Abstract
Objective
We examined functional performance on multiple indicators for two cognitive status groups: (a) not impaired controls (NIC) and (b) mild cognitive impairment (MCI). We identified functional markers associated with differences, changes, and stability in cognitive status.
Method
In the Victoria Longitudinal Study (VLS) we examined cognitive status group effects in (a) cross-sectional functional performance, (b) longitudinal stability, (c) longitudinal functional performance change, and (d) functional marker prediction of later cognitive status. We assembled markers from five continuous clusters of MCI-related functional factors: biological vitality, activity lifestyle, psychosocial affect, subjective health, and global cognition. We used a cross-sectional sample and a two-wave longitudinal sample, stratified by age (mid-old, old-old) and cognitive status (MCI, NIC).
Results
First, cross-sectional results showed that eight markers differentiated MCI and NIC adults, with the latter performing uniformly better. The groups differed on diastolic blood pressure, body mass index, positive and negative affect, MMSE, and the lifestyle indicators of self-maintenance, travel, and novel cognitive activities. Second, Wave1 to Wave2 stabilities in cognitive status classification were high. Third, several markers differentiated the stable (NIC-to-NIC, MCI-to-MCI) from the unstable (NIC-to-MCI, MCI-to-NIC) cognitive status groups. Fourth, five relevant markers for identifying older adults at risk for cognitive status changes were: diastolic blood pressure, self-maintenance activities, novel cognitive activities, positive affect, and global cognitive status.
Conclusion
Selected risk and protective factors differentiate persons classified with MCI from those not currently cognitively impaired, both cross-sectionally and longitudinally.
Keywords: Cognitive Status, Functional Markers, Mild cognitive impairment, Longitudinal
Non-demented older adults vary in cognitive status along several measurable continua. For research and clinical purposes, cognitive status may be circumspectly partitioned into classifications such as cognitively elite or successful (performing at better or more stable levels than matched peers), cognitively normal or typical (displaying modest to moderate levels of deficits or recent declines), and those with mild cognitive disorders or impairments (detectable deficits and variability, accelerated decline, but not dementia) (e.g., de Frias, Dixon, & Strauss, 2009). Across the continua (and the derived classifications), recent research has emphasized the importance of examining a broad range of risk and protection factors that may be associated not only with concurrent cognitive status but also with longitudinal in/stability of status changes (e.g., Dixon, 2011a). These factors include functional markers from a continuum of such epidemiologically important clusters as pathophysiological processes (e.g., Jack et al., 2011), biological vitality (e.g., Anstey, 2008), health and co-morbidities (e.g., Spiro & Brady, 2008), lifestyle activity (e.g., Stern, 2009), and psychosocial affect (e.g., Wilson, Schneider, Boyle, Arnold, Tang, & Bennett, 2007). Some of the above functional markers have the crucial clinical characteristics of being (a) relatively easy to detect and measure, (b) potentially modifiable in their level, number, and impact, and (c) potentially instrumental in influencing timing, rate, direction, and outcome of cognitive status changes and transitions. Relatively few studies have examined associations among a wide and conceptually continuous range (and large number) of functional risk factors in the context of normal aging and mild cognitive impairment (MCI). In this article, we examine both cross-sectional (i.e., concurrent differences) and 2-wave longitudinal associations (e.g., status stability effects) between cognitive status and age (comparing middle-old with old-old adults).
Our conceptualization of MCI refers less to a formal syndrome than to a relatively heterogeneous and transitional set of subclinical (but likely progressive) cognitive deficits or decrements. In emerging conditions, initial deficits may (a) manifest as detectable perturbations across one or another fundamental cognitive domain, (b) be associated with a subset of functional markers, (c) represent initially unknown etiologies, (d) develop along several potential trajectories, and (e) eventually follow relatively progressive decline patterns as consolidation within clinical conditions occurs. Our perspective reflects previous reviews and consensus statements (e.g., Albert et al., 2011; Petersen & Knopman, 2006; Luis, Loewenstein, Acevedo, Barker, & Duara, 2003; Visser & Brodaty, 2006; Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, & Wahlund, 2004). However, for current research purposes, we focus on early phases of cognitive impairment and we use the concept of MCI to reflect a diversity of phenomena associated with transitions from healthy cognitive aging to neurodegenerative diseases. This moderate position is consistent with that of other recent observers (e.g., Christensen, Dear, Anstey, Parslow, Sachdev, & Jorm, 2005; de Frias et al., 2009; Dixon, Garrett, Lentz, MacDonald, Strauss, & Hultsch, 2007; Winblad et al., 2004). In addition, our operational procedures for classifying MCI individuals are similar (a) to other approaches using objective and moderate criteria (e.g., de Frias et al., 2009; Levy, 1994; Ritchie, Artero, & Touchon, 2001; see also Tuokko & Hultsch, 2006) and (b) to previous guidelines and reviews (e.g., Plassman, Williams, Burke, Holsinger, & Benjamin, 2010) that focus on detecting greater cognitive deficits or declines than would have been expected for the person’s age and education level. In general, the present view of MCI suggests the possibility that preclinical impairment may appear as mild deficits in one or more cognitive domains, with the potential for variability in level and domain over time. On the basis of previous research we would expect that some older adults identified prospectively with MCI could experience a progressive, if not precipitous, drop characteristic of neurodegenerative diseases such as AD, whereas others could continue indefinitely in this classification or even improve (e.g., Ganguli, Dodge, Shen, & DeKosky, 2004; Palmer, Wang, Bäckman, Winblad, & Fratiglioni, 2002). The fact of instability of classification underscores the perspective that emerging MCI-like conditions may (at least initially) represent less a single syndrome or clearly defined border zone than a theoretically intriguing, clinically relevant, and diverse phase of aging, initially portending uncertain future directions but eventually knowable outcomes.
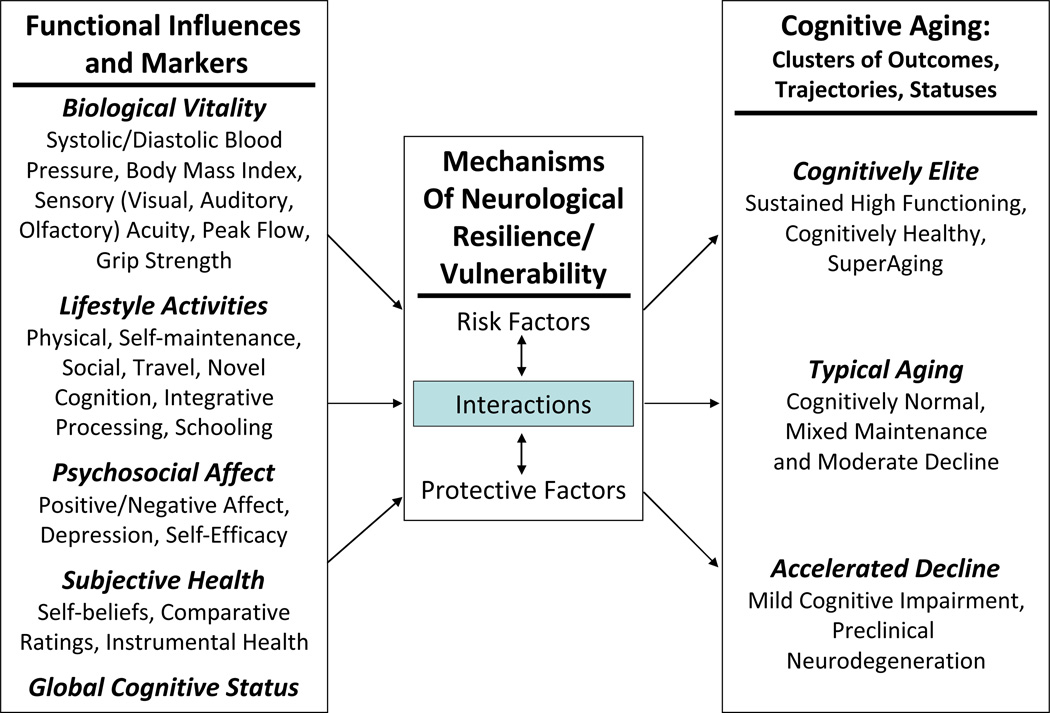
Cognitive functioning, including the trajectories and outcomes of change, in older adults may be susceptible to modification by multiple functional conditions, ranging from proximal comorbidities (e.g., disease-related biological conditions) to more distal influences (contextual or lifestyle factors) (Dixon, 2011a). Because the etiology of cognitive impairment is dynamic and multifactorial, an effective investigative approach may require that multiple domains of functional influences (e.g., biological, cognitive, subjective, experiential) are examined simultaneously. To date, no single category of functional influence has proven to be comparatively superior to differentiating cognitively impaired and normal older adults, nor in predicting cognitive status (Albert et al., 2011; Anstey, 2008; Raz, 2009). Figure 1 portrays the range and mechanisms of influence for a set of functional risk factors commonly associated individually with normal cognitive aging and MCI. The figure reflects the theoretical theme that, in addition to the more proximal neurobiological co-morbidities, even relatively distal functional characteristics may be both modifying and modifiable risk factors (e.g., Cherbuin, Reglade-Meslin, Kumar, Jacomb, Easteal, & Christensen, 2009). In addition, they may influence (a) the magnitude, onset, and changes in relevant neurological mechanisms, genetic predispositions, and neurocognitive reserve, and (b) the trajectories and outcome statuses of subsequent aging-related cognitive changes (see also Anstey et al., 2008; Raz. Rodrigue, Kennedy, & Land, 2009; Stern, 2007, 2009). To date, few studies have examined the comparative influence of functional markers in differentiating or predicting cognitive status changes in normal or cognitively impaired aging.
Figure 1.
Pathways Linking Functional Markers and Principal Trajectories of Cognitive Aging
Potential Functional Markers of Cognitive Impairment and Decline
In addition to neurobiological markers of amyloid deposition, injury, or biochemical change, numerous biological and functional markers have been identified in the MCI literature.
Biological Vitality
Several markers of functional biological vitality have been identified as qualifying observed cognitive deficits in normal aging (Anstey, 2008; Spiro & Brady, 2008) and AD (Buchman, Schneider, Wilson, Bienias, & Bennett, 2006). The present markers representing this domain include vascular (i.e., systolic and diastolic blood pressure, hypertension), anthropometric (body mass index, BMI), sensory (i.e., visual and auditory acuity), pulmonary (peak flow) and musculoskeletal (grip strength) fitness factors. The extent of associations between such functional conditions and age-related cognitive decline may vary by a variety of conditions and modulating factors (Boyle, Buchman, Wilson, Leurgans, & Bennett, 2009; Kivipelto, Ngandu, Fratiglioni, Viitanen, Kareholt, & Winblad, 2005; Molander, Gustafson, & Lovheim, 2010; Reitz, Tang, Manly, Mayeux, & Luchsinger., 2007).
Lifestyle Activities
The level of engagement with the environment (including physical, social, and cognitive activities) has been suggested to functionally influence both normal cognitive performance and change in older adults and the timing of transitions to cognitive impairment and dementia (e.g., Fratiglioni, Paillard-Borg, & Winblad, 2004; Hertzog, Kramer, Wlson, & Lindenberger, 2008; Stern, 2007). The present markers representing this domain include physical, self-maintenance, social, travel, passive, novel, and integrative information processing activities. Activities may help reduce cognitive decline by having beneficial effects on cardio/cerebrovascular health, building brain and cognitive reserve, reducing chronic stress, or promoting a healthy lifestyle (Fratiglioni et al., 2004; Stern, 2009; Verghese et al., 2006). Late-life cognitive changes can also lead to decreased involvement in lifestyle activities (Small, Dixon, McArdle, & Grimm, in press).
Psychosocial Affect
Cognition and emotion in aging are inextricably linked. The current study includes both positive and negative affective dimensions. Negative affect (i.e., depression and the tendency to experience psychological distress) may contribute to MCI by compromising limbic structures that regulate stress-related behaviour and memory systems (Wilson et al., 2007). Positive affect can have direct and beneficial effects on physiological, hormonal and immune function. These, in turn can influence health outcomes and exert important indirect effects on cognitive functioning through their influence on health-promoting behaviours (Moskowitz, Epel, & Acree, 2008).
Subjective Health Status
Subjective health beliefs have been identified as predictors of cognition in relatively healthy older adults (e.g., Wahlin, MacDonald, de Frias, Nilsson, & Dixon, 2006) and predictors of cognitive impairment for up to 10 years after assessment (Bond, Dickinson, Matthews, Jagger, & Brayne, 2006). The present study includes both (a) veridical beliefs about health status (e.g., overall health ratings), and (b) personal or affective impressions of comparative health status (e.g., ratings requiring comparisons to others of same or different ages) (Liang, Bennett, Whitelaw, & Maeda, 1991; Sargent-Cox, Anstey, & Luszcz, 2008).
The Present Study
Most of the previous studies investigating functional risk or protection factors associated with MCI have focused on relatively narrower ranges of domains (e.g., within either biological vitality or lifestyle activities, but usually not both). However, more recent studies have started to consider simultaneously biological, health, behavioural, and environmental variables related to MCI, yielding promising insights into patterns and profiles of brain and cognitive health with aging (e.g., Cherbuin et al., 2009). In the present study we investigate the extent to which differences on multiple functional markers are associated with cognitive functioning, status, and stabilities among typically aging and provisional MCI older adults. The Victoria Longitudinal Study (VLS; Dixon & de Frias, 2004) is well-positioned to contribute to such multifaceted investigations of (a) the differences between normal cognitive aging and MCI, and (b) the factors predicting (or risking/protecting) individuals following such transitions (Artero, Ancelin, Portet, Dupuy, Berr, C., Dartigues, et al., 2008; Dixon et al., 2007). Assembling a multifaceted set of potential functional risk factors in the context of a well-characterized study, we investigate four main research questions. First, using cross-sectional data, can probable MCI individuals be differentiated from normal aging adults on the basis of one or more indicators from the domains of biological vitality, lifestyle activity, personal affect, subjective health factors, and global cognitive status? Second, to what extent are baseline cognitive status classifications stable over the longitudinal interval? Third, are the stable (NIC-to-NIC, MCI-to-MCI) versus unstable (NIC-to-MCI, MCI-to-NIC) cognitive status groups different in the previously identified functional risk factors over the two waves of measurement? Fourth, can these factors be used as transition markers for identifying older adults who are at risk for accelerated cognitive decline?
Method
Participants
This research was conducted under full, active, and continuous human ethics approval from prevailing Institutional Review Boards. Participants were community-dwelling older adults from the Victoria Longitudinal Study (VLS), originally recruited through advertisements in the public media and to community groups. The VLS is an ongoing multi-sample sequential investigation of multiple facets (i.e., cognitive, neuropsychological, health, sensory, and biological) related to human aging. Extensive background information on the VLS general design, measures, and procedures is available (e.g., Dixon & de Frias, 2004). For this study, we assembled a 2-wave (M interval = 4.59 years; SD = 0.50; 99% of returnees were tested in the 4–5-year range) longitudinal data set by combining data collected during the same historical period across VLS Samples 1 and 2. Specifically, the current Wave 1 (W1) data were assembled from VLS Sample 1 (Wave 5) and VLS Sample 2 (Wave 3). Current Wave 2 (W2) data were assembled from VLS Sample 1 (Wave 6) and VLS Sample 2 (Wave 4). Initial exclusionary criteria included history of Alzheimer’s disease, psychiatric disturbance, and serious episodes of cardio/cerebrovascular disease. We developed two study samples; specifically, a large cross-sectional study and a full 2-wave longitudinal design.
The first research question required a cross-sectional sample, as derived from the larger W1 only. We excluded 6 potential participants for scores less than 24 on the Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975). The remaining group (n = 416; 257 women, 159 men), was divided into two age groups. A mid-old (MO) group (n = 168; 102 women, 66 men), ranging from 64 to 73 years (M = 69.35, SD = 2.76), and an old–old (OO) group (n = 248; 155 women, 93 men), ranging from 74 to 95 years (M = 79.61, SD = 4.26). The remaining research questions required a two-wave (fully enrolled) longitudinal data set. At W2, n = 301 participants returned for re-testing. At this point, n = 6 of these returning participants were excluded for MMSE scores of less than 24, and n = 1 for missing scores on the cognitive reference measures. The final two-wave sample consisted of n = 294 participants (184 women and 110 men).
For both the cross-sectional and longitudinal samples, the two age groups were objectively classified into two cognitive status groups: (a) not impaired control (NIC) and (b) provisional mild cognitive impairment (MCI) participants. Cognitive status classification was determined by a three-step procedure adapted from previous VLS and other research (e.g., de Frias et al., 2009; Dixon et al., 2007; Ritchie et al., 2001; Winblad et al., 2004) and emphasized objective and replicable assessments of cohort-relative performance on a conceptually continuous set of five cognitive reference measures (described below). The first step in performing cognitive status classifications was to stratify the sample by both age (64–73 and 74–95 years) and level of education (0–12 years or >=13 years) and placed into one of four cells (2 age levels × 2 education levels). Second, for each of these four groups, mean performance was calculated for each of five cognitive reference measures, representing the theoretical domains of perceptual speed, inductive reasoning, episodic memory, verbal fluency, and semantic memory. Third, these means served as within-sample norms for cognitive status classification. Specifically, participants were classified as MCI if they scored one or more standard deviations below their own age X education group means on one or more of the cognitive tasks (de Frias et al., 2009; Dixon et al., 2007; Ritchie et al., 2001). We applied the same procedure independently at W1 and W2. For W1, the subgroup cell sizes are as follows: MO (low education) n = 38; MO (high education) n = 130; OO (low education) n = 73; OO (high education) n = 175. At W1, the cognitive status classification procedure resulted in a provisional MCI group (n = 196) and an NIC group (n = 220). For W2, the subgroup cell sizes are as follows: MO (low education) n = 31; MO (high education) n = 108; OO (low education) n = 45; OO (high education) n = 110. At W2, the cognitive status classification procedure resulted in a provisional MCI group (n = 131) and an NIC control group (n = 163). Descriptive information about the participants, stratified by cognitive status, shows substantial similarities in background characteristics between the two groups at both W1 and W2 (see Table 1).
Table 1.
Sample Demographics by Cognitive Status and Age Group at Wave 1 and Wave 2
| Mid-Old | Old-Old | |||
|---|---|---|---|---|
| Variable | NIC | MCI | NIC | MCI |
| Age | ||||
| CSL W1 | 69.33 (2.76) | 69.38 (2.79) | 78.91 (3.83) | 80.32 (4.56) |
| LONG W1 | 69.33 (2.76) | 69.15 (2.88) | 78.14 (3.61) | 79.24 (3.98) |
| LONG W2 | 73.61 (2.72) | 74.31(2.85) | 82.47 (3.26) | 84.06 (4.14) |
| Gender (women) | ||||
| CSL W1 | 64.21% | 56.16% | 66.40% | 58.54% |
| LONG W1 | 63.53% | 57.41% | 65.88% | 61.53% |
| LONG W2 | 63.51% | 58.46% | 59.55% | 69.70% |
| Years of Education | ||||
| CSL W1 | 15.53 (2.83) | 15.05 (3.26) | 14.94 (3.01) | 14.20 (3.09) |
| LONG W1 | 15.6 (2.87) | 14.74 (3.11) | 14.67 (2.78) | 14.46 (3.13) |
| LONG W2 | 15.72 (2.98) | 14.75 (2.94) | 14.94 (3.06) | 14.08 (2.70) |
| MMSE | ||||
| CSL W1 | 28.98 (1.05) | 28.56 (1.19) | 28.76 (1.09) | 27.99 (1.47) |
| LONG W1 | 29.04 (1.02) | 28.74 (1.03) | 28.74 (1.13) | 28.13 (1.48) |
| LONG W2 | 28.86 (1.04) | 28.49 (1.57) | 28.29 (1.16) | 27.17 (1.65) |
| Absolute health | ||||
| CSL W1 | 4.23 (0.78) | 4.23 (0.84) | 4.13 (0.68) | 4.02 (0.75) |
| LONG W1 | 4.22 (.76) | 4.44 (.74) | 4.27 (.64) | 4.21 (0.76) |
| LONG W2 | 4.0 (0.77) | 4.29 (0.76) | 4.01 (0.71) | 4.11 (0.71) |
| Relative health | ||||
| CSL W1 | 4.36 (0.71) | 4.29 (0.84) | 4.38 (0.65) | 4.25 (0.70) |
| LONG W1 | 4.35 (.70) | 4.46 (.72) | 4.54 (0.57) | 4.40 (0.67) |
| LONG W2 | 4.26 (0.78) | 4.48 (0.69) | 4.37 (0.61) | 4.43 (0.61) |
| Vocabulary | ||||
| CSL W1 | 46.25 (3.83) | 41.23 (6.35) | 45.99 (4.00) | 42.36 (6.40) |
| LONG W1 | 46.54 (3.61) | 40.74 (6.52) | 45.55 (3.91) | 43.17 (5.36) |
| LONG W2 | 45.68 (3.89) | 41.95 (7.06) | 46.15 (3.49) | 42.12 (5.41) |
| Marital Status(% married) | ||||
| CSL W1 | 67.40% | 71.2%/72.2% | 56%/56.5% | 50.4%/51.2% |
| LONG W1 | 65.90% | 74.1%/75.5% | 57.6%/58.3% | 55.7%/56.5% |
| LONG W2 | 62.20% | 76.9%/78.1% | 60.7%/62.1% | 51.50% |
| Body Mass Index | ||||
| CSL W1 | 27.75 (4.38) | 25.99 (3.92) | 26.59 (5.16) | 26.04 (3.88) |
| LONG W1 | 27.67 (4.28) | 26.14 (3.69) | 26.46 (5.88) | 26.05 (4.32) |
| LONG W2 | 27.64 (4.19) | 26.43 (3.97) | 26.62 (5.77) | 25.79 (4.37) |
| Diabetes | ||||
| CSL W1 | 6.4%/6.8% | 6.8%/7.8% | 6.4%/9.2% | 6.5%/12.1% |
| LONG W1 | 5.9%/6.5% | 7.4%/8.6% | 4.7%/6.8% | 2.8%/5.8% |
| LONG W2 | 6.8%/7.4% | 6.2%/7.6% | 5.6%/8.8% | 1.5%/2.7% |
| Reported Medication Use (%) | ||||
| Antihypertensive | ||||
| CSL W1 | 21.1%/25.6% | 19.2%/25.5% | 21.6%/33.3% | 11.4%/25% |
| LONG W1 | 21.2%/26.1% | 14.8%/20% | 24.7%/38.2% | 8.6%/20% |
| LONG W2 | 23%/27% | 13.8%/19.6% | 20.2%/34.6% | 13.6%/27.3% |
| Antidiabetic | ||||
| CSL W1 | 2.1%/2.3% | 4.1%/4.7% | 7.2%/7.5% | 8.1%/8.8% |
| LONG W1 | 2.4%/2.6% | 3.7%/4.3% | 3.5%/3.7% | 5.7%/6.3% |
| LONG W2 | 2.7%/3% | 3.1%/3.5% | 3.4%/3.6% | 6.1%/6.3% |
| Antidepressant | ||||
| CSL W1 | 8.4%/9.3% | 5.5%/6.3% | 4.8%/5% | 1.6%/1.8% |
| LONG W1 | 9.4%/10.4% | 3.7%/4.3% | 3.5%/3.7% | 1.4%/1.6% |
| LONG W2 | 8.1%/9% | 6.2%/7% | 3.4%/3.6% | 1.5%/1.6% |
Note. CSL W1 = cross-sectional W1 sample; LONG W1 = longitudinal W1 sample; LONG W2 = longitudinal W2 sample. Standard deviations are in parentheses. On a 5-point scale ranging from 1 (very poor) to 5 (very good), absolute health reflects self-rating of health relative to a perfect state with relative health reflecting self-reported health relative to same-aged peers. Vocabulary was indexed by the number of correct responses on a 54-item recognition vocabulary test adapted from Ekstrom et al. (1976). NIC = not impaired controls; MCI = mild cognitive impairment; MMSE = Mini Mental State Exam (Folstein et al., 1975).
Procedure
At each of the two longitudinal waves, participants completed multiple measures of functioning, including biological vitality, activity lifestyle, psychosocial affect, subjective health, and global cognition. We evaluated performance by NIC and MCI groups at both waves.
Measures
Biological Vitality Markers
We used 8 measures of biological performance and attributes to assess participants’ physical fitness, all following standard procedures (MacDonald, Dixon, Cohen, & Hazlitt, 2004; Wahlin et al., 2006). First, mean systolic blood pressure and (second) diastolic blood pressure (mmHg) were calculated over eight readings across two testing sessions. Third, hypertension was determined based on systolic BP > 140 mmHg and diastolic BP > 90 mmHg. Fourth, body mass index (BMI; kg/m2) was calculated from concurrent measurements of weight and height. Fifth, peak expiratory flow (L/minute) was measured (MiniWright Peak Flow Meter) wherein participants were asked to exhale as quickly and forcefully as possible. The score was the highest volume exhaled over three attempts. Sixth, grip strength (kg force) was measured for each hand (Smedley hand dynamometer), using the best score of two attempts for each hand. Seventh, perceived visual acuity, and eighth, perceived auditory acuity, were self-rated relative to a perfect state based on a scale from 1–5 (1 = very good, 5 = very poor).
Activity Lifestyle
We used the standard 67-item VLS Activity Lifestyle Questionnaire (VLS-ALQ) to measure the typical frequency of engagement in multiple examples of everyday activities reflecting the following seven domains: (a) physical, such as jogging or gardening (n = 4); (b) self-maintenance, such as preparing a meal or shopping (n = 6); (c) social, such as attending concerts or visiting friends (n = 7); (d) travel, such as traveling within Canada (n = 3); (e) passive information processing, such as reading the newspaper or watching a documentary (n = 8); (f) integrative information processing, such as using the computer or playing a musical instrument (n = 12); and (g) novel information processing, such as completing income tax forms or playing bridge (n = 27). The frequency of participation is rated on a 9-point scale (never, less than once a year to two or three times a week, and daily). We scaled the responses such that higher scores were associated with greater frequency of activity. Responses on items within each of the seven subscales were summed for the respective subscale scores.
Psychosocial Affect
We used three measures of psychosocial affect: (a) the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977), and (b) the Bradburn Affect Balance Scale (ABS, well-being) positive and negative affect scales (Bradburn, 1969). For the CES-D participants indicated (on a 4-point Likert scale, 20 items, range = 0–60) how they felt in the last week. Higher scores indicated more depressive symptoms. The ABS measures incidence of feeling 10 emotions in the past month, including five positive (e.g., proud) and five negative (e.g., upset), and uses summed scores from both (Maitland, Dixon, Hultsch, & Hertzog, 2001).
Subjective Health
We used two VLS indicators of subjective health (Wahlin et al., 2006). Participants rated their health on a 5-point Likert scale (1 = very good, 5 = poor) relative to (a) a perfect state of health, and (b) their age-peers.
Global Cognition
Global cognitive status was measured using the MMSE (Folstein et al., 1975), with a possible score range of 0–30.
Cognitive Reference Measures
We used indicators of five cognitive domains (i.e., perceptual speed, inductive reasoning, episodic memory, verbal fluency, and semantic memory) to evaluate the participants’ cognitive status (de Frias et al., 2009; Dixon et al., 2007). The psychometric properties of these measures, which are acceptable according to conventional standards, are well-documented elsewhere (Hultsch, Hertzog, Dixon, & Small, 1998). All standardized procedures were followed.
Perceptual Speed
Perceptual processing speed was assessed with the Wechsler Adult Intelligence Scale—Revised Digit Symbol Substitution (DSS) task (Wechsler, 1981). Psychometric characteristics of the DSS are well-established in aging and other populations (e.g., MacDonald, Hultsch, Strauss, & Dixon, 2003). The number of correctly completed items in 90 sec was used as the score.
Inductive Reasoning
Inductive reasoning was assessed with the Letter Series test (Thurstone, 1962). Participants were presented with 20 strings of letters forming a distinct pattern. The score was the total number correct patterns solved inductively.
Episodic Memory
The VLS word recall task, consisting of immediate free recall of two lists of 30 English words (i.e., six words in each of five taxonomic categories) (Dixon, Wahlin, Maitland, Hultsch, Hertzog, & Bäckman, 2004), was used. The average number of correctly recalled words from each list was used as a measure of episodic memory.
Verbal Fluency
The Controlled Associations test from the Educational Testing Service (ETS) kit of factor-referenced cognitive tests (Ekstrom, French, Harman, & Dermen, 1976) was used. The test required the generation of as many synonyms as possible in response to a set of target words within six min. Scored was the total number of correct synonyms.
Vocabulary
The 54-item recognition, multiple-choice vocabulary test was composed by concatenating three 18-item tests from the ETS kit of factor referenced cognitive tests (Ekstrom et al., 1976). The total number of correct items represented the vocabulary score.
Data Analyses
We conducted four sets of analyses designed to evaluate specifically the four research questions. All statistical analyses were performed using SPSS version 17.0 statistical software.
Analyses for Research Question 1
The goal of the first set of analyses was to explore cognitive status group differences in each of the measures of biological vitality, lifestyle activities, psychosocial affect, subjective health, and global cognitive status. We first used the largest available cross-sectional data set (n = 416) to identify specific candidate markers of concurrent cognitive status differences. Because these domains are under-explored in research on cognitive impairment (see Cherbuin et al., 2009), we performed a series of univariate comparisons. Accordingly, we examined the 2 (age) X 2 (cognitive status) analyses of variance (ANOVAs) only for cognitive status main effects and interaction terms. The prevailing hypothesis was that the NIC group would display better performance than the MCI group. Specifically, the analyses and results were intended (a) to contribute to archival information on MCI and its associated functional markers, and (b) to inform subsequent analyses for the remaining three research questions. They were not designed to fully explore theoretical issues pertaining to functional characteristics of aging and MCI. Instead, significant cognitive status group differences in any measure were used to identify that measure as a potential predictor for later use in logistic regression analyses. In this way, the number of predictors used in the later analyses was reduced.
Analyses for Research Question 2
Using the data from the two-wave longitudinal sample, we calculated the stability of classification using all participants who attended both W1 and W2. Given that participants at both waves were independently and objectively classified as NIC or MCI, we calculated the proportion continuing in their W1 classification after the three-year interval. Previous MCI classification procedures have resulted in wide-ranging estimates of 3–5-year stability rates (e.g., Cherbuin et al., 2009; Palmer et al., 2002), but we expected our stability rates would be relatively high.
Analyses for Research Question 3
Performing a secondary classification (i.e., stable or not stable cognitive status) permitted a unique fine-grained set of analyses in which we compared several combinations of the stable/not stable NIC/MCI subgroups to one another in their performance on the candidate functional marker variables over two waves of measurement. Therefore, a series of 2 (age) x 2 (cognitive status stability) x 2 (wave) repeated measures ANOVAs on selected functional markers were conducted. An ANOVA is an appropriate statistical technique given the fact of no missing 2-wave data. The specific performance measures were selected on the basis of the results from the analyses for Research Question 1. In this case, the cognitive status stability factor was operationalized to include comparisons of (a) relatively stable cases of MCI vs. NIC (stability in status) and (b) novel within-status comparisons of stable-unstable subgroups (MCI-Stable vs. MCI-Unstable and NIC-Stable vs. NIC-Unstable).
Analyses for Research Question 4
The goal was to identify relevant functional markers for identifying older adults who are most at risk for accelerated cognitive decline. We used the 2-wave longitudinal sample. Binomial logistic regression analyses were performed to examine whether measures from the four functional factor domains could predict MCI or NIC group status at W2. We computed separate logistic regression analyses for each of our groups: NIC-to-NIC vs. MCI-to-MCI, NIC-to-NIC vs. NIC-to-MCI, and MCI-to-MCI vs. MCI-to-NIC. Only those previously identified functional markers (from Research Question 1) showing significant cognitive status differences were used as predictor variables of cognitive status membership in these logistic regression analyses. Age was used as a control variable.
Results
We report results in four sections according to the research questions. In order to control for multiple statistical tests, we do not report or interpret main effects for age group in this study. In addition, we use the first set of analyses to cull the functional markers considered for subsequent analyses.
Research Question 1: Identification of Baseline Status Group Differences Using Candidate Functional Markers
The results of the W1 ANOVAs (described above) showed significant cognitive status effects for eight candidate functional markers (see Table 2): diastolic blood pressure [F(1,409) = 5.41, p < 0.05, partial η2 = 0.01], BMI [F(1,408) = 6.77, p < 0.05, partial η2 = 0.02], self-maintenance activities [F(1,406) = 12.82, p < 0.001, partial η2 = 0.03], travel activities [F(1,406) = 5.84, p < 0.05, partial η2 = 0.01], novel information processing [F(1,362) = 15.04, p < 0.001, partial η2 = 0.04], positive affect [F(1,391) = 7.57, p < 0.01, partial η2 = 0.02], negative affect [F(1,398) = 5.85, p < 0.05, partial η2 = 0.01], and MMSE [F(1,412) = 23.30, p < 0.001, partial η2 = 0.05]. The NIC group had higher (or better) scores than the MCI group on these measures. The results also revealed significant age X cognitive status interactions for systolic BP [F(1,409) = 4.09, p < 0.05, partial η2= 0.01] and hypertension [F(1,409) = 3.92, p < 0.05, partial η2= 0.01]. As can be seen in the table, the two interactions were due to greater age differences among MCI participants than in the NIC group.
Table 2.
Baseline (W1) Characteristics of the Functional Markers by Age Group and Cognitive Status (NIC and MCI)
| NIC | MCI | ANOVA Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mid-Old | Old-Old | Mid-Old | Old-Old | p value | |||||||
| Factor | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Age | Status | Age X Status |
| Systolic BP | 130.6 | 14.08 | 132.83 | 14.21 | 125.97 | 16.24 | 134.68 | 18.13 | 0.001 | 0.39 | 0.044 |
| Diastolic BP | 78.57 | 9.6 | 75.29 | 9.14 | 74.46 | 10.39 | 74.86 | 9.87 | 0.142 | 0.021 | 0.06 |
| Hypertension | 0.27 | 0.44 | 0.33 | 0.47 | 0.18 | 0.39 | 0.43 | 0.5 | 0.001 | 0.89 | 0.048 |
| BMI | 27.75 | 4.38 | 26.59 | 5.16 | 25.99 | 3.92 | 26.04 | 3.88 | 0.216 | 0.01 | 0.176 |
| Grip strength | 32.19 | 10.15 | 28.08 | 9.48 | 29.61 | 9.45 | 27.22 | 8.1 | 0.001 | 0.077 | 0.374 |
| Peak flow | 431.3 | 122.9 | 407.92 | 107.18 | 408.84 | 132.94 | 390.45 | 108.95 | 0.084 | 0.098 | 0.835 |
| Visual acuity | 2.16 | 0.8 | 2.13 | 0.75 | 1.94 | 0.85 | 2.05 | 0.78 | 0 | 0.067 | 0.074 |
| Auditory acuity | 2.06 | 0.92 | 2.04 | 0.88 | 2.03 | 1.06 | 2 | 0.86 | 0.001 | 0.869 | 0.661 |
| Physical activities | 14.95 | 4.5 | 14.67 | 4.89 | 15.35 | 4.7 | 14.67 | 4.76 | 0.226 | 0.831 | 0.53 |
| Self-maintenance | 30.9 | 5.46 | 29.02 | 5.23 | 27.93 | 5.35 | 28.02 | 5.84 | 0.107 | 0 | 0.078 |
| Travel activities | 6.47 | 2.19 | 6.05 | 2.37 | 5.9 | 1.85 | 5.48 | 2.57 | 0.074 | 0.016 | 1 |
| Passive information | 34.29 | 6.97 | 33.25 | 7.89 | 32.87 | 6.8 | 33.68 | 8.31 | 0.878 | 0.526 | 0.232 |
| Social activities | 22.42 | 6.7 | 22.72 | 7.11 | 21.14 | 7.29 | 22.58 | 7 | 0.227 | 0.32 | 0.425 |
| Integrative information | 18.83 | 8.83 | 18.34 | 8.38 | 19.82 | 8.27 | 16.94 | 7.38 | 0.046 | 0.802 | 0.156 |
| Novel information | 79.72 | 16.8 | 72.26 | 15.62 | 71.57 | 14.84 | 67.17 | 16.41 | 0.001 | 0 | 0.371 |
| CESD | 7.31 | 6.61 | 8.42 | 7.85 | 7.75 | 7.92 | 8.85 | 6.75 | 0.14 | 0.556 | 0.997 |
| Positive affect | 3.78 | 1.42 | 2.89 | 1.78 | 3.53 | 1.66 | 2.18 | 1.79 | 0 | 0.006 | 0.187 |
| Negative affect | 1.03 | 1.31 | 0.61 | 0.95 | 0.8 | 1.23 | 0.33 | 0.69 | 0 | 0.016 | 0.796 |
| Health to perfect | 4.23 | 0.78 | 4.13 | 0.68 | 4.23 | 0.84 | 4.02 | 0.75 | 0.035 | 0.466 | 0.456 |
| Health to age group | 4.36 | 0.71 | 4.38 | 0.64 | 4.29 | 0.84 | 4.25 | 0.7 | 0.947 | 0.159 | 0.667 |
| MMSE | 28.98 | 1.05 | 28.76 | 1.09 | 28.56 | 1.19 | 27.99 | 1.47 | 0.001 | 0 | 0.154 |
Note: p values < .05 are in boldface.
Research Question 2: Stability of Status Group Classifications
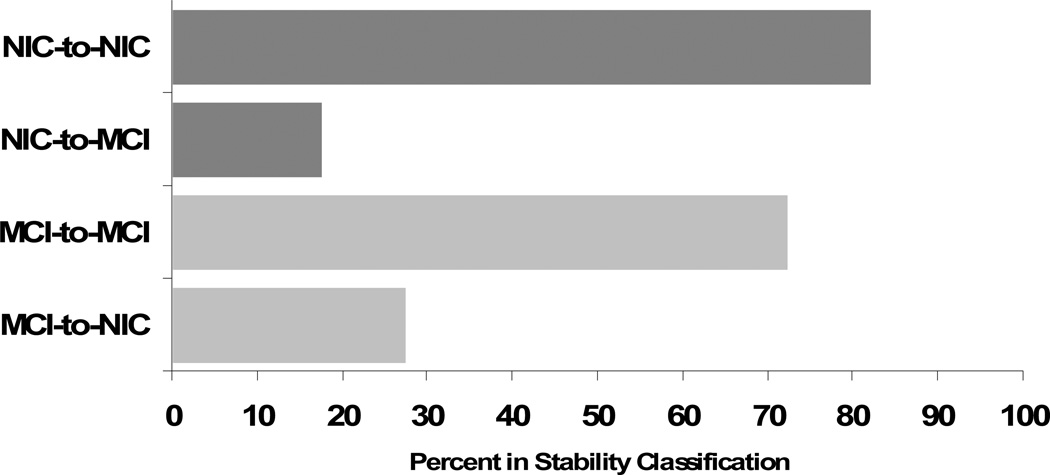
New and independent group classifications were conducted with all participants who returned for W2, and stability rates across the two waves were examined. Independent cognitive status group classifications at both waves permitted calculating the stabilities of initial cognitive status classifications. The cognitive status groups were notably stable over the longitudinal period: NIC = 82.21% (134 of 163 returners remained in baseline status) and MCI = 72.52% (95 of 131 returners remained in baseline status) after the 4+-year interval (see Figure 2).
Figure 2.
Longitudinal Cognitive Status Stability (% remaining in status from W1 to W2)
Research Question 3: Performance Differences Related to Status Stability
As described in the Method section, we followed three phases of analyses to determine whether differences in stability of classification were related to differences in performance on only the eight previously identified functional factor measures across the two waves.
Cognitive Status Stability Results
The cognitive status stability factor involved the relatively “pure” cases of both NIC (i.e., the stable NIC-to-NIC group) and MCI (i.e., the stable MCI-to-MCI group). The results showed significant cognitive status stability effects for seven of the eight previously identified candidate markers: diastolic BP [F(1,222) = 8.02, p = .005, partial η2 = 0.035], self-maintenance activities [F(1,221) = 16.70, p = 0.000, partial η2 = 0.07], travel activities [F(1,219) = 7.19, p < 0.008, partial η2 = 0.03], novel cognitive activities [F(1,200) = 21.85, p = 0.000, partial η2 = 0.10], positive affect [F(1,211) = 4.56, p = 0.034, partial η2 = 0.02], negative affect [F(1,215) = 5.01, p = 0.026, partial η2 = 0.02], and MMSE [F(1,225) = 33.66, p = 0.000, partial η2 = 0.13]. The stable NIC group had higher scores than the stable MCI group on these significant factors (Table 3). For self-maintenance activities, there were also significant two way (cognitive status stability X wave) [F(1,221) = 7.62, p = 0.006, partial η2 = 0.03] and three-way [F(1,221) = 6.89, p = 0.009, partial η2 = 0.03] interactions. The two interactions were due to greater decline between the two waves among stable MCI participants, especially the ones in the older group, as compared to the NIC group.
Table 3.
Wave 1 and Wave 2 Performance on Eight Functional Markers by Cognitive Status and Stability Group
| Risk Factors | NIC-to-NIC | MCI-to-MCI | NIC-to-MCI | MCI-to-NIC |
|---|---|---|---|---|
| Diastolic BP | ||||
| Wave1 | 77.72 (9.88) | 73.63 (10.04) | 76.11 (8.12) | 78.45 (9.18) |
| Wave2 | 73.26 (9.75) | 70.52 (10.51) | 72.01 (8.65) | 71.47 (9.01) |
| BMI | ||||
| Wave1 | 27.21 (5.38) | 26.00 (4.13) | 26.53 (4.27) | 26.66 (3.8) |
| Wave2 | 26.56 (5.19) | 25.82 (4.7) | 25.96 (4.10) | 26.9 (3.8) |
| Self-Maintenance Activities | ||||
| Wave1 | 29.99 (5.54) | 28.12 (5.06) | 30.85 (4.85) | 29.00 (5.25) |
| Wave2 | 29.86 (5.70) | 26.03 (6.55) | 29.65 (6.99) | 29.34 (5.34) |
| Travel Activities | ||||
| Wave1 | 6.53 (2.08) | 5.68 (2.26) | 5.88 (2.36) | 5.82 (2.07) |
| Wave2 | 5.66 (2.43) | 4.94 (2.6) | 5.03 (2.39) | 4.61 (3.37) |
| Novel Cognitive Activities | ||||
| Wave1 | 78.52 (15.92) | 69.31 (16.60) | 67.79 (15.46) | 73.56 (13.32) |
| Wave2 | 75.28 (16.31) | 63.85 (17.31) | 66.06 (16.31) | 71.44 (14.57) |
| Positive Affect | ||||
| Wave1 | 3.40 (1.65) | 2.84 (1.86) | 3.36 (1.65) | 2.92 (1.98) |
| Wave2 | 4.16 (.99) | 4.04 (1.10) | 3.94 (1.12) | 3.37 (1.67) |
| Negative Affect | ||||
| Wave1 | .84 (1.15) | .57 (.98) | .83 (1.22) | .61 (1.20) |
| Wave2 | .86 (1.14) | .57 (.92) | .63 (.84) | .78 (1.07) |
| MMSE | ||||
| Wave1 | 28.92 (1.06) | 28.21 (1.41) | 28.78 (1.17) | 29.00 (.84) |
| Wave2 | 28.57 (1.16) | 27.61 (1.78) | 28.39 (1.52) | 28.48 (1.06) |
NIC Stable-Unstable Results
We conducted the same ANOVAs with the within-NIC group contrast of stable NIC (i.e., NIC-to-NIC) group versus the unstable (and status-declining) NIC-to-MCI group. The results showed overall significant cognitive status stability effects for novel cognitive activities [F(1,148)= 12.73, p = 0.000, partial η2 = 0.08]. As only significant main effects for cognitive status, but not significant interactions were observed, we explored the phenomenon further by performing one-way ANOVAs at each wave. Significant NIC status stability effects for novel cognitive activities were registered at both W1 [F(1,151) = 12.42, p = 0.001, partial η2 = 0.08] and W2 [F(1,163) = 11.86, p = 0.001, partial η2 = 0.07], with higher scores being related to NIC stability (Table 3).
MCI Stable-Unstable Results
We conducted the comparable ANOVAs with the contrasts of stable MCI (MCI-to-MCI group) versus the unstable (but status-improving) MCI (MCI-to-NIC group). The ANOVAs showed a significant cognitive status stability group effect for MMSE [F(1,120) = 10.17, p = 0.002, partial η2 = 0.08]. As expected, the unstable (i.e., apparently improving) group performed better. There were also significant wave X cognitive status interactions for diastolic BP [F(1,117) = 5.22, p = 0.024, partial η2 = 0.04] and positive affect [F(1,111) = 4.35, p = 0.039, partial η2 = 0.04], a significant age X cognitive status interaction for MMSE [F(1,120) = 5.13, p = 0.025, partial η2 = 0.04], and a three-way interaction for self-maintenance activities [F(1,117) = 4.78, p = 0.031, partial η2 = 0.04]. MCI stability was characterized by lower scores at both waves for diastolic BP, MMSE, and self-maintenance activities, and higher W2 scores for positive affect (Table 3).
Research Question 4: Functional Predictors of Status Group Membership
A total of 229 participants who returned at W2 maintained their baseline cognitive status (NIC-to-NIC n = 134, MCI-to-MCI n = 95), with n = 36 initial NIC participants declining to MCI status and n=29 initial MCI participants improving to NIC cognitive status. We used a series of logistic regressions to investigate whether the eight functional factors that showed significant differences between the NIC and the MCI groups at W1 (research question 1 above) would significantly predict cognitive status at W2. Analyses are described in the Method section.
Results of Stability (NIC, MCI) Analyses
We first examined the persistent cases of NIC (i.e., stable NIC-to-NIC group) and MCI (i.e., stable MCI-to-MCI group). The test of the full model with all 8 predictors and age at W1 was statistically significant [λ2(9) = 50.67, p < 0.001], indicating that the W1 predictors, as a group, reliably distinguished the two cognitive status groups (at W2). According to the Nagelkerke R2, the set of predictors accounted for 31.7% of the variance in group membership. Table 4 shows regression coefficients, odds ratios, and 95% confidence intervals for odds ratios, as well as the p values for each of the predictors. The Wald statistic indicated that five W1 markers reliably predicted group membership at W2: diastolic BP [Wald (1) = 7.63, p = 0.006], self-maintenance activities [Wald (1) = 7.66, p = 0.006], novel cognitive activities [Wald (1) = 4.54, p < 0.033], positive affect [Wald (1) = 6.29, p < 0.012], and MMSE [Wald (1) = 9.12, p = 0.003]. Classification rates were high, but better for the NIC-to-NIC group (82.1% correct) than for the MCI-to-MCI group (64.9% correct), for an overall correct classification rate of 75.1%. Subsequently, for these two persistent stability groups, we tested the full concurrent model (at W2) with the 8 functional markers and age, observing an overall statistically reliable effect [λ2(9) = 70.03, p < 0.001]. According to the Nagelkerke R2 the set of predictors accounted for 37.2% of the variance in W2 group membership. The Wald statistic indicated that three concurrent markers reliably separated the two status-stability groups: self-maintenance [Wald (1) = 13.14, p = 0.000], novel cognitive activities [Wald (1) = 9.8, p = 0.02], and MMSE [Wald (1) = 21.38, p = 0.000]]. Classification was better for the NIC-to-NIC group (85.5% correct) than for the MCI-to-MCI group (59.8% correct), for an overall correct classification rate of 75.2%.
Table 4.
Predictors of Cognitive Status (NIC or MCI) and of Transition from NIC to MCI and MCI to NIC, Adjusted for Age
| Status | NIC-to-NIC vs. MCI-to-MCI | NIC-to-NIC vs. NIC-to-MCI | MCI-to-MCI vs. MCI-to-NIC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | B | OR | CI | p value | B | OR | CI | p value | B | OR | CI | p value |
| Diastolic BP | ||||||||||||
| Wave1 | −0.05 | 0.95 | .91–.98 | 0.006 | −0.01 | 0.99 | .94–1.04 | 0.637 | −0.07 | 0.93 | .88–.99 | 0.03 |
| Wave2 | −0.02 | 0.98 | .95–1.01 | 0.287 | −0.01 | 1 | .96–1.04 | 0.976 | 0.01 | 1 | .95–1.06 | 0.952 |
| BMI | ||||||||||||
| Wave1 | −0.03 | 0.97 | .90–1.04 | 0.392 | −0.05 | 0.95 | .85–1.06 | 0.338 | −0.07 | 0.93 | .81–1.07 | 0.324 |
| Wave2 | −0.04 | 0.96 | .91–1.03 | 0.268 | −0.02 | 0.98 | .90–1.06 | 0.603 | −0.06 | 0.94 | .84–1.06 | 0.316 |
| Self-Maintenance | ||||||||||||
| Wave1 | −0.1 | 0.9 | .84–.97 | 0.006 | 0.01 | 1.01 | .93–1.1 | 0.833 | −0.11 | 0.9 | .80–1.00 | 0.062 |
| Wave2 | −0.12 | 0.88 | .83–.95 | 0 | 0.04 | 1.04 | .96–1.13 | 0.284 | −0.11 | 0.9 | .81–.99 | 0.039 |
| Travel Activities | ||||||||||||
| Wave1 | −0.11 | 0.9 | .76–1.07 | 0.231 | −0.09 | 0.91 | .73–1.14 | 0.409 | 0.05 | 1.05 | .79–1.40 | 0.727 |
| Wave2 | −0.07 | 0.93 | .81–1.07 | 0.322 | 0.01 | 1 | .84–1.20 | 0.953 | −0.04 | 0.96 | .78–1.17 | 0.665 |
| Novel Information | ||||||||||||
| Wave1 | −0.02 | 0.98 | .95–1.00 | 0.033 | −0.05 | 0.95 | .92–.98 | 0.004 | −0.01 | 1 | .96–1.04 | 0.856 |
| Wave2 | −0.04 | 0.96 | .94–.99 | 0.002 | −0.04 | 0.96 | .93–.99 | 0.004 | −0.03 | 0.97 | .94–1.01 | 0.102 |
| Positive Affect | ||||||||||||
| Wave1 | −0.27 | 0.76 | .62–.94 | 0.012 | 0.05 | 1.05 | .79–1.41 | 0.719 | −0.42 | 0.66 | .44–.98 | 0.04 |
| Wave2 | −0.01 | 1 | .71–1.40 | 0.98 | −0.21 | 0.81 | .55–1.21 | 0.305 | 0.49 | 1.63 | 1.06–2.51 | 0.027 |
| Negative Affect | ||||||||||||
| Wave1 | −0.07 | 0.94 | .67–1.31 | 0.7 | 0 | 1 | .68–1.49 | 0.986 | 0.18 | 1.2 | .70–2.06 | 0.516 |
| Wave2 | −0.22 | 0.8 | .58–1.11 | 0.186 | −0.29 | 0.75 | .49–1.14 | 0.181 | −0.01 | 0.99 | .61–1.60 | 0.957 |
| MMSE | ||||||||||||
| Wave1 | −0.48 | 0.62 | .45–.84 | 0.003 | −0.14 | 0.86 | .57–1.31 | 0.497 | −0.72 | 0.49 | .27–.88 | 0.017 |
| Wave2 | −0.63 | 0.53 | .41–.69 | 0 | −1 | 0.91 | .66–1.26 | 0.564 | −0.57 | 0.56 | .37–.85 | 0.007 |
Note: p values < .05 are in boldface. OR = odds ratio; CR = confidence interval. Wave1 = Wave 1 predictors of Wave 2 cognitive status; Wave2 = Wave 2 predictors of Wave 2 cognitive status.
Results of NIC (Stable, Unstable) Analyses
Second, we conducted the same logistic regression analyses, beginning with the contrasts of stable NIC (NIC-to-NIC) versus the unstable (NIC-to-MCI) group. A test of the full model with the W1 predictors was close to significant [λ2(9) = 16.84, p = 0.051]. According to the Nagelkerke R2, the set of predictors accounted for 17.1% of the variance in group membership at W2. The Wald statistic indicated that novel cognitive activities at W1 [Wald (1) = 8.47, p = 0.004] reliably predicted group membership at W2. Classification was very good for the NIC-to-NIC group (98.2% correct), but much poorer for the NIC-to-MCI group (19.4% correct), for an overall correct classification rate of 81.1%. Second, a test of the full model with the W2 markers predicting W2 status was statistically reliable [λ2(9) = 17.89, p < 0.05]. According to the Nagelkerke R2, the set of predictors accounted for 16.1% of the variance in group membership. The Wald statistic indicated that novel cognitive activities at W2 [Wald (1) = 8.15, p = 0.004] reliably separated the MCI group from the NIC group. Classification was very good for the NIC-to-NIC group (97.7% correct), but much poorer for the NIC-to-MCI group (5.9% correct), for an overall correct classification rate of 78.8%. Participants were over-classified into the NIC group.
Results of MCI (Stable, Unstable) Analyses
Third, we conducted the same logistic regression analyses with the contrasts of stable MCI (MCI-to-MCI group) versus the unstable MCI (MCI-to-NIC group). First, a test of the full model with the W1 factors (predicting W2 status) was statistically reliable [λ2(9) = 20.71, p < 0.05]. According to the Nagelkerke R2, the set of predictors accounted for 28.9% of the variance in group membership. The Wald statistic indicated that W1 predictors diastolic BP [Wald (1) = 4.73, p = 0.03], positive affect [Wald (1) = 4.2, p = 0.04], and MMSE [Wald (1) = 5.66, p = 0.017] reliably predicted group membership at W2. Classification was very good for the stable MCI-to-MCI group (93.5% correct), but poorer for the MCI-to-NIC group (22.7% correct), for an overall correct classification rate of 77.8%. Second, a test of the full model with the W2 factors (predicting W2 status) was statistically reliable [λ2(9) = 27.83, p < 0.01]. According to the Nagelkerke R2, the set of predictors accounted for 33.1% of the variance in group membership. The Wald statistic indicated that self-maintenance activities [Wald (1) = 4.724 p = 0.039], positive affect [Wald (1) = 4.89, p = 0.027], and MMSE [Wald (1) = 7.26, p = 0.007] reliably separated the MCI group from the NIC group. Classification was better for the MCI-to-MCI group (95.4% correct) than for the MCI-to-NIC group (38.5% correct), for an overall correct classification rate of 82.3%.
Summary
The logistic regression findings indicate that diastolic blood pressure, self-maintenance activities, positive affect, and MMSE comprise a set of particularly important functional markers of MCI in aging populations. Moreover, involvement in novel cognitive activities predicts better preservation of cognitive functioning, and is associated with a reduced risk of developing MCI.
Discussion
The purpose of this study was to systematically compare concurrently and longitudinally two cognitive status groups of older adults on a comprehensive set of functional markers. Such markers are often proposed as potential factors for (a) distinguishing the groups in an early phase of impairment, (b) marking their emerging status transitions, and (c) distinguishing their stability in cognitive status. Conceptually, our approach views emerging cognitive impairment as (a) individually quite mild and developmentally continuous with normal aging, (b) manifested in one or more key cognitive domains, (c) incremental and gradual in its emergence, and (d) following knowable trajectories including those represented eventually by specific subtypes of neurodegenerative disorders. Methodologically, notable features of the approach used in this study include (a) the complementary deployment of cross-sectional and longitudinal designs to examine concurrent differences and changes and stabilities in MCI-related characteristics, (b) the novel multidimensional range of potential risk/protection functional factors (ranging from biological to lifestyle) assembled for comparisons, (c) objective procedures for provisionally classifying the two cognitive status groups, and (d) the sample featuring older adults spanning three decades (60s–90s). We summarize and discuss the results for our four research questions.
Research Question 1
After applying systematic and multi-variable classification procedures, the first goal of the study was to identify baseline status group differences in a comprehensive set of functional factors. The cross-sectional comparisons of NIC and MCI groups at W1 showed that global cognition plus seven factors from three of the four main conceptual domains (biological vitality, lifestyle activities, personal affect, but not subjective health) were associated with significant group differences. The observed differences were in the predicted direction of NIC participants displaying higher performance or activity levels than the MCI participants. Regarding the biological vitality factors, both diastolic blood pressure and BMI were associated with cognitive impairment status. This result is consistent with (and extends) previous research showing normal aging differences in hypertension and obesity and the typical dementia-normal aging differences in select dimensions of cognitive functioning (e.g., Cherbuin et al., 2009; Chu, Tam, Lee, Yik, Song, Cheung, et al 2009; Luchsinger, Patel, Tang, Schupf, & Mayeux, 2007; Nilsson & Nilsson, 2010; Nourhashemi, Deschamps, Larrieu, Letenneur, Dartigues, & Barberger-Gateau, 2003; Raz et al., 2009).
Notably lower levels of the broad-based lifestyle activities (i.e., self-maintenance, travel and novel cognitive activities) were reported by the MCI group than by the cognitively normal older adults. Overall, this result extends the occasional aging- and dementia-related differences reported elsewhere in the literature (e.g., Fratiglioni et al., 2004; Hertzog et al., 2008; Stern, 2009). Conceivably, the cognitive-based practice and stimulation provided by the typical everyday tasks captured by the self-maintenance activities scale (including preparing a meal or shopping) may serve to buttress or support a broader range of cognitive abilities used in everyday life and reflected in neurocognitive assessments of impairment status. Other studies have reported deficits for similar MCI groups among even more outwardly functional or complex activities of daily living (e.g., Bangen, Jak, Schiehser, Delano-Wood, Tuminello, Han, et al., 2010; Burton, Strauss, Bunce, Hunter, & Hultsch, 2009), but this is the first report of personally-oriented support and sustenance activities being related to cognitive status. Whereas self-maintenance activities are practical and essential in everyday life, travel activities (e.g., travelling away from home or outside the province/state) and novel cognitive activities (e.g., completing income tax, playing chess or bridge) are typically optional and depend more on an individual's choice, initiative, and perseverance. Together, travel and novel cognitive activities may support cognitive maintenance in normal aging, and even distinguish provisionally impaired groups from normal aging. If such activities facilitate the maintenance of cognitive functioning with aging, they may do so through mechanisms associated with everyday practice, cognitive complexity, and neurocognitive reserve (e.g., Small, Hughes, Hultsch, & Dixon, 2007; Stern, 2009). Novel cognitive activities have been previously linked to better cognitive performance among healthy older adults (Hultsch, Hertzog, Small, & Dixon, 1999; Kåreholt, Lennartsson, Gatz, & Parker, 2011) but not to MCI groups. To establish the presumed longitudinal sequence of effects—whether such activities “lead” or “lag” cognitive status—future research with more than two waves is advised (e.g., Small et al., in press).
Higher levels of both positive and negative affect were reported by the NIC compared to the MCI participants, but these results should be placed in the context of normal and relatively modest levels of affect for both groups. Positive emotional attitudes may be associated with the preservation of cognitive function in old age, and possibly linked with a tendency for some neurologically healthy individuals to maintain engaged lifestyle activities that positively influence cognitive performance and change. The mild negative affect results complement previous research (Wilson et al., 2007) in that somewhat stronger negative affect is expressed by MCI participants. We used a brief measure targeting milder levels of negative affect (e.g., boredom), and other aspects and intensities of affect could be studied with MCI groups.
Summary
The overall pattern of the present results is complementary to that observed in extant cross-sectional MCI-aging literature. Some studies have observed cognitive impairment-related group differences for an assortment of factors such as: (a) biological vitality, including systolic blood pressure (Cherbuin et al., 2009), hypertension (Cherbuin et al., 2009; Raz et al., 2009), peak expiratory flow (Chyou, White, Yano, Sharp, Burchfiel, Chen, et al., 1996; Cook, Albert, Berkman, Blazer, Taylor, & Hennekens, 1995), grip strength (Boyle et al., 2009), and visual and auditory acuity (Valentijn, van Boxtel, van Hooren, Bosma, Beckers, Ponds, et al., 2005); (b) lifestyle activities, including physical (Verghese et al., 2006), social (Zunzunegui, Alvarado, Del Ser, & Otero, 2003), and passive and integrative (Wang, Zhou, Li, Zhang, Deng, Tang, et al., 2006) activities; (c) psychosocial affect, especially depression (Lopez, Jagust, Dulberg, Becker, DeKosky, Fitzpatrick, et al., 2003); and (d) subjective health (Bond et al., 2006). That there are some inconsistencies across the literature is not surprising from several perspectives. First, the presence of selective effects suggests the importance of developing further consensus in measurement, methodology, and classification regarding MCI (e.g., Albert et al., 2011; Winblad et al., 2004). The present study is designed to contribute to this effort in that it examines multiple cognitive and functional factors simultaneously in objectively classified groups that achieved high levels of status stability. Second, some of the previous prospective studies investigating risk factors of MCI were carried out with different populations, including both younger and older groups (Lopez et al., 2003; Tervo, Kivipelto, Hanninen, Vanhanen, Hallikainen, Mannermaa, et al., 2004). The effects may vary by age and accumulated co-morbidities, perhaps as a result of selective survival and recruitment. Third, although we found interesting results with three of our cognitive-based lifestyle activity scales, the physical and social activity scales have a limited number of items and may not reflect those activities that differentiate mildly impaired from normally declining older adults (Hughes & Ganguli, 2009; Larson, Wang, Bowen, McCormick, Teri, Crane, et al., 2006). It is instructive that multiple researchers are detecting impairment group differences for a complementary set of indicators from the domains of biological vitality (e.g., vascular), lifestyle activities (e.g., cognitive), and psychosocial affect. At the cross-sectional level, these modest similarities encourage further research, especially with longitudinal and predictive designs.
Research Questions 2 and 3
The next two goals were to examine (a) longitudinal stability of the cognitive status group classification and (b) the potential role of longitudinal cognitive status stability on functional marker performance and change. For research question 2, we independently and objectively classified the participants who returned for W2 as either MCI or NIC. We then computed stability information for these continuing participants. The stability of classification was notably high (cf. with those observed in previous research with cognitively impaired participants: de Frias et al., 2009; Palmer et al., 2002; Tuokko & Hultsch, 2006). Specifically, about 82% of our returning W1 NIC group and 72.5% of our W1 MCI group retained their status over the longitudinal interval.
Given these high (but not perfect) stabilities, we then pursued a fine-grained series of analyses, as stipulated in research question 3. Specifically, we compared in several combinations the stable/not stable NIC/MCI subgroups to one another in their performance on the candidate functional marker variables over the two waves of measurement. The stable NIC-to-NIC versus MCI-to-MCI contrast showed that better scores on diastolic BP, self-maintenance activities, travel activities, novel cognitive activities, positive and negative affect, and MMSE differentiated the stable NIC group from the stable MCI group. Compared to the stable NIC, the stable MCI participants, especially the ones in the older age group, showed a greater decline over the two waves of measurement in their involvement in self-maintenance activities, such as preparing a meal or shopping. These results extend previous research suggesting that the relationships between lifestyle activity and cognition may be slightly greater in the later half of older adulthood (Bielak et al., 2007), and perhaps especially for cognitively impaired older adults. The stable NIC group had better levels of both positive and negative affect, compared to the stable MCI group. Consistent with the idea of positivity bias in aging (Carstensen & Mikels, 2005), positive affect increased over the two waves of measurement in both groups. Interestingly, negative affect changed in the unstable MCI-to-NIC and NIC-to-MCI groups, with participants showing higher levels of negative affect at the waves in which they were classified as NIC (i.e., negative affect decreased over the two waves in the NIC-to-MCI group and increased in the MCI-to-NIC group).The NIC group is more cognitively and socially active, and possibly more likely to encounter cognitive challenges that lead to early awareness of deficits which, in turn, may contribute to slight (subclinical) elevations in negative affect.
The comparison of the stable NIC (i.e., NIC-to-NIC) group versus the unstable (status-declining) NIC-to-MCI group extends previous research showing that a higher level of engagement in mentally stimulating activities was associated with those not experiencing notable longitudinal cognitive decline (i.e., the NIC-NIC group; Hultsch et al., 1999; Schooler & Mulatu, 2001; Wang et al., 2006). Finally, the comparison of the stable MCI (MCI-to-MCI group) versus the unstable (but improving) MCI (MCI-to-NIC) group showed that individuals whose cognitive status improved, especially those in the mid-old group, had overall better global cognitive performance compared to the ones who remained in their MCI classification status. Interestingly, the MCI-to-NIC subgroup also had higher diastolic BP at W1, similar to the stable NIC-to-NIC group, but at W2 the scores decreased, becoming more similar overall to the stable MCI group. Self-maintenance activities were influenced by age, cognitive status, and wave of measurement. They were preserved in the improved (MCI-to-NIC) group, while they decreased for the stable MCI group, especially for the oldest participants in this group.
Research Question 4
The fourth goal of the study was to examine whether cognitive group status could be predicted by concurrent performance on the eight significant functional risk factors identified previously. In the stable NIC-to-NIC versus MCI-to-MCI group, higher baseline scores on diastolic BP, self-maintenance and novel cognitive activities, positive affect and global cognitive status were identified as significant predictors of decreased risk of cognitive decline by wave 2. The comparison of the stable NIC (i.e., NIC-to-NIC) group versus the unstable (status-declining) NIC-to-MCI group provided results consistent with previous research identifying novel cognitive activities as one of the few domains that significantly predicted longitudinal change (Bielak et al., 2007; Small et al., 2011). Finally, the comparison of the stable MCI (MCI-to-MCI group) versus the unstable (but improving) MCI (MCI-to-NIC) group identified higher baseline scores on diastolic BP and positive affect, as well as better global cognition (MMSE), as significant predictors of cognitive status improvements at the second wave.
The precise mechanisms through which these functional risk factors might affect cognition are still unclear, but there are a number of reasonable hypotheses. We explore, in turn, possible mechanisms associated with each significant risk factor. Regarding diastolic blood pressure, two mechanisms have been proposed to interpret the association between diastolic BP and cognitive decline: blood pressure levels decrease during the course of the cognitive decline process, and lower diastolic BP might induce or accelerate cognitive decline by lowering cerebral blood flow (hypoperfusion/hypoxia (Guo, Viitanen, Fratiglioni, & Winblad, 1996; Henry-Feugeas, 2008). In addition, midlife elevation of BP is a risk factor for later cognitive decline (Hughes & Ganguli, 2009). The beneficial effect of cognitively based lifestyle indicators, such as self-maintenance and novel cognitive activities, might operate through cognitive reserve (Small et al., 2007; Stern, 2009a), which may be enabled by more efficient use of brain networks or a better ability to recruit alternative brain networks as needed. In addition, participation in self-maintenance activities fulfils a meaningful role, which could potentially sustain a person’s self-concept of competence and usefulness, which in turn may lead to more practice, increased effort, and perhaps lower rates of cognitive decline (Seeman, McAvay, Merrill, Albert, & Rodin, 1996). Regarding psychosocial affect, positive affect can have direct and beneficial effects on physiological, hormonal and immune function which in turn influence health outcomes. It may also have important indirect effects on cognitive functioning through its influence on health-promoting behaviours (Moskowitz et al., 2008), helping individuals maintain the kinds of physical, social and intellectual activities that could buffer cognitive decline in old age.
Conclusion and Limitations
The results of this study show that (a) eight relatively distal (but functional) risk/protection factors may influence concurrent status and longitudinal stabilities in both cognitively normal and mildly impaired older adults, and (b) five of these functional markers performed as predictors of cognitive status group membership several years later. Although all of the baseline candidate factors were previously mentioned or tested in the literature, they have not been considered together, or tested within the same population. We emphasize that cognitive impairment in older adults is also affected by proximal neurobiological factors (see Figure 1), which are unmeasured in this study (see Albert et al., 2011). Nevertheless, the results of the present study demonstrate that functional markers from the domains of biological vitality, lifestyle activities, psychosocial affect, as well as global cognition, may also play an important role in modulating these effects (Anstey, 2008; Cherbuin et al., 2009). Moreover, although other risk factors show beneficial relationships in the short term, activities that challenge cognitive skills seem to offer the best predictive power for longer term cognitive functioning (Small et al., 2007; Small et al., in press; Stern 2007).
Regarding the present study and the future research it implies, several specific strengths and limitations should be noted. First, the extensive VLS sample and battery provided an opportunity to (a) objectively identify and characterize large groups of provisionally normal and mildly impaired older adults and (b) systematically test a comprehensive set of candidate functional markers of MCI, including both protection and risk factors, well-matched to a growing literature. Second, the methodological strengths of examining relatively large samples with complete data on multifaceted indicators measured in both cross-sectional and longitudinal designs should be noted. Third, our fully objective classification procedures produced notably high longitudinal status stabilities as well as the opportunity to examine differential profiles by stability status.
Despite the robust set of results, several limitations should be listed. First, as noted in the introduction, we used an objective, multi-variable, well-defined, and documented classification procedure to create two clusters of cognitive status. There are, however, other legitimate procedures, clinical approaches, and methodological concerns in the literature, any one of which may constrain the generalizability of single studies, including our own. For example, there are multiple clinical and operational definitions of the phenomena of cognitive impairment. Ours reflects in part the relatively intact and pre-clinical samples of the available study populations, as well as the perspective emphasizing some continuity from normal aging to MCI conditions. Second, we should note that our 5-test cognitive reference battery proved to be a sensitive, effective, and reliable measurement instrument for cognitive impairment. Nevertheless, future research may be directed at refining the specific contributions of each subtest to the overall battery, including the relative performances by the subtests in (a) classifying MCI participants concurrently and (b) producing classifications that are stable. Third, one important limitation of our procedure is that we do not yet have data available on the future outcomes of these status transitions; that is, to date, these VLS participants have not yet produced substantial numbers of incident cases of dementia. Therefore, linking our neurocognitive indicators to eventual neurodegenerative diseases awaits future longitudinal research. In addition, the available two waves do not permit optimal tracking of longer-term individualized trajectories. Fourth, although we tested a wide range of functional variables, we included neither proximal brain-related indicators nor genetic markers. Future research would benefit from including carefully selected neuroimaging (e.g., volumetric measures), neuro-related biomarkers (e.g., cerebrospinal fluid derived beta-amyloid indicators), and genetic (e.g., APOE) markers in the context of suitably large samples and longitudinal data. Fifth, we acknowledge the potential relevance of sample selection and attrition. In the present case, there is a general sample selection introduced in the VLS sampling frame. The VLS was designed to begin at baseline with relatively healthy older adults, following them as they developed neurocognitive conditions of interest (e.g., MCI). Notably, this is both a limitation and an advantage, in that we are able to track emerging cases of impairment. Regarding attrition, drop-outs occur in any 2-wave design, but a stability study requires two complete waves of data.
The present study focuses on functional markers, transitions, and stabilities in both normal aging and probable cognitive impairment. Our findings offer a comprehensive perspective of potentially modifiable functional risk factors associated with MCI, with ramifications for future research across several fields of study and clinical applications. Such evidence highlights the importance of positive affect, global cognitive status, self-maintenance and intellectually stimulating activities to preserve cognitive functioning in old age. Interventions targeting these risk factors may be beneficial in modulating normal decline and emerging impairment in otherwise healthy older adults.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (National Institute on Aging) to Roger A. Dixon (R37 AG008235). Dr. Dixon is also supported by the Canada Research Chairs program. Stuart MacDonald was supported by a Scholar Award from the Michael Smith Foundation for Health Research. We appreciate the many important contributions of VLS participants and staff (including Jill Friesen, Terry Perkins, and Bonnie Whitehead) to the present research. We gratefully acknowledge the helpful contributions of the late Dr. Esther Strauss to an initial conceptualization of this project. Dr. Dolcos is now at Department of Psychology, University of Illinois at Urbana-Champaign. Further information about the VLS may be accessed via: http://www.ualberta.ca/~vlslab/index.html.
References
- Albert MS, DeKoskey ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease. Alzheimer's & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K. Cognitive aging and functional biomarkers: What do we know, and where from here? In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Los Angeles: Sage; 2008. pp. 327–340. [Google Scholar]
- Anstey KJ, Cherbuin N, Chirstensen H, Burns R, Reglade-Meslin C, Salim A, Kumar R, Jorm AF, Perminder S. Follow-up of Mild Cognitive Impairment and related disorders over four years in adults in their sixties: The PATH Through Life Study. Dementia and Geriatric Cognitive Disorders. 2008;26:226–233. doi: 10.1159/000154646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Smith GA. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychology and Aging. 1999;14:605–618. doi: 10.1037//0882-7974.14.4.605. [DOI] [PubMed] [Google Scholar]
- Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, Dartigues JF, Tzourio C, Rouaud O, Poncet M, Pasquier F, Auriacombe S, Touchon J, Ritchie K. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:979–1084. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- Bangen KJ, Jak AJ, Schiehser DM, Delano-Wood L, Tuminello W, Han SD, et al. Complex activities of daily living vary by mild cognitive impairment subtype. Journal of the International Neuropsychological Society. 2010;16:630–639. doi: 10.1017/S1355617710000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak AA, Hughes TF, Small BJ, Dixon RA. It's never too late to engage in lifestyle activities: significant concurrent but not change relationships between lifestyle activities and cognitive speed. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2007;62:P331–P339. doi: 10.1093/geronb/62.6.p331. [DOI] [PubMed] [Google Scholar]
- Bond J, Dickinson H, Matthews F, Jagger C, Brayne C. Self-rated health status as a predictor of death, functional and cognitive impairment: a longitudinal cohort study. European Journal of Ageing. 2006;3:193–206. doi: 10.1007/s10433-006-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Archives of Neurology. 2009;66:1339–1344. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburn NM. The structure of psychological well-being. Oxford, England: Aldine; 1969. [Google Scholar]
- Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55:570–81. doi: 10.1159/000228918. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Cherbuin N, Reglade-Meslin C, Kumar R, Jacomb P, Easteal S, Christensen H, et al. Risk factors of transition from normal cognition to mild cognitive disorder: the PATH through Life Study. Dementia and Geriatric Cognitive Disorders. 2009;28:47–55. doi: 10.1159/000229025. [DOI] [PubMed] [Google Scholar]
- Christensen H, Dear KBG, Anstey KJ, Parslow RA, Sachdev P, Jorm AF. Within occasion intra-individual variability and pre-clinical diagnostic status: Is intra-individual variability an indicator of mild cognitive impairment? Neuropsychology. 2005;19:309–317. doi: 10.1037/0894-4105.19.3.309. [DOI] [PubMed] [Google Scholar]
- Chu LW, Tam S, Lee PW, Yik PY, Song Y, Cheung BM, et al. Late-life body mass index and waist circumference in amnestic mild cognitive impairment and Alzheimer's disease. Journal of Alzheimer's Disease. 2009;17:223–232. doi: 10.3233/JAD-2009-1043. [DOI] [PubMed] [Google Scholar]
- Chyou P-H, White LR, Yano K, Sharp DS, Burchfiel CM, Chen R, et al. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. American Journal of Epidemiology. 1996;143:750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- Cook NR, Albert MS, Berkman LF, Blazer D, Taylor JO, Hennekens CH. Interrelationships of peak expiratory flow rate with physical and cognitive function in the elderly. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1995;50:317–323. doi: 10.1093/gerona/50a.6.m317. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: From cognitively elite to cognitively impaired. Neuropsychology. 2009;23:778–791. doi: 10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA. Enduring theoretical themes in psychological aging: Derivation, functions, perspectives, and opportunities. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7th ed. San Diego, CA: Academic Press; 2011a. pp. 3–23. [Google Scholar]
- Dixon RA. Evaluating everyday competence in older adult couples: Epidemiological considerations. Gerontology. 2011b;57:173–179. doi: 10.1159/000320325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11:346–376. [Google Scholar]
- Dixon RA, Garrett DD, Lentz TL, MacDonald SW, Strauss E, Hultsch DF. Neurocognitive markers of cognitive impairment: exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Wahlin Å, Maitland SB, Hultsch DF, Hertzog C, Bäckman L. Episodic memory change in late adulthood: generalizability across samples and performance indices. Memory & Cognition. 2004;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JEW, Harman HH, Dermen D. Manual for the kit of factor-referenced cognitive tests. Princeton, N.J.: Educational Testing Service; 1976. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Bickel JF, Lovden M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2006;61:P253–261. doi: 10.1093/geronb/61.5.p253. [DOI] [PubMed] [Google Scholar]
- Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: the Kungsholmen project. British Medical Journal. 1996;312:805–808. doi: 10.1136/bmj.312.7034.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry-Feugeas MC. Alzheimer's disease in late-life dementia: a minor toxic consequence of devastating cerebrovascular dysfunction. Medical Hypotheses. 2008;70:866–875. doi: 10.1016/j.mehy.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development. Psychological Science in the Public Interest. 2008;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hughes TF, Ganguli M. Modifiable midlife risk factors for late-life cognitive impairment and dementia. Current Psychiatry Reviews. 2009;5:73–92. doi: 10.2174/157340009788167347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory change in the aged. New York: Cambridge University Press; 1998. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kåreholt I, Lennartsson C, Gatz M, Parker MG. Baseline leisure time activity and cogniiton more than two decades later. International Journal of Geriatric Psychiatry. 2011;26:65–74. doi: 10.1002/gps.2490. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Levy R. Aging-associated cognitive decline. International Psychogeriatrics. 1994;6:63–68. [PubMed] [Google Scholar]
- Liang J, Bennett J, Whitelaw N, Maeda D. The structure of self-reported physical health among the aged in the United States and Japan. Medical Care. 1991;29:1161–1180. doi: 10.1097/00005650-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Lin MY, Gutierrez PR, Stone KL, Yaffe K, Ensrud KE, Fink HA, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. Journal of the American Geriatrics Society. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Archives of Neurology. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Archives of Neurology. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R. Mild cognitive impairment: directions for future research. Neurology. 2003;61:438–444. doi: 10.1212/01.wnl.0000080366.90234.7f. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: findings from the Victoria Longitudinal Study. Gerontology. 2004;50:64–81. doi: 10.1159/000075557. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Hultsch DF, Strauss E, Dixon RA. Age-related slowing of digit symbol substitution revisited: what do longitudinal age changes reflect? Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2003;58:P187–194. doi: 10.1093/geronb/58.3.p187. [DOI] [PubMed] [Google Scholar]
- Maitland SB, Dixon RA, Hultsch DF, Hertzog C. Well-being as a moving target: measurement equivalence of the Bradburn Affect Balance Scale. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2001;56:P69–P77. doi: 10.1093/geronb/56.2.p69. [DOI] [PubMed] [Google Scholar]
- Molander L, Gustafson Y, Lovheim H. Low Blood Pressure Is Associated with Cognitive Impairment in Very Old People. Dementia and Geriatric Cognitive Disorders. 2010;29:335–341. doi: 10.1159/000289821. [DOI] [PubMed] [Google Scholar]
- Moskowitz JT, Epel ES, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychology. 2008;27:S73–S82. doi: 10.1037/0278-6133.27.1.S73. [DOI] [PubMed] [Google Scholar]
- Nilsson L-G, Nilsson E. Overweight and cognition. Scandinavian Journal of Psychology. 2010;50:660–667. doi: 10.1111/j.1467-9450.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- Palmer K, Wang HX, Backman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. American Journal of Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Knopman DS. MCI is a clinically useful concept. International Psychogeriatrics. 2006;18:394–402. doi: 10.1017/S1041610206003929. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raz N. Decline and compensation in aging brain and cognition: Promises and constraints. Neuropsychology Review. 2009;19:411–415. doi: 10.1007/s11065-009-9122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the Risk of Mild Cognitive Impairment. Archives of Neurology. 2007;64:1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Ortiz CA, Kuo YF, DiNuzzo AR, Ray LA, Raji MA, Markides KS. Near vision impairment predicts cognitive decline: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. Journal of the American Geriatrics Society. 2005;53:681–686. doi: 10.1111/j.1532-5415.2005.53219.x. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- Sargent-Cox KA, Anstey KJ, Luszcz MA. Determinants of self-rated health items with different points of reference: implications for health measurement of older adults. Journal of Aging and Health. 2008;20:739–761. doi: 10.1177/0898264308321035. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS. The reciprocal effects of leisure time activities and intellectual functioning in older people: a longitudinal analysis. Psychology and Aging. 2001;16:466–482. doi: 10.1037//0882-7974.16.3.466. [DOI] [PubMed] [Google Scholar]
- Seeman T, McAvay G, Merrill S, Albert M, Rodin J. Self-efficacy beliefs and change in cognitive performance: MacArthur Studies of Successful Aging. Psychology and Aging. 1996;11:538–551. doi: 10.1037//0882-7974.11.3.538. [DOI] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Neuropsychology. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Hughes TF, Hultsch DF, Dixon RA. Lifestyle activities and late-life changes in cognitive performance. In: Stern Y, editor. Cognitive reserve. New York: Psychology Press; 2007. pp. 173–186. [Google Scholar]
- Spiro AI, Brady CB. Integrating health into cognitive aging research and theory: Quo Vadis? In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging. Interdisciplinary perspectives. Los Angeles: Sage Publications, Inc; 2008. pp. 260–284. [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009a;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, editor. Cognitive reserve. New York: Psychology Press; 2007. [Google Scholar]
- Tervo S, Kivipelto M, Hanninen T, Vanhanen M, Hallikainen M, Mannermaa A, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dementia Geriatric Cognitive Disorders. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- Thurstone TG. Primary mental abilities: Grades 9–12, 1962 revision. Chicago: Science Research Associates; 1962. [Google Scholar]
- Tuokko HA, Hultsch DF, editors. Mild cognitive impairment. New York: Taylor & Francis; 2006. [Google Scholar]
- Uhlmann RF, Larson EB, Rees TS. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. Journal of the American Geriatrics Society. 2004;52:1996–2002. [Google Scholar]
- Valentijn SA, van Boxtel MP, van Hooren SA, Bosma H, Beckers HJ, Ponds RW, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. Journal of the American Geriatrics Society. 2005;53:374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, Hall C, Buschke H, Lipton RB. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Brodaty H. MCI is not a clinically useful concept. International Psychogeriatrics. 2006;18:402–409. [PubMed] [Google Scholar]
- Wahlin Å, MacDonald SWS, de Frias CM, Nilsson L-G, Dixon RA. How do health and biological age influence chronological age and sex differences in cognitive aging: Moderating, mediating, or both? Psychology and Aging. 2006;21:318–332. doi: 10.1037/0882-7974.21.2.318. [DOI] [PubMed] [Google Scholar]
- Wang JY, Zhou DH, Li J, Zhang M, Deng J, Tang M, et al. Leisure activity and risk of cognitive impairment: the Chongqing aging study. Neurology. 2006;66:911–913. doi: 10.1212/01.wnl.0000192165.99963.2a. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal Of the American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Zunzunegui MV, Alvarado BE, Del Ser T, Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2003;58:S93–S100. doi: 10.1093/geronb/58.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]