Abstract
Successful treatment and control of HIV/AIDS is one of the biggest challenges of 21st century. More than 33 million individuals are infected with HIV worldwide and more than 2 million new cases of HIV infection have been reported. The situation demands development of effective prevention strategies to control the pandemic of AIDS. Due to lack of availability of an effective HIV vaccine, antiretroviral drugs and nucleic acid therapeutics like siRNA have been explored for HIV prophylaxis. Clinical trials shave shown that antiretroviral drugs, tenofovir and emtricitabine can offer some degree of HIV prevention. However, complete prevention of HIV infection has not been achieved yet. Nanotechnology has brought a paradigm shift in the diagnosis, treatment and prevention of many diseases. The current review discusses potential of various nanocarriers such as dendrimers, polymeric nanoparticles, liposomes, lipid nanocarriers, drug nanocrystals, inorganic nanocarriers and nanofibers in improving efficacy of various modalities available for HIV prophylaxis.
Keywords: HIV, Nanotechnology, Polymeric nanoparticles, Dendrimers, Prophylaxis, Vaginal delivery
1. Introduction
Approximately three decades ago, human immunodeficiency virus (HIV) was found to be the cause of acquired immunodeficiency syndrome (AIDS) [1]. Since its discovery, HIV is responsible for more than 25 million deaths worldwide [1–7]. At the moment, more than 33 million individuals are infected with HIV across the globe. During last 5 years, more than 2 million new HIV infections have been reported every year and around same number of individuals have died because of HIV [7]. The scenario is projected to get worse in the next decade mainly in Asia, Africa and Eastern Europe [2]. Thus, HIV represents one of the insurmountable problems of the 21st century. HIV/AIDS has caused significant socioeconomic damage worldwide. With the advent of highly active antiretroviral therapy (HAART) that uses a cocktail of antiretroviral drugs, improvements have been achieved. HAART has been responsible for significant improvement in life expectancy and quality [1–6]. However, HAART is associated with disadvantages such as emergence of drug resistant viral strains, inconvenient dosage regimen (daily administration of one or more pills for lifetime), serious adverse effects, and inability to eradicate HIV from reservoirs [1–6]. In view of this, significant attempts have been focused on prevention of HIV infection.
The majority of HIV infections are transmitted through sexual contact. Hence, early efforts were focused on advocating the use of physical barriers such as condoms and behavioral modifications (ABC, abstinence, be faithful, and correct consistent use of condom). However, these methods have not been very successful [7]. Studies have shown that male circumcision can considerably reduce chances of contracting HIV but this approach has its own limitations and is not a female controlled method of HIV prophylaxis [7]. Thus, it is important to develop more effective modalities for prevention of HIV. This review focuses on the applications of nanotechnology for HIV/AIDS prevention.
2. Modalities for prophylaxis of HIV
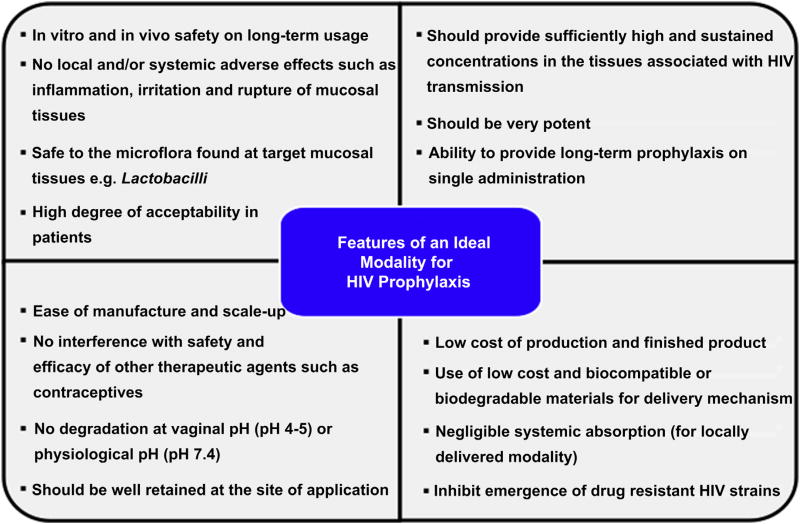
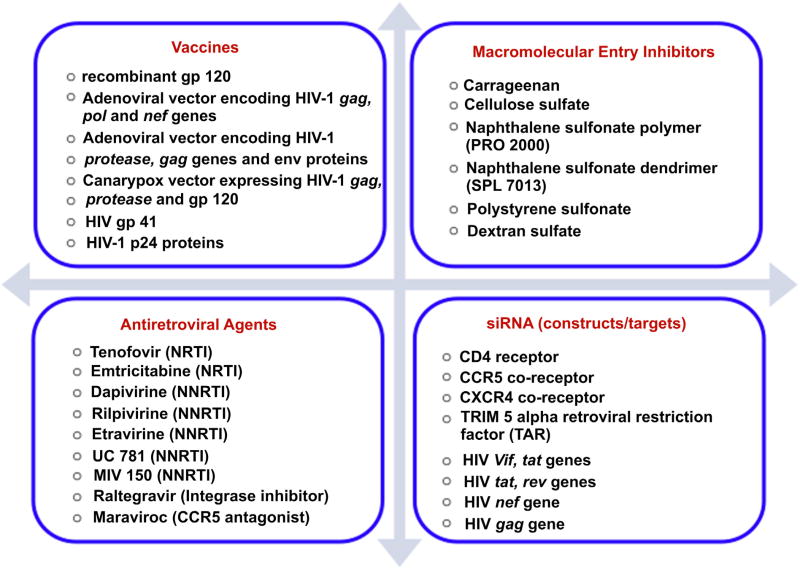
The process of development of prophylactic modality is always focused on making it widely available for disease prevention. Over the years, various modalities have been and are being developed for prophylaxis of HIV. The desired properties of an ideal modality for HIV prophylaxis are shown in Fig. 1[8–10]. Broadly, prophylactic modalities can be divided into four categories viz. vaccines, macromolecular HIV entry inhibitors, antiretroviral drugs, and nucleic acid-based therapeutics (Fig. 2). There are numerous reviews that discuss each of these modalities in detail [2,7,8–10]. In this review, we intend to give an overview of various modalities available for HIV prophylaxis.
Fig. 1.
Desired properties of an ideal prophylactic modality.
Fig. 2.
List of various modalities available for HIV prophylaxis.
2.1. Vaccines
Development of vaccines has been the most prominent prophylactic strategy for a variety of viral infectious diseases. However, developing safe and effective vaccines against HIV is a very challenging task. The vast genetic diversity and high mutation rate are the major hurdles in the development of a HIV vaccine [11–14]. Moreover, structural characteristics of HIV envelope glycoprotein (gp120) such as variable loops, glycosylated N-terminus and flexible conformation are responsible for evasion of host immune response by virus [11–13]. Despite a plethora of challenges, scientists are continuously exploring various strategies to develop an effective vaccine. Until today, few clinical trials have been carried out to evaluate efficiency of vaccines in HIV prevention (Table 1). It is noteworthy that the first three clinical trials failed to show any prophylactic effect against HIV infection. RV144 was the first trial that demonstrated 31% protection from HIV infection in the Phase III [11–13]. Although some progress has been made, complete prophylaxis with a HIV vaccine still remains out of reach.
Table 1.
Details of completed and ongoing human clinical trials of various modalities for HIV prophylaxis [9–25].
| Trial name | Prophylactic modality | Study size | Regimen | Clinical phase | Results |
|---|---|---|---|---|---|
| VAX003 (AIDSVAX B/E) | Recombinant gp120 + alum | 2527 | 7 i.m. Injections over 30 months (dose: 300 μg) | Phase III | No efficacy |
| VAX004 (AIDSVAX B/B) | Recombinant gp120 + alum | 5403 | 7 i.m. Injections over 30 months (dose: 300 μg) | Phase III | No efficacy |
| STEP | Ad5 vector encoding HIV-1 gag, pol and nef genes | 3000 | 3 i.m. Injections (day 0, week 4 and week 26) containing 1.5 × 1010 adenovirus genomes | Phase III | No efficacy |
| RV144 | ALVAC-HIV (canarypox vector expressing HIV-1 gag, protease and gp120) + AIDSVAX (B/E) | 16,395 | 4 i.m. Injections of ALVAC-HIV (day 0, week 4 12 and 24) at the dose of 106.5 TCID50 + 2 i.m. injections of 300 μg AIDSVAX B/E on week 12 and 24 | Phase III | 31.2% protection |
| HVTN 505 | Ad5 vector encoding HIV-1 gag, polgenes and env A, B, C + DNA vaccine encoding nef | 2200 | 3 i.m. Injections of DNA vaccine over 8 weeks + single injection of Ad5 vector on week 24 | Phase II | Results awaited |
| Carraguard® (PC 515) | 3% Carrageenan | 6202 | Vaginal application of 4 ml of 3% Carrageenan gel 1 h before intercourse | Phase III | No efficacy; Carraguard was found to be safe |
| Ushercell | 6% Cellulose sulfate | 1398 | Vaginal application of 3.5 ml of 6% cellulose sulfate gel 1 h before intercourse | Phase III | No efficacy, increased risk of HIV acquisition |
| MDP 301 | 0.5% and 2% PRO 2000 (naphthalene sulfonate polymer) | 9385 | Vaginal application of 0.5% or 2% gel before intercourse | Phase III | No efficacy |
| CAP | 13% cellulose acetate phthalate gel | 6 | Vaginal application of gel | Phase I | Mucosal irritation due to hyperosmolarity |
| MTN 004 | VivaGel (3% SPL7013, dendrimers containing naphthalene sulfonate) end groups | 61 | Twice daily application of 3.5 g VivaGel for 14 days | Phase I | VivaGel was well tolerated although higher incidences of low grade genitourinary adverse effects were observed |
| VivaGel | VivaGel (3% SPL7013) | 11 | One time application of 3.5 g VivaGel | Phase I | Cervicovaginal fluid collected at 3 h after application of VivaGel showed complete inhibition of HIV-1 and cervicovaginal fluid collected at 24 h after application showed 88% protection |
| VOICE 004 | 1% Tenofovir gel | 889 | Vaginal application of 4 ml gel up to 12 h before and after sex | Phase III | 39% Protection; 54% protection in women with high adherence |
| iPrEx | Tenofovir (300 mg) + Emtricitabine (200 mg) (Truvada®) | 2499 (MSM) | Daily oral Truvada® | Phase III | 44% Protection |
| TDF2 | Tenofovir (300 mg) + Emtricitabine (200 mg) (Truvada®) | 1200 | Daily oral Truvada® | Phase III | 63% Protection |
| PIP | Tenofovir (300 mg) + Emtricitabine (200 mg) (Truvada®) or Tenofovir (300 mg) | 4747 | Daily oral Tenofovir or Truvada® | Phase III | 62% Protection for tenofovir group and 73% protection with Truvada® |
| FEM-PrEP | Tenofovir (300 mg) + Emtricitabine (200 mg) (Truvada®) | 1951 | Daily oral Truvada® | Phase III | Trial stopped due to lack of efficacy |
| VOICE | Tenofovir (300 mg) + Emtricitabine (200 mg) (Truvada®) or Tenofovir (300 mg) or 1% tenofovir gel | 5029 | Daily oral Tenofovir or Truvada® or once daily application of 1% tenofovir gel | Phase III | Oral tenofovir and 1% tenofovir gel did not show efficacy |
| IPM 012 | Dapivirine gel | 36 | Once daily vaginal application of two different 0.05% dapivirine gels (2.5 g) for a period of 11 days | Phase I | Dapivirine concentration in cervicovaginal fluid was five logs higher than in vitro IC50 |
| IPM 014A | Dapivirine gel | 280 | Once daily vaginal application of 0.05% dapivirine gel (2.5 g) for 6 weeks | Phase I/II | Ongoing |
| IPM 014B | Dapivirine gel | 100 | Once daily vaginal application of 0.05% dapivirine gel for 6 weeks | Phase I/II | Results awaited |
| IPM 020 | Dapivirine gel | 128 | Once daily vaginal application of two different 0.05% dapivirine gels for a period of 12 weeks | Phase I/II | |
| IPM 013 | Dapivirine (25 mg) vaginal ring | 48 | Group A: dapivirine ring inserted on day 0 and 31 Group B: dapivirine ring inserted on day 0, 38, 59 | Phase I | Peak dapivirine concentrations reached in a day and dapivirine released at a concentration above IC50 for up to 35 days |
| IPM 015 | Dapivirine (25 mg) vaginal ring | 280 | Dapivirine ring inserted once in 28 days over a period of 12 weeks | Phase I/II | Results awaited |
| IPM 027 | Dapivirine (25 mg) vaginal ring | 1650 | N.A. | Phase II | Ongoing |
| MTN 013/ IPM 026 | Maraviroc (100 mg), dapivirine (25 mg) and maraviroc (100 mg) + dapivirine (25 mg) vaginal rings | 48 | Insertion of vaginal ring and checking of drug levels and safety for 28 days | Phase I | Results awaited |
| UC 781 | UC 781 gel | 25 | Twice daily application of 3.5 ml 0.1% or 0.25% UC 781 gel for 14 days | Phase I | Cervicovaginal lavage collected from 13 (out of 15) women treated with 0.25% UC 781 gel showed inhibition of HIV |
2.2. Macromolecular entry inhibitors
Several anionic macromolecules were found to inhibit binding of HIV-1 to CD4 cells by interacting with envelope glycoproteins [3]. Hence, their potential as prophylactic modality has been evaluated in many clinical trials (Table 1). Although macromolecular entry inhibitors showed a great promise in animal models, clinical trials in humans failed to show any significant protection as compared to placebo [3,9,10]. In fact, certain entry inhibitors like cellulose sulfate showed an increased risk of HIV acquisition due to destruction of vaginal epithelium [10]. At the moment, nanotechnology based macromolecular entry inhibitor (VivaGel®) is being evaluated in clinical trials [14–17]. Further details are discussed in a later section.
2.3. Antiretroviral agents
Due to lack of efficacy of vaccines and macromolecular entry inhibitors, there is a growing consensus on the use of drugs with proven antiretroviral activity for prophylaxis of HIV. It is anticipated that the presence of sufficient concentrations of antiretroviral drugs at the site would help to prevent HIV infection. It should be noted that drugs which act before integration of HIV with the human DNA are deemed to be useful for HIV prophylaxis [18]. Hence, drugs which act on HIV entry, HIV fusion, HIV reverse transcriptase and HIV integrase are being explored for the HIV prophylaxis (Fig. 2). For HIV prophylaxis, antiretroviral agents have been delivered either orally or locally. At the moment, nucleoside (or nucleotide) reverse transcriptase inhibitors (NRTIs) such as tenofovir and emtricitabine have been widely explored for HIV prophylaxis in various clinical trials (Table 1) [4,15,19–22]. Although results of these clinical trials are promising, none of the trials showed complete protection. In fact, some trials were discontinued due to lack of efficacy [19–22]. At the moment, several trials are ongoing that evaluate potential of non-nucleoside reverse transcriptase inhibitor (NNRTI) such as dapivirine and UC 781 (vaginal gel and/or ring) [23,24]. Use of antiretroviral drug combination is a commonly employed strategy for HIV therapy to increase efficacy and reduce resistance and side effects. In view of this, clinical trial using combination of antiretroviral drugs (dapivirine and maraviroc) has also been initiated for HIV prophylaxis[23,25].
2.4. Nucleic acid therapeutics (siRNA)
Discovery of RNA interference (RNAi) mechanism has brought a revolution in many fields including medicine. RNAi employs short RNA constructs to induce degradation of mRNA machinery in a sequence specific manner [26–29]. RNAi can be accomplished by using a short double stranded RNA (21–25 nucleotides in length; siRNA) or a short hairpin RNA (shRNA), a stably expressed hairpin like precursor. As siRNAs can achieve sequence specific gene silencing at a very small concentration, they are being actively pursued as therapeutic agents for a variety of indications [26–29]. In the last few years, considerable efforts have been made to develop RNAi constructs for prophylaxis of HIV by systemic and/or local delivery. siRNA targeting various HIV-1 encoded genes like tat, rev, pol, nef, gag, vif, env, vpr and LTR have shown potential to inhibit HIV-1 infection or suppression of HIV-1 infection in the cells. Transmission of HIV occurs through utilization of various cellular receptors from the host (CD4, CCR5, CXCR4) [27–29]. Hence, siRNAs targeting expression of these receptors have also shown potential in HIV prophylaxis. Although RNAi therapeutics has potential for HIV prophylaxis, there are several challenges associated with the delivery of RNAi therapeutics. Extreme hydrophilicity and anionic charge of siRNAs significantly hamper their cellular uptake [28–30]. Moreover, siRNAs should be released in the cytoplasm of the cells in order to achieve silencing. Hence, it is important that the delivery mechanism of siRNA should prevent lysosomal degradation of siRNA. siRNAs are also susceptible to nuclease mediated degradation in the body [28–30]. All these challenges pose a great difficulty in successful delivery of siRNAs. Until today, siRNA mediated HIV prophylaxis has been explored only in animals.
Various routes (oral, nasal, intramuscular, subcutaneous, intravenous, vaginal and rectal) have been employed to administer modalities for HIV prophylaxis. Prophylactic modalities (mainly chemical products) applied locally to either vagina or rectum are termed ‘microbicides’. Importance of routes of administration on the efficacy of prophylactic modalities will be discussed later.
3. Factors affecting efficacy of prophylactic modalities
For effective prophylaxis, it is important to attain sufficient concentrations of prophylactic modality at the potential site of infection. Various physicochemical properties such as aqueous solubility, permeability and log P value have considerable influence on the local and/or systemic distribution of the prophylactic modality. According to biopharmaceutics classification system (BCS), therapeutic agents are classified as Class I (high solubility, high permeability), Class II (low solubility and high permeability), Class III (high solubility and low permeability) and Class IV (low solubility and low permeability). Table 2 enlists physicochemical properties and BCS Classification of the antiretroviral drugs that are being considered for prophylaxis [31–39]. Prophylactic modalities such as vaccines and siRNA should be considered as BCS Class III drugs.
Table 2.
| Drug | Solubility | pKa | log P | BCS classification | Half-life | % Bioavailability |
|---|---|---|---|---|---|---|
| Tenofovir | 13.4 mg/ml | 1.3; 7.9; 3.0; 5.3 | −1.1 | Class III | 17 | 25–39 |
| Emtricitabine | 112 mg/ml | 2.63 | −0.43 | Class I | 10 | 93 |
| Efavirenz | <10 μg/ml | 10.2 | 3.68 | Class II | 40–50 | 42–80 |
| Nevirapine | 100 μg/ml | 2.8 | 2.05 | Class II | 25–30 | >90 |
| Dapivirine | <10 μg/ml | 5.8 | 5.27 | Class II | N.A. | N.A. |
| Etravirine | <10 μg/ml | 3.5 | 5.2 | Class IV | 30–40 | N.A. |
| Rilpivirine | <10 μg/ml | 5.6 | 4.86 | Class II | 34–55 | 24 (monkeys) |
| Raltegravir | <1 mg/ml | ∼1.25 | 1.06 | Class II | 9 | ∼65% |
| Maraviroc | ∼1 mg/ml | 3.3, 7.9 | 4.37 | Class III | 14–18 | 23–33 |
N.A., not available.
Tenofovir has been extensively evaluated as a prophylactic modality. Tenofovir is a BCS Class III drug with high solubility but low permeability [40]. In order to improve permeability of tenofovir, a prodrug (tenofovir disoproxilfumarate) was synthesized for oral administration. Studies indicate that tenofovir prodrug yields 1000-fold higher intracellular concentration of tenofovir diphosphate as compared to tenofovir base [40]. Moreover, tenofovir prodrug has 100-fold lower IC50 as compared to tenofovir base which clearly indicates the importance of permeability [41]. Interestingly, tenofovir gel used in clinical trials have employed tenofovir base instead of tenofovir prodrug. CAPRISA004 trial which employed pre-and post-coital administration of 1% tenofovir gel showed 39% protection from HIV in women [21]. However, the VOICE trial that employed coitus-independent once daily application of 1% tenofovir gel was stopped due to fulity. One of the reasons for this failure could be low permeability of tenofovir base. It is possible that due to low permeability, once daily application of tenofovir base may not yield sufficient concentration of tenofovir diphosphate in various tissues of reproductive tract. A recent study showed that less than 5% of the tenofovir base permeated through HEC-1A cells (endometrial adenocarcinoma cells) placed on a transwell membrane [33], which may corroborate this hypothesis. Thus, strategies that could improve permeability and local distribution of tenofovir would be very advantageous. At the moment, dapivirine (NNRTI) is also being evaluated as a microbicide in various clinical trials [25]. Due to high permeability and intracellular half-life [33], dapivirine has high potency. However, dapivirine is a hydrophobic drug. For solubilization of dapivirine, a considerable amount of cosolvents like glycerine and ethanol are required [42]. Studies indicate that use of high concentrations of cosolvents can increase osmolarity of gels which can affect integrity of vaginal epithelium and lead to increased susceptibility to HIV infection [43]. Furthermore, dapivirine tends to form aggregates in solution and/or at acidic pH [44]. No studies have been carried out to study formation of dapivirine aggregates in the vaginal milieu. Higher generation hydrophobic NNRTIs like etravirine and rilpivirine also share similar problem which may pose difficulties in their use as a microbicide. UC 781 is a hydrophobic NNRTI that is being evaluated as a microbicide. UC 781 was found to undergo degradation in aqueous solution [45]. UC 781 is also light sensitive and undergoes metal catalyzed oxidation [45]. Thus, developing suitable and stable formulation of microbicides such as UC 781 is a challenging task. Raltegravir is an integrase inhibitor that belongs to BCS Class II. However, a recent study has shown the permeability of raltegravir is significantly dependent on the pH, which would have an impact on its use as a microbicide [46]. Various studies have shown that drugs like tenofovir, raltegravir and maraviroc are substrates for drug efflux transporters [47–49]. Until today, there are no in vitro studies on the expression of drug efflux transporters on the cells relevant to vaginal and/or rectal delivery of microbicides. It could be interesting to study the use of P-glycoprotein modulators on the in vitro and in vivo distribution of microbicides.
Vaccines, peptides and siRNA-based modalities are continuously being explored for HIV prophylaxis. However, these modalities have poor tissue permeability due to their extreme hydrophilicity and/or anionic charge [26–30]. Furthermore, these modalities are quite susceptible to chemical and metabolic instability. Acidic environment of vagina and cervicovaginal mucus are also major barriers for effectiveness of peptides, vaccines and siRNA. It is important to identify strategies that would increase permeability, chemical and metabolic stability and immunogenicity of vaccines and/or siRNA therapeutics.
High osmolarity of delivery vehicle (gel) is another factor that hinders/compromises efficacy of prophylactic modalities. Tenofovir gel used for CAPRISA004 trial contained 20% w/v glycerine. Usually, 2.25% w/v glycerine is sufficient to attain anosmolarity similar to cervicovaginal fluids. The tenofovir gel used in the CAPRISA004 trial had osmolarity of 3111 mmol/kg and in vitro studies showed that it caused epithelial stripping of polarized explant [50]. Recently, a Phase 1 trial of an HIV-1 entry inhibitor (cellulose acetate phthalate) was halted due to significant mucosal irritation caused by hyperosmolarity of the gel [51]. In order to avoid mucosal irritation and damage of vaginal epithelium, microbicide gels should have osmolarity less than 1000 mmol/kg [43]. Thus, suitable strategies need to be designed to tackle the problem of hyperosmolarity without compromising efficacy of microbicides.
4. Nanotechnology for HIV prophylaxis
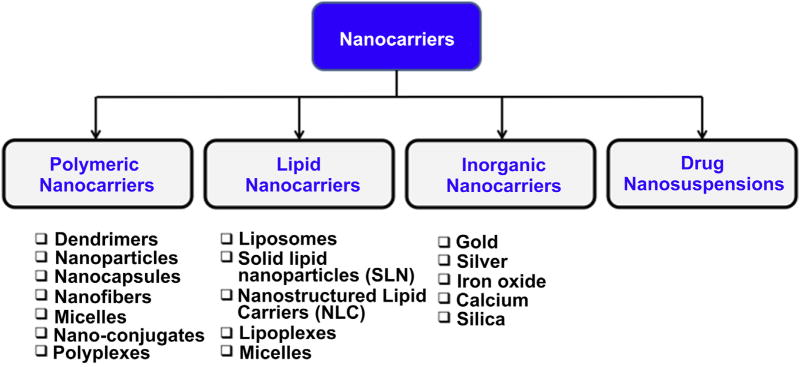
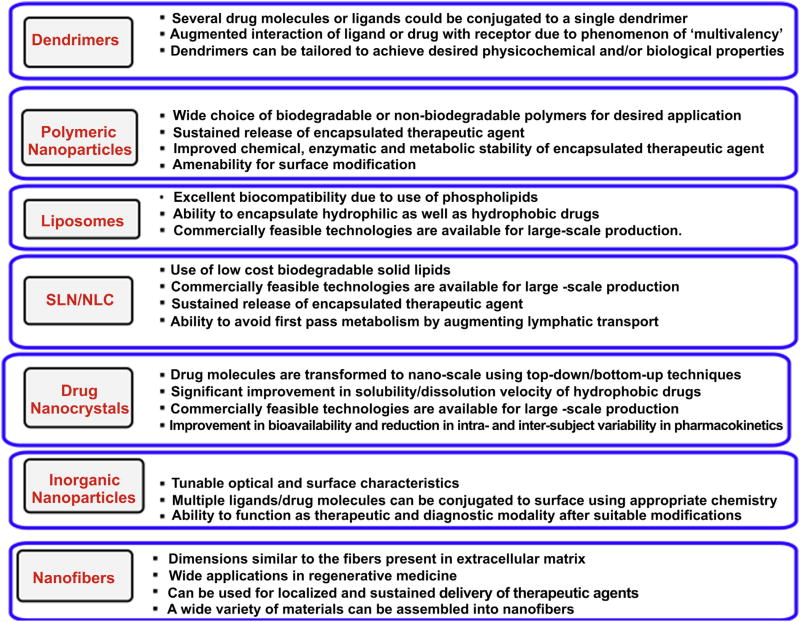
According to the National Nanotechnology Initiative, nanotechnology involves study of materials/architectures of size 1–100 nm in at least one dimension [1,5,52–54]. However, materials with size up to several hundred nanometers are also included under nanotechnology. At the moment, several nanotechnology based products are being used and evaluated in clinical practice. Since last decade, efforts are being made to improve diagnosis, therapy and prophylaxis of HIV/AIDS with the help of nanotechnology. In last few years, several reviews have been published which mainly focus on application of nanotechnology for improving therapeutic effect and targeting of antiretroviral modalities [1,5,32,52–56]. In the present review, we have mainly focused on application of nanotechnology pertaining to prophylaxis of HIV. Until now, various types of nanocarriers have been developed for improving HIV prophylaxis (Fig. 3). Nanocarriers offer various advantages which would be useful to overcome challenges/problems associated with current prophylactic modalities. Various types of nano-architectures developed for HIV prophylaxis are discussed in the following sections and their salient features are shown in Fig. 4.
Fig. 3.
Various types of nano-architectures available for HIV prophylaxis.
Fig. 4.
Salient features of various nano-architectures.
5. Nano-architectures for HIV prophylaxis
5.1. Dendrimers
Dendrimers are a versatile class of polymeric nano-structures with unique architectural and topological features. Unlike conventional linear polymers, dendrimers exhibit three-dimensional tree-like structures, narrow polydispersity index and precise number of terminal groups [52,55,57]. Interestingly, it is possible to synthesize dendrimers with precise physicochemical and desired biological properties by manipulating the structure of central core, structure and number of branching units and composition and number of surface functional groups [52,55,57].
Due to presence of numerous surface functional groups, it is possible to conjugate multiple drug or targeting ligand molecules to a single dendrimer. Dendrimers also have the ability to encapsulate hydrophobic drugs in their interior cavity[52,55,57]. However, due to limitations on the cavity size, drug payload obtained after encapsulation is significantly lower than that obtained with conjugation [52,55,57]. Dendrimers with the right composition and number of surface functional groups can form complexes with cell or viral receptors through multivalent interactions resulting in inhibition of viral fusion to target cells [52,58]. In fact, the potential of dendrimers to prevent viral infection was demonstrated back in 1996 [59]. Thus far, dendrimers based on diverse building blocks such as polyamidoamine (PAMAM), poly-amino acids, polypropylene imine, polyethers and carbosilane have been evaluated.
5.1.1. Dendrimers as topical microbicides
Due to a lack of efficacy of polyanionic natural, semi-synthetic or synthetic linear polymers in the clinical trials (Table 1), dendrimers were actively pursued as topical microbicides. Until today, dendrimers is the only nanotechnology that has advanced to human clinical trials for HIV prophylaxis[52]. Extensive studies on the linear polyanionic inhibitors provided a basis for the design of suitable dendrimers. It is well known that the anionic moieties of the linear polyanionic inhibitors inhibit viral fusion to cell membrane by binding to viral glycoproteins such as gp120 and/or gp41 [3]. Hence, dendrimers with anionic surface functional groups have been designed and explored to maximum extent. Although most of the linear polyanionic entry inhibitors exhibit sulfated end groups, dendrimers with various other anionic end groups have also been designed [58, 60]. Dendrimers that are being evaluated as HIV entry inhibitors can be broadly classified into (1) classical dendrimers with anionic end groups; (2) carbohydrate terminated dendrimers (glycodendrimers); and (3) dendrimers based on inorganic–organic backbones.
5.1.1.1. Classical dendrimers with anionic end groups
Dendrimers with classical branching units such as polyamidoamine (PAMAM) and poly-l-lysine, but different anionic end groups have been extensively investigated by researchers to identify optimal design parameters for dendrimers with maximum antiviral activity and minimal toxicity [58,60,61]. These systematic investigations have resulted in development of first topical nanomicrobicide, SPL7013 (also referred as VivaGel®). SPL7013 is a g-4 poly-l-lysine dendrimers synthesized from divalent benzhydrylamine amide of l-lysine and it contains 32 sodium 1-(carboxymethoxy) naphthalene-3,6-disulfonate as terminal anionic functional groups [58,62]. The precise chemistry and process design have ensured large-scale synthesis of SPL7013 as single molecular entity with molecular weight of 16,581 Da. Various in vitro studies have established broad spectrum antiviral activity of SPL7013 against HIV and HSV (EC50 ranging from 0.5 to 3 μg/mL) whereas SPL7013 was not cytotoxic to Vero cells at a concentration as high as 10,000 μg/mL [62]. Thus, SPL7013 showed excellent selectivity index. Unlike dextran sulfate and other linear polyanionic entry inhibitors, SPL7013 showed similar potency against entry of CXCR4-(X4) and CCR5-using (R5) HIV-1 strains [60]. Telwatte et al. have demonstrated that SPL7013 has potent virucidal activity against the CXCR4-tropic HIV-1 strains [63]. SPL7013 (3% w/w) was formulated into an aqueous mucoadhesive Carbopol® gel buffered to aphysiologically acceptable pH (VivaGel®). Single intravaginal application of VivaGel to pigtailed macaques completely prevented simian-human immunodeficiency virus infection. Moreover, VivaGel® was found to be safe in pigtailed macaques after vaginal as well as rectal administration [64].
VivaGel® is the first nanotechnology based microbicide to enter human clinical trials. During the Phase I clinical trial, VivaGel was found to be safe and well tolerated in women after once daily intravaginal application for 7 days. Moreover, no systemic absorption of SPL7013 was observed indicating good vaginal retention and absence of systemic toxicity [65]. An additional Phase I randomized placebo controlled trial was carried out to evaluate safety of VivaGel® in healthy young women after twice daily application for 14 days. Women in VivaGel® arm showed higher incidences of grade 1 or 2 genitourinary adverse events as compared to control but no serious adverse events were observed in both the arms [15]. Price et al. evaluated antiviral efficacy of SPL7013 present in the cervicovaginal fluids of the women treated with VivaGel®. VivaGel demonstrated activity against HIV and HSV for at least 3 h post-dose. This is the first clinical evidence of the efficacy of the VivaGel® and it may be possible to administer VivaGel® 3 h before coitus [16]. In a recent Phase I trial, Mosicicki et al., evaluated the effect of VivaGel treatment on various mucosal biomarkers associated with epithelial damage [17]. It was observed that VivaGel treated women showed higher levels of IFN-γ, IL-2, IL-5, IL-6 and IL-10 after 7-day and/or 14-day administration. Incidentally, the VivaGel treated group also showed higher population of CD8+/CD69+ T cells, CD4+/CD69+ cells and CD4+/CCR5+ T cells. However, all these changes abolished 7 days after discontinuation of VivaGel treatment. Thus, changes observed after VivaGel treatment appeared to be reversible. However, this trial indicated that subsequent VivaGel cytotoxicity should be monitored meticulously.
5.1.1.2. Glycodendrimers
Carbohydrates are an integral part of several complex biological processes such as cell differentiation, metastasis and infection. Carbohydrate receptors are expressed on variety of immune cells such as macrophages as well as epithelial cells such as vaginal epithelial cells [66–69]. Carbohydrate receptors such as C-type lectin receptors present on the antigen presenting cells (dendritic cells) and mannose receptors present on the vaginal epithelial cells and human spermatozoa have important role in the HIV infection [66–69]. Cell surface glycosphingolipids such as galactosylceramide can act as a receptor for the HIV gp120 and facilitate HIV entry in the absence of CD4 receptors [70]. On the other hand, a variety of linear sulfated polysaccharides have shown the ability to inhibit HIV entry in vitro although they were unsuccessful in clinical trials [3]. In view of this, it was hypothesized that multivalent presentation of carbohydrates or sulfated carbohydrates on the dendrimeric scaffold could be useful for inhibition of HIV entry. Tabarani et al. demonstrated that mannose hyper-branched dendritic polymers can inhibit interaction between HIV gp120 and DC-SIGN (dendritic cell-specific ICAM-3 grabbing non-integrin), a C-type lectin receptor present on dendritic cells [71].
Kensinger et al. designed various generations of polypropylenimine (PPI) glycodendrimers with galactose or sulfated galactose as terminal groups and evaluated their ability to prevent HIV infection [70,72]. It was observed that dendrimers terminated with sulfated galactose were more active as compared to galactose terminated dendrimers. In fact, sulfated galactose generation 5 (G-5) dendrimers were found to be more potent than linear poly-saccharide dextran sulfate in certain cases [72]. This indicates the potential of glycodendrimers. In a series of investigations, Han et al. have evaluated potential of various types of sulfated cellobiose terminated poly-l-lysine dendrimers to inhibit HIV entry [73,74]. Generation 3 (G-3) sulfated cellobiose terminated poly-l-lysine dendrimers showed similar potency to the NRTI (nucleosidereverse transcriptase inhibitor) ddC (2′–3′-dideoxycytidine) [73]. The same group evaluated effect of derivatization of lysine core with stearylamide on the anti-HIV effect of sulfated cellobiose terminated dendrimers. The fatty acid derivatization reduced the potency of the dendrimers [74]. Hence, fatty acid derivatization was not found to be useful for increasing potency. Clayton et al. synthesized sialic acid and sulfated sialic acid terminated PAMAM dendrimers and evaluated their anti-HIV activity in comparison to dextran sulfate. The sialic acid dendrimers were much less potent as compared to dextran sulfate [75]. Schengrund et al. designed G-5 PPI dendrimers terminated with either 3′-sialyllactose (GM3) or globotriose (Gb3). Depending on the type of sugar, cell and virus, the IC50 value of dendrimers ranged from 0.1 to 15 μg/mL [76]. Sattin et al., demonstrated that tetravalent Boltron-type dendrimers terminated with linear trimannoside mimics (termed as Dendron-12) can inhibit infection of lymphocytes (up to 90%) by CXCR4-(X4) and CCR5-using (R5) HIV-1 strains at 50 μM concentrations [77]. Moreover, the dendrimers did not show any signs of toxicity at 4-fold higher concentrations. The authors also carried out in vitro studies that involved pre-treatment of cells with Dendron-12 for 30 min or 2 h, thorough washing to remove Dendron-12 followed by HIV infection at 0, 6, or 12 h. In all cases, Dendron-12 significantly reduced HIV infection. Thus, glycodendrimers could have potential for long-term prophylaxis [77]. The same group evaluated the potential of Dendron-12 in the cervical explants. Dendron-12 could successfully prevent infection of cervical tissue by various clades of R5- and X4-tropic viruses and was found to be well tolerated by cervical explants at tested concentrations [78].
Mannose and lactose terminated dendrimers based on gallic acid core have been evaluated for ability to inhibit dimerization of HIV-1 capsid protein [79]. Unfortunately, none of the glycodendrimers showed inhibition of capsid protein dimerization whereas benzoic acid sodium terminated gallic acid dendrimers showed considerable inhibition of capsid protein dimerization [79]. From various studies on the glycodendrimers, it can be inferred that the anti-HIV activity considerably depends on the nature of the terminal carbohydrate group and its subsequent derivatization. The influence of the type of core and branching units on the activity of glycodendrimers has not been explored yet.
5.1.1.3. Dendrimers based on inorganic-organic backbones
In recent years, dendrimers based on inorganic compounds such as phosphorus and silicon have been designed as HIV entry inhibitors. Blanzat et al. have evaluated anti-HIV potential of phosphorus dendrimers based on hexachlorocyclotriphosphazene or thiophosphoryltrichloride core in a series of investigations [80–82]. Phosphorous dendrimers with either cinnamic acid or phosphonic acid end groups were prepared. These anionic dendrimers interacted with cationic galactosylceramide analogue (amino lactitol) to yield self-assembled catanionic dendrimers [80,81]. The anti-HIV activity of these dendrimers was evaluated. Interestingly, all the dendrimers demonstrated the ability to inhibit HIV infection at nm or μm concentrations. However, all the dendrimers yielded considerably low selectivity index (less than 100) [80,81]. Thus, this series of dendrimers was deemed to be unsuitable for further development. The same group designed phosphonate terminated poly-(phosphorhydrazone) dendrimers with different pendant alkyl chains [82]. Unlike earlier investigation, no attempts were made to fabricate cataninonic dendrimers. Interestingly, these dendrimers did not show any cytotoxicity up to 10 μm concentration whereas IC50 against HIV was in the nm range [82]. This study clearly indicated the influence of dendrimers architecture and pendant alkyl groups on the anti-HIV activity of dendrimers. Munoz-Fernandez et al. have designed a series of dendrimers containing carbosilane (Si–C) or carbosiloxane (Si–O) core and various cationic or anionic end functional groups for various applications [83–85]. Recently, the same group designed second generation carbosilane dendrimers with anionic sulfonate end groups and evaluated their ability to inhibit HIV [90]. The dendrimers were non-toxic to various epithelial cell lines and PBMC at concentrations ranging from 20–100 μM. Moreover, carbosilane dendrimers were able to prevent HIV-1 and HIV-2 infection in activated PBMC. Carbosilane dendrimers also partially inhibited translocation of HIV through trans-epithelial monolayer in vitro. Carbosilane dendrimers were also safe to rabbits after 2 weeks of intravaginal application [85]. Thus, dendrimers based on inorganic–organic core do have potential to become topical microbicides. However, there have been no studies to establish their safety on chronic administration.
5.1.2. Dendrimers as carriers
A small number of studies have been reported in the literature that explores the potential of dendrimers as a carrier for antiretroviral modalities. Jain et al. have designed poly(propylene imine; PPI) dendrimers terminated with tuftsin (a macrophage activating tetrapeptide) or mannose (ligand for lectin receptors present on dendritic cells and macrophages) as targeting ligands [86,87]. Antiretroviral drugs such as efavirenz and lamivudine were incorporated in the dendrimers and the efficacy and safety of these dendritic nanocarriers was evaluated. The PPI dendrimers functionalized with targeting ligands resulted in greater in vitro anti-HIV activity and reduced cytotoxicity as compared to PPI dendrimers without targeting ligands. Functionalization of PPI dendrimers with targeting ligands masked their cationic charge leading to reduced cytotoxicty and greater efficacy.
Munoz-Fernandez et al. evaluated the potential of water-soluble amine terminated cationic carbosilane dendrimers to deliver various siRNAs to PBMC and lymphocytic SupT1 cells [84]. The carbosilane dendrimers prevented siRNAs from RNAse mediated degradation and dendrimers–siRNA comeplexes were non-toxic to cells up to 30 μg/mL. The dendrimers could successfully transfect PBMC (which are usually hard to transfect) and SupT1 cells with siRNA and prevented HIV infection [84]. In an interesting investigation, Navath et al., fabricated in-situ forming hydrogel based on crosslinking of thiopyridine terminated G-4 PAMAM dendrimers and thiol terminated 8-arm PEG [88]. Vaginal delivery of dendrimeric hydrogels to guinea pigs revealed that hydrogels were retained in the vaginal cavity for at least 72 h and did not cause any alteration in the vaginal pH. The hydrogels did not show any signs of local toxicity and started biodegradation after 72 h. Thus, dendrimeric hydrogels could be used for sustained delivery of water-soluble antiretroviral drugs such as tenofovir or emtricitabine into vaginal cavity. In short, dendrimers can be successfully used as carriers for antiretroviral agents.
5.2. Polymeric nanoparticles
Polymeric nanoparticles (or nanospheres) are solid colloidal nano-scale particles composed of macromolecular substances of natural or synthetic origin and have size range between 10 and 1000 nm [89]. The therapeutic agent(s) can be dissolved, encapsulated, adsorbed or conjugated to polymeric nanoparticles by means of various methods [89]. Over the years, various methods have been developed to engineer polymeric nanoparticles that can carry a variety of hydrophobic or hydrophilic drugs as well as biomolecules like proteins and siRNA [90]. A variety of natural or synthetic biodegradable polymers as well as non-biodegradable polymers have been employed for fabrication of polymeric nanoparticles. However synthetic polymers such as poly-lactic-co-glycolic acid (PLGA), poly-caprolactone (PCL), polyalkylcyanoacrylates, polymethylmethacrylates (Eudragits) and natural polymers like chitosan are most widely used for fabrication of polymeric nanoparticles. Although there are several literature reports and reviews about delivery of antiretroviral drugs [32,53,54], we will discuss reports relevant to prophylaxis of HIV. We have also included some recent reports on potential of polymeric nanoparticles for HSV prophylaxis as they have implications in vaignal delivery of microbicides using polymeric nanoparticles.
5.2.1. Polymeric nanoparticles for delivery of antiretroviral drugs and siRNA for HIV prophylaxis
Polymeric nanoparticles received great attention for (vaginal) delivery of microbicides after investigations by Ham et al., and Woodrow et al., were published in 2009 [91,92]. Until today, most of the investigations focusing on HIV prophylaxis have employed PLGA nanoparticles to deliver microbicides. PLGA is a US FDA approved biodegradable polymer and is acceptable for delivering drugs via all major routes of administration. PLGA nanoparticles can undergo endolysosomal escape and deliver encapsulated cargo into cytoplasm [93]. This aspect is very important for successful delivery of antiretroviral drugs as well as RNAi therapeutics. There are numerous examples in the literature that demonstrate sustained release potential of PLGA nanoparticles. In view of this, PLGA nanoparticles were thought to have potential in achieving long-term HIV prophylaxis. Ham et al., engineered PLGA nanoparticles containing PSC-RANTES, a CCR5 chemokine receptor inhibitor [91]. Encapsulation of the PSC-RANTES in the PLGA nanoparticles did not affect its anti-HIV activity as compared to PSC-RANTES solution. Interestingly, ex-vivo permeation studies in human ectocervical tissue demonstrated that PSC-RANTES-PLGA nanoparticles have significantly higher (4.8 times) uptake as compared to non-encapsulated PSC-RANTES within a period of 4 h. Furthermore, PSC-RANTES-PLGA nanoparticles could reach the basal layer of the cervical epithelium, which is critical component of the HIV infection process [91]. The augmented delivery of PSC-RANTES from the nanoparticles could be due to greater cellular uptake of nanoparticles as well as protection of PSC-RANTES from the acidic/ enzymatic degradation in cellular milieu due to its encapsulation into nanoparticles. Saltzman et al. have carried out various investigations on the vaginal delivery of PLGA nanoparticles. In the first investigation, Saltzman et al. fabricated PLGA nanoparticles (<200 nm) containing siRNA–spermidine complex in their core. In vitro studies confirmed ability of PLGA nanoparticle encapsulated siRNA to silence target genes in a cell- and dose-dependent manner [92]. In vivo efficacy of the PLGA nanoparticles carrying siRNA (that targets enhanced green fluorescent protein expression; eGFP) was evaluated in female mice expressing eGFP in their reproductive tract. Interestingly, single intravaginal application of siRNA carrying PLGA nanoparticles induced sustained eGFP silencing in the entire reproductive tract for the period of 14 days [92]. PLGA-siRNA nanoparticles achieved deep penetration into the epithelial tissue and were better tolerated than sirna-lipoplexes. In the second investigation, the authors evaluated distribution of various types of fluorescent nanoparticles in the reproductive tract of female mice after single intravaginal administration [94]. It was observed that surface characteristics of the PLGA nanoparticles significantly governed their intravaginal distribution and retention. PLGA nanoparticles without any surface modification and PLGA nanoparticles bearing avidin on their surface showed significantly lower retention in the reproductive tract as compared to PLGA nanoparticles bearing PEG chains on their surface [94]. This study clearly indicated need for surface PEGylation of PLGA nanoparticles to achieve optimal intravaginal delivery. Surface PEGylated PLGA nanoparticles were recovered from cervicovaginal lavage even after 24 h. However, the study was not carried beyond 1 day to study further fate of the PLGA nanoparticles. The same group has developed PLGA nanoparticles containing siRNA that can target genes (UL29.2 or nectin-1) relevant to HSV-2 infection [95]. The authors evaluated in vitro and in vivo efficacy of siRNA containing PLGA nanoparticles and siRNA-lipoplexes. PLGA nanoparticles containing siRNA showed significantly higher gene silencing (in vitro) as compared to siRNA-lipoplexes. The PLGA nanoparticles and lipoplexes containing siRNA were intravaginally administered to mice before and after lethal HSV-2 challenge and disease progression and mortality in mice was observed for 28 days. Interestingly, PLGA nanoparticles treated mice showed higher survival as compared to lipoplexes [95]. Moreover, histopathological studies indicated that siRNA-lipoplexes treatment caused a greater accumulation of polymorphonuclear neutrophils in the vaginal mucosa whereas no gross inflammation and epithelial damage was observed after delivery of PLGA-siRNA nanoparticles. The in vivo silencing of target genes by PLGA-siRNA nanoparticles was confirmed 7 days after the treatment. The highlight of the study is that for the first time, siRNA carrying nanosystems were able to increase the survival of HSV infected mice for 28 days [95]. This investigation clearly demonstrates the ability of PLGA nanoparticles to become a carrier for variety of microbicides.
Hanes et al. have recently evaluated potential of acyclovir containing PLGA nanoparticles to prevent HSV infection [96]. The authors engineered acyclovir loaded conventional PLGA nanoparticles as well as PLGA nanoparticles with ability to rapidly penetrate vaginal mucus. The authors demonstrated that simple coating of PLGA nanoparticles with Pluronic F127 (a US FDA approved polymeric emulsifier) imparts rapid mucus penetrating ability to PLGA nanoparticles. Interestingly, a significant amount (more than 60%) of mucus penetrating PLGA nanoparticles was retained in the reproductive tract of female mice as compared to the conventional PLGA nanoparticles. Moreover, conventional PLGA nanoparticles caused acute inflammatory events like Non-xynol 9 after administration whereas mucus penetrating nanoparticles did not show any such events. Intravaginal administration of acyclovir containing mucus penetrating nanoparticles was found to protect significantly higher number of mice (53%) as compared to acyclovir solution (16%). Furthermore, administration of acyclovir solution containing 10 times higher concentration of acyclovir than acyclovir nanoparticles offered only 30% protection [96]. This clearly establishes utility of mucus penetrating nanoparticles in vaginal delivery.
Youan et al. focused on developing polymeric nanoparticles for delivering tenofovir. In view of the lack of success with VOICE trial, developing smart carrier for delivery of tenofovir is an urgent need. Youan et al. developed pH-sensitive PLGA nanoparticles as well as mucoadhesive chitosan nanoparticles for vaginal delivery of tenofovir [97,98]. Tenofovir loaded pH-sensitive PLGA nanoparticles were developed by blending different ratios of PLGA with Eudragit S100, a pH-sensitive polymer that dissolves at pH 7.4. The developed nanoparticles were well tolerated by vaginal epithelial cells and Lactobacillus sp. Interestingly, due to presence of Eudragit S100, the NPs released minimal amount of drug in simulated vaginal fluids (pH 4.5) and significantly higher release of drug was observed in the simulated seminal fluid (pH 7.6). The form of tenofovir (tenofovir base or tenofovir disoproxilfumarate) affected the rate of drug release. Although these nanoparticles released higher amount of drug at pH 7.6, the drug release was sustained for 24 h due to presence of PLGA. The S100-PLGA nanoparticles demonstrated 50% uptake in the vaginal epithelial cells over a period of 24 h [97]. Tenofovir loaded chitosan nanoparticles were also evaluated for drug release, cytotoxicity and mucoadhesivity. Size of the chitosan nanoparticles was found to have considerable impact on the encapsulation efficiency and release rate of tenofovir. Chitosan nanoparticles with larger size yielded greater encapsulation efficiency and slower release rate. The chitosan nanoparticles were tolerated by vaginal epithelial cells and Lactobacillus sp. The mucoadhesive nature of chitosan nanoparticles was confirmed using porcine vaginal mucosa [98]. Recent evidence suggests that nanoparticles should preferably have mucus penetrating ability to reach to vaginal epithelial cells and deliver the drug. Although chitosan is a mucoadhesive polymer, it also imparts positive surface charge to nanoparticles due to free primary amine groups. It has been demonstrated that positively charged nanoparticles with certain features can penetrate the mucus. Thus, whether chitosan nanoparticles have mucoadhesive and/or mucus penetrating ability still remains to be clearly elucidated.
Beletti et al. fabricated tenofovir loaded hybrid nanoparticles containing chitosan and PLGA [99]. The nanoparticles were prepared by multiple emulsion method and chitosan was added in the inner phase of the primary emulsion. The presence of chitosan helped in increasing the encapsulation efficiency of tenofovir in the nanoparticles as compared nanoparticles prepared without chitosan. Although the nanoparticles showed a tendency to sustain release of tenofovir in the pH 7.4 media, the release was not studied for more than 9 h [99]. Although smart delivery strategy for tenofovir is warranted, the extreme hydrophilicity of the tenofovir poses a major problem for its nanoencapsulation. None of the reported investigations on tenofovir polymeric nanoparticles have been able to achieve encapsulation efficiency greater than 40%. This is a major challenge that still needs to be overcome.
dasNeves et al. developed poly-caprolactone (PCL) nanoparticles containing dapivirine, a hydrophobic NNRTI under development as a vaginal microbicide [100]. PCL nanoparticles with anionic, cationic and neutral surface charge were developed. It was observed that cationic nanoparticles had greater cell uptake but they were also more cytotoxic. Poloxamer 338 containing neutral PCL nanoparticles were found to be well tolerated by cells. PCL nanoparticles significantly improved intracellular delivery of dapivirine as compared to dapivirine solution in a variety of cells. However, no appreciable difference in IC50 values has been reported. Moreover, no attempts were made to test the intracellular concentrations after 8 h [100]. Yoo et al. fabricated nanoparticles of Eudragit S100 due to its pH-sensitive nature [106]. The Eudragit S100 nanoparticles were non-toxic to vaginal epithelial cells even at higher concentrations (1 mg/mL). Due to their pH-sensitive behavior, nanoparticles showed very less drug release at vaginal pH whereas immediate release was observed at physiological pH. The nanoparticles were abundantly taken up by the vaginal epithelial cells [101]. Thus, S100 nanoparticles could be pursued further as carriers for antiretroviral drugs.
Use of antiretroviral drug combination is gaining popularity in the field of HIV prophylaxis mainly to maximize success of prophylaxis and to obviate possibilities of resistance. We have earlier demonstrated that it is possible to fabricate PLGA nanoparticles containing a combination of three antiretroviral drugs, viz. lopinavir, ritonavir and efavirenz [102,103]. Moreover, these combination nanoparticles demonstrated sustained release of antiretroviral drugs in vitro as well as in vivo. However for prophylaxis, it is important to develop formulations of drugs that act before integration of HIV to host DNA. Thus, we focused our investigation on developing PLGA nanoparticles containing different antiretroviral modalities. Until today, there are no reports on use of HIV integrase inhibitors for vaginal prophylaxis of HIV infection. Raltegravir (RAL) is the only HIV-1 integrase inhibitor approved by US FDA. There were two recent reports demonstrating potential of RAL in oral preexposure prophylaxis [104,105]. Moreover, Koh et al. demonstrated that RAL pre-treated HeLa-T4 cell and primary human cells can resist HIV-1 infection even after washout of RAL from culture medium [106]. Thus, RAL was thought to have potential for vaginal pre-exposure prophylaxis of HIV infection. We aimed at developing nanoparticles containing a combination of RAL, an integrase inhibitor and efavirenz, a NNRTI. Although there are concerns about the use of efavirenz as a microbicide due to resistance issues and teratogenicity [107,108], we used it as a model NNRTI to establish a proof-of-concept. We fabricated PLGA nanoparticles containing a combination of raltegravir and efavirenz (RAL-EFV-NPs) using simple and scalable emulsion-solvent evaporation technique [109]. Furthermore, nanoparticles were fabricated using Pluronic F127 as a stabilizer, which is already known to impart mucus-penetrating properties to nanoparticles [96]. It was observed that RAL and EFV exhibited different encapsulation efficiency due to differences in their physicochemical properties. The RAL-EFV-NPs demonstrated significantly less cytotoxicity as compared to RAL + EFV solution at the same concentration. Thus, PLGA nanoparticles can improve selectivity index of antiretroviral drugs. RAL-EFV-NPs (at RAL and EFV concentration of 5 μg/mL) did not show any signs of toxicity to HeLa cells over a period of 14 days. The ability of RAL-EFV-NPs in the prophylaxis was evaluated in vitro using TZM-bl HIV indicator cells. The cells were pre-treated with RAL-EFV-NPs or RAL-EFV solution for 1 day and cells were infected with HIV after complete removal of treatments. Interestingly, RAL-EFV-NPs demonstrated lower EC90 value as compared to RAL + EFV solution [109]. This clearly indicated the ability of PLGA nanoparticles to augment delivery of drugs to the cells. The intracellular concentrations of RAL and EFV in RAL-EFV-NPs treated HeLa cells were observed over a period of 14 days. RAL intracellular concentration was maintained for a period of 6 days whereas EFV was detected in the cells (concentration 100 times greater than reported IC50 of EFV) even at the end of 14 days [22]. The intracellular concentrations of the drugs seemed to be dependent on the metabolic enzymes expressed in the HeLa cells. In summary, PLGA nanoparticles can offer sustained intracellular delivery of encapsulated drugs and can be useful for long-term prophylaxis.
It is also important to develop a suitable vehicle for the delivery of PLGA nanoparticles. Unfortunately, none of the literature reports describe development of suitable vaginal delivery vehicle for the polymeric nanoparticles. The gelling agent can considerably affect the size and colloidal stability of the nanocarriers as observed by Patravale et al. [110]. Instead of developing a conventional gel, we aimed at developing a thermosensitive gel containing RAL-EFV-NPs. Thermosensitive gels are liquid at room temperature and form a highly viscous gel at 37 °C once delivered inside the body [111]. Thermosensitive gels are easy to handle and deliver as compared to conventional gels due to their liquid nature, can spread evenly on gelation whereas their high viscosity at body temperature can minimize chances of gel leakage [112]. We successfully developed thermosensitive gel based on combination of Pluronic F127 and Pluronic F68. Incorporation of nanoparticles in the gel did not affect their size or other physicochemical characteristics. We carried out transwell experiments on the thermosensitive gel containing Rhodamine 6G labeled fluorescent PLGA nanoparticles to check whether incorporation of PLGA nanoparticles in the thermosensitive gel has any effect on their release or uptake profile. Interestingly, fluorescent PLGA nanoparticles traversed through transwell membrane and were taken up by the HeLa cells within 30 min [109]. This clearly indicated that thermosensitive gel enabled quick release of the PLGA nanoparticles from the matrix. Thermosensitive gel containing fluorescent PLGA nanoparticles were administered to mice by intravaginal route and various tissues were excised after 24 h to observe presence of fluorescence in the tissues. Interestingly, tissues such as vaginal epithelium showed considerable fluorescence even after 24 h (unpublished data). Thus, RAL-EFV-NPs carrying thermosensitive gel showed promise for further development.
5.2.2. Polymeric nanoparticles for delivery of vaccines for HIV prophylaxis
In view of the failures observed with most of the HIV vaccine clinical trials, development of smart vaccine delivery system is highly warranted. Polymeric nanoparticles (especially biodegradable nanoparticles) have shown a great potential to become the next generation vaccine adjuvants. Moreover, biodegradable polymeric nanoparticles offer a safer alternative to conventional adjuvants such as alum salts. Polymeric nanoparticles can (1) protect encapsulated antigen from proteolytic degradation; (2) yield sustained and enhanced cross-presentation of antigen to immune cells; (3) undergo endolysosomal escape after uptake by immune cells; and (4) be tailored by various methods to elicit a desired immune response [113–115]. There are several reviews in the literature discussing advantages of nano-scale carriers as vaccine adjuvants for a variety of vaccines [113–115]. Readers are requested to refer to these excellent reviews for further information. Although various polymers are available for nanoparticle fabrication, polystyrene, PLA, poly-glutamic acid (PGA) and polymethylmethacrylate (PMMA) have been mainly used for fabrication of the nanoparticles. In most of the studies, electrostatic interactions between anionic nanoparticles and cationic HIV vaccines have been utilized for enabling delivery of vaccines to target cells/organs. Various routes of administration (oral, nasal, dermal and vaginal) have been employed for achieving mucosal immunization.
Ataman and colleagues fabricated surfactant-free anionic PLA nanoparticles by a simple diafiltration technique [116]. HIV p24 protein was electrostatically adsorbed onto nanoparticles and the process did not result in loss of its antigenicity and immunogenicity. The antigen loaded nanoparticles were subcutaneously injected to mice, rabbits and macaques. Interestingly, p24-PLA nanoparticles elicited significantly higher antibody titers including strong cytotoxic T-lymphocyte (CTL) responses in mice as compared to soluble antigen or alum/Freund adjuvanted antigen. The similar observations were noted in rabbits and macaques. Moreover, the PLA nanoparticles induced high levels of IFN-γ-producing T cell responses. These T cell responses were similar to that observed with viral vectors such as Modified Vaccinia virus Ankara against the p24 antigen in macaques [116]. In another study, the authors explored feasibility of developing divalent HIV vaccines based on PLA nanoparticles. Interestingly, authors succeeded in co-adsorbing p24 antigen and gp120 envelope glycoprotein onto PLA nanoparticles [117]. Both the antigens were found to have similar affinity for PLA nanoparticles and their structural and functional integrity was preserved even after adsorption onto PLA nanoparticles. Furthermore, subcutaneous administration of this divalent nanovaccine yielded strong antibody responses against both the antigens [117]. This clearly indicates the feasibility of designing smart vaccine delivery systems with the use of polymeric nanoparticles. The immunogenic ability of antigen carrying PLA nanoparticles has also been compared to MF59, a nanoemulsion based adjuvant. Various HIV antigens (p24gag, wild-type Tat and a mutated and detoxified version of Tat) were delivered to rabbits with either PLA nanoparticles or MF59 and immune responses were monitored [118]. It was observed that the nature of adjuvant as well as type of antigen influenced the type or extent of immune responses. In case of the p24 antigen, the immune response induced by PLA nanoparticles was focused on immunodominant domain as compared to MF59. No such differences in immune responses were observed in the case of wild-type Tat. For mutated detoxified Tat, PLA nanoparticles augmented the number of epitopes recognized by serum IgG as compared to MF59 [118]. Thus, efficacy of vaccine adjuvants would have to be evaluated on a case-by-case basis and no generalization can be made about superiority of particular type of adjuvant.
Aline et al. investigated the ability of p24-PLA nanoparticles to boost adjuvant capacity of dendritic cells. Dendritic cells were incubated (pulsed) with p24 antigen, blank PLA nanoparticles and p24 antigen adsorbed PLA nanoparticles [119]. Only p24-PLA nanoparticles were capable of inducing maturation of dendritic cells leading to enhance expression of cell surface markers such as MHC classes I and II, CD40, CD80 and CD86 [119]. Moreover, p24-PLA nanoparticles treated dendritic cells were capable of releasing cytokines such as IL-4 and IL-7. Dendritic cells treated with p24-PLA nanoparticles were capable of inducing high antibody titers in the blood and intestine (mucosal immunity) of mice. Dendritic cells treated with p24 antigen or blank PLA nanoparticles alone were incapable of inducing mucosal immune response. Thus, PLA nanoparticles can also be used to boost capacity of adjuvants [119]. Liard et al. evaluated systemic and mucosal immune responses after administration of p24-PLA nanoparticles in mice at different sites of the skin viz. subcutaneous, intradermal and transcutaneous route [120]. Subcutaneous delivery of p24-PLA nanoparticles was associated with generation of HIV-1 p24-specific IgG in the absence of antigen-specific CD8 T cells whereas intradermal administration yielded cellular and humoral responses. Administration of p24-PLA nanoparticles by transcutaneous route (hair follicle mediated transport of nanoparticles) induced major CD8 effector cells in the absence of IgG. Moreover, transcutaneous delivery also yielded generation of IgA in the stratified epithelium of the vagina (mucosal immunity). Thus, site of administration in skin seemed to as MHC classes I and II, CD40, CD80 and CD86 [119]. Moreover, p24-PLA nanoparticles treated as MHC classes I and II, CD40, CD80 and CD86 [119]. Moreover, p24-PLA nanoparticles treated delivery.
Poly-amino acids such as Poly-γ-glutamic acid (PGA) have been actively pursued as an adjuvant for HIV vaccines. PGA is a highly anionic biodegradable polymer. HIV antigens can be adsorbed onto PGA by electrostatic interaction. Wang et al. evaluated immune responses elicited after intranasal administration of HIV gp120, HIV gp120 + cholera toxin B subunit and HIV gp120 adsorbed onto PGA nanoparticles. Interestingly, only HIV gp120 adsorbed onto PGA nanoparticles induced significant antigen-specific lymphocyte proliferation [121]. The PGA nanoparticles also induced long-lived memory CD8+ T cells. Moreover, after single intranasal immunization with PGA nanoparticles, the central memory T cells remained relatively constant from day 30 to day 238 although decay in the effector memory T cells was observed. In another study, PGA nanoparticles were evaluated for in vitro and in vivo immunostimulation and for ability to deliver ovalbumin to dendritic cells (DC) [122]. PGA nanoparticles yielded significant increase in uptake of ovalbumin by dendritic cells and also offered sustain release of ovalbumin in the cells. Moreover, PGA nanoparticles were capable of inducing the maturation of DCs which is indicative of their adjuvant potential. PGA nanoparticles containing HIV p24 were subcutaneously administered to mice. PGA nanoparticles induced antigen-specific IFN-γ-producing T cells in spleen cells and p24-specific serum antibodies. The levels of p24-specific serum antibodies induced by PGA nanoparticles were comparable to complete Freund's adjuvant. However, PGA nanoparticles predominantly activated p24-specific IFN-γ-producing T cells which were not seen in case of complete Freund's adjuvant.
Uto et al. compared adjuvant effect of PGA nanoparticles with alum salt [123]. Ovalbumin containing PGA nanoparticles were more efficiently taken up by dendritic cells as compared to alum adjuvanted ovalbumin. Mice immunized with PGA nanoparticles induced significantly higher antigen-specific CD8+ T cells as compared to alum and monophosphoryl lipid A (MPLA) adjuvanted ovalbumin [123]. This indicates potential of PGA nanoparticles in vaccine delivery. Himeno et al., compared immune responses after administration of HIV gp120 and PGA nanoparticles containing HIV gp120 to rhesus macaques [124]. PGA nanoparticles elicited stronger gp120-specific cellular and humoral immune responses than gp120 alone. However, PGA nanoparticles could not offer protection against challenge by simian/human immunodeficiency chimeric virus (SHIV) in the macaques. Further studies would be required to clarify these results.
Surface modified polystyrene nanoparticles (PS-NP) have also been evaluated as vaccine adjuvants. Baba et al. designed poly-methacrylic acid decorated polystyrene nanoparticles. The nanoparticles were coated with concanavalin A (a lectin with high affinity for HIV gp120). These surface modified nanoparticles were capable of capturing infectious HIV-1 (irrespective of cell tropism) as well as heat inactivated HIV-1 [125,126]. In the first study, the authors compared immunogenic potential of heat inactivated HIV-1, concanavalin A decorated PS-NP and heat inactivated HIV-1 capturing concanavalin A PS-NP (HIV-PS-NP) after intravaginal administration to mice [126]. Vaginal fluids of immunized mice were evaluated for presence of anti-HIV-1 IgG and IgA. No detectable levels of anti-HIV-1 IgG were observed in all treatment groups. However, HIV-PS-NP showed significantly higher anti-HIV-1 IgA levels as compared to other groups. Moreover, vaginal fluids obtained from HIV-PS-NP immunized mice were able to neutralize immunizing HIV-1 strain. HIV-PS-NP showed different intravaginal distribution as compared to other treatments [126]. In another study, the authors compared the effect of route of administration on the immunogenicity of HIV-PS-NP. HIV-PS-NPs were administered to mice by oral, nasal, vaginal and intraperitoneal route and HIV-1 specific IgA levels in the vaginal tissues were evaluated [127]. It was observed that intranasal administration of HIV-PS-NP yielded highest levels HIV-1 specific IgA in vagina and vaginal fluids of these mice were able to neutralize HIV-1IIIB. Furthermore, intranasal immunization of mice with HIV-PS-NP also showed presence of HIV-1 specific cytotoxic T-cells in the spleen [128]. Finally, immunization potential of SHIV capturing PS-NP was studied in macaques. Intranasal administration of SHIV-PS-NP resulted in detectable levels of vaginal anti-HIV-1 gp120 IgA and IgG antibodies in all the macaques [129]. Although these studies are promising, PS-NPs are non-biodegradable in nature. Moreover, PS-NPs were found to be less effective in inducing antigen-specific CD8+T-cell responses as compared to biodegradable PGA nanoparticles [121].
Delair et al. developed nanoparticles based on ionic interaction of two natural polymers viz. chitosan and dextran sulfate. The authors developed positively as well as negatively charged polysaccharide nanoparticles by altering the ratio of chitosan to dextran sulfate and studied adsorption of HIV-1 p24 antigen on these nanocarriers. Interestingly, negatively charged nanoparticles yielded higher binding and stability of the adsorbed p24 [130]. The nanocarriers were efficiently internalized by dendritic cells. Subcutaneous administration of polysaccharide nanoparticles yielded significant p24-specific cellular and humoral immune response in mice [131].
Researchers have evaluated polymethylmethacrylate (PMMA) based nanocarriers for delivering HIV antigens or HIV DNA vaccines. PEGylatedpolymethylmethacrylates have been evaluated for delivery of HIV gag and tatDNA vaccines [132,133]. Intramuscular immunization with PMMA nanoparticles carrying HIV tat gene DNA vaccine yielded significantly higher Th-1 type T-cell response and HIV-1 Tat-specific cytotoxic T cell response [132]. Intranasal administration of PMMA nanoparticles carrying HIV gag DNA vaccine resulted in significantly higher number of Gag-specific IFN-γ secreting cells as well as Gag-specific IgG as compared naked DNA vaccine [133]. Nanoparticles with cationic PMMA core and anionic Eudragit L 100-55 corona have been evaluated as vaccine adjuvants [134,135]. The nanoparticles were able to adsorb significant amount of HIV-1 Tat (antigen) and also increased stability of Tat. HIV-1 Tat adsorbed nanoparticles were administered by intramuscular, subcutaneous or intranasal route and immune response was monitored. Nanoparticles were able to induce potent and long-lasting immune response (Tat-specific cytotoxic T lymphocytes as well as IgG) and were well tolerated by animals.
Zhu et al. evaluated immunization potential of Eudragitmicroparticles encapsulating HIV vaccine carrying PLGA nanoparticles on oral delivery [136]. The authors developed PLGA nanoparticles encapsulating PCLUS3–18IIIB (a CD4+ T cell helper epitope fused with an HIV Env CD8+ cytotoxic T lymphocyte epitope) and TLR ligands (MALP2 + poly(I:C) + CpG) and these nanoparticles were encapsulated in Eudragit L 100-55 or Eudragit FS-30D micro-speheres. Eudragit L 100-55 and Eudragit FS-30D microparticles were orally administered to mice and immune responses were compared with intracolorectal administration of HIV antigens. Interestingly, Eudragit FS-30D microparticles induced significant colorectal immunity after oral administration and protected animals against rectal and vaginal viral challenge. Thus, it is possible to develop smart carriers that can induce mucosal immunity and can protect peptide antigens from harsh environment of gastrointestinal tract. In short, polymeric nanoparticles have a great potential to be successful in delivery of HIV vaccines.
5.3. Liposomes
Liposomes were the first to be developed as well as commercialized among all the nanocarriers and have a longest history of research and development. Liposomes are vesicular carriers composed of phosholipid bilayers and aqueous core. The size of the liposomes can range between 80 nm to 10 μm depending upon preparation method and composition. There are numerous reviews that provide detailed description of liposome components, fabrication methods, types of liposome, biophysical properties, characterization and their applications [137–141]. The tendency of liposomes to get rapidly recognized by phagocytic cells of liver and spleen after intravenous administration (passive targeting) and at least partly localize in lysosomes has been utilized for augmenting prophylaxis and/or therapy of various infectious diseases [138,142]. Moreover, the surface of the liposomes can be easily engineered with various moieties to enhance their recognition as well as uptake by macrophages or other components of immune system. Liposomes have mainly been explored for delivering HIV vaccines although few studies have focused on delivering antiretroviral agents or siRNA.
5.3.1. Liposomes for delivery of HIV vaccines and siRNA
The first attempt to deliver HIV antigens using liposomes was reported almost two decades ago [143]. Since then, influence of several aspects such as liposome components, fabrication methods, HIV antigen, route of administration and type of adjuvant has been evaluated to develop liposomeal HIV vaccines and the research is still in progress. Philips et al., evaluated the influence of liposome components on the immune responses to HIV gp120 after subcutaneous administration [144]. Liposomes were fabricated with various phospholipids such as dipalmitoylphosphatidylcholine (DPPC), dimyristoylphosphatidylglycerol (DMPG), dipalmitoylphosphatidylethanolamine (DPPE) and phosphatidylserine (PS) and their effect on immunization was monitored. It was observed that liposomes composed of DPPC/DMPG were able to elicit the highest levels of HIV gp120-specific IgG as compared to the other liposomes and the IgG levels were significantly higher than HIV gp120 adjuvanted with alum. It was also observed that liposomes composed of DPPC/PS yielded IgG levels considerably lower than HIV gp120 adjuvanted alum [144]. This indicated the influence of liposome composition on the immune response. The potential of cationic liposomes for delivery of HIV-1 DNA vaccine was evaluated by Okuda et al. [145]. Intranasal administration of HIV DNA vaccine (encoding env gp160 and rev genes) via cationic liposomes resulted in significantly higher levels of mucosal IgA in feces and vaginal fluids and antibodies against HIV-1 were detected for at least 10 months. Moreover, co-administration of HIV DNA vaccine with plasmid encoding for interleukin-12 (IL-12) and granulocyte/macrophage-CSF through cationic liposomes induced high levels of HIV-1 specific cytotoxic T-lymphocytes (CTL) [145].
It is well known that antigen presenting cells such as dendritic cells and macrophages express receptors that can bind to mannose and mannose containing polysaccharides. Hence, liposomes coated with mannose containing moieties (mannan) were evaluated for targeted delivery of HIV vaccines. Toda et al., evaluated immunization potential of DNA vaccine (encoding HIV-IIIB env and rev genes) loaded onto cationic liposomes with or without mannan coating [146]. The immune responses obtained after intramuscular and intranasal administration of different liposomal vaccines were compared. Interestingly, mannan coated liposomes showed significantly higher serum IgG, fecal IgA and IFN-γ levels in mice as compared to liposomes without mannan coating. This clearly indicated the importance of targeting of liposomal vaccines [146]. Mannosylated glycolipids have also been used for coating liposomal HIV vaccines. Liposomes containing either peptide sequence from HIV gp120 or SIV GST-Nef protein were functionalized with mannosylated glycolipids and their immunization potential was evaluated [147,148]. Once again, targeted liposomes were associated with better immune response as compared to other controls.
Usually, exogenous antigen is presented to MHC Class II molecules through endosomes which leads to CD4+ T-cell response. However, for efficacy of HIV vaccines, strong CTL response is required. Hence, utility of the pH-sensitive liposomes was explored for delivering HIV vaccines. It is known that liposomes containing pH-sensitive lipids like dioleoylphosphatidylethanolamine (DOPE) can fuse with endosomal membrane and release the antigens into the cytosol [149]. The endosomal escape of antigen facilitates its presentation to MHC Class-I that can lead to CTL response [150]. Superiority of pH-sensitive liposomes (containing ovalbumin as a model antigen) over pH-insensitive liposomes has been established by in vitro and in vivo studies [150,151]. Chang et al. prepared various pH-sensitive liposomes by using either DOPE or 1-Palmitoyl-2-Oleoyl-Phosphatidylethanolamine (POPE) as a pH-sensitive lipid and incorporated peptide sequences from V3 loop of HIV-1 gp120 into these liposomes [152]. It was observed that POPE containing liposomes elicited significantly higher immune response as compared to DOPE containing liposomes indicating importance of liposome composition.
Liposomes containing microbial adjuvants (Cholera toxin B subunit) or inactivated virus (hemagglutinating virus of Japan; HVJ) have been explored for potentiating mucosal immune response to HIV antigens [150–155]. Cholera toxin B subunit exerts adjuvant effect by increasing localization of antigen to the galglioside GM1 present on surface of the antigen presenting cells [153]. Lian et al. formulated liposomes containing GM1 and incorporated CTB as well as recombinant HIV envelope protein into the liposomes [153]. It was hypothesized that CTB bound to surface of GM1 containing liposomes would also be able to bind to GM1 present on the mucosal cells; thereby increasing presentation of HIV antigen to mucosal cells. Intranasal administration of CTB decorated GM1 liposomes was associated with significantly higher HIV envelope protein-specific mucosal IgA and IgG as compared to liposomes devoid of GM1 or antigen adjuvanted with alum [153]. Similarly, HIV gp160 loaded liposomes decorated with inactivated HVJ and HIV gp41 peptide loaded liposomes containing adjuvant MA729 (analogue of muramyl dipeptide) elicited significantly higher antigen-specific antibody titers as compared to antigen adjuvanted with alum [154,155].
Dendritic cells express several microbial pattern recognition receptors such as Toll-like receptors (TLR). Hence, adjuvants that can bind to TLR were incorporated in liposomes for increasing immunogenicity [156]. Rao et al. used this approach for vaccine delivery. Liposomes containing monophosphoryl lipid A (MPLA; binds to TLR4) and oligodeoxynucleotides containing cytosine phosphate guanosine (CpG ODN; bind toTLR9) were fabricated. The ability of these liposomes to elicit immune response to HIV-1IIIB gp140 was evaluated [156]. It was observed that MPLA and CpG ODN containing liposomes yielded highest level of immunization as compared to the treatment groups that contained individual adjuvants. The study also concluded that incorporation of MPLA in the liposomes is important for maintaining high level of immunization [156]. Singh and Bisen reported the importance of surface characteristics of liposomes on the immune response to HIV antigen [157]. The authors fabricated pH-sensitive liposomes with or without surface PEGylation and conjugated HIV gp41 epitopes to the liposomes. Interestingly, surface PEGylation of liposomes was found to have considerable influence on the immunization. Surface PEGylated pH-sensitive liposomes elicited significantly higher anti-gp41 antibodies as compared to the pH-sensitive liposomes without surface PEGylation [157].
Watson et al. synthesized various lipid derivatives of peptides from membrane proximal region (MPR) of HIV gp41 and incorporated them into MPLA containing liposomes [158]. The effect of type of lipid anchor on the immunogenicity of the antigen was evaluated by immunizing animals with the different liposomes. Liposomes containing lipid conjugated antigen elicited significantly higher antibody titer as compared to non-conjugated antigen. The type of the lipid anchor had significant effect on the secondary structure of the antigen as well as extent of immune response. Antigen conjugated to a pH-sensitive lipid cholesterylhemisuccinate (CHEMS) was found to yield highest immune response [158]. The authors also studied effect of type of antigen association to liposomes on the immune response [159]. For this purpose, two strategies were employed. In the first strategy, ability of chelated divalent metal ions such as nickel to form coordinate bonds with short histidine residues was utilized. Liposomes containing nickel chelating lipids (Ni-Lipo) were developed and HIV antigen (MPR peptides) with short sequence of histidine residues (his-tag) was employed to augment interaction of HIV antigen with liposomes. In the second strategy, liposomes containing CHEMS conjugated antigen were fabricated. It was observed that Ni-Lipo elicited significantly higher immune response in comparison to liposomes devoid of nickel chelating lipids [159]. This indicated effect of type of antigen association to liposomes on immune response.
Fairman et al. designed complex of cationic liposomes and DNA containing CpG motifs (CLDC). Two SIV antigens viz. simian immunodeficiency virus (SIV) gag protein and SIVmac239 were delivered using the CLDC and immune responses were evaluated in macaques [160]. Intramuscular administration of CLDC yielded stronger SIV-specific T-cell and B-cell responses as compared to antigens delivered without CLDC. Moreover, CLDC treated macaques showed better memory responses several months later following boosting by SIVmac239 [160]. Recently, application of liposomes in delivering self-amplifying RNA vaccines has been reported [161]. The liposomes employed for this purpose had important features such as presence of cationic lipid (for condensing RNA vaccine), ionizable lipid (for pH triggered release) and PEGylated lipids [161]. As described earlier, all these features were shown to improve immune responses. The authors used 9-kb self-amplifying RNA derived from an alphavirus for designing vaccines against various viruses including HIV and the vaccines were incorporated into liposomes with a very high encapsulation efficiency of 85%. Self-amplifying RNA encoding HIV envelope protein gene (Env, SF162 gp140) was delivered using liposomes by intramuscular, intradermal and subcutaneous routes. Interestingly, liposomal vaccine delivered through intramuscular route showed significantly higher antigen-specific immune response as compared to the other routes. The authors also established utility of liposomal vaccines against various other viruses [161].
Liposomes have also been used for systemic or intravaginal delivery of siRNA targeting HIV-1 or HSV-2 [162–164]. Intra-vaginally applied liposomes carrying siRNA targeting HSV-2 UL27 gene (encode an envelope glycoprotein) and UL29 gene (encode DNA binding protein) were efficiently taken up by epithelial and lamina propria cells and also showed sustained gene silencing in vagina and ectocervix of mice for at least nine days [162]. The liposomal siRNA formulation did not cause induction of interferon-responsive genes or inflammation in the reproductive tract. Moreover, the liposomal siRNA protected mice from lethal HSV-2 challenge [26]. Wu et al. developed alginate based scaffolds for delivery of muco-inert PEGylated cationic liposomes containing fluorescently labeled siRNA [163]. It was observed that liposomal siRNA could reach to vaginal epithelium and silence gene expression [163]. Kim et al. developed neutral liposomes decorated with hyaluronic acid on the surface. Monoclonal antibodies targeting human integrin LFA-1 were conjugated to the liposomes through hyaluronic acid [164]. An siRNA with ability to silence leukocyte-specific HIV co-receptor CCR5 expression was incorporated into liposomes. Due to presence of anti-integrin antibody, systemically administered liposomal siRNA was selectively taken up by integrin receptors present on T-cells and macrophages and showed in vivo gene silencing for as long as 10 days. Interestingly, humanized BLT mice pre-treated with liposomal anti-CCR5 siRNA showed enhanced resistance to infection after HIV challenge [164]. Thus, liposomal siRNA formulations have a great potential in HIV prophylaxis.
5.3.2. Liposomes for delivery of antiretroviral agents
Phospholipids such as cardiolipin have shown ability to inhibit HIV-1 in vitro. However, cardiolipid has relatively low selectivity index [165]. Malavia et al. formulated various liposomes containing cardiolipin and synthetic phospholipids by simple ethanol injection method. The composition of liposomes exhibited considerable effect on the anti-HIV activity as well as selectivity index of cardiolipin [170]. Fluorescently labeled cardiolipin liposomes were found to be retained in the vaginal cavity of mice for approximately 24 h after intravaginal administration and liposomal formulation did not cause any adverse effects [165]. MC-1220, a hydrophobic non-nucleoside reverse trascriptase inhibitor was encapsulated in liposomes and liposomes were encapsulated in Carbopol® gel [171]. Pre-treatment of macaques with liposomal gel showed 50–60% protection after SHIV challenge whereas control group showed 100% infection. The RNA viral load at necropsy was significantly lower in the macaques treated with liposomal gels as compared to control group [166]. In summary, liposomes can be used for augmenting efficacy of prophylactic modalities of HIV.
5.4. Lipid nanocarriers
Lipid nanocarriers such as solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) are extensively being explored for a variety of applications for the last two decades. SLN are composed of low cost and biodegradable solid lipid(s) which are nanosized with the help of a suitable stabilizer and emulsification technique [142,167,168]. Lipids that are solid at body temperature (fatty acids and their mono-, di- or tri-glycerides, monoglycerides, hard fat or even waxes) are employed for fabrication of SLN. NLC are next generation lipid nanocarriers that contain a mixture of solid lipids and liquid lipids (oil) in appropriate proportions as a matrix. Various liquid lipids such as medium chain tri-glycerides, mono- and di-glycerides of short chain fatty acids and propylene glycol esters of short chain fatty acids are employed for fabrication of NLC [142,167,168]. The presence of liquid lipid in the NLC confers long-term colloidal stability and greater drug encapsulation and loading unlike SLN [142,167,168]. Although several fabrication techniques have been developed for fabrication of SLN/ NLC; melt-emulsification followed by high-pressure homogenization is most commonly used. SLN/NLC have already been used in few commercial topical preparations [142]. Despite these advantages, SLN/NLC have not been explored to their full potential for HIV treatment and prophylaxis. Most of the investigations reported hitherto, focus on development of SLN of protease inhibitors for improving their permeability and in vitro transport through blood-brain barrier and these investigations are out of scope of this review. Alukda et al. have reported development of SLN of tenofovir for vaginal prophylaxis [169]. The SLN were fabricated by a phase inversion method and surface of the SLN was functionalized with multiple layers of poly-l-lysine and heparin. Poly-l-lysine coat was introduced to augment cellular uptake of SLN whereas functionalization with heparin was carried out for targeting of SLN to natural cytotoxic receptors of natural killer cells. However, the authors did not establish any proof for this hypothesis. The functionalized SLN were well tolerated by vaginal epithelial cells even at a high concentration (900 μg/mL) [169].
In past few years, researchers have evaluated potential of SLN as an adjuvant for HIV vaccines. Mumper et al. have carried out several investigations to establish potential of lipid nanocarriers as HIV vaccine carriers [170–174]. These investigators have developed a ‘microemulsion template’ method to fabricate emulsifying wax based anionic SLN. The anionic SLN were coated with HIV-1 Tat (1–72) protein [175]. The HIV-1 Tat (1–72) coated SLN were injected twice to mice at an interval of 2 weeks by subcutaneous route and immune responses were compared to Tat adjuvanted with alum or Lipid A. On day 28, Tat-specific IgG and IgM levels observed with SLN were comparable to that of Tat adjuvanted with alum. However, splenocytes isolated from Tat coated SLN immunized mice showed 3-fold higher release of IFN-γ as compared to mice immunized with Tat adjuvanted with alum [170]. This indicates potential of Tat coated SLN in eliciting cellular and humoral responses for HIV vaccine. Studies were also carried out to evaluate immune responses of Tat coated SLN and Tat adjuvanted with alum at various doses of Tat [171]. Tat coated SLN carrying lower amount of Tat (1 μg) showed similar Tat-specific total IgG titers as compared to Tat coated SLN carrying higher amount of Tat (5 μg). However, Tat-specific total IgG titers obtained with SLN carrying lower amount of Tat were significantly higher than Tat adjuvanted with alum. This indicated potential of SLN over conventional vaccine adjuvants. The study also established that all Tat (1–72) immunized groups elicited Tat-neutralizing antibodies capable of inhibiting Tat-initiated long terminal repeat (LTR) and that anti-sera from Tat-SLN immunized mice showed higher reactivity towards N-terminal and basic regions of Tat as compared to that of Tat adjuvanted with alum [171].
Mumper et al. also devised strategies to increase interaction between HIV antigen and nanoparticles and studied the effect on the immune responses. The authors explored two strategies for increasing interaction of HIV antigens with the nanoparticles viz. (1) chelated divalent metal based coordination of HIV antigen to SLN and (2) conjugation of HIV antigen to SLN. In the first strategy, the ability of chelated divalent metal ions such as nickel to form coordinate bonds with short histidine residues was utilized. SLN containing nickel chelating lipid (Ni-SLN) were developed and HIV antigen (HIV-1 Gag p24) with short sequence of histidine residues (his-tag) were employed to augment interaction of HIV antigen with SLN [172]. Immune responses to his-tag HIV-1 Gag p24 bound Ni-SLN were compared to HIV-1 Gag p24 adjuvanted with alum and HIV-1 Gag p24 adsorbed onto anionic SLN. Interestingly, Ni-NP yielded significantly higher Gag p24-specific serum IgG and IgG2a levels in mice as compared to alum as well as anionic SLN. These results clearly demonstrated effect of increasing antigen-nanoparticle interaction on the immunization [172]. The ability of Ni-SLN to co-deliver two HIV-1 antigens (his-p24 and his-Nef) was also evaluated in comparison to his-p24 and his-Nefadjuvanted alum [173]. Interestingly, co-delivery of his-p24 and his-Nef through Ni-SLN resulted in significantly higher p24-specific and Nef-specific serum IgG as compared to alum. Moreover, co-delivery of his-p24 and his-Nef through Ni-SLN elicited significantly higher IgG levels as compared to delivery of individual antigen through Ni-SLN [178]. This shows advantage associated with co-delivery of HIV antigens. Conjugation of HIV antigen to SLN was employed as a second strategy to augment immune responses. In this study, HIV-1 p24 was conjugated to Brij 700 (a stabilizer for SLN) by means of a facile tresyl conjugation [174]. The immune response to Brij 700-p24 conjugate and Brij 700-p24 conjugate incorporated in SLN was studied. SLN containing conjugated p24 showed significantly higher p24-specific IgG levels as compared Brij 700-p24 conjugate alone and p24 adjuvanted with alum [174]. This demonstrates advantage associated with the SLN. However, whether antigen conjugation is more immunogenic as compared to nickel mediated antigen coordination still remains to be evaluated.
Arias et al. have reported potential of carnauba wax based SLN as an adjuvant for HIV antigens [175]. The authors developed carnauba wax SLN with different surface characteristics and excellent long-term colloidal stability. HIV gp140 antigen and TLR-9 (Toll-like receptor-9) ligands such as CpGB or PolyI:C were adsorbed on SLN. Intradermal administration of these SLN in mice yielded significantly higher levels of HIV gp140-specific IgG as compared to antigen alone. Moreover, intranasal administration of these SLN in mice resulted in significantly higher serum and vaginal IgG and IgA levels as compared to antigen alone [175]. Carnauba wax SLN could induce systemic as well as mucosal immunity to HIV antigens. Thus, lipid nanocarriers may emerge as low cost and high efficacy adjuvants for HIV vaccines.
5.5. Drug nanocrystals or nanosuspensions
As the name suggests, drug nanocrystals are essentially nanoscale drug particles dispersed in an aqueous (water or buffers) or non-aqueous (polyhydric alcohols or oils) medium. Drug nanocrystals also contain a suitable stabilizer or mixture of stabilizers to maintain long-term colloidal stability [55,176]. Drug nanocrystals can be generated using methods based on top–down approach or bottom-up approach. Top–down approaches such as media milling and high-pressure homogenization are the most preferred methods for generation of nanocrystals due to their amenability for large-scale production. Various platform technologies such as Nano-Crystal®, Nanopure® and NANOEDGE® have been developed for fabrication of drug nanocrystals [55,176]. Various products based on drug nanocrystals are currently available in the pharmaceutical market. Thus, ‘drug nanocrystals’ is an industrially feasible nanotechnology. Conversion of pure drug to nano-scale particles leads to dramatic increase in the surface area and dissolution velocity leading to increase in bioavailability and reduction in pharmacokinetic variability [55,176]. Drug nanocrystals have mainly been developed for (1) extremely hydrophobic drugs that are difficult to deliver as a solution by intravenous route and (2) low dose hydrophobic drugs exhibiting dissolution rate limited oral bioavailability.
Baret et al. developed nanocrystals of rilpivirine (a non-nucleoside reverse transcriptase inhibitor) with the help of media milling technique [177]. Rilpivirine nanocrystals of 200, 400 and 800 nm were injected in mice and dogs by intramuscular and subcutaneous routes and their pharmacokinetic behavior was monitored. Interestingly, all the treatments showed detectable rilpivirine levels up to 90 days in dogs and 3 weeks in mice indicating their utility in long-term prophylaxis of HIV. The pharmacokinetic behavior of rilpivirine was influenced by the size of the nanocrystals as well as route of administration. It was observed that 200 nm nanocrystals yielded higher and less variable rilpivirine plasma concentration as compared to 400 and 800 nm nancocrystals [177]. In another study, the authors compared 200 nm rilpivirine nanocrystals delivered to rats and dogs at 5 mg/kg dose by IM and SC routes [178]. The IM administration resulted in higher initial peak plasma concentration as well as higher clearance as compared to SC administration of rilpivirine nanocrystals. The SC administration yielded stable plasma concentration for at least 6 weeks in dogs. Rilpivirine concentrations were also detected in lymphoid tissues throughout the treatment, indicating uptake of nanocrystals by macrophages. However, rilpivirine levels in lymphoid tissues were considerably higher for 1 month for IM route as compared to SC route in dogs[178]. Thus, drug nanocrystals can serve as a long-term prophylactic modality for HIV infection.
5.6. Inorganic nanoparticles
Inorganic metals such as antimony, iron, platinum, calcium, gold and silver have long history as therapeutic agents. Since last decade, nano-structures based on various inorganic metals are actively being pursued as therapeutic and imaging modalities. Moreover, inorganic nanomaterials have also been tailored to act as carriers for drugs and RNAi therapeutics.
Elicheguerra et al. were the first to demonstrate the effect of silver nanoparticles on HIV-1 [179]. Polyvinyl pyrrolidone coated silver nanoparticles were found to be effective against an array of HIV-1 strains including M tropic strains, T tropic strains and drug resistant strains [179,180]. The silver nanoparticles exhibited significantly higher selectivity index as compared to silver salts (silver nitrate and silver sulfadiazine) indicating the importance of the nanosizing of the particles. Mechanistic studies indicated that silver nanoparticles bind to disulfide bonds in the CD4 binding domain of gp120 and inhibit CD4 mediated viral fusion to host cells [180]. Interestingly, silver nanoparticles were also found to interfere with other stages of viral replication cycle, which may minimize emergence of resistant viral strains. Lara et al. evaluated HIV inhibitory potential and tolerability of silver nanoparticles in cervical explants [181]. Silver nanoparticles were well tolerated by cervical explants at the concentration of 0.15 mg/mL Pre-treatment of cervical explant with silver nanoparticles prevented its infection by cell free as well as cell-associated virus. The anti-HIV activity of silver nanoparticles was evident within 1 min after topical treatment of the cervical explant. Moreover, 20-min pre-treatment of cervical explants with silver nanoparticles gel (followed by thorough rinsing to remove gel) was able to prevent HIV infection for 48 h [181]. This clearly indicates the potential of silver nanoparticles in the long-term prophylaxis of HIV. Silver nanoparticles have also shown to have an additive effect with neutralizing antibodies developed against HIV-1 envelope proteins [182]. Thus, silver nanoparticles can also be used for combination therapy. Recently, the ability of silver nanoparticles coated polyurethane condoms were evaluated for prevention of HIV and HSV infection [183]. Interestingly, condoms impregnated with silver nanoparticles did not show cytotoxicity to HeLa cells and T cells after 3 h. Furthermore, silver nanoparticles impregnated condoms were able to inhibit macrophage (M)- and T lymphocyte (T)-tropic HIV as well as HSV. This study opens a new door to male controlled microbicides.
Gold nanoparticles have been extensively explored for an array of biomedical applications [184]. The advancement in fabrication methodologies has enabled generation of gold nanoparticles with varying sizes and tunable optical and surface properties. It is well known that appropriately designed gold nanoparticles can enable multivalent presentation of the materials present on their surface; thus, enabling maximal interaction with the target receptors. It is possible to functionalize surface of gold nanoparticles with a variety of biomolecules such enzymes, antibodies, carbohydrates, siRNAs and even drugs without loss of their inherent activity [184]. In fact, gold nanoparticles carrying TNF-α (Aurimune™, CytImmune Sci. Inc.) are currently being evaluated in clinical trials for cancer therapy. Paradoxically, very few studies have focused on applications of gold nanoparticles for the HIV treatment or prophylaxis. Melander et al. observed that conjugation of SDC-1721 (a derivative of HIV fusion inhibitor TAK-779) to gold nanoparticles exhibited IC50 value against HIV-1 at concentration as low as 10 nM. Interestingly, neither gold nanoparticles nor SDC-1721 alone were able to demonstrate HIV-1 inhibitory potential [185]. This study clearly indicates that multivalent presentation of biologically inactive modality with the help of gold nanoparticles can transform them into biologically active therapeutic agents.
Penades et al. have carried out a series of experiments to explore the effect of multivalent presentation of various oligomannosides on the gold nanoparticles (Manno-Glyconanoparticles) for the inhibition of DC-SIGN mediated HIV trans-infection [186–188]. Te-50, a manno-glyconanoparticles containing 56 units of linear tetra-saccharide were found to be active against infection by both R5 and X4 tropic HIV at nM concentrations [187]. The experiment clearly showed that manno-glyconanoparticles prevented attachment of gp120 to DC-SIGN resulting in HIV inhibition. Moreover, these nanoparticles were found to mimic carbohydrate epitope of the 2G12, a broadly neutralizing monoclonal antibody against HIV [188]. The same group evaluated anti-HIV potential of gold nanoparticles bearing anionic sulfate or phosphate end functional groups [189]. Gold nanoparticles with phosphate end groups did not show any anti-HIV activity whereas gold nanoparticles decorated with sulfate groups and inert monosaccharide (5-(thio)pentyl d-glucopyranoside) on the surface inhibited HIV at nM concentrations [189]. A recent study explored cytotoxicity and antiviral activity of PEG coated gold nanoparticles [190]. Gold nanoparticles were well tolerated by cells and they were able to inhibit M-tropic, T-tropic, dual-tropic and resistant strains of HIV-1. The authors observed that gold nanoparticles prevented HIV-1 entry by binding to gp120. Shiang et al. fabricated gold nanoparticles functionalized with apatamers that can recognize polymerase and RNAse H region of HIV-1 reverse transcriptase [191]. Interestingly, aptamer functionalized gold nanoparticles were stable to nuclease mediated degradation and also inhibited HIV-1 infection by blocking reverse transcriptase. Thus, suitably functionalized gold nanoparticles could have potential as topical microbicides, vaccines, vaccine adjuvants and delivery systems for vaccine and nucleic acid.
Magnetic nanoparticles (Resovist®) are currently being used as MRI contrast agents. However, several studies have established that magnetic nanoparticles can also be functionalized to deliver drugs and biomolecules to target cells or organs [192]. Saiyad et al. studied the ability of magnetic nanoparticles to deliver azidothymidine triphosphate (AZTTP) to PBMC [193]. AZTTP was bound to magnetic nanoparticles by simple ionic interaction. In vitro studies indicated that the AZTTP bound nanoparticles did not offer any significant advantage over free AZTTP. Extensive studies need to be carried out to realize full potential of magnetic nanoparticles in the HIV prophylaxis.
Silica nanoparticles are being explored for a variety of biomedical applications such as diagnostics and delivery of therapeutic agents [194]. Recently, Vasilyeva et al. have fabricated 2′-deoxy-ribonucleoside triphosphate (dNTP) functionalized silica nanoparticles using click chemistry [195]. Interestingly, dNTP functionalized silica nanoparticles were able to get integrated into growing DNA chain. Furthermore, these nanoparticles were capable of reaching to nuclei after incubation with cells [195]. Thus, NRTI conjugated silica nanoparticles could be an interesting approach for prophylaxis of HIV.
Calcium nanoparticles have attracted great attention as a delivery system for oligonycleotides and siRNAs [196]. The excellent biocompatibility of calcium nanoparticles is of particular interest to researchers. He et al. carried out studies to check the ability of calcium phosphate nanoparticles to function as mucosal adjuvants [197]. Mice were treated with calcium phosphate nanoparticles and HSV-2 antigen by intravaginal and intranasal route and generation of mucosal immunity was evaluated. Interestingly, nanoparticle + HSV-2 antigen combination generated HSV-specific mucosal IgA and IgG with concomitant increase in systemic IgG responses. Moreover, intravaginal administration of nanoparticle + HSV-2 antigen combination yielded higher antibody titers at mucosal surfaces as compared to intransal administration [197]. Thus, calcium nanoparticles could have utility as vaccine adjuvants for HIV vaccines.
5.7. Nanofibers
As the name suggests, nanofibers are the sphagetti-like like masses (fibers) with diameters ranging from 1 to 1000 nm [198–200]. Various materials such as polymers, peptides, polysaccharides can be tailored to form nanofibers. Usually, nanofibers are fabricated using electrospinning process. It is possible to vary diameter, length and pore size of nanofibers by controlling parameters of the electrospinning process. The nano-scale dimensions of nanofibers are quite close to that of extracellular matrix (ECM) fibers [198–200]. Hence, nanofibers have been extensively explored in the field of regenerative medicine. Applications of nanofibers as tissue engineering scaffolds, wound dressings and vascular grafts have been widely investigated [198–200]. Furthermore, nanofibers are also being explored for the localized and controlled delivery of drugs and biotechnology based therapeutics (peptides, nucleic acids) [198–200].
Recently, nanofibers have been explored for delivering various microbicides for HIV prophylaxis [201]. Huang et al., have fabricated nanofibers of cellulose acetate phthalate (CAP), a macromolecular HIV-1 entry inhibitor. CAP nanofibers were well tolerated by vaginal epithelial cells and vaginal microflora. Due to pH-sensitive nature of CAP, nanofibers maintained integrity in acidic pH (vaginal environment). However, addition of semen to nanofibers led to immediate dissolution of CAP. CAP nanofibers retained ability of CAP to prevent HIV-1 entry. Moreover, incorporation of tenofovir in CAP nanofibers significantly improved its antiviral activity [201]. CAP nanofibers can release antiretroviral drugs on coitus which would help in preventing sexual transmission of HIV. Low cost of CAP and ease of scale-up of electro-spinning process indicate that drug loaded CAP nanofibers have good potential in HIV prophylaxis.
Woodrow et al. have fabricated nanofibers of various biodegradable polymers such as PLA and PCL [202]. Nanofibers of various dimensions were easily formulated by using blends of poly-ethyleneoxide (PEO) and biodegradable polymers. Nanofibers did not show any toxicity to various cells and explants. Several microbicides such as maraviroc, azadothymidine, acyclovir, glycerylmonolaurate were successfully incorporated into the nanofibers. Nanofibers sustained release of the microbicides and showed similar antiviral activity as compared to free drugs. Intravaginal administration of fluorescent nanofibers showed that they were very well retained in the reproductive tract. This study also established spermicidal activity of GML loaded nanofibers [202]. Thus, polymeric nanofibers could be used for delivering combination of microbicides and contraceptives.
6. Factors governing in vitro and in vivo fate of nano-architectures
There are several factors that influence in vitro and in vivo fate of nano-architectures. Several studies have shown that factors such as material properties, size, shape, surface charge and surface chemistry can affect cellular uptake, intracellular distribution, toxicity, immunogenicity and biodistribution of nano-architectures. Hence, the process of design and fabrication of nano-architectures is very crucial.
6.1. Properties of material used for fabrication
The nano-architectures can be fabricated using various materials such as polymers, lipids and inorganic metals. Inherent properties of these materials can significantly influence cellular and/or tissue uptake and immunological properties of nano-architectures. For example, due to the intrinsic properties of metals like gold and silica, fluorescent nano-architectures can be generated without using any fluorescent dye whereas nano-architectures based on polymers and lipids do not have such capability [184,203]. It has been observed that phospholipid coated polymeric nanoparticles and gold nanoparticles showed significantly higher intracellular uptake as compared to nanoparticles without phospholipid coating [204,205]. Similar results were found for albumin coated polymeric nanoparticles [206]. Studies have also shown that micelles based on polyoxyethylene oxide (PEO)-polypropylene oxide (PPO) co-polymers are capable of complement activation whereas PEG-phospholipid micelles do not exhibit this property [207]. These investigations clearly indicate importance of inherent properties of materials. Furthermore, composition of materials belonging to same class (polymer or lipid or metal) can influence the in vitro and in vivo performance. Studies have shown that the chemical structure of cationic lipids used can dramatically impact efficacy, cellular uptake, transfection and toxicity of cationic nano-architectures. Nano-architectures composed of cationic lipids with single alkyl chain were more toxic and incapable of transport through cervicovaginal mucus and transfection [208,209]. On the contrary, nano-architectures composed of cationic lipids with two alkyl chains showed enhanced transport through mucus and cells with concomitant reduction in cytotoxicity [208–210]. Similarly, Sunshine et al., have shown that the end groups of the polymer can substantially impact cellular uptake and extent of transfection [211]. Molecular weight is another important property that can govern performance and/or fate of nano-architectures. The process of glomerular filtration (renal clearance) has a molecular weight cutoff of ∼48 kDa [212–214]. Thus, non-biodegradable polymers with molecular weight higher than the cutoff would not be eliminated by kidneys and should be avoided for fabrication. Hanes et al. have shown that molecular weight of PEO-PPO block copolymers (Pluronics) and/or length of PPO chain can have significant impact on the transport of nano-architectures through cervicovaginal mucus [215]. The authors report that coating of nanocarriers with Pluronic F127 (high molecular weight and PPO chain length > 3000 Da) yielded quick transport through cervicovaginal mucus as compared to Pluronic F68 (low molecular weight and PPO chain length < 3000 Da) coated nanocarriers. Polymer molecular weight can influence particle size, rate of in vitro and in vivo drug release which in turn affects efficacy of nano-architectures [216,217]. In case of polyethylenimines (PEI), polymer molecular weight has a dramatic impact on their transfection capability, antiviral activity and cytotoxicity [218,219]. Thus, properties of material used for fabrication of nano-architectures are of great importance to obtain an optimal effect.
6.2. Size
The size of nanoparticles is a very important feature that governs their biodistribution and uptake in tissues and cells. Physiological barriers such as mucus, blood capillaries, endothelium, renal vasculature and fenestrations in the liver and spleen have different size cut-offs and have a key role in determining in vivo biodistribution and clearance of nanocarriers [214]. Understanding the size limits of various physical barriers is an important aspect of the nanocarrier designing process. The size of the nanocarriers has also been found to influence their transport through mucosal barriers like cervicovaginal mucus [220]. Cervicovaginal mucus is one of the key components that determine the success of vaginal prophylaxis of HIV. Cervicovaginal mucus has a nanoscopic mesh of mucin fibers and various other components. The mesh size of the ovulatory cervical mucus is anticipated to be ∼100 nm [53,220]. In view of this, the effect of size of the nanocarriers on their transport through cervicovaginal mucus has been studied. Initial studies indicated that viruses with size less than 100 nm (polio, rotaviruses) easily diffuse through cervicovaginal mucus whereas viruses like herpes simplex virus (HSV, particle size ∼ 180 nm) get trapped into the mucus [221]. Hence, it was believed that nanoparticles with size ≤ 100 nm would show rapid mucosal transport. However, Lai et al. observed that particles with size between 200 and 500 nm were able to quickly diffuse through cervicovaginal mucus whereas particles with average size of 100 nm were trapped in the mucus [222]. However, a recent study from the same group showed that nanoparticles with size less than 100 nm can also rapidly diffuse through mucus after suitable surface engineering [96].
While designing nanocarriers for prophylaxis of HIV through systemic routes, one should be aware that cutoff size for renal clearance is ∼ 10 nm and 48 kDa [212–214]. It is well known that the width of the sinusoidal space in liver and spleen is about 50–100 nm and the size limit for blood–brain barrier is considered to be ∼12 nm [212,214]. Studies have shown that particles less than 10 nm undergoes rapid renal clearance and particles higher than 1000 nm are rapidly opsonized by reticuloendothelial system [212–214,223,224]. Size of the nanoparticles also influences plasma protein adsorption pattern. PEGylated polyhexadecyl cyanoacrylate (PHDCA) nanoparticles of different size showed differential adsorption of serum proteins. Smaller PHDCA nanoparticles (80 nm) showed very less protein adsorption (6%) whereas larger nanoparticles (243 nm) showed higher protein adsorption (34%) [212]. Studies have shown that smaller nanoparticles (<100 nm) have higher residence time in blood than larger nanoparticles [212–214,223,224].
The size of nanocarriers also governs intracellular uptake, receptor interaction, immune response and toxicity. Size dependent uptake of PLGA nanoparticles has been studied in Caco-2 and HT-29 cells. Interestingly, particles in the size range of 100–150 nm showed highest cellular uptake in the cells whereas particles with size ≤50 nm and ≥200 nm showed lesser cellular uptake [225,226]. Similar results have been observed for mesoporous silica nanoparticles [227]. The relation between nanocarrier size and cellular uptake is considered to be dependent on the competition between the bending energy and the stretching energy of cell membrane [226]. Huang et al. showed that polyvinyl pyrrolidone coated iron oxide nanoparticles of size 100 nm exhibited highest MRI contrast in liver [228]. In case of HIV prophylaxis, uptake of nanocarriers by macrophages is an important criterion for the successful results. Yu et al. observed that Pluronic coated iron oxide nanoparticles of size 100 nm had higher uptake in THP-1 human leukemic monocytes as compared to nanoparticles of size 40 nm [229]. Similar results have been observed for uptake of gold nanoparticles (90 nm) in mouse macrophages [230]. In short, studies indicate that nanocarriers of size ∼ 100 nm would have higher propensity for cellular uptake. Binding affinity of a ligand to receptor was found be dependent on the particle size. Binding affinity of Herceptin to the ErbB2 receptor was higher for 70 nm particles as compared to 10 nm particles [224].
Various in vitro and/or in vivo studies have been carried out to evaluate the impact of nanocarrier size on immune responses. This is of particular interest in development of HIV nano-vaccines. Gutierro et al. evaluated PLGA nanoparticles of size 200, 500 and 1000 nm for their ability to elicit immune response [231]. It was observed that 1000 nm particles yielded higher total serum IgG levels as compared to smaller particles. Interestingly, there was no significant difference in the serum IgG2a/IgG1 ratio for particles of all size. Hence, it was concluded that antigen processing and presentation was similar in case of all the particles [231]. Cohen et al. observed that acid sensitive hydrogel particles of size 35 nm and 35 μm show similar extent of T-cell activation in vitro as well as in vivo[232]. On the contrary, polystyrene nanoparticles of different size showed size dependent immune responses. Polystyrene particles with size <200 nm were found to be more efficient in generating cytotoxic T lymphocytes as compared to 2 μm particles [233]. Recently, Wang et al. noticed that mesoporous silica nanoparticles of size 430 nm elicited higher serum IgG and IgA levels than 130 nm and 1–2 μm particles [234]. Studies have shown that 250 nm dextran coated iron oxide nanoparticles showed significant complement activation whereas 600 nm particles did not activate complement [223]. In an interesting study, Xiong et al. observed that macrophages treated with PLGA nanoparticles of size 60 nm and 100 nm show significantly higher TNF-α release (indicator of inflammatory response) as compared to 200 nm particles [235]. However, these observations were also dependent on the concentration of the nanoparticles. Size dependent toxicity and free radical generation has been observed for silver nanoparticles. Silver nanoparticles of size 15 nm were significantly toxic to macrophages as compared to 30 and 55 nm silver nanoparticles [236]. On the contrary, PLGA nanoparticles did not show size dependent toxicity in macrophages [235]. All these studies indicate that other factors such as material type are also crucial in size dependent responses.
6.3. Shape
In last 10 years, extensive studies have been carried out to understand the effect of nanocarrier shape on cellular uptake, intra-cellular trafficking, in vivo circulation and/or biodistribution. There are many methods available to generate nanocarriers with different geometry and aspect ratio [237,238]. Various in vitro studies have been carried out to study the effect of nanocarrier shape on uptake in different cells. For the process of cell uptake, attachment of nanocarrier to cell surface is the first step. Internalization of nanocarriers can occur by various mechanisms such as phagocytosis, macropinocytosis, clathrin or caveolae-mediated endocytosis and clathrin/caveolae-independent endocytosis [237,238]. The mechanism of cellular internalization is governed by size and nature of the extracellular object. Hence, morphology of the nano-carrier has significance in the internalization process [237,238]. Phagocytosis is a process of internalization of foreign particles by macrophages through rearrangement of the actin skeleton. Uptake of nanocarriers by macrophages is an important aspect in drug delivery. For effective treatment of bacterial and viral infections, nanocarriers should be effectively taken up by macrophages. Studies indicate that particles with oblate ellipsoid shape exhibit higher macrophage uptake as compared to the spherical and prolate ellipsoid shape particles [239–241]. Lin et al. observed that sphere and hexagon shaped polymeric nanoparticles of 70 nm do not show a difference in the macrophage uptake. However, hexagonal shape particles of 120 nm showed considerably lower uptake [242]. Nowacek et al. studied uptake of antiretroviral drug nanocrystals (nanoART) of different shapes in macrophages. It was observed that nanocrystals with rounded particles and irregular edges showed quite low macrophage uptake as compared to rods with smooth and regular edges [243]. Between rods, longer rods were more efficiently taken up as compared to shorter rods. Similar results have been found for silica nanorods [244]. On the contrary, gold nanospheres were found to have much greater uptake in macrophages as compared to gold nanorods [245]. Champion and Mitragotri showed that polystyrene nanospheres exhibit higher macrophage uptake than worm-like polystyrene particles of equal volume [240]. It should be also noted that nonphagocytic cells might show different preference towards nanocarrier shape. Studies have also shown importance of aspect ratio (ratio of width to height) of particles with similar shape on the cellular uptake. Monodisperse hydrogel nanoparticles of different aspect ratios were fabricated using PRINT technology. It was observed that particles with higher aspect ratio were internalized much quicker than particles with lower aspect ratio. Also, cylinders with larger diameter were taken up faster than cylinders with smaller diameter although their aspect ratios were same [237,238]. Thus, one should keep in mind that factors other than size are also important in the cellular uptake.
In vivo studies have shown that gold nanorods exhibit longer circulation in the blood as compared to gold nanospheres [245]. Furthermore, gold nanospheres showed significantly higher accumulation in the liver as compared to gold nanorods. Similar results have been observed for the polymeric nanospheres and hexagonal shaped particles [242]. In an interesting work, in vivo circulation of biodegradable filamentous micelles was compared to stealth liposomes. Interestingly, filamentous micelles resided in circulation for a week whereas liposomes were cleared within 2 days [237,238]. Intravascular behavior of spherical, quasi-hemispherical, cylindrical and discoidal silica particles was examined in various studies [246–248]. It was observed that discoidal particles were preferentially taken up by lungs, spleen and heart but not by liver [248]. Devrajan et al. showed that irregular shape polymer–lipid hybrid nanoparticles (LIPOMERs) undergo selective splenic uptake (in mice, rabbit and dog) as compared to spherical particles [249]. Szoka et al. have shown that PEGylated cyclic polymers have significantly higher residence time as compared to the linear polymers of the same molecular weight [38]. Thus, it may be possible to modulate biodistribution of particles by manipulating shape. These studies might help in designing nanocarriers for HIV prophylaxis through systemic route but until today, distribution of nanocarriers with different shape on local (vaginal/rectal) delivery has not been studied.
6.4. Surface characteristics
Surface characteristics such as surface charge, surface coating, surface functional groups and surface hydrophilicity (PEGylation) have significant impact on in vitro as well as in in vivo behavior of nanocarriers [213,214]. The surface charge of nanocarriers has a great impact on their colloidal stability, interaction with blood components, cellular uptake, toxicity and mucosal transport [213,214]. Generally, a minimum zeta potential (surface charge) of ±30 mV is required for good colloidal stability of nanocarriers prepared via electrostatic interactions whereas in the case of nanocarriers fabricated via combination of electrostatic and steric interactions, a minimum zeta potential of ±20 mV is desirable [176]. Studies have shown that nanocarriers with high negative surface charge (liposomes; zeta potential of ∼ −40 mV) are rapidly cleared from blood as compared to the neutral liposomes. Moreover, negatively charged liposomes showed increased mononuclear phagocytic system (MPS) uptake in the liver as compared to neutral liposomes [213]. Hanes et al. have demonstrated that highly anionic polymeric nanoparticles (zeta potential of ∼−40 mV or higher) have very slow mucosal transport as compared to particles with zeta potential close to neutral charge [222]. The presence of anionic proteoglycans in the mucus is believed to be responsible for repulsion of anionic nanocarriers leading to reduce mucosal transport. Generally, positively charged nanocarriers are known to have significantly higher cellular uptake as compared to negatively charged nanocarriers [213,214]. This is mainly due to their increased electrostatic interaction with the anionic cell membrane. Furthermore, cationic nanocarriers may also exhibit different mechanism of cell uptake as compared to anionic nanocarriers. Kannan et al. observed that anionic dendrimers exhibit caveolae-mediated endocytosis whereas cationic dendrimers show non-clathrin, non-caveolae dependent endocytosis [250]. Until now, cationic nanocarriers are widely used for delivering nucleic acid therapeutics in vitro and in vivo[212,214]. Studies also indicate that cationic nanocarriers show selective uptake in the angiogenic endothelium of tumors, which led to clinical development of paclitaxel containing cationic liposomes (EndoTAG-1®) [251]. Positively charged liposomes have been shown to have activity against parasites like leishmania and viruses like HSV [142,228]. Positively charged nanocarriers containing appropriate cationic components (like dicotadecyldimethyl ammonium bromide and PEI) are also known to act as vaccine adjuvants [252,253]. However, positive charge is also associated with increased cytotoxicity. Many studies indicate that positively charged nanocarriers have higher cytotoxicity as compared to anionic and neutral nanocarriers [213,214]. It has been observed that positively charged nanocarriers form aggregates with serum proteins after i.v. administration, which may increase the risk of transient embolism in lung capillaries in certain cases [212,214]. Furthermore, positively charged nanocarriers are rapidly cleared from blood stream and accumulate in liver and lung to a greater extent. Recently, oral administration of positively charged dendrimers to mice was found to cause adverse effects such as hemobilia and splenomegaly [254]. On the contrary, negatively charged dendrimers did not show such adverse effects and were well tolerated even at 10-fold higher dose [254]. Recent study indicates that polymeric nanoparticles containing cationic surfactant cetyltrimethylammonium bromide (CTAB) have significantly lower diffusivity through cervicovaginal mucus as compared to anionic and neutral nanoparticles [208]. Extensive interactions of positively charged nanoparticles with anionic mucin proteoglycans was believed to be responsible for their entrapment in mucus. Although surface charge is not the only determinant of the behavior of nanocarriers, one can expect that nanocarriers with close to neutral surface charge could have optimal in vitro and in vivo performance.
Studies have shown that charge dependent in vitro and in vivo behavior of nanocarriers can be easily modulated by decorating surface of the nanocarriers with moieties (such as PEG) that can provide steric barrier. It has been observed that surface charge of negatively charged nanocarriers (surface charge: −40 mV) can be brought close to neutral by use of PEGylation [212,213,223]. PEGylation can also reduce cytotoxicity and/or clearance of positively charged nanocarriers [212,213,223,255]. In fact, recently, positively charged dendritic micelles with a very high PEG density showed minimal interaction with the cells (indicative of minimal or no cytotoxicity) [256]. Surface PEGylation of nanocarriers has been widely used for reducing their opsonization and MPS uptake, which leads to longer in vivo circulation of nanocarriers. Interestingly, PEGylation of nanocarriers has also shown to improve their mucosal transport. Hanes et al. have shown that decorating surface of anionic polymeric nanocarriers with PEG can significantly improve their transport through cervicovaginal mucus [222, 257]. Saltzman et al. have shown that surface PEGylated PLGA nanoparticles have longer retention time in vaginal cavity than non-PEGylated nanocarriers [94]. It should be noted that PEG chain length (or molecular weight of PEG used for PEGylation) and density of PEG chains on nanocarriers are also very important factors governing in vitro and in vivo behavior of nanocarriers. Typically, very low (≤1000 Da) or very high (>20,000 Da) molecular weight PEGs have not been found to be suitable for increasing in vivo circulation and/or transport through cervicovaginal mucus [258–260]. Polymeric nanoparticles coated with vitamin E TPGS (PEG chain length 1000 Da) get trapped in cervicovaginal mucus whereas increasing chain length of PEG in vitamin E TPGS to 5000 Da significantly increased mucosal transport of nanocarriers [260]. In another study, the authors compared mucosal transport of nanoparticles coated with PEG-2000 and PEG-10000. It was observed that nanocarriers coated with PEG-2000 had significantly higher mucosal transport as compared to that of PEG-10000 coated nanocarriers [257]. It should be noted that PEG chain length required for optimal in vitro and in vivo behavior would also depend upon material properties, surface charge and type of nano-architecture.
7. Importance of route of administration
The route of administration can have an immense impact on the disposition of the therapeutic agent and/or nanocarriers. Additionally, the route of administration can have considerable effect on the extent of therapeutic efficacy, immunological effects and/or duration of action depending upon the anatomical site and its physiology [214]. It is important to understand various barriers and challenges associated with various route of administration while selecting appropriate route for drug delivery. In order to achieve prophylaxis against infectious diseases including HIV, drugs or vaccines have been administered through various routes such as intramuscular, intravenous, peritoneal, dermal, subcutaneous, nasal, oral, vaginal and rectal [261–263]. Table 3 discusses various advantages and disadvantages associated with the different routes of administration. It is evident that each route of administration has pros and cons. The choice of route of administration depends on many factors such as type of infectious disease, properties of therapeutic agent, type of prophylaxis (systemic or local), concentration required for prophylaxis and type of immune responses (systemic and/or mucosal). There are relatively few studies that compare the effect of various routes of administration on the extent of prophylaxis and until today, there is no study that compares all the routes of administration at once. The majority of these studies have focused on studying the effect of route of administration on the extent of systemic and/or mucosal immune responses for various vaccines. Based on these studies, it is still difficult to predict most appropriate vaccination strategy among local vaccination (vaginal or rectal), systemic (intramuscular) and distant mucosal vaccination (nasal) to obtain desired level of protection against HIV infection. Conventionally, intramuscular (i.m.) route is widely used for delivery of vaccines. However, for an infection like HIV or HSV-2 which requires mucosal immunization (or Th1 response), i.m. route may not always be desirable. Jazayeri et al. compared immune responses to DNA vaccine encoding HSV-gD2 (glycoprotein D of Herpes Simplex Virus-2) after i.m. and s.c. immunization [264]. Interestingly, the type and extent of immune response was found to be dependent on route of administration. It was observed that s.c. administration of vaccine elicited significantly higher IL-2 production and cytotoxic T-lymphocytes (Th1 response) as compared to i.m. injection. The i.m. route showed significantly higher HSV-2 neutralizing antibodies (Th2 response) as compared to s.c. administration [264]. In an another study, immune responses to PLGA nanoparticles containing plasmid DNA encoding HIV gp160 were compared after oral and i.m. administration. Interestingly, oral route yielded systemic as well as mucosal immune responses whereas i.m. route yielded only systemic immune responses [265]. These observations indicate that i.m. route may not always be desirable for mucosal immunization. Additionally, an interesting study evaluated the impact of type of nanocarrier and route of administration on the immune responses [266]. Cationic liposomes and trimethyl chitosan nanoparticles (TMC-NPs) containing ovalbumin with or without adjuvant were administered via i.m., s.c. and intradermal (i.d.) routes and immune responses were monitored. Liposomes and TMC-NPs containing ovalbumin (OVA) with or without adjuvant elicited similar OVA-IgG1 titers (Th2 response) irrespective of route of administration. However, OVA-IgG2a and IFN-γ titers (Th1 response) were found to be dependent upon route of administration, type of nanocarrier and/or presence of adjuvant [266]. IFN-γ titers were highest for TMC-NPs containing OVA with or without adjuvant administered by the s.c. route and lowest for the i.m. route whereas no such differences were observed in case of liposomes. OVA-IgG2a titers were highest for cationic liposomes containing OVA after administration by the i.d. route and lowest for the s.c. route. However, no such differences were found in case of TMC-NPs [266]. Recently, a small scale human clinical trial evaluated difference in immune responses after delivery of DNA vaccine and recombinant replication-defective adenovirus type 5 (rAd5) vaccine boost by i.m., s.c. and i.d. routes [267]. The trial could not identify best route of administration for vaccines. Thus, choice of i.m., i.d. and s.c. as route of administration for mucosal immunization may have to be evaluated on a case-by-case basis.
Table 3.
Brief overview of advantages and disadvantages associated with various routes of administration.
| Route of administration | Advantages | Disadvantages |
|---|---|---|
| Oral |
|
|
| Intravenous |
|
|
| Intramuscular |
|
|
| Subcutaneous |
|
|
| Nasal |
|
|
| Intradermal |
|
|
| Vaginal |
|
|
| Rectal |
|
|
Sexual transmission is the most common cause for HIV infection and vaccination strategies that can induce significant mucosal immunization in vagina and/or rectum are desirable. There have been few studies to identify best route of administration for generating immunization in vaginal and/or rectal mucosa. Arias et al. evaluated immune responses in the vagina after nasal, vaginal and rectal administration of carnauba wax nanoparticles containing HIV gp140 antigen [180]. Interestingly, intranasal administration of nanoparticles elicited potent systemic immune response and mucosal immune response in the vagina whereas vaginal and rectal administration showed very weak immune response [175]. It is well known that vaginal and rectal mucosa can offer a barrier for effective delivery of antigens. Furthermore, vaginal acidic pH can also have a negative influence on antigen stability [175,268]. In addition, vaginal and rectal mucosal tissues have dearth of follicle-associated epithelium (FAE). On the contrary, intranasal administration enables delivery of antigen to nasopharynx associated lymphoid tissue (NALT) containing large population of microfold cells (M cells) and FAE bearing high densities of immunologically active cells [175,268]. Thus, intranasal delivery can offer greater antigen delivery and presentation to immune cells as compared to vaginal and rectal administration, which may be reflected in the immune responses. Pattani et al. developed microneedles containing HIV gp140 antigen and primed animals with microneedles by the i.d. route [268]. Afterwards, animals were boosted with antigen by intranasal (i.n.), intra-vaginal and s.c. route and systemic and vaginal immunization were evaluated. Once again, the nasal route was found to be superior as compared to the other routes of administration [268]. Buffa et al. have noted similar observations for delivery of HIV gp140 [269]. Thus, nasal administration could be beneficial for acquiring highest level of immunization in vaginal mucosa but further evidence with different antigens and different nanocarriers would be required to validate these observations.
In the case of prophylaxis using antiretroviral drugs, oral, subcutaneous and vaginal routes have mainly been explored. Oral route is the most convenient and preferred route of administration. However, oral route also presents maximum barriers and challenges for drug delivery. In order to show an optimal effect, therapeutic agent has to survive in the harsh acidic environment of stomach and various digestive enzymes and be absorbed at an optimal level. Thus, often-high doses of therapeutic agents have to be administered to achieve effective systemic as well as mucosal concentrations. Currently, a once a day oral administration of Truvuda® (tablet containing 200 mg of emtricitabine and 300 mg of tenofovir disoproxilfumarate) has been approved for pre-exposure prophylaxis [4]. It is well known that NRTIs such as emtricitabine and tenofovir can have dose related side effects such as mitochondrial toxicity. As sexual contact is major mode of transmission of HIV infection, it is advisable to develop strategies that can offer effective concentrations of antiretroviral drugs in vaginal and/or rectal tissues. Albeit orally delivered antiretroviral drugs would be compartmentalized into vaginal and rectal tissues but the dose required to achieve effective concentration would be much higher as compared to local and/or systemic delivery. In view of this, researchers compared vaginal and rectal delivery of tenofovir with oral administration of tenofovir [4,270]. Vaginal and rectal delivery yielded dramatically higher mucosal concentrations of tenofovir diphosphate (active metabolite of tenofovir) as compared to oral administration of tenofovir. Nutall et al. compared pharmacokinetics of the 1% tenofovir gel in macaques after intravaginal and intrarectal administration. Interestingly, tenofovir concentrations (in vaginal fluid) achieved after intravaginal administration was considerably lower than concentrations (in rectal fluid) obtained after intrarectal administration [271]. These studies clearly indicated importance of route of administration in the prophylaxis using antiretroviral drugs. Furthermore, s.c. route has also been compared to oral route. Garcia-Lerma et al. compared once weekly s.c. administration of tenofovir and emtricitabine with daily oral dosing of tenofovir and emtricitabine in macaques [272]. Interestingly, once weekly s.c. administration of antiretroviral drugs yielded similar degree of protection from HIV infection as compared to daily oral dosing of antiretroviral drugs. Thus, simple change in route of administration can have dramatic impact on the HIV prophylaxis. It is important to note that s.c. route is invasive but requires minimal training whereas oral route is very convenient for patients. A recent multinational study compared attitudes and acceptance of patients towards oral and parenteral pre-exposure prophylaxis [273]. Interestingly, the survey indicated willingness to experience inconvenience and expense associated with parenteral delivery indicating potential for development of long-lasting parenteral formulations of antiretroviral drugs for HIV prophylaxis. Finally, it should be kept in mind that there are several other factors in addition to route of administration that would govern direction for an optimal HIV prophylaxis.
8. Future perspectives and conclusion
HIV/AIDS is responsible for death of more than 25 million individuals worldwide since its discovery and every year more than 2 million individuals acquire HIV infection. As a result, prevention of HIV infection has become a global priority. Until now, development of conventional prophylactic modalities like HIV vaccines has not been successful due to intricate structural features of HIV. Hence, antiretroviral agents and nucleic acid-based therapeutics are being actively explored as prophylactic modalities for HIV. Recent clinical trials have shown that oral or local (vaginal) administration of antiretroviral agents like tenofovir offers moderate prevention from HIV infection. Extreme hydrophilicity or hydrophobicity, poor permeability, poor chemical or enzymatic stability are some of the major reasons for moderate success of antiretroviral agents as prophylactic modalities. Nanotechnology has demonstrated a potential to bring change in this scenario. Over the years, various nano-architectures have been developed and evaluated for augmenting prophylactic activity of HIV vaccines, antiretroviral agents and nucleic acid therapeutics. These nano-architectures have shown ability to improve solubility, permeability, stability and pharmacokinetics (systemic or local) of the prophylactic modalities. The ability of certain nano-architectures such as polymeric nanoparticles, lipid nanoparticles and nanofibers to offer sustained release of prophylactic modality is of great benefit to reduce number of dosing and to improve patient adherence. Smartly assembled nano-architectures such as nanofibers and dendrimeric-based hydrogels could be useful for sustained and local delivery of hydrophilic antiretroviral modalities like tenofovir and emtricitabine. It is also possible to develop nano-architectures with inherent ability to inhibit HIV by intelligent manipulation of composition and surface characteristics. Combination of antiretroviral agents has demonstrated a great success in improving management of HIV infections. Recently, evaluation of vaginal rings containing combination of antiretroviral agents (acting on different stages of HIV replication) has been initiated. In view of this, it would be interesting to develop nano-architectures containing a combination of antiretroviral drugs or prophylactic modalities (antiretroviral agent + siRNA). The performance of nano-architectures is also dependent on the route of administration selected for prophylaxis.
At the moment, only one nanotechnology based product (VivaGel®, a Carbopol based gel containing dendrimers with ability to inhibit HIV-1 entry) is being evaluated in Phase I clinical trials. Although nanotechnology has a great potential in HIV prophylaxis, several aspects need to be considered and optimized for successful translation of nanotechnology from lab to clinical settings. Various aspects such as biocompatibility, safety and cost of materials, regulatory status of the materials ease of scale-up and large-scale-manufacture, reproducibility of in vitro and in vivo effects of large-scale batches are very important for successful translation of nanotechnology from lab to clinical settings. It is also important that the developed nanotechnology should offer significant cost to benefit ratio in order to gain wide acceptability. This is very important as the majority of HIV affected individuals are from economically poor and underdeveloped countries. A recent Phase I trial has shown that VivaGel® could be effective in preventing HIV infection at least 3 h before coitus. However, it is expected that nanotechnology based prophylactic modalities should offer coitus-independent and long-term (more than 1 day) prophylaxis in order to justify the added cost associated with use of nanotechnology. In view of this, it would be interesting to know the fate of VivaGel®, the first nano-microbicide.
Acknowledgments
This work was supported by National Institute of Health grants 1R56AI095115-01 (to C.J.D.). The authors have no conflict of interest.
References
- 1.Mamo T, Moseman EA, Kolishetti N, Salvador-Morales C, Shi J, Kuritzkes DR, et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine (London) 2010;5:269–85. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckheit RW, Jr, Watson KM, Morrow KM, Ham AS. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Res. 2010;85:142–58. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirrone V, Wigdahl B, Krebs FC. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 2011;90:168–82. doi: 10.1016/j.antiviral.2011.03.176. [DOI] [PubMed] [Google Scholar]
- 4.Majid A, Redfield R, Gilliam B. The use of preexposure prophylaxis treatments for HIV prophylaxis. HIV/AIDS Res Palliative Care. 2012;4:17–28. doi: 10.2147/HIV.S25082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan SD, Aalinkeel R, Law WC, Reynolds JL, Nair BB, Sykes DE, et al. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int J Nanomedicine. 2012;7:5301–14. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hankins CA, Dybul MR. The promise of pre-exposure prophylaxis with antiretroviral drugs to prevent HIV transmission: a review. Curr Opin HIV AIDS. 2013;8:50–8. doi: 10.1097/COH.0b013e32835b809d. [DOI] [PubMed] [Google Scholar]
- 7.Morris GC, Lacey CJ. Microbicides and HIV prevention: lessons from the past, looking to the future. Curr Opin Infect Dis. 2010;23:57–63. doi: 10.1097/QCO.0b013e328334de6d. [DOI] [PubMed] [Google Scholar]
- 8.Turpin J. Topical microbicides to prevent the transmission of HIV: formulation gaps and challenges. Drug Deliv Transl Res. 2011;1:194–200. doi: 10.1007/s13346-011-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JL, Kashuba AD. Formulation, pharmacokinetics and pharmacodynamics of topical microbicides. Best Pract Res Clin Obstet Gynaecol. 2012;26:451–62. doi: 10.1016/j.bpobgyn.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdool Karim SS, Baxter C. Overview of microbicides for the prevention of human immunodeficiency virus. Best Pract Res Clin Obstet Gynaecol. 2012;26:427–39. doi: 10.1016/j.bpobgyn.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wren L, Kent SJ. HIV vaccine efficacy trial: glimmers of hope and the potential role of antibody-dependent cellular cytotoxicity. Hum Vaccin. 2011;7:466–73. doi: 10.4161/hv.7.4.14123. [DOI] [PubMed] [Google Scholar]
- 12.Koff WC. HIV vaccine development: challenges and opportunities towards solving the HIV vaccine-neutralizing antibody problem. Vaccine. 2012;30:4310–5. doi: 10.1016/j.vaccine.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 13.McKinnon LR, Card CM. HIV vaccine efficacy trials: a brief history, and options for going forward. AIDS Rev. 2010;12:209–17. [PubMed] [Google Scholar]
- 14.Cohen CR, Brown J, Moscicki AB, Bukusi EA, Paull JR, Price CF, et al. A phase I randomized placebo controlled trial of the safety of 3% SPL7013 Gel (VivaGel®) in healthy young women administered twice daily for 14 days. PLoS One. 2011;6:e16258. doi: 10.1371/journal.pone.0016258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGowan I, Gomez K, Bruder K, Febo I, Chen BA, Richardson BA, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004) AIDS. 2011 May 15;25(8):1057–64. doi: 10.1097/QAD.0b013e328346bd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, et al. SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One. 2011;6:e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moscicki AB, Kaul R, Ma Y, Scott ME, Daud II, Bukusi EA, et al. Measurement of mucosal biomarkers in a phase 1 trial of intravaginal 3% StarPharma LTD 7013 gel (VivaGel) to assess expanded safety. J Acquir Immune Defic Syndr. 2012;59:134–40. doi: 10.1097/QAI.0b013e31823f2aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramjee G, Kamali A, McCormack S. The last decade of microbicide clinical trials in Africa: from hypothesis to facts. AIDS. 2010;24(Suppl. 4):S40–9. doi: 10.1097/01.aids.0000390706.81383.f3. [DOI] [PubMed] [Google Scholar]
- 19.Ramjee G. Microbicide research: current and future directions. Curr Opin HIV AIDS. 2010;5:316–21. doi: 10.1097/COH.0b013e32833a9f66. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MS, Muessig KE, Smith MK, Powers KA, Kashuba ADM. Antiviral agents and HIV prevention: controversies, conflicts, and consensus. AIDS. 2012;26:1585–98. doi: 10.1097/QAD.0b013e3283543e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis. 2012;25:51–7. doi: 10.1097/QCO.0b013e32834ef5ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchbindber SP, Liu A. Pre-exposure prophylaxis and the promise of combination prevention approaches. AIDS Behav. 2011;15(Suppl. 1):S72–9. doi: 10.1007/s10461-011-9894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg ZF, Devlin B. Future strategies in microbicide development. Best Pract Res Clin Obstet Gynaecol. 2012;26:503–13. doi: 10.1016/j.bpobgyn.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Haaland RE, Evans-Strickfaden T, Holder A, Pau CP, McNicholl JM, Chaikummao S, et al. UC781 microbicide gel retains anti-HIV activity in cervicovaginal lavage fluids collected following twice-daily vaginal application. Antimicrob Agents Chemother. 2012;56:3592–6. doi: 10.1128/AAC.00452-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. [accessed on February 25, 2013]; http://www.ipmglobal.org/our-work/research/clinical-trial.
- 26.Katakowski JA, Palliser D. siRNA-based topical microbicides targeting sexually transmitted infections. Curr Opin Mol Ther. 2010;12:192–202. [PMC free article] [PubMed] [Google Scholar]
- 27.Symonds GP, Johnstone HA, Millington ML, Boyd MP, Burke BP, Breton LR. The use of cell-delivered gene therapy for the treatment of HIV/AIDS. Immunol Res. 2010;48:84–98. doi: 10.1007/s12026-010-8169-7. [DOI] [PubMed] [Google Scholar]
- 28.Zeller SJ, Kumar P. RNA-based gene therapy for the treatment and prevention of HIV: from bench to bedside. Yale J Biol Med. 2011;84:301–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Chen Y, Ahmadie R, Ho EA. Advancements in the field of intravaginal siRNA delivery. J Control Release. 2013;167:29–39. doi: 10.1016/j.jconrel.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Guzman-Villanueva D, El-Sherbiny IM, Herrera-Ruiz D, Vlassov AV, Smyth HD. Formulation approaches to short interfering RNA and MicroRNA: challenges and implications. J Pharm Sci. 2012;101:4046–66. doi: 10.1002/jps.23300. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62:491–502. doi: 10.1016/j.addr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Khalil NM, Carraro E, Cótica LF, Mainardes RM. Potential of polymeric nanoparticles in AIDS treatment and prevention. Expert Opin Drug Deliv. 2011;8:95–112. doi: 10.1517/17425247.2011.543673. [DOI] [PubMed] [Google Scholar]
- 33.Grammen C, Augustijns P, Brouwers J. In vitro profiling of the vaginal permeation potential of anti-HIV microbicides and the influence of formulation excipients. Antiviral Res. 2012;96:226–33. doi: 10.1016/j.antiviral.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Arab-Alameddine M, Fayet-Mello A, Lubomirov R, Neely M, di Iulio J, Owen A, et al. Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals. Antimicrob Agents Chemother. 2012;56:2959–66. doi: 10.1128/AAC.05424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forbes CJ, Lowry D, Geer L, Veazey RS, Shattock RJ, Klasse PJ, et al. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J Control Release. 2011;156:161–9. doi: 10.1016/j.jconrel.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1:209–22. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weuts I, Van Dycke F, Voorspoels J, De Cort S, Stokbroekx S, Leemans R, et al. Physicochemical properties of the amorphous drug, cast films, and spray dried powders to predict formulation probability of success for solid dispersions: etravirine. J Pharm Sci. 2011;100:260–74. doi: 10.1002/jps.22242. [DOI] [PubMed] [Google Scholar]
- 38.Schöller-Gyüre M, Kakuda TN, De Smedt G, Vanaken H, Bouche MP, Peeters M, et al. A pharmacokinetic study of etravirine (TMC125) co-administered with ranitidine and omeprazole in HIV-negative volunteers. Br J Clin Pharmacol. 2008;66:508–16. doi: 10.1111/j.1365-2125.2008.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathias A, Menning M, Wiser L, Wei X, Dave A, Chuck S, et al. Bioequivalence of the emtricitabine/rilpivirine/tenofovir disoproxil fumarate single tablet regimen. J Bioequiv Availab. 2012;4:100–5. [Google Scholar]
- 40.Naesens L, Bischofberger N, Augustijns P, Annaert P, Van den Mooter G, Arimilli MN, et al. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl) adenine in mice. Antimicrob Agents Chemother. 1998;42:1568–73. doi: 10.1128/aac.42.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesquita PM, Rastogi R, Segarra TJ, Teller RS, Torres NM, Huber AM, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012;67:1730–8. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Fabio S, Van Roey J, Giannini G, van den Mooter G, Spada M, Binelli A, et al. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS. 2003;17:1597–604. doi: 10.1097/00002030-200307250-00003. [DOI] [PubMed] [Google Scholar]
- 43.Friend DR. Pharmaceutical development of microbicide drug products. Pharm Dev Technol. 2010;15:562–81. doi: 10.3109/10837450903369879. [DOI] [PubMed] [Google Scholar]
- 44.Frenkel YV, Clark AD, Jr, Das K, Wang YH, Lewi PJ, Janssen PA, et al. Concentration and pH dependent aggregation of hydrophobic drug molecules and relevance to oral bioavailability. J Med Chem. 2005;48:1974–83. doi: 10.1021/jm049439i. [DOI] [PubMed] [Google Scholar]
- 45.Damian F, Fabian J, Friend DR, Kiser PF. Approaches to improve the stability of the antiviral agent UC781 in aqueous solutions. Int J Pharm. 2010;396:1–10. doi: 10.1016/j.ijpharm.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Moss DM, Siccardi M, Murphy M, Piperakis MM, Khoo SH, Back DJ, et al. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother. 2012;56:3020–6. doi: 10.1128/AAC.06407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Gelder J, Deferme S, Naesens L, De Clercq E, van den Mooter G, Kinget R, et al. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab Dispos. 2002;30:924–30. doi: 10.1124/dmd.30.8.924. [DOI] [PubMed] [Google Scholar]
- 48.Zembruski NC, Büchel G, Jödicke L, Herzog M, Haefeli WE, Weiss J. Potential of novel antiretrovirals to modulate expression and function of drug transporters in vitro. J Antimicrob Chemother. 2011;66:802–12. doi: 10.1093/jac/dkq501. [DOI] [PubMed] [Google Scholar]
- 49.Weatherley B, McFadyen L. Maraviroc modelling strategy: use of early phase 1 data to support a semi-mechanistic population pharmacokinetic model. Br J Clin Pharmacol. 2009;68:355–69. doi: 10.1111/j.1365-2125.2009.03455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dezzutti CS, Rohan LC, Wang L, Uranker K, Shetler C, Cost M, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother. 2012;67:2139–42. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lacey CJ, Woodhall S, Qi Z, Sawant S, Cowen M, McCormack S, et al. Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int J STD AIDS. 2010;21:714–7. doi: 10.1258/ijsa.2010.010215. [DOI] [PubMed] [Google Scholar]
- 52.du Toit LC, Pillay V, Choonara YE. Nano-microbicides: challenges in drug delivery, patient ethics and intellectual property in the war against HIV/AIDS. Adv Drug Deliv Rev. 2010;62:532–46. doi: 10.1016/j.addr.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Mallipeddi R, Rohan LC. Nanoparticle-based vaginal drug delivery systems for HIV prevention. Expert Opin Drug Deliv. 2010;7:37–48. doi: 10.1517/17425240903338055. [DOI] [PubMed] [Google Scholar]
- 54.Mallipeddi R, Rohan LC. Progress in antiretroviral drug delivery using nanotechnology. Int J Nanomed. 2010 Aug 9;5:533–47. [PMC free article] [PubMed] [Google Scholar]
- 55.Desai P, Date AA, Patravale VB. Overcoming poor oral bioavailability using nanoparticle formulations – opportunities and limitations. Drug Discov Today Technol. 2012;9:e87–95. doi: 10.1016/j.ddtec.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Kim PS, Read SW. Nanotechnology and HIV: potential applications for treatment and prevention. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010 Nov-Dec;2(6):693–702. doi: 10.1002/wnan.118. [DOI] [PubMed] [Google Scholar]
- 57.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15:171–85. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 58.McCarthy TD, Karellas P, Henderson SA, Giannis M, O'Keefe DF, Heery G, et al. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharm. 2005;2:312–8. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
- 59.Zanini D, Roy R. Novel dendritic alpha-sialosides: synthesis of glycodendrimers based on a 3,3′-iminobis(propylamine) core. J Org Chem. 1996;61:7348–54. doi: 10.1021/jo961047z. [DOI] [PubMed] [Google Scholar]
- 60.Tyssen D, Henderson SA, Johnson A, Sterjovski J, Moore K, La J, et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS One. 2010;5:e12309. doi: 10.1371/journal.pone.0012309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witvrouw M, Fikkert V, Pluymers W, Matthews B, Mardel K, Schols D, et al. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol Pharmacol. 2000;58:1100–8. [PubMed] [Google Scholar]
- 62.Rupp R, Rosenthal SL, Stanberry LR. VivaGel (SPL7013 Gel): a candidate dendrimer-microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine. 2007;2:561–6. [PMC free article] [PubMed] [Google Scholar]
- 63.Telwatte S, Moore K, Johnson A, Tyssen D, Sterjovski J, Aldunate M, et al. Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1. Antiviral Res. 2011;90:195–9. doi: 10.1016/j.antiviral.2011.03.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patton DL, Cosgrove Sweeney YT, McCarthy TD, Hillier SL. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006;50:1696–700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Loughlin J, Millwood IY, McDonald HM, Price CF, Kaldor JM, Paull JR. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel): a dose ranging, phase I study. Sex Transm Dis. 2010;37:100–4. doi: 10.1097/OLQ.0b013e3181bc0aac. [DOI] [PubMed] [Google Scholar]
- 66.Owais M, Gupta CM. Targeted drug delivery to macrophages in parasitic infections. Curr Drug Deliv. 2005;2:311–8. doi: 10.2174/156720105774370177. [DOI] [PubMed] [Google Scholar]
- 67.Baribaud F, Doms RW, Pöhlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin Ther Targets. 2002;6:423–31. doi: 10.1517/14728222.6.4.423. [DOI] [PubMed] [Google Scholar]
- 68.Fanibunda SE, Modi DN, Gokral JS, Bandivdekar AH. HIV gp120 binds to mannose receptor on vaginal epithelial cells and induces production of matrix metalloproteinases. PLoS One. 2011;6:e28014. doi: 10.1371/journal.pone.0028014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fanibunda SE, Velhal SM, Raghavan VP, Bandivdekar AH. CD4 independent binding of HIV gp120 to mannose receptor on human spermatozoa. J Acquir Immune Defic Syndr. 2008;48:389–97. doi: 10.1097/QAI.0b013e318179a0fb. [DOI] [PubMed] [Google Scholar]
- 70.Kensinger RD, Yowler BC, Benesi AJ, Schengrund CL. Synthesis of novel, multivalent glycodendrimers as ligands for HIV-1 gp120. Bioconjug Chem. 2004;15:349–58. doi: 10.1021/bc034156a. [DOI] [PubMed] [Google Scholar]
- 71.Tabarani G, Reina JJ, Ebel C, Vivès C, Lortat-Jacob H, Rojo J, et al. Mannose hyperbranched dendritic polymers interact with clustered organization of DC-SIGN and inhibit gp120 binding. FEBS Lett. 2006;580:2402–8. doi: 10.1016/j.febslet.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 72.Kensinger RD, Catalone BJ, Krebs FC, Wigdahl B, Schengrund CL. Novel polysulfated galactose-derivatized dendrimers as binding antagonists of human immunodeficiency virus type 1 infection. Antimicrob Agents Chemother. 2004;48:1614–23. doi: 10.1128/AAC.48.5.1614-1623.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han S, Yoshida D, Kanamoto T, Nakashima H, Uryu T, Yoshida T. Sulfated oligosaccharide cluster with polylysine core scaffold as a new anti-HIV dendrimer. Carbohydr Poly. 2010;80:1111–5. [Google Scholar]
- 74.Han S, Kanamoto T, Nakashima H, Yoshida T. Synthesis of a new amphiphilic glycodendrimer with antiviral functionality. Carbohydr Polym. 2012;90:1061–8. doi: 10.1016/j.carbpol.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 75.Clayton R, Hardman J, LaBranche CC, McReynolds KD. Evaluation of the synthesis of sialic acid-PAMAM glycodendrimers without the use of sugar protecting groups, and the anti-HIV-1 properties of these compounds. Bioconjug Chem. 2011;22:2186–97. doi: 10.1021/bc200331v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosa Borges A, Wieczorek L, Johnson B, Benesi AJ, Brown BK, Kensinger RD, et al. Multivalent dendrimeric compounds containing carbohydrates expressed on immune cells inhibit infection by primary isolates of HIV-1. Virology. 2010;408:80–8. doi: 10.1016/j.virol.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sattin S, Daghetti A, Thépaut M, Berzi A, Sánchez-Navarro M, Tabarani G, et al. Inhibition of DC-SIGN-mediated HIV infection by a linear trimannoside mimic in a tetravalent presentation. ACS Chem Biol. 2010;5:301–12. doi: 10.1021/cb900216e. [DOI] [PubMed] [Google Scholar]
- 78.Berzi A, Reina JJ, Ottria R, Sutkeviciute I, Antonazzo P, Sanchez-Navarro M, et al. A glycomimetic compound inhibits DC-SIGN-mediated HIV infection in cellular and cervical explant models. AIDS. 2012;26:127–37. doi: 10.1097/QAD.0b013e32834e1567. [DOI] [PubMed] [Google Scholar]
- 79.Doménech R, Abian O, Bocanegra R, Correa J, Sousa-Herves A, Riguera R, et al. Dendrimers as potential inhibitors of the dimerization of the capsid protein of HIV-1. Biomacromolecules. 2010;11:2069–78. doi: 10.1021/bm100432x. [DOI] [PubMed] [Google Scholar]
- 80.Blanzat M, Turrin CO, Aubertin AM, Couturier-Vidal C, Caminade AM, Majoral JP, et al. Dendritic catanionic assemblies: in vitro anti-HIV activity of phosphorus-containing dendrimers bearing galb1cer analogues. Chem-biochem. 2005;6:2207–13. doi: 10.1002/cbic.200500203. [DOI] [PubMed] [Google Scholar]
- 81.Pérez-Anes A, Stefaniu C, Moog C, Majoral JP, Blanzat M, Turrin CO, et al. Multivalent catanionic GalCer analogs derived from first generation dendrimeric phosphonic acids. Bioorg Med Chem. 2010;18:242–8. doi: 10.1016/j.bmc.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 82.Pérez-Anes A, Spataro G, Coppel Y, Moog C, Blanzat M, Turrin CO, et al. Phosphonate terminated PPH dendrimers: influence of pendant alkyl chains on the in vitro anti-HIV-1 properties. Org Biomol Chem. 2009;7:3491–8. doi: 10.1039/b908352a. [DOI] [PubMed] [Google Scholar]
- 83.Chonco L, Bermejo-Martín JF, Ortega P, Shcharbin D, Pedziwiatr E, Klajnert B, et al. Water-soluble carbosilane dendrimers protect phosphorothioate oligo-nucleotides from binding to serum proteins. Org Biomol Chem. 2007;5:1886–93. doi: 10.1039/b703989a. [DOI] [PubMed] [Google Scholar]
- 84.Weber N, Ortega P, Clemente MI, Shcharbin D, Bryszewska M, de la Mata FJ, et al. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J Control Release. 2008;132:55–64. doi: 10.1016/j.jconrel.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 85.Chonco L, Pion M, Vacas E, Rasines B, Maly M, Serramía MJ, et al. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. J Control Release. 2012;161:949–58. doi: 10.1016/j.jconrel.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 86.Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur J Pharm Sci. 2008;34:181–9. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Dutta T, Jain NK. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta. 2007;1770:681–6. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 88.Navath RS, Menjoge AR, Dai H, Romero R, Kannan S, Kannan RM. Injectable PAMAM dendrimer-PEG hydrogels for the treatment of genital infections: formulation and in vitro and in vivo evaluation. Mol Pharm. 2011;8:1209–23. doi: 10.1021/mp200027z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F, Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation II. Biomedical applications and current status of peptide and protein nanoparticulate delivery systems. Nanomedicine. 2006;2:53–65. doi: 10.1016/j.nano.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26:502–11. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endolysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–26. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 94.Cu Y, Booth CJ, Saltzman WM. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. J Control Release. 2011;156:258–64. doi: 10.1016/j.jconrel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steinbach JM, Weller CE, Booth CJ, Saltzman WM. Polymer nanoparticles encapsulating siRNA for treatment of HSV-2 genital infection. J Control Release. 2012;162:102–10. doi: 10.1016/j.jconrel.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med. 2012;4:138–79. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang T, Sturgis TF, Youan BB. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur J Pharm Biopharm. 2011;79:526–36. doi: 10.1016/j.ejpb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng J, Sturgis TF, Youan BB. Engineering tenofovir loaded chitosan nanoparticles to maximize microbicide mucoadhesion. Eur J Pharm Sci. 2011;44:57–67. doi: 10.1016/j.ejps.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Belletti D, Tosi G, Forni F, Gamberini MC, Baraldi C, Vandelli MA, et al. Chemico-physical investigation of tenofovir loaded polymeric nanoparticles. Int J Pharm. 2012;436:753–63. doi: 10.1016/j.ijpharm.2012.07.070. [DOI] [PubMed] [Google Scholar]
- 100.das Neves J, Michiels J, Ariën KK, Vanham G, Amiji M, Bahia MF, et al. Polymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirine. Pharm Res. 2012;29:1468–84. doi: 10.1007/s11095-011-0622-3. [DOI] [PubMed] [Google Scholar]
- 101.Yoo JW, Giri N, Lee CH. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int J Pharm. 2011;403:262–7. doi: 10.1016/j.ijpharm.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 102.Destache CJ, Belgum T, Christensen K, Shibata A, Sharma A, Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect Dis. 2009;9:198. doi: 10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA. Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice. J Antimicrob Chemother. 2010;65:2183–7. doi: 10.1093/jac/dkq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neff CP, Ndolo T, Tandon A, Habu Y, Akkina R. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS One. 2010;5:e15257. doi: 10.1371/journal.pone.0015257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clavel C, Peytavin G, Tubiana R, Soulié C, Crenn-Hebert C, Heard I, et al. Raltegravir concentrations in the genital tract of HIV-1-infected women treated with a raltegravir-containing regimen (DIVA 01 study) Antimicrob Agents Chemother. 2011;55:3018–21. doi: 10.1128/AAC.01460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koh Y, Haim H, Engelman A. Identification and characterization of persistent intracellular human immunodeficiency virus type 1 integrase strand transfer inhibitor activity. Antimicrob Agents Chemother. 2011;55:42–9. doi: 10.1128/AAC.01064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghosn J, Chaix ML, Delaugerre C. HIV-1 resistance to first- and second-generation non-nucleoside reverse transcriptase inhibitors. AIDS Rev. 2009 Jul-Sep;11(3):165–73. [PubMed] [Google Scholar]
- 108.Ford N, Calmy A, Mofenson L. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2011 Nov 28;25(18):2301–4. doi: 10.1097/QAD.0b013e32834cdb71. [DOI] [PubMed] [Google Scholar]
- 109.Date AA, Shibata A, Goede M, Sanford B, La Bruzzo K, Belshan M, et al. Development and evaluation of a thermosensitive vaginal gel containing raltegravir + efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral Res. 2012;96:430–6. doi: 10.1016/j.antiviral.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007;345:163–71. doi: 10.1016/j.ijpharm.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 111.Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels-review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–26. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 112.Roy S, Gourde P, Piret J, Désormeaux A, Lamontagne J, Haineault C, et al. Thermoreversible gel formulations containing sodium lauryl sulfate or n-Lauroylsarcosine as potential topical microbicides against sexually transmitted diseases. Antimicrob Agents Chemother. 2001;45:1671–81. doi: 10.1128/AAC.45.6.1671-1681.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63:943–55. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 114.De Temmerman ML, Rejman J, Demeester J, Irvine DJ, Gander B, De Smedt SC. Particulate vaccines: on the quest for optimal delivery and immune response. Drug Discov Today. 2011;16:569–82. doi: 10.1016/j.drudis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 115.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ataman-Onal Y, Munier S, Ganée A, Terrat C, Durand PY, Battail N, et al. Surfactant-free anionic PLA nanoparticles coated with HIV-1 p24 protein induced enhanced cellular and humoral immune responses in various animal models. J Control Release. 2006;112:175–85. doi: 10.1016/j.jconrel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 117.Lamalle-Bernard D, Munier S, Compagnon C, Charles MH, Kalyanaraman VS, Delair T, et al. Coadsorption of HIV-1 p24 and gp120 proteins to surfactant-free anionic PLA nanoparticlespreserves antigenicity and immunogenicity. J Control Release. 2006;115:57–67. doi: 10.1016/j.jconrel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 118.Guillon C, Mayol K, Terrat C, Compagnon C, Primard C, Charles MH, et al. Formulation of HIV-1 Tat and p24 antigens by PLA nanoparticles or MF59 impacts the breadth, but not the magnitude, of serum and faecal antibody responses in rabbits. Vaccine. 2007;25:7491–501. doi: 10.1016/j.vaccine.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 119.Aline F, Brand D, Pierre J, Roingeard P, Séverine M, Verrier B, et al. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27:5284–91. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 120.Liard C, Munier S, Arias M, Joulin-Giet A, Bonduelle O, Duffy D, et al. Targeting of HIV-p24 particle-based vaccine into differential skin layers induces distinct arms of the immune responses. Vaccine. 2011;29:6379–91. doi: 10.1016/j.vaccine.2011.04.080. [DOI] [PubMed] [Google Scholar]
- 121.Wang X, Uto T, Akagi T, Akashi M, Baba M. Induction of potent CD8+ T-cell responses by novel biodegradable nanoparticles carrying human immunodeficiency virus type 1 gp120. J Virol. 2007;81:10009–16. doi: 10.1128/JVI.00489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang X, Uto T, Akagi T, Akashi M, Baba M. Poly(gamma-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: potential for an AIDS vaccine. J Med Virol. 2008;80:11–9. doi: 10.1002/jmv.21029. [DOI] [PubMed] [Google Scholar]
- 123.Uto T, Akagi T, Toyama M, Nishi Y, Shima F, Akashi M, et al. Comparative activity of biodegradable nanoparticles with aluminum adjuvants: antigen uptake by dendritic cells and induction of immune response in mice. Immunol Lett. 2011;140:36–43. doi: 10.1016/j.imlet.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 124.Himeno A, Akagi T, Uto T, Wang X, Baba M, Ibuki K, et al. Evaluation of the immune response and protective effects of rhesus macaques vaccinated with biodegradable nanoparticles carrying gp120 of human immunodeficiency virus. Vaccine. 2010;28:5377–85. doi: 10.1016/j.vaccine.2010.04.110. [DOI] [PubMed] [Google Scholar]
- 125.Hayakawa T, Kawamura M, Okamoto M, Baba M, Niikawa T, Takehara S, et al. Concanavalin A-immobilized polystyrene nanospheres capture HIV-1 virions and gp120: potential approach towards prevention of viral transmission. J Med Virol. 1998;56:327–31. doi: 10.1002/(sici)1096-9071(199812)56:4<327::aid-jmv7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 126.Kawamura M, Naito T, Ueno M, Akagi T, Hiraishi K, Takai I, et al. Induction of mucosal IgA following intravaginal administration of inactivated HIV-1-capturing nanospheres in mice. J Med Virol. 2002;66:291–8. doi: 10.1002/jmv.2144. [DOI] [PubMed] [Google Scholar]
- 127.Akagi T, Kawamura M, Ueno M, Hiraishi K, Adachi M, Serizawa T, et al. Mucosal immunization with inactivated HIV-1-capturing nanospheres induces a significant HIV-1-specific vaginal antibody response in mice. J Med Virol. 2003;69:163–72. doi: 10.1002/jmv.10279. [DOI] [PubMed] [Google Scholar]
- 128.Kawamura M, Wang X, Uto T, Sato K, Ueno M, Akagi T, et al. Induction of dendritic cell-mediated immune responses against HIV-1 by antigen-capturing nanospheres in mice. J Med Virol. 2005;76:7–15. doi: 10.1002/jmv.20317. [DOI] [PubMed] [Google Scholar]
- 129.Miyake A, Akagi T, Enose Y, Ueno M, Kawamura M, Horiuchi R, et al. Induction of HIV-specific antibody response and protection against vaginal SHIV transmission by intranasal immunization with inactivated SHIV-capturing nanospheres in macaques. J Med Virol. 2004;73:368–77. doi: 10.1002/jmv.20100. [DOI] [PubMed] [Google Scholar]
- 130.Drogoz A, Munier S, Verrier B, David L, Domard A, Delair T. Towards biocompatible vaccine delivery systems: interactions of colloidal PECs based on polysaccharides with HIV-1 p24 antigen. Biomacromolecules. 2008;9:583–91. doi: 10.1021/bm701154h. [DOI] [PubMed] [Google Scholar]
- 131.Weber C, Drogoz A, David L, Domard A, Charles MH, Verrier B, et al. Poly-saccharide-based vaccine delivery systems: Macromolecular assembly, interactions with antigen presenting cells, and in vivo immunomonitoring. J Biomed Mater Res A. 2010;93:1322–34. doi: 10.1002/jbm.a.32605. [DOI] [PubMed] [Google Scholar]
- 132.Castaldello A, Brocca-Cofano E, Voltan R, Triulzi C, Altavilla G, Laus M, et al. DNA prime and protein boost immunization with innovative polymeric cationic core-shell nanoparticles elicits broad immune responses and strongly enhance cellular responses of HIV-1 tat DNA vaccination. Vaccine. 2006;24:5655–69. doi: 10.1016/j.vaccine.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 133.Qiao Y, Huang Y, Qiu C, Yue X, Deng L, Wan Y, et al. The use of PEGylated poly [2-(N, N-dimethylamino) ethyl methacrylate] as a mucosal DNA delivery vector and the activation of innate immunity and improvement of HIV-1-specific immune responses. Biomaterials. 2010;31:115–23. doi: 10.1016/j.biomaterials.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 134.Voltan R, Castaldello A, Brocca-Cofano E, Altavilla G, Caputo A, Laus M, et al. Preparation and characterization of innovative protein-coated poly(-methylmethacrylate) core-shell nanoparticles for vaccine purposes. Pharm Res. 2007;24:1870–82. doi: 10.1007/s11095-007-9310-8. [DOI] [PubMed] [Google Scholar]
- 135.Caputo A, Castaldello A, Brocca-Cofano E, Voltan R, Bortolazzi F, Altavilla G, et al. Induction of humoral and enhanced cellular immune responses by novel core–shell nanosphere- and microsphere-based vaccine formulations following systemic and mucosal administration. Vaccine. 2009;27:3605–15. doi: 10.1016/j.vaccine.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 136.Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genito-rectal viral infect. Nat Med. 2012;18:1291–6. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Allen TM. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs. 1998;56:747–56. doi: 10.2165/00003495-199856050-00001. [DOI] [PubMed] [Google Scholar]
- 138.Szebeni J. The interaction of liposomes with the complement system. Crit Rev Ther Drug Carrier Syst. 1998;15:57–88. [PubMed] [Google Scholar]
- 139.Désormeaux A, Bergeron MG. Liposomes as drug delivery system: a strategic approach for the treatment of HIV infection. J Drug Target. 1998;6:1–15. doi: 10.3109/10611869808997877. [DOI] [PubMed] [Google Scholar]
- 140.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 141.Elizondo E, Moreno E, Cabrera I, Córdoba A, Sala S, Veciana J, et al. Liposomes and other vesicular systems: structural characteristics, methods of preparation, and use in nanomedicine. Prog Mol Biol Transl Sci. 2011;104:1–52. doi: 10.1016/B978-0-12-416020-0.00001-2. [DOI] [PubMed] [Google Scholar]
- 142.Date AA, Joshi MD, Patravale VB. Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv Drug Deliv Rev. 2007;59:505–21. doi: 10.1016/j.addr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 143.Bui T, Dykers T, Hu SL, Faltynek CR, Ho RJ. Effect of MTP-PE liposomes and interleukin-7 on induction of antibody and cell-mediated immune responses to a recombinant HIV-envelope protein. J Acquir Immune Defic Syndr. 1994;7:799–806. [PubMed] [Google Scholar]
- 144.Phillips NC, Gagné L, Ivanoff N, Riveau G. Influence of phospholipid composition on antibody responses to liposome encapsulated protein and peptide antigens. Vaccine. 1996;14:898–904. doi: 10.1016/0264-410x(96)82949-5. [DOI] [PubMed] [Google Scholar]
- 145.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, et al. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J Immunol. 1997;159:3638–47. [PubMed] [Google Scholar]
- 146.Toda S, Ishii N, Okada E, Kusakabe KI, Arai H, Hamajima K, et al. HIV-1-specific cell-mediated immune responses induced by DNA vaccination were enhanced by mannan-coated liposomes and inhibited by anti-interferon-gamma antibody. Immunology. 1997;92:111–7. doi: 10.1046/j.1365-2567.1997.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fukasawa M, Shimizu Y, Shikata K, Nakata M, Sakakibara R, Yamamoto N, et al. Liposome oligomannose-coated with neoglycolipid, a new candidate for a safe adjuvant for induction of CD8+ cytotoxic T lymphocytes. FEBS Lett. 1998;441:353–6. doi: 10.1016/s0014-5793(98)01577-4. [DOI] [PubMed] [Google Scholar]
- 148.Ben Haij N, Mzoughi O, Planès R, Bahraoui E. Cationic nanoglycolipidic particles as vector and adjuvant for the study of the immunogenicity of SIV Nef protein. Int J Pharm. 2012;423:116–23. doi: 10.1016/j.ijpharm.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 149.Simões S, Moreira JN, Fonseca C, Düzgüneş N, de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56:947–65. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 150.Reddy R, Zhou F, Huang L, Carbone F, Bevan M, Rouse BT. pH sensitive li-posomes provide an efficient means of sensitizing target cells to class I restricted CTL recognition of a soluble protein. J Immunol Methods. 1991;141:157–63. doi: 10.1016/0022-1759(91)90142-3. [DOI] [PubMed] [Google Scholar]
- 151.Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991;147:2860–3. [PubMed] [Google Scholar]
- 152.Chang JS, Choi MJ, Kim TY, Cho SY, Cheong HS. Immunogenicity of synthetic HIV-1 V3 loop peptides by MPL adjuvanted pH-sensitive liposomes. Vaccine. 1999;17:1540–8. doi: 10.1016/s0264-410x(98)00353-3. [DOI] [PubMed] [Google Scholar]
- 153.Lian T, Bui T, Ho RJ. Formulation of HIV-envelope protein with lipid vesicles expressing ganglioside GM1 associated to cholera toxin B enhances mucosal immune responses. Vaccine. 1999;18:604–11. doi: 10.1016/s0264-410x(99)00315-1. [DOI] [PubMed] [Google Scholar]
- 154.Sakaue G, Hiroi T, Nakagawa Y, Someya K, Iwatani K, Sawa Y, et al. HIV mucosal vaccine: nasal immunization with gp160-encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. J Immunol. 2003;170:495–502. doi: 10.4049/jimmunol.170.1.495. [DOI] [PubMed] [Google Scholar]
- 155.Agrawal L, Haq W, Hanson CV, Rao DN. Generating neutralizing antibodies, Th1 response and MHC non restricted immunogenicity of HIV-I env and gag peptides in liposomes and ISCOMs with in-built adjuvanticity. J Immune Based Ther Vaccines. 2003;1:5. doi: 10.1186/1476-8518-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rao M, Matyas GR, Vancott TC, Birx DL, Alving CR. Immunostimulatory CpG motifs induce CTL responses to HIV type I oligomeric gp140 envelope protein. Immunol Cell Biol. 2004;82:523–30. doi: 10.1111/j.0818-9641.2004.01283.x. [DOI] [PubMed] [Google Scholar]
- 157.Singh SK, Bisen PS. Adjuvanticity of stealth liposomes on the immunogenicity of synthetic gp41 epitope of HIV-1. Vaccine. 2006;24:4161–6. doi: 10.1016/j.vaccine.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 158.Watson DS, Szoka FC., Jr Role of lipid structure in the humoral immune response in mice to covalent lipid-peptides from the membrane proximal region of HIV-1 gp41. Vaccine. 2009;27:4672–83. doi: 10.1016/j.vaccine.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Watson DS, Platt VM, Cao L, Venditto VJ, Szoka FC., Jr Antibody response to polyhistidine-tagged peptide and protein antigens attached to liposomes via lipid-linked nitrilotriacetic acid in mice. Clin Vaccine Immunol. 2011;18:289–97. doi: 10.1128/CVI.00425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fairman J, Moore J, Lemieux M, Van Rompay K, Geng Y, Warner J, et al. Enhanced in vivo immunogenicity of SIV vaccine candidates with cationic liposome-DNA complexes in a rhesus macaque pilot study. Hum Vaccin. 2009;5:141–50. doi: 10.4161/hv.5.3.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA. 2012;109:14,604–14,609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 163.Wu SY, Chang HI, Burgess M, McMillan NA. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. J Control Release. 2011;155:418–26. doi: 10.1016/j.jconrel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 164.Kim SS, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18:370–6. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Malavia NK, Zurakowski D, Schroeder A, Princiotto AM, Laury AR, Barash HE, et al. Liposomes for HIV prophylaxis. Biomaterials. 2011;32:8663–8. doi: 10.1016/j.biomaterials.2011.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Caron M, Besson G, Etenna SL, Mintsa-Ndong A, Mourtas S, Radaelli A, et al. Protective properties of non-nucleoside reverse transcriptase inhibitor (MC1220) incorporated into liposome against intravaginal challenge of Rhesus macaques with RT-SHIV. Virology. 2010;405:225–33. doi: 10.1016/j.virol.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 167.Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–84. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 168.Müller RH, Shegokar R, Keck CM. 20 Years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr Drug Discov Technol. 2011;8:207–27. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- 169.Alukda D, Sturgis T, Youan BB. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. J Pharm Sci. 2011;100:3345–56. doi: 10.1002/jps.22529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cui Z, Patel J, Tuzova M, Ray P, Phillips R, Woodward JG, et al. Strong T cell type-1 immune responses to HIV-1 Tat (1–72) protein-coated nanoparticles. Vaccine. 2004;22:2631–40. doi: 10.1016/j.vaccine.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 171.Patel J, Galey D, Jones J, Ray P, Woodward JG, Nath A, et al. HIV-1 Tat-coated nanoparticles result in enhanced humoral immune responses and neutralizing antibodies compared to alum adjuvant. Vaccine. 2006;24:3564–73. doi: 10.1016/j.vaccine.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 172.Patel JD, O'Carra R, Jones J, Woodward JG, Mumper RJ. Preparation and characterization of nickel nanoparticles for binding to his-tag proteins and antigens. Pharm Res. 2007;24:343–52. doi: 10.1007/s11095-006-9154-7. [DOI] [PubMed] [Google Scholar]
- 173.Yan W, Jain A, O'Carra R, Woodward JG, Li W, Li G, et al. Lipid nanoparticles with accessible nickel as a vaccine delivery system for single and multiple His-tagged HIV antigens. HIV AIDS (Auckl) 2009;1:1–11. doi: 10.2147/HIV.S5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jain A, Yan W, Miller KR, O'Carra R, Woodward JG, Mumper RJ. Tresyl-based conjugation of protein antigen to lipid nanoparticles increases antigen immunogenicity. Int J Pharm. 2010;401:87–92. doi: 10.1016/j.ijpharm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arias MA, Loxley A, Eatmon C, Van Roey G, Fairhurst D, Mitchnick M, et al. Carnauba wax nanoparticles enhance strong systemic and mucosal cellular and humoral immune responses to HIV-gp140 antigen. Vaccine. 2011;29:1258–69. doi: 10.1016/j.vaccine.2010.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–40. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 177.Baert L, van't Klooster G, Dries W, François M, Wouters A, Basstanie E, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm. 2009;72:502–8. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 178.van't Klooster G, Hoeben E, Borghys H, Looszova A, Bouche MP, van Velsen F, et al. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010;54:2042–50. doi: 10.1128/AAC.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lara HH, Ixtepan-Turrent L, Garza-Treviño EN, Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J Nanobiotechnol. 2010;8:15. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lara HH, Ixtepan-Turrent L, Garza Treviño EN, Singh DK. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. J Nanobiotechnol. 2011;9:38. doi: 10.1186/1477-3155-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mohammed Fayaz A, Ao Z, Girilal M, Chen L, Xiao X, Kalaichelvan P, et al. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: a new approach to inhibit HIV- and HSV-transmitted infection. Int J Nanomed. 2012;7:5007–18. doi: 10.2147/IJN.S34973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41:2256–82. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 185.Bowman MC, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J Am Chem Soc. 2008;130:6896–7. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martínez-Avila O, Hijazi K, Marradi M, Clavel C, Campion C, Kelly C, et al. Gold manno-glyconanoparticles: multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chemistry. 2009;15:9874–88. doi: 10.1002/chem.200900923. [DOI] [PubMed] [Google Scholar]
- 187.Martínez-Avila O, Bedoya LM, Marradi M, Clavel C, Alcamí J, Penadés S. Multivalent manno-glyconanoparticles inhibit DC-SIGN-mediated HIV-1 trans-infection of human T cells. ChemBioChem. 2009;10:1806–9. doi: 10.1002/cbic.200900294. [DOI] [PubMed] [Google Scholar]
- 188.Marradi M, Di Gianvincenzo P, Enríquez-Navas PM, Martínez-Ávila OM, Chiodo F, Yuste E, et al. Gold nanoparticles coated with oligomannosides of HIV-1 glycoprotein gp120 mimic the carbohydrate epitope of antibody 2G12. J Mol Biol. 2011;410:798–810. doi: 10.1016/j.jmb.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 189.Di Gianvincenzo P, Marradi M, Martínez-Avila OM, Bedoya LM, Alcamí J, Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg Med Chem Lett. 2010;20:2718–21. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 190.Vijayakumar S, Ganesan S. Gold nanoparticles as an HIV entry inhibitor. Curr HIV Res. 2012;10:643–6. doi: 10.2174/157016212803901383. [DOI] [PubMed] [Google Scholar]
- 191.Shiang YC, Ou CM, Chen SJ, Ou TY, Lin HJ, Huang CC, et al. Highly efficient inhibition of human immunodeficiency virus type 1 reverse transcriptase by aptamers functionalized gold nanoparticles. Nanoscale. 2013;5:1678–86. doi: 10.1039/c3nr33403a. [DOI] [PubMed] [Google Scholar]
- 192.Frimpong RA, Hilt JZ. Magnetic nanoparticles in biomedicine: synthesis, functionalization and applications. Nanomedicine (Lond) 2010;5:1401–14. doi: 10.2217/nnm.10.114. [DOI] [PubMed] [Google Scholar]
- 193.Saiyed ZM, Gandhi NH, Nair MP. AZT 5′-triphosphate nanoformulation suppresses human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells. J Neurovirol. 2009;15:343–7. doi: 10.1080/13550280903062813. [DOI] [PubMed] [Google Scholar]
- 194.Bitar A, Ahmad NM, Fessi H, Elaissari A. Silica-based nanoparticles for biomedical applications. Drug Discov Today. 2012;17:1147–54. doi: 10.1016/j.drudis.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 195.Vasilyeva SV, Silnikov VN, Shatskaya NV, Levina AS, Repkova MN, Zarytova VF. SiO2 nanoparticles as platform for delivery of nucleoside triphosphate analogues into cells. Bioorg Med Chem. 2013;21:703–11. doi: 10.1016/j.bmc.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 196.Uskoković V, Uskoković DP. Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J Biomed Mater Res B Appl Biomater. 2011;96:152–91. doi: 10.1002/jbm.b.31746. [DOI] [PubMed] [Google Scholar]
- 197.He Q, Mitchell A, Morcol T, Bell SJ. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin Diagn Lab Immunol. 2002;9:1021–4. doi: 10.1128/CDLI.9.5.1021-1024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lim SH, Mao HQ. Electrospun scaffolds for stem cell engineering. Adv Drug Deliv Rev. 2009;61:1084–96. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 199.Gunn J, Zhang M. Polyblend nanofibers for biomedical applications: perspectives and challenges. Trends Biotechnol. 2010;28:189–97. doi: 10.1016/j.tibtech.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 200.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev. 2011;17:349–64. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang C, Soenen SJ, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33:962–9. doi: 10.1016/j.biomaterials.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 202.Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS One. 2012;7:e49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee JE, Lee N, Kim T, Kim J, Hyeon T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc Chem Res. 2011;44:893–902. doi: 10.1021/ar2000259. [DOI] [PubMed] [Google Scholar]
- 204.Hao Y, Yang X, Song S, Huang M, He C, Cui M, et al. Exploring the cell uptake mechanism of phospholipid and polyethylene glycol coated gold nanoparticles. Nanotechnology. 2012;23:045103. doi: 10.1088/0957-4484/23/4/045103. [DOI] [PubMed] [Google Scholar]
- 205.Chavanpatil MD, Handa H, Mao G, Panyam J. Incorporation of phospholipids enhances cellular uptake and retention of surfactant-polymer nanoparticles. J Biomed Nanotechnol. 2007;3:291–6. [Google Scholar]
- 206.Cu Y, LeMoëllic C, Caplan MJ, Saltzman WM. Ligand-modified gene carriers increased uptake in target cells but reduced DNA release and transfection efficiency. Nanomedicine. 2010;6:334–43. doi: 10.1016/j.nano.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moghimi SM, Andersen AJ, Ahmadvand D, Wibroe PP, Andresen TL, Hunter AC. Material properties in complement activation. Adv Drug Deliv Rev. 2011;63:1000–7. doi: 10.1016/j.addr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 208.das Neves J, Rocha CM, Gonçalves MP, Carrier RL, Amiji M, Bahia MF, et al. Interactions of microbicide nanoparticles with a simulated vaginal fluid. Mol Pharm. 2012;9:3347–56. doi: 10.1021/mp300408m. [DOI] [PubMed] [Google Scholar]
- 209.Tabatt K, Sameti M, Olbrich C, Müller RH, Lehr CM. Effect of cationic lipid and matrix lipid composition on solid lipid nanoparticle-mediated gene transfer. Eur J Pharm Biopharm. 2004;57:155–62. doi: 10.1016/j.ejpb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 210.Dawson M, Krauland E, Wirtz D, Hanes J. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnol Prog. 2004;20:851–7. doi: 10.1021/bp0342553. [DOI] [PubMed] [Google Scholar]
- 211.Sunshine JC, Peng DY, Green JJ. Uptake and transfection with polymeric nanoparticles are dependent on polymer end-group structure, but largely independent of nanoparticle physical and chemical properties. Mol Pharm. 2012;9:3375–83. doi: 10.1021/mp3004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 214.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–61. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yang M, Lai SK, Wang YY, Zhong W, Happe C, Zhang M, et al. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew Chem Int Ed Engl. 2011;50:2597–600. doi: 10.1002/anie.201006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MN. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119:77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 217.Tsai YM, Chang-Liao WL, Chien CF, Lin LC, Tsai TH. Effects of polymer molecular weight on relative oral bioavailability of curcumin. Int J Nanomed. 2012;7:2957–66. doi: 10.2147/IJN.S32630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Morimoto K, Nishikawa M, Kawakami S, Nakano T, Hattori Y, Fumoto S, et al. Molecular weight-dependent gene transfection activity of unmodified and galactosylated polyethyleneimine on hepatoma cells and mouse liver. Mol Ther. 2003;7:254–61. doi: 10.1016/s1525-0016(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 219.Maitani Y, Ishigaki K, Nakazawa Y, Aragane D, Akimoto T, Iwamizu M, et al. Polyethylenimine combined with liposomes and with decreased numbers of primary amine residues strongly enhanced therapeutic antiviral efficiency against herpes simplex virus type 2 in a mouse model. J Control Release. 2013;166:139–46. doi: 10.1016/j.jconrel.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 220.das Neves J, Amiji M, Sarmento B. Mucoadhesive nanosystems for vaginal microbicide development: friend or foe? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:389–99. doi: 10.1002/wnan.144. [DOI] [PubMed] [Google Scholar]
- 221.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81:1930–7. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci. 2007;104:1482–7. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Annu Rev Pharmacol Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 224.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 225.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–22. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 226.Xu A, Yao M, Xu G, Ying J, Ma W, Li B, et al. A physical model for the size-dependent cellular uptake of nanoparticles modified with cationic surfactants. Int J Nanomed. 2012;7:3547–54. doi: 10.2147/IJN.S32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gan Q, Dai D, Yuan Y, Qian J, Sha S, Shi J, et al. Effect of size onhe cellular endocytosis and controlled release of mesoporous silica nanoparticles for intracellular delivery. Biomed Microdevices. 2012;14:259–70. doi: 10.1007/s10544-011-9604-9. [DOI] [PubMed] [Google Scholar]
- 228.Huang J, Bu L, Xie J, Chen K, Cheng Z, Li X, et al. Effects of nanoparticle size on cellular uptake and liver MRI with polyvinylpyrrolidone-coated iron oxide nanoparticles. ACS Nano. 2010;4:7151–60. doi: 10.1021/nn101643u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yu SS, Lau CM, Thomas SN, Jerome WG, Maron DJ, Dickerson JH, et al. Size-and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages. Int J Nanomed. 2012;7:799–813. doi: 10.2147/IJN.S28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–47. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 231.Gutierro I, Hernández RM, Igartua M, Gascón AR, Pedraz JL. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine. 2002;21:67–77. doi: 10.1016/s0264-410x(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 232.Cohen JA, Beaudette TT, Tseng WW, Bachelder EM, Mende I, Engleman EG, et al. T-cell activation by antigen-loaded pH-sensitive hydrogel particles in vivo: the effect of particle size. Bioconjug Chem. 2009;20:111–9. doi: 10.1021/bc800338n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 234.Wang T, Jiang H, Zhao Q, Wang S, Zou M, Cheng G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: effect of silica architecture on immunological properties. Int J Pharm. 2012;436:351–8. doi: 10.1016/j.ijpharm.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 235.Xiong S, George S, Yu H, Damoiseaux R, France B, Ng KW, et al. Size influences the cytotoxicity of poly (lactic-co-glycolic acid) (PLGA) and titanium dioxide (TiO(2)) nanoparticles. Arch Toxicol. 2013 doi: 10.1007/s00204-012-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13,608–13,619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 237.Venkataraman S, Hedrick JL, Ong ZY, Yang C, Ee PL, Hammond PT, et al. The effects of polymeric nanostructure shape on drug delivery. Adv Drug Deliv Rev. 2011;63:1228–46. doi: 10.1016/j.addr.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 238.Daum N, Tscheka C, Neumeyer A, Schneider M. Novel approaches for drug delivery systems in nanomedicine: effects of particle design and shape. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:52–65. doi: 10.1002/wnan.165. [DOI] [PubMed] [Google Scholar]
- 239.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–9. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sharma G, Valenta DT, Altman Y, Harvey S, Xie H, Mitragotri S, et al. Polymer particle shape independently influences binding and internalization by macrophages. J Control Release. 2010;147:408–12. doi: 10.1016/j.jconrel.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lin SY, Hsu WH, Lo JM, Tsai HC, Hsiue GH. Novel geometry type of nanocarriers mitigated the phagocytosis for drug delivery. J Control Release. 2011;154:84–92. doi: 10.1016/j.jconrel.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 243.Nowacek AS, Balkundi S, McMillan J, Roy U, Martinez-Skinner A, Mosley RL, et al. Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages. J Control Release. 2011;150:204–11. doi: 10.1016/j.jconrel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Huang X, Teng X, Chen D, Tang F, He J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials. 2010;31:438–48. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 245.Arnida Janát-Amsbury MM, Ray A, Peterson CM, Ghandehari H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm. 2011;77:417–23. doi: 10.1016/j.ejpb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lee SY, Ferrari M, Decuzzi P. Design of bio-mimetic particles with enhanced vascular interaction. J Biomech. 2009;42:1885–90. doi: 10.1016/j.jbiomech.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 247.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26:235–43. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 248.Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010;141:320–7. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 249.Devarajan PV, Jindal AB, Patil RR, Mulla F, Gaikwad RV, Samad A. Particle shape: a new design parameter for passive targeting in splenotropic drug delivery. J Pharm Sci. 2010;99:2576–81. doi: 10.1002/jps.22052. [DOI] [PubMed] [Google Scholar]
- 250.Perumal OP, Inapagolla R, Kannan S, Kannan RM. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials. 2008;29:3469–76. doi: 10.1016/j.biomaterials.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 251.Eichhorn ME, Ischenko I, Luedemann S, Strieth S, Papyan A, Werner A, et al. Vascular targeting by EndoTAG-1 enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int J Cancer. 2010;126:1235–45. doi: 10.1002/ijc.24846. [DOI] [PubMed] [Google Scholar]
- 252.McNeil SE, Rosenkrands I, Agger EM, Andersen P, Perrie Y. Subunit vaccines: distearoylphosphatidylcholine-based liposomes entrapping antigen offer a neutral alternative to dimethyldioctadecylammonium-based cationic liposomes as an adjuvant delivery system. J Pharm Sci. 2011;100:1856–65. doi: 10.1002/jps.22427. [DOI] [PubMed] [Google Scholar]
- 253.Garzón MR, Berraondo P, Crettaz J, Ochoa L, Vera M, Lasarte JJ, et al. Induction of gp120-specific protective immune responses by genetic vaccination with linear polyethylenimine-plasmid complex. Vaccine. 2005;23:1384–92. doi: 10.1016/j.vaccine.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 254.Thiagarajan G, Greish K, Ghandehari H. Charge affects the oral toxicity of poly(amido amine) dendrimers Eur J Pharm Biopharm 2013 in press. doi: 10.1016/j.ejpb.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang W, Xiong W, Zhu Y, Xu H, Yang X. Protective effect of PEGylation against poly(amidoamine) dendrimer-induced hemolysis of human red blood cells. J Biomed Mater Res B Appl Biomater. 2010;93:59–64. doi: 10.1002/jbm.b.31558. [DOI] [PubMed] [Google Scholar]
- 256.Pearson RM, Patra N, Hsu HJ, Uddin S, Král P, Hong S. Positively charged dendron micelles display negligible cellular interactions. ACS Macro Lett. 2013;2:77–81. doi: 10.1021/mz300533w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci USA. 2009;106:19,268–19,273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Moghimi SM. The effect of methoxy-PEG chain length and molecular architecture on lymph node targeting of immuno-PEGliposomes. Biomaterials. 2006;27:136–44. doi: 10.1016/j.biomaterials.2005.05.082. [DOI] [PubMed] [Google Scholar]
- 259.Kaminskas LM, Boyd BJ, Karellas P, Krippner GY, Lessene R, Kelly B, et al. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly L-lysine dendrimers. Mol Pharm. 2008;5:449–63. doi: 10.1021/mp7001208. [DOI] [PubMed] [Google Scholar]
- 260.Mert O, Lai SK, Ensign L, Yang M, Wang YY, Wood J, et al. A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. J Control Release. 2012;157:455–60. doi: 10.1016/j.jconrel.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Demberg T, Robert-Guroff M. Mucosal immunity and protection against HIV/ SIV infection: strategies and challenges for vaccine design. Int Rev Immunol. 2009;28:20–48. doi: 10.1080/08830180802684331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Duerr A. Update on mucosal HIV vaccine vectors. Curr Opin HIV AIDS. 2010;5:397–403. doi: 10.1097/COH.0b013e32833d2e39. [DOI] [PubMed] [Google Scholar]
- 263.Yu M, Vajdy M. Mucosal HIV transmission and vaccination strategies through oral compared with vaginal and rectal routes. Expert Opin Biol Ther. 2010;10:1181–95. doi: 10.1517/14712598.2010.496776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jazayeri M, Soleimanjahi H, Fotouhi F, Pakravan N. Comparison of intramuscular and footpad subcutaneous immunization with DNA vaccine encoding HSV-gD2 in mice. Comp Immunol Microbiol Infect Dis. 2009;32:453–61. doi: 10.1016/j.cimid.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 265.Kaneko H, Bednarek I, Wierzbicki A, Kiszka I, Dmochowski M, Wasik TJ, et al. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology. 2000;267:8–16. doi: 10.1006/viro.1999.0093. [DOI] [PubMed] [Google Scholar]
- 266.Mohanan D, Slütter B, Henriksen-Lacey M, Jiskoot W, Bouwstra JA, Perrie Y, et al. Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–9. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 267.Koblin BA, Casapia M, Morgan C, Qin L, Wang ZM, Defawe OD, et al. Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: a randomized clinical trial. PLoS One. 2011;6:e24517. doi: 10.1371/journal.pone.0024517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pattani A, McKay PF, Garland MJ, Curran RM, Migalska K, Cassidy CM, et al. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release. 2012;162:529–37. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Buffa V, Klein K, Fischetti L, Shattock RJ. Evaluation of TLR agonists as potential mucosal adjuvants for HIV gp140 and tetanus toxoid in mice. PLoS One. 2012;7(12):e50529. doi: 10.1371/journal.pone.0050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28:1412–21. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nuttall J, Kashuba A, Wang R, White N, Allen P, Roberts J, et al. Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob Agents Chemother. 2012;56:103–9. doi: 10.1128/AAC.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.García-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul MR, Piot PK. Attitudes and acceptance of oral and parenteral HIV preexposure prophylaxis among potential user groups: a multinational study. PLoS One. 2012;7:e28238. doi: 10.1371/journal.pone.0028238. [DOI] [PMC free article] [PubMed] [Google Scholar]