Abstract
Background
Enteroendocrine K cells secrete the incretin hormone glucose-dependent insulinotropic peptide (GIP) and are predominately located in the duodenum. GIP levels should decrease after gastric bypass due to duodenal exclusion; however, studies have found conflicting data regarding the changes in GIP secretion after gastric bypass and duodenal–jejunal bypass (DJB).
Methods
We performed a DJB or Sham surgery on Wistar rats followed by an oral glucose tolerance test on postoperative (post-op) day 12 and superior mesenteric lymphatic cannulation on post-op day 14. We measured meal-stimulated GIP concentrations and small bowel GIP and GLP-1 protein content after DJB or Sham surgery.
Results
There was no difference in glucose tolerance by 12 days post-op. We found no difference in lymphatic GIP concentration area under the curve between DJB and Sham rats (15,240 pg/ml min±2,651 vs. 17,201 pg/ml min± 2,763, respectively, p=0.62). GIP and GLP-1 protein contents were both significantly increased only in the midjejunum in DJB rats compared to Sham rats (p=0.009 and p=0.01, respectively).
Conclusions
Plasma and lymphatic GIP concentrations did not significantly change after DJB in Wistar rats. DJB increased GIP protein content in the midjejunum at the new site of nutrient absorption, but this was surprisingly not countered by a decrease in GIP protein content in the bypassed duodenum. Further studies are needed to determine the mechanisms that account for the discrepancy in GIP production and subsequent secretion after DJB as well as what role GIP plays in the effect of gastrointestinal surgery on glucose homeostasis.
Keywords: Duodenal–jejunal bypass, Glucose-dependent insulinotropic polypeptide, Incretin, Lymph, Roux-en-Y gastric bypass, K cell
Introduction
Roux-en-Y gastric bypass (RYGB) results in the rapid improvement of type 2 diabetes in approximately 80% of morbidly obese patients [1–4]. Changes in secretion of one or both incretin hormones have been a commonly explored mechanism to explain the observed improvements in glucose homeostasis after RYGB. The changes in incretin secretion are likely yielded from the rerouting of the gastrointestinal tract after RYGB with exclusion of the majority of the stomach and entire duodenum and early delivery of nutrients to the distal small bowel.
The incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are responsible for 50–70% of postprandial insulin secretion from the pancreas [5,6]. GIP is secreted from the enteroendocrine K cells in response to enteral nutrients. K cells are located throughout the entire gastrointestinal tract but are primarily concentrated in the duodenum [7]. Despite a primary role as an incretin hormone, recent evidence suggests that GIP oversecretion with a subsequent enhancement in receptor signaling may propagate the development of obesity, insulin resistance, and type 2 diabetes. This is supported by studies of GIP receptor mice, which are resistant to diet-induced obesity and have preserved insulin sensitivity compared to high-fat fed controls [8]. Further, the antagonism of the GIP receptor prevents the development of diabetes in ob/ob mice and reverses obesity-associated metabolic disturbances in high-fat fed mice [9, 10].
Given the proximal focus of GIP secreting K cells, bypassing the entire duodenum should theoretically result in a dramatic reduction in nutrient-mediated GIP secretion. However, there have been a large number of conflicting reports regarding the changes noted in GIP secretion after RYGB, including increased, decreased, or no change in secretion. In a published review of longitudinal studies of RYGB or biliopancreatic diversion (BPD) and the effect on incretin secretion, results varied from a decrease in fasting and postprandial GIP as early as 1 week after surgery, to no change at 12 months, to an increase in postprandial levels 1 month after surgery [11–14]. Many of these discrepancies may be related to differences in surgical bypass limb length, time from surgery, fasting or postprandial assessment, and the assay used for GIP measurement.
In this study, we utilized lymphatic incretin sampling as a more sensitive medium than plasma [15] to detect postprandial changes in GIP secretion induced by duodenal–jejunal bypass (DJB; also known as duodenal–jejunal exclusion) surgery, an experimental surgery used initially in rodents to model RYGB [16–18]. DJB does not exclude the stomach as in a RYGB but shares duodenal and proximal jejunal exclusion to nutrient flow, a jejunal Roux-en-Y reconstruction, and early nutrient delivery to the distal small bowel. We have previously published that in Goto–Kakizaki rats, the primary mechanism for a modest improvement in glucose tolerance after DJB appears to be mediated by the surgical rerouting of the small bowel with a resultant increase in GLP-1 secretion and receptor signaling [19]. However, it is unclear if in other models of type 2 diabetes, hormonal mechanisms beyond the GLP-1 receptor, such as a reduction in GIP secretion or signaling, are involved.
We performed DJB or Sham surgery in chow fed Wistar rats, followed 2 weeks later by lymphatic cannulation for the assessment of gastrointestinal GIP secretion in response to a mixed meal. We hypothesized that bypassing the duodenum, the major site of enteroendocrine K cells, would result in a reduction in lymphatic GIP concentrations. To further characterize the changes in GIP secretion mediated by the gastrointestinal surgery, we compared GIP protein content within separate anatomic segments of small bowel of both surgical groups. In addition, GLP-1 protein content was analyzed within the small bowel of DJB and Sham rats as a comparison gastrointestinal hormone.
Methods
Animals
Eleven- to 12-week-old male Wistar rats (Charles River Laboratories, Wilmington, MA, USA) were allowed to acclimate to their environment for 1 week prior to the beginning of the experiment. Rats had free access to chow (Harlan Teklad standard rodent diet 7012, Indianapolis, IN, USA) except as noted for the experimental protocols below. All procedures were approved by the University of Cincinnati’s Institutional Animal Care and Use Committee.
Duodenal–Jejunal Bypass
DJB (n=10) and Sham surgeries (n=9) were performed as previously described under 18-h fasting conditions [19]. In brief, the duodenum was divided just distal to the pylorus. The jejunum was divided 10 cm distal to the ligament of Treitz (LOT). A duodenojejunostomy was created by anastomosing the proximal segment of divided duodenum to the distal segment of divided jejunum. A jejujejunostomy was made 15 cm distal from the duodenojejunostomy in an end-to-side fashion. Postoperatively, rats were allowed access to water for the first 24 h. On postoperative day (POD)1, rats were started on a liquid diet of regular vanilla Ensure ad lib. On POD2, all rats were returned to their standard chow diet. For Sham surgeries, the bowel was divided and re-anastomosed at the proximal duodenum and 10 and 25 cm distal to the LOT. All DJB and Sham rats received the same pre- and postoperative care. Body weight and food intake were measured daily for 2 weeks following surgery.
Oral Glucose Tolerance Test
An oral glucose tolerance test (OGTT) was performed on all rats at approximately POD 12. D-Glucose (2 g/kg) was administered by oral gavage to overnight fasted animals. Glucose was measured in duplicate from the tail vein using a hand-held glucometer at 0, 15, 30, 60, and 120 min after the gavage. Blood samples were simultaneously collected in EDTA-coated collecting tubes for the measurement of insulin concentration. Samples were spun at 4,000×g for 10 min at 4°C, and plasma was stored at −20°C for later analysis.
Lymph and Stomach Cannulation
Lymphatic cannulation was performed approximately 2 weeks postoperatively from the metabolic surgery (POD14–16). Rats were fasted overnight but allowed free access to water. Lymphatic cannulation of the superior mesenteric lymphatic duct was performed as previously described [20]. A gastrotomy was made along the greater curvature of the stomach, and a silicone tube (0.04 in ID and 0.085 in OD; VWR International, West Chester, PA, USA) was placed into the stomach and secured with 4–0 silk suture. Both the lymphatic and gastric tubes were exteriorized thru the lateral abdominal wall. Animals were kept in Bollman, restraint cages for the remainder of the study and housed in temperature regulated isolettes at 25°C. Successful lymphatic cannulation was performed in 8 of 10 DJB rats and 8 of 9 Sham rats. Once the rat was fully awake after lymphatic cannulation, an infusion of 0.9% NaCl solution was started through the gastric tube at 3 ml/h to replenish the fluid and electrolyte loss from continuous lymphatic drainage. Nutrient administration and lymph collections were performed the same day, allowing the animals to recover from cannulation surgery for at least 2 h.
Nutrient Administration and Sample Collection
Lymph was continuously collected on ice for 10 min prior to the intragastric administration of a 7.68-ml/kg regular vanilla Ensure bolus. Lymph was subsequently collected in 10-min increments for 1 h total. Blood samples were simultaneously collected from the tail vein corresponding to the lymph sample time points at fasting, 15, 35, and 55 min. Both lymph and blood samples were collected in EDTA-coated tubes with the addition of a 1% dipeptidyl peptidase-4 inhibitor (Millipore, St. Charles, MO, USA). Blood samples were spun at 4°C at 4,000×g for 10 min, and the supernatant was stored at −20°C until later use.
Hormone Assays
Total GIP, active GLP-1, and insulin concentrations were determined using separate commercially available rat/mouse, sandwich ELISA kits (Millipore, St. Charles, MO, USA). The GIP ELISA measures both active GIP (1–42) and inactive GIP (3–42) and does not cross react with glucagon, oxyntomodulin, GLP-1, and GLP-2. The GLP-1 ELISA measures the active forms of GLP-1, including GLP-1 (7–36) and GLP-1 (7–37), and does not cross react with inactive forms of GLP-1, glucagon, or GLP-2. The insulin ELISA is 100% specific for rat insulin and does not cross react with rat C-peptide.
Intestinal GIP and GLP-1 Protein Content
Four separate segments of small bowel were isolated for GIP and GLP-1 protein content. These segments included (1) the second segment of the duodenum, (2) the proximal jejunum 10 cm distal to the LOT (Sham rats) or just distal to the duodenojejunostomy (enteral limb of DJB rats), (3) the midjejunum 25 cm distal to the LOT (Sham rats) or just distal to the jejujejunostomy (common channel in DJB rats), and (4) the distal ileum. Intestinal isolation and processing was performed as previously described [19]. After homogenization in acetic acid and lyophilization, the dried segments were resuspended in dH20, diluted, and analyzed for total protein content, total GIP, and active GLP-1 concentrations.
Statistics
Area under the curve (AUC) was calculated using the trapezoidal rule. Glucose, insulin, and GIP AUC as well as incretin protein content for each intestinal segment were compared using a t test. Comparisons of results between the two experimental groups over time (body weight, food intake, glucose, insulin, and GIP concentrations) were performed using a two-way repeated-measures ANOVA. A Holm–Sidak test was used for post hoc analysis. All values are presented as the mean±SE. Values were determined statistically significant if p<0.05.
Results
Body Weight and Food Intake
We found no difference in daily postoperative body weights between the two surgical groups for the 2-week study duration. All animals lost weight until resumed on their chow diet and had returned to their preoperative body weights by POD5 (Fig. 1a). At the completion of the study, DJB rats weighed 379.0 g±2.4 and Sham rats weighed 386.3 g±2.6 (p=0.44). As shown in Fig. 1b, DJB rats ate significantly less chow than Sham rats from POD3–8 (excluding POD6). By POD9, the DJB and Sham rats had no difference in daily food intake (30.04 g±0.96 vs. 30.51 g±1.02, respectively, p=0.77).
Fig. 1.
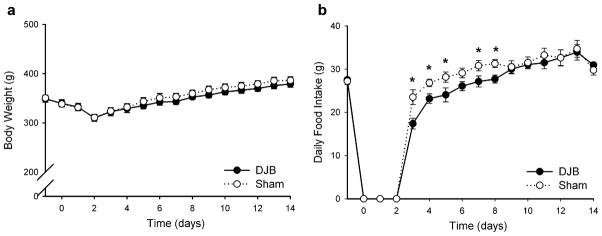
Body weight and food intake after gastrointestinal surgery in Wistar rats. Body weights (a) and food intake (b) were assessed daily preoperatively and for 2 weeks postoperatively. *p<0.05, statistically different between the two surgical groups. Data are presented as mean±SE
Glucose Homeostasis
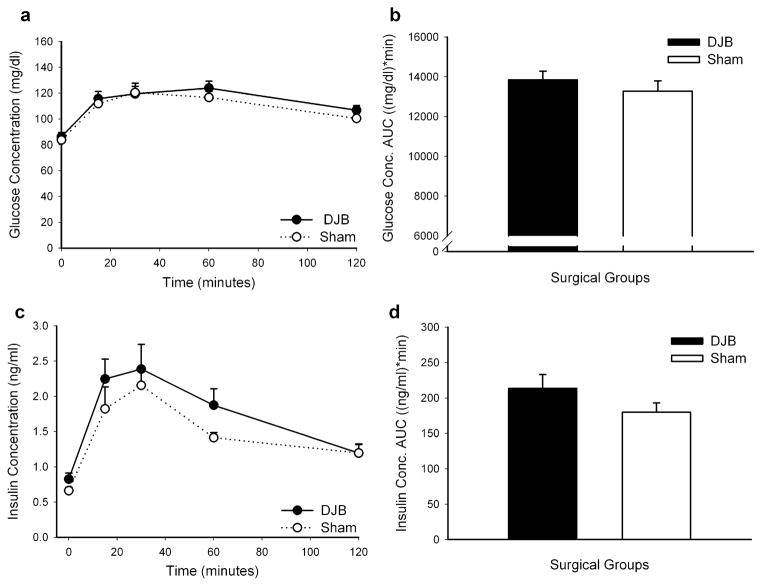
A 2-g/kg D-glucose OGTT was performed approximately 12 days after DJB or Sham surgery. There was no difference in plasma glucose concentrations between the two groups for any time point measured (Fig. 2a). Similarly as shown in Fig. 2b, there was no difference in glucose concentration AUC between DJB and Sham rats (13,851 mg/dl min±442 vs. 13,280 mg/dl min±518, respectively, p=0.41). As shown in Fig. 2c, there was an insignificant trend for increased insulin secretion to an oral glucose load in DJB rats compared to Sham rats less than 2 weeks after surgery. The greatest difference was noted at 60 min after the administration of an oral glucose gavage (DJB 1.87 ng/ml±0.19, Sham 1.41 ng/ml±0.20; p=0.14). This trend was reflected in the insulin concentration AUC in Fig. 2d. DJB rats had an insulin concentration AUC of 213.6 ng/ml min±19.4 compared to Sham rats of 180.1 ng/ml min±13.1 (p=0.18).
Fig. 2.
An oral glucose tolerance test (2 g/kg D-glucose) was performed 12 days after DJB or Sham surgery. Plasma glucose concentrations (a) and insulin concentrations (c) were measured at fasting and 15, 30, 60, and 120 min after the glucose gavage. Glucose concentration AUC (b) and insulin concentration AUC (d) were determined using the trapezoidal rule. Data are presented as mean±SE
Plasma and Lymphatic GIP Concentration
We performed superior mesenteric lymphatic cannulation with simultaneous placement of a gastric tube for nutrient delivery approximately 2 weeks after DJB or Sham surgery. Lymph and tail vein plasma samples were collected at fasting and for 1 h after the administration of mixed meal gastric bolus. We found no difference in the lymphatic flow rates between DJB and Sham rats (data not shown). As shown in Fig. 3a, we found no difference in plasma GIP concentrations at any time point measured between DJB and Sham rats. Supporting this finding, there was no difference in intestinal lymphatic GIP concentrations after a mixed meal bolus despite this being a more sensitive medium for incretin detection (Fig. 3a). There was an insignificant trend toward a lower GIP peak concentration reached at 25 min after the nutrient bolus in DJB rats compared to Sham rats (353 pg/ml±63 vs. 466 pg/ml±110, respectively, p= 0.18). There was also no difference between the two groups as shown in Fig. 2b in plasma (DJB 3,996 pg/ml min±399, Sham 4,772 pg/ml min±559; p=0.27) or lymphatic GIP concentration AUC (DJB 15,240 pg/ml min±2,651, Sham 17,201 pg/ml min±2,763; p=0.62).
Fig. 3.
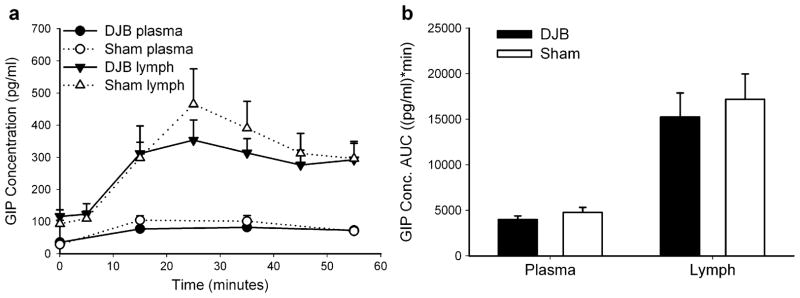
Total GIP concentrations were measured from plasma or gastrointestinal lymph (a) at fasting and for 1 h following the administration of a mixed meal (7.68 ml/kg Ensure). Plasma and lymphatic GIP concentration AUC (b) was calculated using the trapezoidal rule. Data are presented as mean±SE
Small Bowel GIP and GLP-1 Protein Content
To determine how GIP protein content changed after DJB surgery, we examined four separate segments of the small bowel after duodenal bypass and the corresponding anatomical segments of small bowel in Sham rats. As shown in Fig. 4a, at 2 weeks after surgery, there was a significant increase in midjejunal GIP protein content in DJB rats (the “common channel” for nutrient and bile/pancreatic secretions) compared to Sham rats (percentage of total GIP protein/total protein; 0.0037%± 0.0003 vs. 0.0026%±0.0002, p=0.009). Despite bypassing the duodenum with DJB surgery, there was a trend for increased GIP protein content in the duodenum of DJB rats compared to Sham rats (0.0029%±0.0008 vs. 0.0021%± 0.0003, respectively, p=0.07). We found no difference in proximal jejunal (the “enteral limb” of DJB rats) or ileal GIP protein content between the two surgical groups.
Fig. 4.
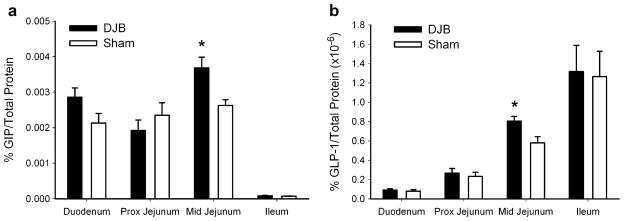
Percentage intestinal GIP (a) or GLP-1 protein content (b) was determined at 2 weeks after DJB or Sham surgery in Wistar rats. Intestinal segments were taken from the duodenum, proximal jejunum (distal to the duodenojejunostomy in DJB rats or 10 cm distal to the ligament of Treitz in Sham rats), midjejunum (distal to the jejujejunostomy in DJB rats or 25 cm distal to the ligament of Treitz in Sham rats), and distal ileum. *p<0.05, statistically different for the tested segment of small bowel between DJB and Sham rats. Data are presented as mean±SE
To see if a separate GI hormone followed the same pattern of production as GIP after duodenal bypass, we tested the effect of DJB on small bowel GLP-1 protein content, a potent incretin hormone previously implicated in improving glucose tolerance after metabolic surgery [7, 13, 14, 19]. DJB, as shown in Fig. 4b, again significantly increased GLP-1 protein content in the midjejunum compared to sham rats (0.81×10−6% ± 0.048 vs. 0.58×10−6% ± 0.063, respectively, p=0.01). No difference in GLP-1 protein content was found among any of the other three intestinal segments analyzed (duodenum: p=0.65, jejunum: p=0.58, and ileum: p=0.84).
Discussion
Given the conflicting clinical reports on the changes in GIP secretion after gastric bypass, we studied the effects of DJB not only on plasma GIP secretion but also gastrointestinal lymphatic GIP, a newly recognized, highly sensitive medium for the measurement of postprandial incretins in rodents. We found that DJB does not significantly alter systemic GIP concentrations in plasma or lymph 2 weeks after DJB surgery compared to Sham rats despite bypassing the proximal small bowel, which is the major focus of GIP-secreting K cells. Intestinal GIP, as well as GLP-1, protein content was increased in the midjejunum at the common channel of DJB rats compared to Sham rats. Surprisingly, duodenal GIP protein content was not decreased by intestinal bypass of duodenum and cannot account for the minimal differences noted in systemic GIP concentrations after DJB surgery.
In this study, we were interested specifically in what effect DJB has on plasma and lymphatic GIP concentrations given the hormone’s proximal small bowel site of secretion and the recent literature highlighting the beneficial effects noted with reduction of GIP signaling on obesity propagation and glucose intolerance [8–10]. There is an exaggerated secretion of GIP from K cells of obese type 2 diabetic patients and animals [21, 22]. In support of a relationship between enhanced GIP signaling and the development of obesity and insulin resistance, Irwin et al. has shown that antagonism of the GIP receptor in ob/ob mice prevents the development of obesity-associated diabetes with significant improvements in glucose concentrations and insulin sensitivity [10]. Rubino et al., among others, have published animal and clinical data to support that a major mediator of the effect of gastric bypass might derive from a change in secretion of a probable hormone originating from the proximal small bowel that affects insulin sensitivity and glucose homeostasis [17, 23–26], leading us to speculate if GIP could be mediating these effects. While we found minimal effects of DJB on systemic GIP secretion, further studies are needed to determine if the sensitivity of the GIP receptor to stimulation changes after DJB and RYGB surgery. In addition, this study examined the effects of DJB on lean, glucose-tolerant, Wistar rats. It may be that the effect of DJB on GIP secretion and signaling differs between lean, obese, and diabetic phenotypes.
The mechanism/s to the resolution of diabetes after gastric bypass has/have often been considered in terms of the proximal (“foregut”) and distal (“hindgut”) bowel hypothesis. While the improvement in glucose tolerance after RYGB is undoubtedly a multifactorial process, including the significant impact of postoperative weight loss and calorie restriction on glucose homeostasis, gastrointestinal specific mechanisms could include (1) exclusion of the majority of the endocrine stomach from nutrient flow, (2) proximal small bowel exclusion from nutrient stimulus, and (3) enhanced nutrient delivery to the distal small bowel. While DJB is unable to address any hormone or neural factors that may be affected by gastric nutrient exclusion, it is an extremely useful experimental model of RYGB to study the effects of proximal small bowel exclusion and enhanced distal small bowel stimulation on glucose homeostasis independent of calorie restriction and weight loss. Supporting our previously published findings with DJB surgery in GK rats, we found in this study only modest changes in early postoperative food intake between the two groups, with no difference in food intake noted by POD9 and no affect of DJB on body weight compared to Sham surgery [19]. While we are not aware of any published reports that document an affect of acute changes in food intake on subsequent postprandial GIP secretion, it is possible that the early postoperative feeding differences between the two groups is masking an effect of the surgery on GIP secretion that would be revealed by examining a later time point.
We found no improvement in glucose tolerance in DJB rats by 2 weeks after surgery. We noted an insignificant trend toward increased insulin secretion to an oral glucose load in DJB rats, but this did not result in a physiologically relevant reduction in glucose concentrations. Rubino et al. have published similar results in lean Wistar rats, showing no significant early effects of DJB on glucose concentrations over time [17]. Given the early time point of evaluation in our study and a glucose tolerant phenotype, it is unclear if a longer observation period would result in a significant improvement in glucose tolerance even in “normal”, lean rats as documented by other investigators with the use of ileal interposition [27].
Our laboratory and collaborators have previously published the enhanced sensitivity of lymphatic incretin sampling in rodents compared to plasma. Lu et al. found that the duodenal administration of a mixed meal results in a 3-fold higher secretion of lymphatic GIP compared to portal blood [15]. D’Alessio et al. have published that postprandial GLP-1 levels are 5- to 6-fold higher in lymph compared to portal plasma [28]. In this study, we found that lymphatic GIP concentration curves parallel the pattern of secretion in plasma. However, there was a 3.3-fold average higher concentration of fasting lymphatic GIP compared to plasma GIP. We also demonstrated a 4.3- and 4.5-fold increase in peak GIP secretion of lymph compared to peak plasma GIP levels after a gastric administration of Ensure for DJB and Sham rats, respectively, highlighting the increased sensitivity of postprandial lymphatic sampling of GI hormones in rodents.
Our model of DJB bypasses approximately 30% of the small intestine (the entire duodenum and 25 cm total of jejunum before the jejujejunostomy). This is similar to the percent of bypassed intestine in a standard RYGB which bypasses the duodenum, first 30 cm of jejunum with a 100–150-cm enteral limb (approximately 25–30% of bypassed small bowel). Supporting an animal model that bypasses a similar amount of small bowel as RYGB, we found comparable lymphatic flow rates after a mixed meal. Lymph flow is predominately driven by enteral fat absorption and chylomicron secretion suggesting that DJB does not produce a significant amount of fat malabsorption. The conflicting reports on GIP secretion in the literature may well be related to the surgical length of bypass performed by the primary surgeon. Although GIP-secreting K cells are located throughout the gastrointestinal tract, as seen in this study, there is a marked reduction in GIP content of the ileum. Studies of BPD reporting changes in GIP secretion almost universally demonstrate a decrease in postprandial GIP secretion possibly due to primary nutrient absorption occurring in the ileum of BPD patients rather than the jejunum of RYGB patients [11, 26, 29].
We used intestinal protein content as a surrogate marker of segmental K cell secretion after DJB surgery. While we anticipated that DJB would increase common channel GIP production due to increased nutrient absorption within the jejunum and decrease duodenal GIP production due to lack of nutrient stimulation, we found the surprising trend of increased GIP protein production in not only the jejunum but also the duodenum. This finding suggests that there is not a decrease in intracellular GIP protein production due to the lack of enteral nutrient stimulation of the duodenum as we had hypothesized to account for the minimal systemic changes found in GIP concentrations. Unfortunately, mesenteric lymphatic cannulation cannot be used to segregate what percentage the different segments of small bowel contribute to the systemic concentration. Lymphatic concentrations instead represent total gastrointestinal secretion. Because we failed to find a difference between GIP concentrations of the two groups, we wonder to what extent the increased production of GIP in the duodenum translates into secretion changes. Studies of GIP secretion in response to fat suggest a role for not just mucosal nutrient exposure but also cellular uptake of the nutrient by the K cell (which would not occur in the excluded duodenal segment) [30]. Further research is needed to determine how the mechanisms for K cell intracellular GIP production and subsequent extracellular secretion are regulated, as well as how the duodenal K cell population contributes to systemic GIP secretion after gastric bypass surgery. In addition, we wonder what mechanisms govern the increased production of GIP and GLP-1 within the common channel including proximal stimulation affected by duodenal exclusion, early nutrient delivery to the distal small bowel, or the new concentration of bile and pancreatic secretions entering the common channel after DJB.
In conclusion, we found that 2 weeks after DJB surgery in chow-fed Wistar rats, there is a minimal change in systemic GIP concentrations despite bypassing the proximal small bowel. GIP protein content of the midjejunum was significantly increased after DJB with a surprising trend toward increased GIP production within the duodenum despite the intestinal bypass. Mechanisms responsible for the discrepancy in GIP protein content of the duodenum and subsequent nutrient-driven GIP secretion requires further investigation. Additional studies are also needed to determine if GIP receptor sensitivity changes after RYGB even if no systemic changes in GIP concentration occur following surgery.
Acknowledgments
Portions of this work were presented in abstract form at the Society for Surgery of the Alimentary Tract 2009 meeting in conjunction with Digestive Disease Week. This work was supported in part by the National Institute of Health (F32 DK082205 to TK, DK076928 and DK056863 to PT).
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Tammy L. Kindel, Email: venematl@email.uc.edu, Department of Pathology and Laboratory Medicine, Genome Research Institute, University of Cincinnati, 2180 E. Galbraith Road, Cincinnati, OH 45237, USA
Stephanie M. Yoder, Department of Pathology and Laboratory Medicine, Genome Research Institute, University of Cincinnati, 2180 E. Galbraith Road, Cincinnati, OH 45237, USA
David A. D’Alessio, Department of Internal Medicine, University of Cincinnati, Cincinnati, OH, USA
Patrick Tso, Department of Pathology and Laboratory Medicine, Genome Research Institute, University of Cincinnati, 2180 E. Galbraith Road, Cincinnati, OH 45237, USA.
References
- 1.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjöström CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–84. doi: 10.1002/j.1550-8528.1999.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. NEJM. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 5.Nauck MA, Bartels E, Orskov C, et al. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912–7. doi: 10.1210/jcem.76.4.8473405. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–40. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 7.Vilsboll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47:357–66. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 8.Hansotia T, Maida A, Flock G, et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117:143–52. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClean PL, Irwin N, Cassidy RS, et al. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E1746–55. doi: 10.1152/ajpendo.00460.2007. [DOI] [PubMed] [Google Scholar]
- 10.Irwin N, McClean PL, O’Harte FPM, et al. Early administration of the glucose-dependent insulinotropic polypeptide receptor antagonist (Pro3)GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia. 2007;50:1532–40. doi: 10.1007/s00125-007-0692-2. [DOI] [PubMed] [Google Scholar]
- 11.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–31. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 12.Schrumpf E, Bergan A, Djoseland O, et al. The effect of gastric bypass operation on glucose tolerance in obesity. Scand J Gastroenterol Suppl. 1985;107:24–31. doi: 10.3109/00365528509099748. [DOI] [PubMed] [Google Scholar]
- 13.Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;31:1709–16. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bose M, Olivan B, Teixeira J, et al. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg. 2009;19:217–29. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu WJ, Yang Q, Sun W, et al. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1130–8. doi: 10.1152/ajpgi.00400.2007. [DOI] [PubMed] [Google Scholar]
- 16.Rubino F, Marescaux J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacheco D, de Luis DA, Romero A, et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto–Kakizaki rats. Am J Surg. 2007;194:221–4. doi: 10.1016/j.amjsurg.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Kindel TL, Yoder SM, Seeley RJ, et al. Duodenal–jejunal exclusion improves glucose tolerance in the diabetic, Goto–Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13:1762–72. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 20.Kindel TL, Yang Q, Yoder SM, et al. Nutrient-driven incretin secretion into intestinal lymph is different between diabetic Goto–Kakizaki rats and Wistar rats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G168–74. doi: 10.1152/ajpgi.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flatt PR, Bailey CJ, Kwasowski P, et al. Plasma immunoreactive gastric inhibitory polypeptide in obese hyperglycaemic (ob/ob) mice. J Endocrinol. 1984;101:249–56. doi: 10.1677/joe.0.1010249. [DOI] [PubMed] [Google Scholar]
- 22.Vilsbøll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 23.Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25:527–31. doi: 10.1007/s002680020348. [DOI] [PubMed] [Google Scholar]
- 24.Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31:S290–6. doi: 10.2337/dc08-s271. [DOI] [PubMed] [Google Scholar]
- 25.Bikman BT, Zheng D, Pories WJ, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93:4656–63. doi: 10.1210/jc.2008-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salinari S, Bertuzzi A, Asnaghi S, et al. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–80. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strader AD, Clausen TR, Goodin SZ, et al. Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes Surg. 2008;19:96–104. doi: 10.1007/s11695-008-9754-x. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessio DA, Lu W, Sun W, et al. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2163–9. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- 29.Mingrone G, Nolfe G, Castagneto Gissey G, et al. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia. 2009;52:873–81. doi: 10.1007/s00125-009-1288-9. [DOI] [PubMed] [Google Scholar]
- 30.Schirra J, Katschinski M, Weidmann C, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]