Abstract
Abiraterone acetate is an orally administered potent inhibitor of cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17A1 or CYPc17), which is essential for synthesis of testosterone from cholesterol. While decreasing serum testosterone through inhibition of testicular function is the first line of treatment for men with metastatic prostate cancer, residual androgens may still be detected in patients treated with LHRH agonists. Treatment with abiraterone results in rapid, and complete, inhibition of androgen synthesis in the adrenal glands and within the tumor itself. An overall survival benefit of maximal androgen suppression was recently demonstrated in a randomized placebo controlled phase III clinical trial of abiraterone with prednisone versus prednisone in men with metastatic castrate resistant prostate cancer previously treated with docetaxel chemotherapy. Abiraterone’s efficacy demonstrates the importance of androgen signaling in patients with castrate resistant metastastic disease, and the importance of studies of other novel agents such as MDV3100, an androgen receptor inhibitor, that additionally targets androgen receptor translocation. These promising results now pose a new angle to an old problem regarding hormonal therapy and raise new questions about how resistance develops, how to best sequence therapy, and how to optimize combinations with other emerging novel targeted agents.
Keywords: Abiraterone, Prostate Cancer, CYP17A1, CYP17, CYPc17
Introduction
“To raise new questions, new possibilities, to regard old problems from a new angle, requires creative imagination and marks real advance in science”
-Albert Einstein
Solving a key problem of androgen growth stimulation in prostate cancer, the findings by Huggins and Hodges in 1941, with a subsequent Nobel Prize, forever changed our approach on the treatment of prostate cancer and improved the lives of thousands of men diagnosed with prostate cancer. With some improvement on the use of androgen ablation therapy at different points of disease progression, over 70 years passed without a distinctly new angle in the hormonal treatment of prostate cancer. With the approval of abiraterone acetate (abiraterone) for the treatment of castrate resistant prostate cancer, there is now evidence that targeting the androgen receptor axis in men with advanced castrate resistant prostate cancer can lead to improved survival. This significantly changed our central assumptions of castrate-resistant prostate cancer progression and opened the potential for multiple advances as we better understand additional targets, agents, and mechanisms of resistance to maximal castration therapy.
Rationale for targeting CYPc17
Although it would seem that the rationale of targeting CYPc17 would be obvious, the importance of androgen dependence for prostate tumor growth has been understood in the past through a framework of prior studies of castrating therapies (1, 2). Unfortunately, surgical and medical castrating therapies that decrease the synthesis of testosterone (T) or dihydrotestosterone (DHT) are only temporarily effective in patients with advanced disease, and do not abrogate all sources of androgen exposure to tumor tissue (3, 4). While the dominant source of ligand for the androgen receptor (AR) is testosterone produced by the testicular Leydig cells, approximately 10% of circulating androgen is synthesized in the adrenal gland (5). Accumulating evidence demonstrates that intratumoral synthesis of the potent AR ligands T and DHT may occur, using weak adrenal androgens as substrates (6–8). Additional studies suggest that castrate tumors can synthesize intratumoral androgens de novo from cholesterol (9–11) and can oxidize the progesterone derivative androstanediol directly to DHT via the “backdoor pathway”(12). Therefore, inhibiting androgen synthesis despite inhibition of testicular function has a compelling rationale in the treatment of castrate resistant prostate cancer.
As an approach to inhibit androgen synthesis, a focus on the essential role of CYP17 in sex steroid syntheses provided the rationale needed for developing agents to treat men with castrate resistant prostate cancer. The pathway for synthesis of T and DHT is well characterized, as shown in Figure 1. The cytochrome P450 system is a superfamily of enzymes responsible for catalyzing numerous biosynthesis and detoxification pathways. CYPc17 (or CYP17A1-cytochrome P450, family 17, subfamily A, polypeptide 1) is a dual functional enzyme with activity as both a 17-alpha-hydroxylase and a 17,20 lyase. Activity of CYPc17 is essential for synthesis of T and DHT from cholesterol (13, 14). The physiological consequences of abrogating CYPc17 activity is demonstrated in children with congenital adrenal hyperplasia who lack sex steroid and cortisol production, while experiencing ACTH mediated overproduction of mineralocorticoids leading to hypertension and hypokalemia (14, 15).
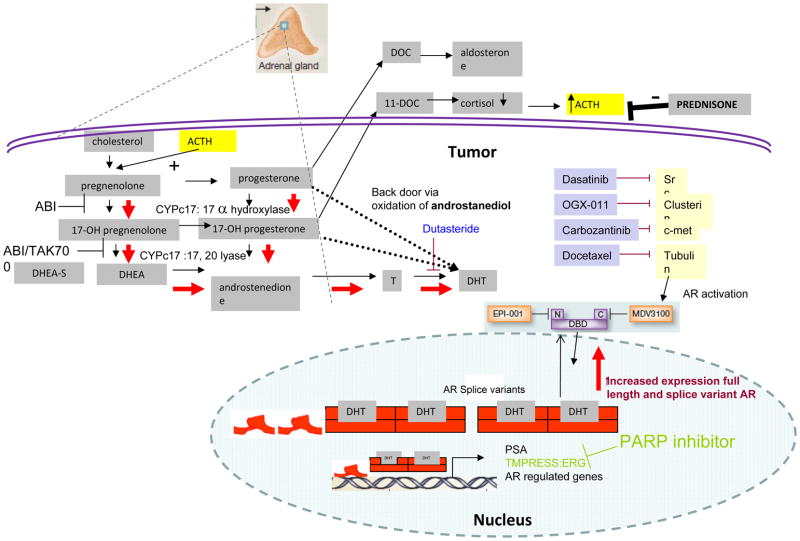
Figure 1.
Therapies targeting the androgen signaling axis. Abiraterone is a potent and selective inhibitor of CYPc17, blocking synthesis of testosterone and DHT. Other agents such as MDV3100 target the AR directly. Resistance to abiraterone is proposed to occur through upregulation of intratumoral CYPc17 and other genes involved in synthesis of intratumoral androgens to restore DHT levels and through increased levels of AR receptor and receptor splice variants. Abiraterone resistance pathways are depicted in red, agents that may be combined with abiraterone are indicated in blue.
As proof of principal, it has long been recognized that ketoconazole decreases the levels of multiple CYP enzymes involved in steroid synthesis including CYP17, but with a relatively weak IC50 while being associated with significant toxicity (13). The clinical activity of ketoconazole has been demonstrated in multiple phase II studies (reviewed in Yap et al (16)) and a phase III trial (CALGB 9583) in men with castrate resistant disease randomized to antiandrogen withdrawal or antiandrogen withdrawal plus ketoconazole (17). PSA response (decrease in PSA by 50% from baseline) was achieved in 11% and 27% respectively. No significant difference in overall survival was noted, although this analysis was limited by the substantial crossover to ketoconazole by patients in the control arm. Ketoconazole toxicities include fatigue, hepatotoxicity, nausea and rash. Its utility is also often limited by drug interactions due to the non-specific inhibition of CYP450 mediated drug metabolism.
Clinical development of Abiraterone
Given the compelling rationale for development of more potent and specific inhibitors of CYPc17, medicinal chemists explored a variety of compounds to inhibit the CYPc17 enzyme (14). Abiraterone acetate was synthesized at the Institute for Cancer Research in London and is structurally related to pregnenolone, a natural substrate of CYPc17 (18). Placement of a nitrogen containing pridyl group at carbon 17 of pregnenolone led to potent inhibition of CYPc17 while a double bond at the 16,17 position lead to irreversible binding and inhibition of CYPc17. An acetate pro-drug of abiraterone was developed to increase oral bio-availability (14). Early phase I studies demonstrated good bioavailability at doses of greater than 200 mg, a half life of approximately 28 hours, and significantly increased absorption with food (19). Abiraterone is metabolized by CYP3A4 and is an inhibitor of CYP2D6. Therefore, caution with co-administration of abiraterone with other drugs is important especially for drugs that inhibit or induce CYP3A4, which may alter abiraterone levels and drugs that are substrates of CYP2D6, which may be affected by abiraterone. Included in the initial studies were also men who were not on an LHRH agonist. In this population, a compensatory surge in LH led to a rise in testosterone by day 4 of treatment with abiraterone, confirming the need for abiraterone to be given concomitantly with suppression of testicular function (19).
These early proof of principal studies were followed by a continuous dosing, phase I dose escalation trial in chemotherapy naive men with castrate resistant prostate cancer who had not received prior ketoconazole (20). Doses of up to 2000 mg per day were well tolerated. Testosterone became rapidly undetectable within 8 days at all dose levels and remained undetectable even at the time of disease progression, indicating durable CYP17 inhibition by abiraterone. PSA decline of >50% occurred in 57% of patients. As anticipated, based on knowledge from patients with congenital CYP17 deficiency, absence of CYP17 led to loss of feedback mediated ACTH suppression by cortisol. ACTH driven synthesis of deoxycorticosterone and corticosterone, upstream from CYP17, prevented adrenocortical insufficiency and led to excess mineralcorticoid effects of hypertension, hypokalemia and edema. These effects could be reversed by administration of dexamethasone to restore ACTH suppression. In 4 of 15 patients who developed progression while on study, dexamethasone caused a PSA response suggesting, as one possiblity, that promiscuous AR activation by steroids upstream of CYP17 led to abiraterone resistance in some patients. A second phase I trial also included patients who had received prior ketoconazole (21). A 50% decrease in PSA was seen in nine (47%) of 19 patients with prior ketoconazole therapy and nine (64%) of 14 patients without prior ketoconazole therapy.
Phase II studies
Phase II trials of abiraterone in men with CRPC were conducted in chemotherapy naïve (22, 23) and chemotherapy resistant patients (24, 25), with either prednisone or the mineralcorticoid antagonist eplereonone to manage mineralocorticoid excess. In the studies of patients with no prior chemotherapy, PSA decline of ≥ 50% ranged from 67% (23) to 79% (22) with a median time to progression of 32 to 71 weeks, respectively. In the trials of patients with prior chemotherapy, declines of ≥ 50% were seen in 36% and 51% of subjects (24, 25) with a notable ≥ 90% decline in approximately 15% of patients. Median time to PSA progression was 24 weeks in both trials. Correlative studies in the phase II trials included evaluation of circulating tumor cells (CTC), providing important preliminary data for use of CTCs as a biomarker. CTC number, using the cell search system, has previously been shown to be an independent prognostic factor for survival (26). In two trials of abiraterone after chemotherapy, CTC counts of ≥ 5 cells/7.5 ml were seen in 70% to 79% of subjects. While on treatment with abiraterone, 34% to 41% of patients converted to the more favorable subgroup with < 5 cells/7.5 ml, providing a rationale for incorporating CTC into randomized phase III trials with abiraterone (24, 25). Molecular profiling of CTCs was also performed, demonstrating the feasibility of detecting TMPRSS2-ERG fusions in CTC (27). In one study, the presence of an ERG rearrangement in CTC or archival tissue was associated with an increased chance of having a 90% or greater decrease in PSA, suggesting an association between ERG rearrangement and benefit from abiraterone. In a recent study of 48 men treated with abiraterone after chemotherapy, TMPRSS2-ERG status did not predict for a decline in PSA or improved survival leading the authors to suggest that TMPRSS2-ERG fusion status may have a limited role as a biomarker (28). Additional ongoing studies will be important to better understand predictive biomarkers.
Phase III results
The clinical utility of abiraterone was definitively demonstrated in a randomized phase III trial in which 1195 men with castrate resistant prostate cancer, previously treated with docetaxel chemotherapy, were randomized in a 2:1 ratio to receive 5 mg of prednisone twice daily with either 1000 mg of abiraterone acetate or placebo (29). Patients treated with prior ketoconazole, uncontrolled hypertension, and cardiac ejection fraction (EF) below 50% were excluded. The primary endpoint of the study was overall survival, and the planned interim analysis performed after 67% of on study deaths occurred (median follow-up of 12.8 months) demonstrated that median survival improved from 10.9 months to 14.8 months (HR=0.646, p<0.0001) for patients treated with abiraterone (29). In an updated survival analysis performed after 775 deaths, median overall survival was 15.8 months vs. 11.2 months (30). Patients receiving abiraterone also had an increase in confirmed PSA response (29.1% vs 5.5%, p<0.0001) and progression free survival as determined by PSA increase of ≥ 25% (10.2 vs 6.6 months, p<0.0001). Recent data presented at the American Society of Clinical Oncology (ASCO) in 2011 confirmed that abiraterone doubled the time to the first skeletal related event (10 vs 5 months, p=0.0006) (31).
Abiraterone side effects were primarily related to elevated mineralcorticoid effects, as a result of CYP17 inhibition. Common toxicities included fluid retention and edema (31%, all grades), hypokalemia (17%, all grades), increased hepatic transaminases (10% all grades), hypertension (10% all grades) and cardiac events (13% all grades, 3% grade 3/4). Cardiac events included tachycardia (3% all grades) and atrial fibrillation (2% all grades); however, there was no significant increase in fatal cardiac events in the abiraterone group. Abiraterone was not tested in patients with baseline EF<50%. A post baseline EF of <50% was seen in 7.7% of patients on abiraterone and 5% of patients on placebo leading the FDA to recommend caution when using abiraterone in patients with a history of cardiovascular disease with monitoring of blood pressure, serum potassium and symptoms of fluid retention at least monthly (32).
The approval of abiraterone for treatment of metastatic CRPC has encouraged new questions that may lead to additional future options. While initially tested and proven to improve survival in men with disease that progressed with chemotherapy, the benefit of abiraterone in castrate resistant men prior to chemotherapy is of great interest. This question is the subject of a phase III trial of abiraterone in men with asymptomatic or mildly symptomatic castrate resistant disease without prior chemotherapy or ketoconazole (NCT00887198). Accrual to this trial, with progression free and overall survival endpoints, has been completed and results are pending. In the absence of phase III data, the completed phase II trial by Ryan et al. with abiraterone plus prednisone prior to chemotherapy (with time to PSA progression of 71 weeks) demonstrated promising activity in the pre-chemotherapy space (22). Recent consensus guidelines appropriately recognize that patients with significant co-morbidities, who may not be suitable for cytotoxic chemotherapy, could be considered for treatment with abiraterone if they have castrate resistant metastatic disease as it has substantially less toxicity than cytotoxic chemotherapy (33). Currently there are also studies comparing neo-adjuvant castration with abiraterone/prednisone plus leuprolide to single agent leuprolide prior to prostatectomy in men with localized high-risk prostate cancer (NCT01088529 and NCT00924469). These studies will be important to compare changes in levels of androgens and other potential biomarkers of androgen signaling (pre, during, and post treatment) in the serum, primary tumor microenvironment and bone marrow.
Mechanisms of resistance to abiraterone
Our current understanding of mechanism of resistance to abiraterone remains in the early stages; however, relevant pre-clinical and clinical data is beginning to emerge. In the majority of patients in the phase II studies, published data demonstrated that PSA increased at the time of disease progression. Expression of PSA, which is under control of the AR, implies that progression while on abiraterone is related to ongoing activity of the AR. Activation of the AR in turn may occur through ligand dependent and independent mechanisms (3). While abiraterone activity may be related to inhibition of intratumoral synthesis of T and DHT, it also appears that abiraterone resistance may be mediated through upregulation of intratumoral CYPc17 (9, 11). In a castrate resistant VCap xenograft model, treatment with abiraterone resulted in an initial tumor response and decreased AR gene activation followed by relapse at 4 to 6 weeks. In all relapsing xenografts, CYPc17 expression markedly increased. These data imply the abiraterone exerts a selective pressure leading to abiraterone resistance via intratumoral androgen synthesis (9). Using two LuCap prostate xenografts treated with abiraterone, Mosetegheal and colleagues, likewise, showed the abiraterone resulted in induction of CYPc17 and other genes involved in denovo synthesis of intratumoral androgens (11). Additionally, some tumors demonstrated increases in expression of the full length AR as well as AR variants lacking the c-terminal ligand binding domain.
Taken together, these data suggest that resistance to abiraterone may be overcome by direct targeting of the AR. MDV3100 is a potent AR antagonist that may be able to overcome the effects of increased transcription of the full length AR (34, 35) as well as AR splice variants that function to potentiate the effect of full length AR(36). Recently, results from an independent data monitoring committee interim analysis of a phase III trial of MDV3100 vs. placebo in men with castrate resistant prostate cancer previously treated with docetaxel were released by the manufacturer. This report showed that MDV3100 treatment led to an increase in overall survival from 13.6 to 18.4 months (HR=0.631, P<0.0001), but full peer-reviewed results and analysis are pending (37). Additional studies of MDV3100 effects on bone marrow androgen levels in patients with bone metastases suggest that MDV3100 caused a compensatory increase in bone marrow testosterone, a potential surrogate for intratumoral testotersone (38). Thus, individually, abiraterone and MDV3100 induce antagonistic changes in the androgen axis that could potentially be abrogated with combination therapy. Another AR antagonist in development is EPI-001. This drug disrupts activity of the AR N-terminal domain, thereby maintaining activity against AR splice variants with c-terminal ligand binding domain deletions (39).
Based on the promising activity of ketoconazole given in combination with dutasteride, which inhibits conversion of testosterone to the more active DHT (40), it could be hypothesized that the combination of abiraterone plus a second drug targeting DHT synthesis may help to overcome abiraterone resistance. Drugs targeting other enzymes involved DHT synthesis such as AKR1C3 are also in development (41). Other potential mechanisms for targeting the AR include inhibiting AR chaperone proteins such as clusterin (42). In pre-clinical models, treatment with the clusterin antisense molecule OGX-011 in combination with MDV-3100 led to accelerated AR degradation and repressed AR transcriptional activity, as well as significantly delayed time to progression compared to treatment with MDV-3100 alone. Finally, an emerging appreciation of the role of c-met signaling in the castrate resistant state, based on the clinical experience with the dual c-met/VEGF inhibitor cabozantinib will likely drive evaluation of combined AR and c-met blockade in patients with CRPC (43).
In addition to upregulation of ligand synthesis, non-ligand based mechanisms of AR activation and true AR independent carcinogenesis are both clinically relevant mediators of androgen independence. A drugable target that may potentially activate the AR in a ligand independent manner includes Src kinase (44). In this regard, dasatinib, a Src kinase inhibitor is currently being evaluated in combination with abiraterone as a means of overcoming resistance to abiraterone (NCT01254864). Recent data also suggests that continued blockade of the AR receptor is necessary when targeting the PI3 kinase signaling pathway, a important mediator of castrate resistance (45). In PTEN negative prostate cancer with resultant activation of the PI3 kinase-AKT-mTOR signaling pathway, pharmacologic inhibition mTORC1 (everolimus) or PI3Kand mTORC1/2 (BEZ225) resulted in reciprocal increase in AR protein levels and AR signaling, while inhibition of AR led to increased activation of AKT by inhibition of an AKT phosphatase. Therefore, dual AR/PI3kinase pathway inhibition will be an important area of investigation. The optimal combination strategy for abiraterone may also be informed by our emerging understanding of the role that the high frequency gene fusion between the AR regulated TMPRSS2 gene and the ETS family of transcription factors. The TMPRSS2-ERG fusion is the most common of the fusions, and is found in approximately 50% of localized prostate cancers (46). Multiple lines of evidence have shown that overexpression of the ETS related transcription factors increases prostate cancer development and invasiveness. In an effort to target ETS transcription factors by identifying essential proteins that interact with ETS, the DNA repair protein PARP was found to interact with ERG implying that PARP1 inhibitors may block ETS-mediated transcription (47). In support of this finding, pharmacological inhibition of PARP1 blocked ETS-positive, but not ETS-negative, prostate cancer xenograft growth. Therefore, abiraterone mediated inhibition of AR, and downstream TMPRSS2-ETS transcription combined with PARP1 inhibition may overcome the deleterious effects of ETS transcription. Furthermore, preliminary data suggests that inhibition of PARP1 enzymatic activity suppresses ligand-dependent AR transcriptional activity. Therefore, coordinate PARP and AR inhibition may be a means of maximal AR inhibition (48). Finally, as other mechanisms of general resistance to tumor cell stress such as autophagy are better understood in the context of androgen ablation in prostate cancer, targeting these mechanisms may also hold promise to improve upon current results (49).
Considerations of Sequence and Space
At the current time abiraterone is approved for use after treatment with docetaxel based chemotherapy. Therefore, it remains to be determined if earlier initiation of treatment with abiraterone will result in an increased duration of benefit compared to treatment after chemotherapy. Similarly, it is also not known if treatment with abiraterone and subsequent progression will impact response to docetaxel chemotherapy. Recent studies have demonstrated that some of the benefit from taxane based therapy may be due to taxane mediated suppression of AR, raising the possibility of cross resistance to maximal castration therapy and taxane based therapy (50, 51). Additional studies regarding sequencing of taxane based treatment in association with abiraterone may be informative in this regard. Furthermore, it remains unknown if there is a detrimental effect from discontinuing abiraterone or other second generation hormonal agents such as MDV3100 or TAK-700, as opposed to continuing these agents in the setting of progression, as is routinely done with LHRH agonists. Other considerations regarding use of abiraterone prior to chemotherapy relate to optimal integration with sipuleucel-T, which was approved based on prolongation of survival in men who received this therapy prior to chemotherapy. The immune implications of maximal castration therapy are of potential interest, given studies that suggest a benefit of vaccination in conjunction with hormonal therapy. A phase II trial in men with castrate non-metastatic prostate cancer demonstrated a median survival of 6.2 years for patients who received vaccine prior to nilutamide, compared to 3.7 years for patients who started on nilutamide followed by vaccine (p=0.045). This study suggested that vaccine therapy initiates a host immune response that can be augmented by subsequent complete androgen blockade (52). Finally, the metabolic implications of maximal androgen suppression on bone health, insulin resistance and cardiovascular health remain to be determined. Of particular interest will be the long term metabolic effects of maximal castration therapy on patients who receive LHRH agonists and maximal androgen suppression in the early disease setting with high risk localized disease or PSA relapse after definitive therapy.
Summary
The compelling survival benefit seen with abiraterone use in patients with heavily pretreated, castrate resistant disease, has refocused attention on the value of inhibition of the AR axis throughout the entire natural history of the prostate cancer disease process: a real advance in the treatment of prostate cancer. As a result of dramatic advances in our understanding of prostate pathogenesis, regulation of the AR, and novel drug development, rationally designed abiraterone combination studies hold great additional promise. At the same time, fundamental questions regarding integration of abiraterone into our current treatment paradigms present multiple quandaries for the practicing oncologist and provide the impetus for expedient development of clinical trials to optimize our use of abiraterone for improvement of patient care. Additional challenges include prioritizing among multiple compelling trial concepts and the potential need for integration of new agents targeting the androgen axis, resulting in an urgent need for increased cooperation amongst investigators, industry and regulatory agencies to bring the greatest benefit to the patients in the most efficient manner possible. Finally, as we continue to look for strategies to develop innovative options to continue to win the war on cancer, it is important to reflect on our how our 70 year old view of an old problem regarding hormonal therapy and resistance changed with a creative new angle and approach that led to a significant advance and positive impact on countless lives.
Footnotes
Disclosures: Johnson and Johnson-Research support (MNS, RSD, SG)
References
- 1.Huggins C, Hodges C. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–7. [Google Scholar]
- 2.Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–44. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 3.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–8. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458–62. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 6.Cai C, Balk SP. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocr Relat Cancer. 2011;18:R175–82. doi: 10.1530/ERC-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 9.Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–13. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 11.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 Inhibition with Abiraterone in Castration-Resistant Prostate Cancer: Induction of Steroidogenesis and Androgen Receptor Splice Variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohler JL, Titus MA, Wilson EM. Potential prostate cancer drug target: bioactivation of androstanediol by conversion to dihydrotestosterone. Clin Cancer Res. 2011;17:5844–9. doi: 10.1158/1078-0432.CCR-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakki T, Bernhardt R. CYP17- and CYP11B-dependent steroid hydroxylases as drug development targets. Pharmacol Ther. 2006;111:27–52. doi: 10.1016/j.pharmthera.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–6. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 15.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am. 2001;30:101–19. vii. doi: 10.1016/s0889-8529(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 16.Yap TA, Carden CP, Attard G, de Bono JS. Targeting CYP17: established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8:449–57. doi: 10.1016/j.coph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–71. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–88. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan CJ, Shah S, Efstathiou E, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–61. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in postdocetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 27.Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clin Cancer Res. 2011;17:3903–12. doi: 10.1158/1078-0432.CCR-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danila DC, Anand A, Sung CC, et al. TMPRSS2-ERG Status in Circulating Tumor Cells as a Predictive Biomarker of Sensitivity in Castration-Resistant Prostate Cancer Patients Treated With Abiraterone Acetate. Eur Urol. 2011:897–904. doi: 10.1016/j.eururo.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ning YM, Kluetz P, Liu K. CENTER FOR DRUG EVALUATION AND RESEARCH: Clinical Review of NDA 202379. Zytiga™ (abiraterone acetate) for Metastatic Castration-Resistant Prostate Cancer after Prior Chemotherapy. 2011 [Google Scholar]
- 31.Logothetis C, De Bono JS, Molina A, et al. Effect of abiraterone acetate (AA) on pain control and skeletal-related events (SRE) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) post docetaxel (D): Results from the COU-AA-301 phase III study. ASCO Meeting Abstracts. 2011;29:4520. [Google Scholar]
- 32.Zytiga Prescribing Information.
- 33.NCCN (National Comprehensive Cancer Network) Prostate Cancer Guidelines 4.2011. 2011. [Google Scholar]
- 34.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medivation. [Accessed November 10 2011];Survival Data From Interim Analysis of Phase 3 AFFIRM Trial of MDV3100 in Men With Advanced Prostate Cancer. http://investorsmedivationcom/releasedetailcfm?ReleaseID=620500.
- 38.Efstathiou E, Titus MA, Tsavachidou D, et al. MDV3100 effects on androgen receptor (AR) signaling and bone marrow testosterone concentration modulation: Apreliminary report. ASCO Meeting Abstracts. 2011;29:4501. [Google Scholar]
- 39.Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 40.Taplin ME, Regan MM, Ko YJ, et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7099–105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mostaghel EA, Plymate S. New hormonal therapies for castration-resistant prostate cancer. Endocrinol Metab Clin North Am. 2011;40:625–42. doi: 10.1016/j.ecl.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto H, Kuruma H, Zoubeidi A, Fazli L, Gleave ME. An evaluation of clusterin antisense inhibitor OGX-011 in combination with the second-generation antiandrogen MDV3100 in a castrate-resistant prostate cancer model. ASCO Meeting Abstracts. 2011;29:4502. [Google Scholar]
- 43.Hussain M, Smith MR, Sweeney C, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. ASCO Meeting Abstracts. 2011;29:4516. [Google Scholar]
- 44.Cai H, Babic I, Wei X, Huang J, Witte ON. Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res. 2011;71:862–72. doi: 10.1158/0008-5472.CAN-10-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenner JC, Ateeq B, Li Y, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–78. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiewer MJ, Goodwin J, Knudsen KA. Impact of PARP1 on AR signaling and therapeutic response in prostate cancer presented at AACR International Conference on Frontiers in Cancer Prevention Research, 2010. Cancer Prevention Research. 2010;3:Abstract A26. [Google Scholar]
- 49.Amaravadi RK, Lippincott-Schwartz J, Yin XM, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–66. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–31. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]