, Elsevier; 2008. pp. 124–126. [Google Scholar]
- 14.Shtatland ES, Kleinman K, Cain EM. Stepwise methods in using SAS proc logistic and SAS enterprise miner for prediction. Presented at the SUGI ’28 Proceeding, Paper 258-28; Cary, NC: SAS Institute, Inc; 2003. [Google Scholar]
- 15.Shtatland ES, Kleinman K, Cain EM. SAS Institute I, editor. SUGI’29 Proceeding, Paper 191-29. Cary, NC: SAS Institute, Inc; 2004. A new strategy of model building in proc logistic with automatic variable selection, validation, shrinkage and model averaging. [Google Scholar]
- 16.Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600. doi: 10.1056/NEJMsa067735. [DOI] [PubMed] [Google Scholar]
- 17.Kulinskaya E. Length of stay as a performance indicator: robust statistical methodology. IMA J Management Math. 2005;16(4):369–381. [Google Scholar]
- 18.Wang EE, Law BJ, Boucher FD, et al. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study of admission and management variation in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1996;129(3):390–395. doi: 10.1016/s0022-3476(96)70071-9. [DOI] [PubMed] [Google Scholar]
- 19.Prais D, Schonfeld T, Amir J. Admission to the intensive care unit for respiratory syncytial virus bronchiolitis: a national survey before palivizumab use. Pediatrics. 2003;112(3 pt 1):548–552. doi: 10.1542/peds.112.3.548. [DOI] [PubMed] [Google Scholar]
- 20.Bloemers BL, van Furth AM, Weijerman ME, et al. Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis: a prospective birth-cohort study. Pediatrics. 2007;120(4) doi: 10.1542/peds.2007-0788. Available at: www.pediatrics.org/cgi/content/full/120/4/e1076. [DOI] [PubMed] [Google Scholar]
- 21.Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322–324. doi: 10.1001/archpedi.156.4.322. [DOI] [PubMed] [Google Scholar]
- 22.Oray-Schrom P, Phoenix C, St Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0 –90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314–319. doi: 10.1097/01.pec.0000092576.40174.28. [DOI] [PubMed] [Google Scholar]
- 23.Kern S, Uhl M, Berner R, Schwoerer T, Langer M. Respiratory syncytial virus infection of the lower respiratory tract: radiological findings in 108 children. Eur Radiol. 2001;11(12):2581–2584. doi: 10.1007/s003300100887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.