Abstract
Polyhydroxybutyrate (PHB) is a biological polymer which belongs to the class of polyesters and is ubiquitously present in all living organisms. Mammalian mitochondrial membranes contain PHB consisting of up to 120 hydroxybutyrate residues. Roles played by PHB in mammalian mitochondria remain obscure. It was previously demonstrated that PHB of the size similar to one found in mitochondria mediates calcium transport in lipid bilayer membranes. We hypothesized that the presence of PHB in mitochondrial membrane might play a significant role in mitochondrial calcium transport. To test this, we investigated how the induction of PHB hydrolysis affects mitochondrial calcium transport. Mitochondrial PHB was altered enzymatically by targeted expression of bacterial PHB hydrolyzing enzyme (PhaZ7) in mitochondria of mammalian cultured cells. The expression of PhaZ7 induced changes in mitochondrial metabolism resulting in decreased mitochondrial membrane potential in HepG2 but not in U87 and HeLa cells. Furthermore, it significantly inhibited mitochondrial calcium uptake in intact HepG2, U87 and HeLa cells stimulated by the ATP or by the application of increased concentrations of calcium to the digitonin permeabilized cells. Calcium uptake in PhaZ7 expressing cells was restored by mimicking calcium uniporter properties with natural electrogenic calcium ionophore - ferutinin. We propose that PHB is a previously unrecognized important component of the mitochondrial calcium uptake system.
Keywords: Poly-3-hydroxybutyrate, Inorganic polyphosphate, Calcium, PhaZ7, Mitochondria
1. Introduction
Poly-3-hydroxybutyrate (PHB) is a polyester made of monomers of 3-hydroxybutyric acid (HB). PHB is ubiquitously present in all living organisms ranging from bacteria to humans. In mammalian organisms PHB is found in the form of a short chain or “complexed” PHB (cPHB), consisting of polymers with chain lengths ranging from 2 to 120 monomers [1–3]. cPHB has been found in all living organisms examined, suggesting its important biological function [2]. While physiological roles of PHB of higher eukaryotes, including mammals, are in general poorly understood, several studies suggest that it might be involved in the regulation of membrane transport. Specifically, it has been demonstrated that cPHB is directly involved in the formation of bacterial cation selective channels through the formation of a polyphosphate (polyP) polyP/Ca2+/PHB complex [4] and is closely associated with such protein channels as KcsA [5], OmpA [6] and mammalian TRPM8 [7].
In mammalian cells most of the cPHB is found in the mitochondria and is primarily localized in the mitochondrial membrane fraction [3]. It was previously demonstrated that cPHB isolated from rat liver mitochondria is complexed with calcium and polyP and can form stable ion channels when reconstituted into artificial lipid bilayers [8]. The properties of this channel were similar to the properties of mitochondrial permeability transition pore (mPTP) [9,10], suggesting that mitochondrial cPHB might be an important target for the polyP – a potent activator of mPTP [11–13]. Furthermore, earlier studies have shown that synthetic PHB of a size similar to that found in mitochondria can induce cation selective ion transport in model lipid membranes. Importantly, in these experiments ion transport can occur by both channel [14] and carrier [15] mechanisms and does not require the presence of polyP. Specifically, it was demonstrated that liposomes containing small amount of PHB can accumulate calcium and that the kinetics of this process are similar to the action of calcium ionophores [2,16,17]. Taking this into account we hypothesized that cPHB may play an important role in mitochondrial ion transport. To test this hypothesis, we investigated the activity of the calcium uptake system of mammalian mitochondria expressing bacterial PHB depolymeraze (PhaZ7), an enzyme that specifically hydrolyses cPHB [18]. We found that the expression of this enzyme significantly inhibits mitochondrial calcium uptake. Our data suggest that cPHB is a previously unrecognized important player in mitochondrial calcium uptake.
2. Materials and methods
2.1. Generation of mitochondrial PhaZ7 (mPhaZ7) construct
pAcGFP1-Mito cDNA (MTS-GFP), which encodes a fusion protein consisting of a mitochondrial targeting sequence, derived from the precursor of subunit VIII of human cytochrome c oxidase, and the GFP from Aequorea coerulescens, was purchased (Clontech, Mountain View, CA) and modified by the removal of the GFP stop codon and its replacement with an in-frame XhoI/NotI linker. A PHB depolymeraze cDNA, PhaZ (kindly provided by Dieter Jendrossek), was amplified by high-fidelity PCR (Phusion polymeraze, NEB), with primers containing 5′ XhoI and 3′ NotI restriction sites, and subcloned into the modified pAcGFP1-Mito vector by standard techniques. The DNA sequences of all constructs were determined to be correct before transfection experiments. All plasmid DNAs to be transfected into mammalian cells we prepared using the Endo-Free Plasmid Maxi Kit (Qiagen).
2.2. Cell culture
Human hepatocellular carcinoma HepG2 cell line, human glioblastoma U87 cell line and HeLa cervical cancer cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (Gibco) containing 10% FBS (Invitrogen) and 1% Pen/Strep (Invitrogen) at 37°C with 5% CO2. Cells were transfected with a standard calcium-phosphate procedure.
2.3. Confocal experiments
For measurements of mitochondrial membrane potential (Am), cells were loaded with 25 nM tetramethylrhodamine methylester (TMRM) for 30 min at room temperature and the dye was present during the experiment. TMRM is used in the redistribution mode to assess ΔΨm, and therefore a reduction in TMRM fluorescence represents mitochondrial depolarization.
Cells were loaded for 30 min at room temperature with 5 µM X-rhod-1 AM or Fluo-3 AM, (Molecular Probes, Invitrogen) prior to imaging in HEPES-buffered salt solution (HBSS) composed of (mM): 156 NaCl, 3 KCl, 2MgSO4, 1.25 KH2PO4, 2 CaCl2, 10 glucose and 10 HEPES, pH adjusted to 7.35 with NaOH. For cell permeabilization experiments HBSS was replaced with pseudo intracellular buffer solution composed of (mM) 120 KCl, 10 NaCl, 1 KH2PO4, 2 MgCl2, 20 HEPES–KOH pH 7.4 containing 5 mM succinate and 1 µM rotenone and supplemented with 1 mM EGTA and 20 µM digitonin. Media was then replaced by the same pseudo intracellular buffer without digitonin and containing increasing concentrations of calcium. Concentration of free calcium was estimated using the Ca2+–EGTA Calculator v1.3 [19], software available at: http://www.stanford.edu/~cpatton/CaEGTA-TS.htm. Confocal images were obtained using a 510 CLSM (Zeiss, Thornwood, NY) equipped with a META detection system and a X40 oil-immersion objective. The 488 nm Argon laser line was used to excite fluo-3 fluorescence, which was measured using a bandpass filter from 505 to 550 nm. Illumination intensity was kept to a minimum (at 0.1–0.2% of laser output) to avoid phototoxicity and the pinhole was set to give an optical slice of ~2 µm. TMRM and X-rhod-1 were excited using the 543 nm laser line and fluorescence measured using a 560 nm longpass filter.
Ferutinin (Enzo Life Sciences) was added to a final concentration of 10–50 µM and carbonyl cyanide p-trifluoromethoxyphenylhydrazone (CCCP) was added to a final concentration of 1 µM.
All the imaging data presented in the paper are representative of at least 3 experiments.
2.4. Cell survival experiments
HeLa and HepG2 cells were transfected with either the mito-GFP or the mPhaZ7 expression construct by a standard calcium phosphate procedure. The number of green cells in ten fields per dish was counted at 24 and 48 h post transfection and the percentage of green cells at 48 h was expressed as a percentage of the 24 h count. Cells were stained with propidium iodide (5 µM) and only cells that failed to stain were counted as viable.
2.5. Statistical analyses
Statistical analysis was performed using Origin 8.5 software (Microcal Software, Northampton, MA). Results are expressed as means ±SEM.
3. Results
3.1. Targeted expression of PHB-depolymeraze (PhaZ7) in mitochondria of mammalian cells
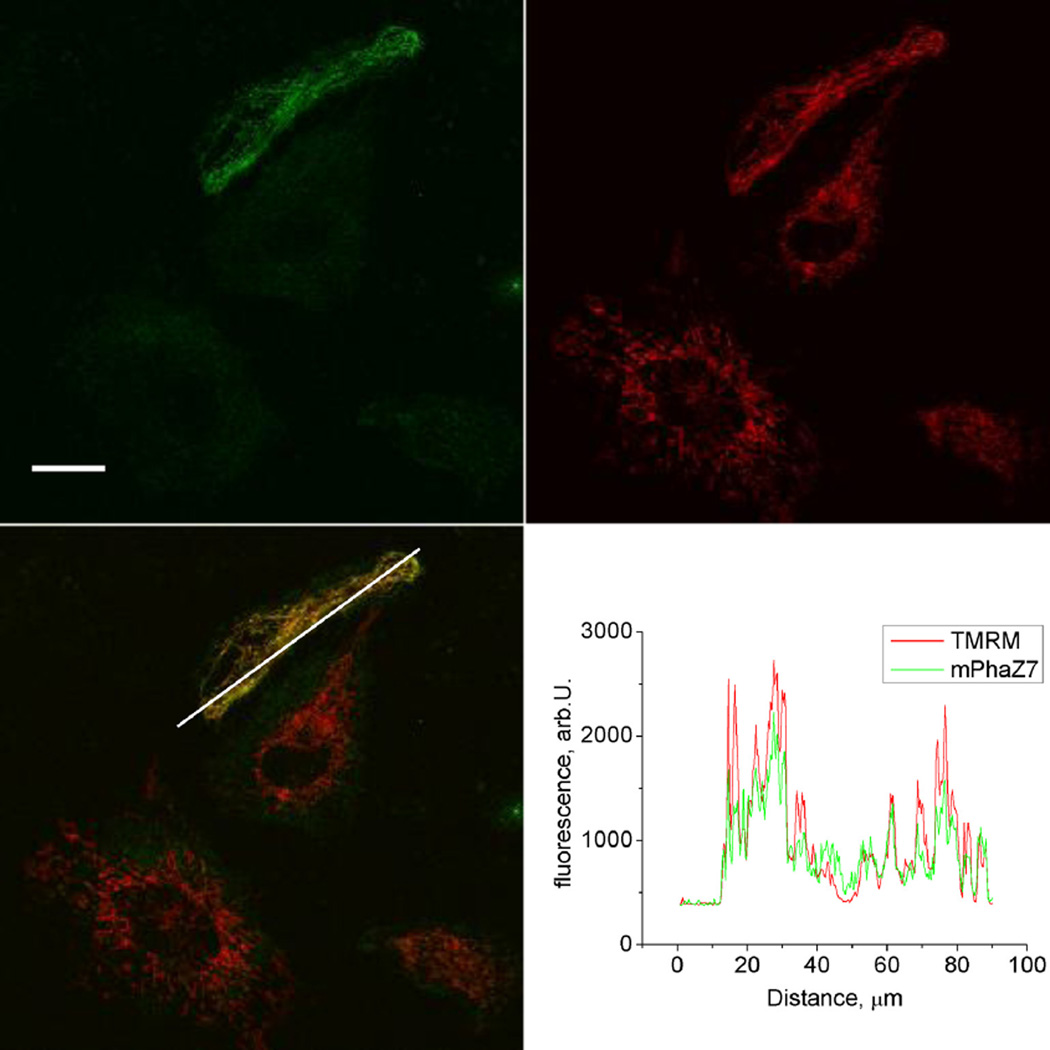
The endogenous enzymes responsible for mitochondrial PHB metabolism are currently unknown. For that reason, we chose to address the potential role of PHB in mitochondrial function by expressing a bacterial enzyme that is known to specifically hydrolyze the PHB polymer. For these experiments we used the bacterial PHB-depolymeraze, PhaZ7. Although a number of bacterial PHB-depolymerazes have been identified, PhaZ7 is a unique enzyme which specifically hydrolyzes short-chain or “complexed” cPHB present in mammalian mitochondria, but not storage sPHB [18,20]. Here, we generated a DNA construct through fusion of PhaZ7 coding DNA with DNA coding a mitochondrially targeted GFP protein. Targeted expression of mPhaZ7 was confirmed by the appearance of the green fluorescent signal in the mitochondria of transfected cells (Fig. 1). As can be seen from the images of HeLa cells, the GFP signal is co-localized with the red fluorescent signal originating from the potential sensitive mitochondrial probe, TMRM. A similar mitochondrial expression pattern was found in all cell types used in the present study. In our experiments we used a mitochondrial targeting sequence specific to the matrix targeted proteins, which suggests that the expressed enzyme was likely localized in the matrix of mitochondria.
Fig. 1.
Mitochondrial localization of the mPhaZ7 protein. Confocal images of live cells showing green signal from mPhaZ7, red signal from TMRM, and superimposed mPhaZ7 and TMRM images collected from the cultured HeLa cells; Intensity profiles (lower right panel) were collected along the line of the white arrow. mPhaZ7: mitochondrially targeted PHB depolymeraze fused with the GFP protein.
3.2. Effect of mPhaZ7 overexpression on mitochondrial membrane potential
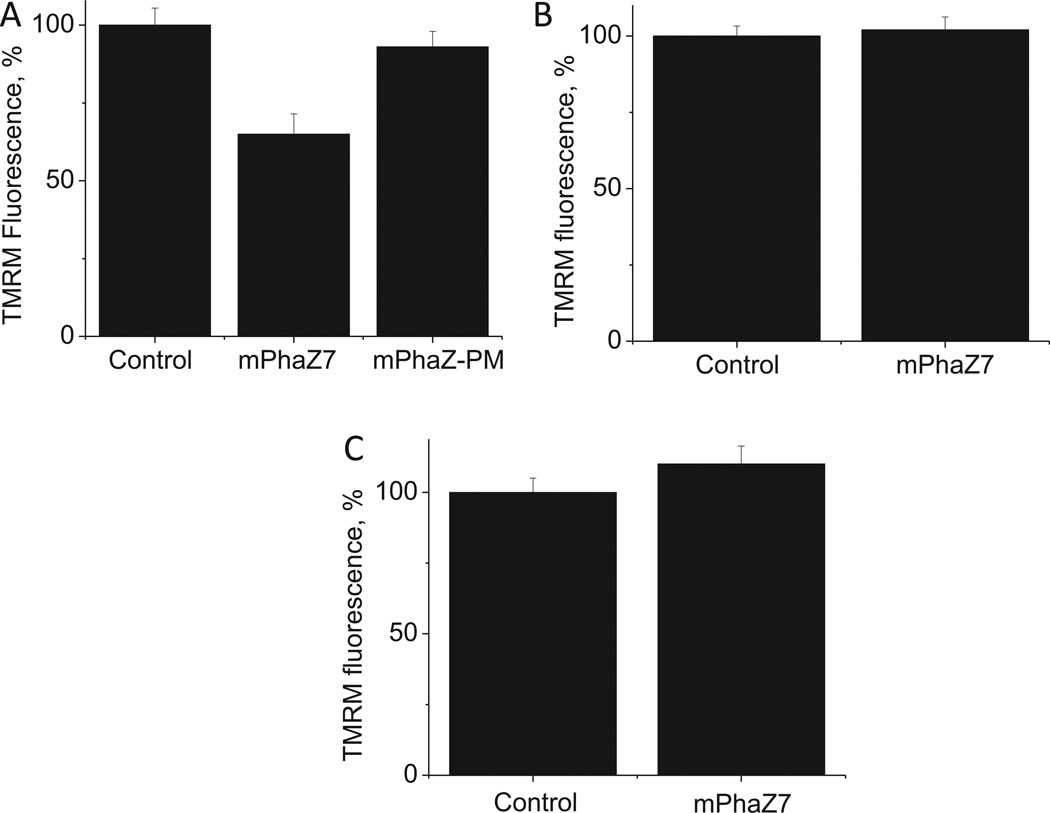
Next we investigated how the expression of mPhaZ7 affects ΔΨm. We found that the expression of mPhaZ7 caused a significant reduction of mitochondrial membrane potential in HepG2 cells, as evidenced by the decrease of the TMRM fluorescence by 65 ±6.5% of control (n = 55 control cells; n = 55 mPhaZ7 cells; p < 0.001; see Fig. 2A). However, we did not detect any significant effect of mPhaZ7 expression on mitochondrial membrane potential in HeLa (n = 25 control cells; n = 25 mPhaz7 cells; see Fig. 2B) or U87 cells (n = 35 control cells; n = 35 mPhaZ7 cells, see Fig. 2C). Thus, the expression of PHB depolymerazing enzyme in mitochondria alters mitochondrial metabolism in HepG2 cells, but not in U87 or HeLa cells. Notably, in control experiments where HepG2 cells were transfected with a point mutant (Ser136Ala) of mPhaZ7 (mPhaZ7-PM), which is known to lack PHB depolymeraze activity [18], the membrane potential was not changed (Fig. 2A). This suggests that the decrease of the membrane potential seen in HepG2 cells is linked to the PHB depolymerazing activity of the enzyme. We should note here that although the PHB role has never been studied in mammalian mitochondria, it is known that in bacterial organisms this polymer can play an integral role in energy metabolism [21]. In these organisms, the PHB metabolism is directly linked to the metabolism of acetyl-CoA - the key substrate of the Krebs cycle. Thus one of the possible explanations of the effects on the membrane potential seen in HepG2 cells is that the endogenous PHB plays a significant role in their bioenergetics.
Fig. 2.
Influence of mPhaZ7 expression on mitochondrial membrane potential. HepG2 cells (A) expressing mPhaZ7 show lower mitochondrial membrane potential than controls, as measured by TMRM fluorescence, whereas HeLa (B) or U87 (C) cells did not.
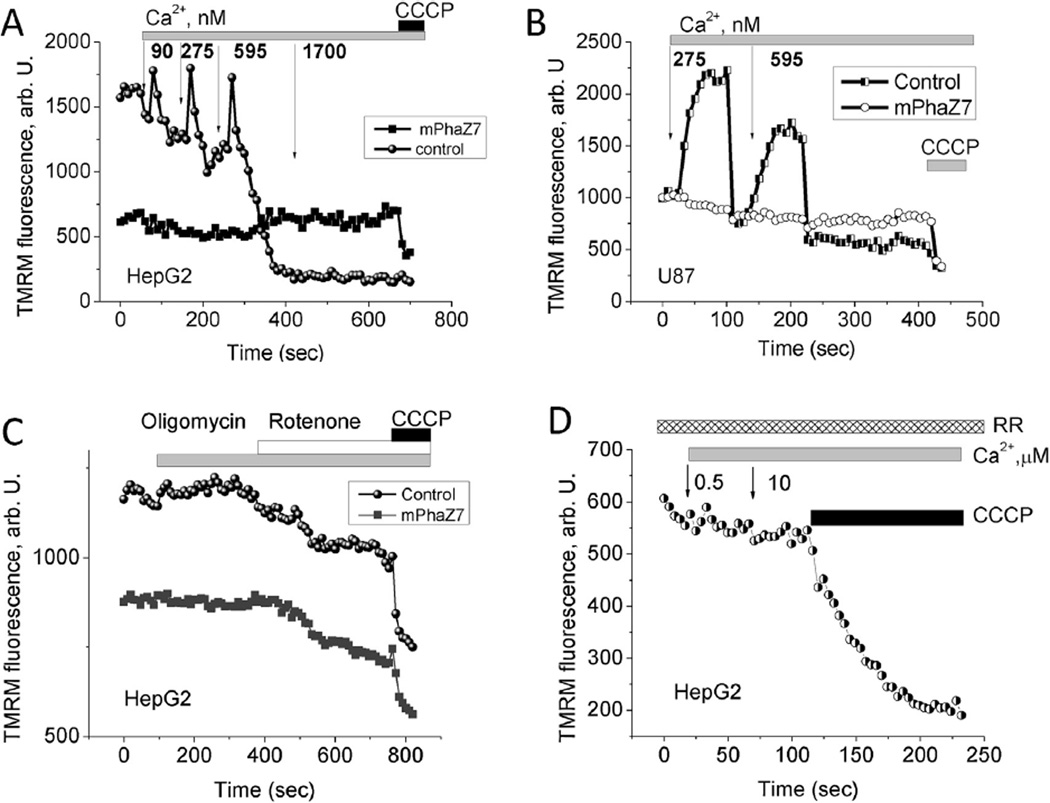
Next, we tested the effect of the mPhaZ7 expression on the kinetics of calcium−induced changes to the mitochondrial membrane potential. To do this we monitored changes in TMRM fluorescence in mitochondria of permeabilized cells in pseudo-intracellular medium. In HepG2 control cells, which did not demonstrate GFP fluorescence, the addition of submicromolar concentrations of calcium caused transient increase in mitochondrial membrane potential, presumably due to calcium-induced activation of the mitochondrial electron transport chain (Fig. 3A) [22]. Further additions of calcium eventually led to a fast and complete drop in ΔΨm, caused either by the opening of the calcium induced mPTP or by the higher rates of calcium uniporter in the presence of increased amounts of calcium. Neither transient increases of membrane potential nor mitochondrial depolarization were detected in mitochondria expressing mPhaZ7 (Fig. 3A). A similar response to the calcium addition was observed in the preparation of U87 cells (Fig. 3B). It should be noted that these mitochondria did respond to the addition of the protonophore CCCP, suggesting that they were able to maintain the ΔΨm constant even in the presence of micro-molar concentrations of calcium. Thus, cells expressing mPhaZ7 demonstrated insensitivity to micromolar concentrations of calcium as observed by the lack of changes in ΔΨm. Importantly, no transient increase in the membrane potential was observed in the presence of the uniporter inhibitor – Ruthenium Red (Fig. 3D). This observation suggests that an increase in membrane potential requires calcium entry into the mitochondria, which is consistent with the idea about its stimulatory role on mitochondrial metabolism.
Fig. 3.
Effect of calcium addition on mitochondrial membrane potential in digitonin-permeabilized HepG2 (A) and U87 (B) cells. Calcium concentrations at panels (A) and (B) are in nM. Panel (C) shows the effect 2 µg/ml oligomycin and 5 µM rotenone on the mitochondrial membrane potential in HepG2 cells, CCCP was added in concentration of 1 µM. (D) Lack of changes in the mitochondrial membrane potential of digitonin-permeabilized HepG2 cells in response to calcium addition and in the presence of the inhibitor of mitochondrial calcium uptake ruthenium red.
Immortal cell lines may show abnormal mitochondrial metabolism [23]. Moreover, mPhaZ7 expression can change the maintenance mechanism of mitochondrial potential. In cells with normal oxidative phosphorylation, ΔΨm is maintained by the proton pumping activity of the respiratory chain. If respiration is impaired, then hydrolysis of the ATP by the F1-F0-ATPase may take place. The application of oligomycin (2 µg/ml) (an inhibitor of the F1-F0-ATP synthase) to HepG2 control cells or to HepG2 expressing mPhaZ7 induced a small hyperpolarization of the membrane potential (Fig. 3C). Subsequent inhibition of complex I with 5 µM rotenone induced a decrease in ΔΨm in both cell types (Fig. 3C) suggesting that HepG2 cells maintained ΔΨm by respiration, which was not affected by lack of PHB.
3.3. Effect ofmPhaZ7 overexpression on mitochondrial calcium uptake
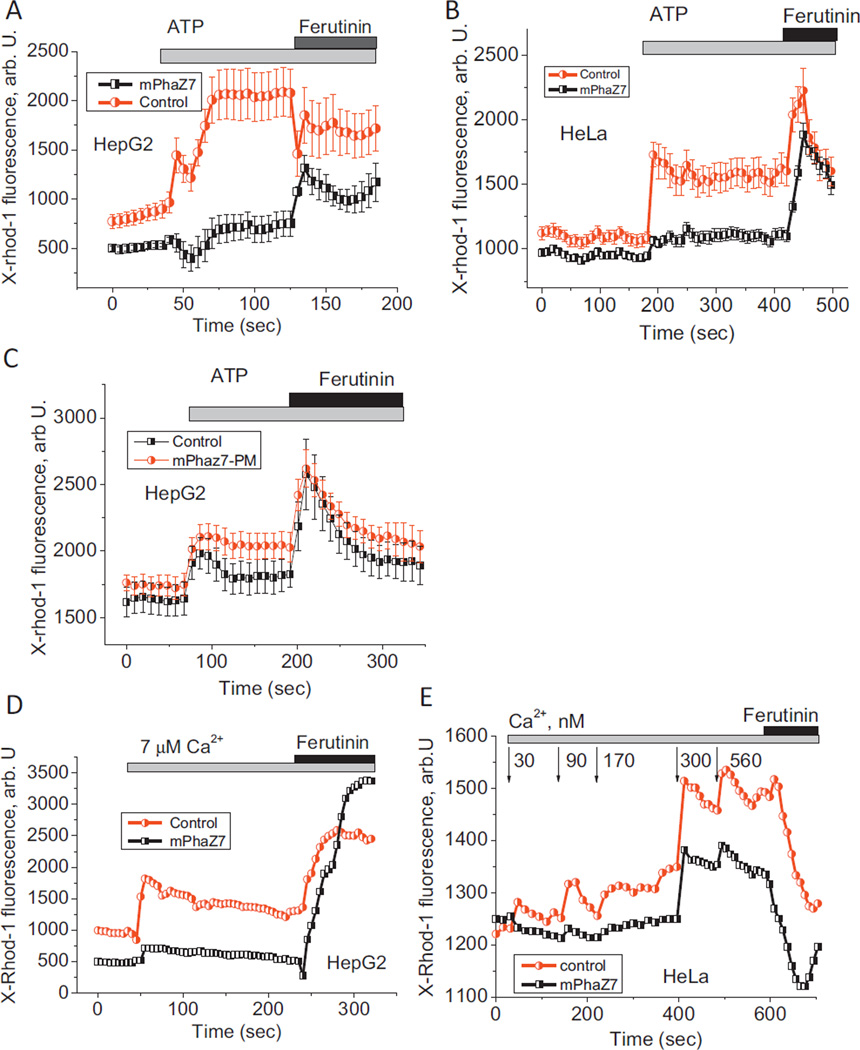
Taking into account that the addition of calcium to permeabilized cells did not cause detectable changes in mitochondrial membrane potential in cells transfected with mPhaZ7, we hypothesized that mitochondria of these cells have an altered calcium uptake system. To test this idea we measured the kinetics of calcium uptake in these cells. Calcium uptake was monitored using the calcium sensitive dye X-rhod-1, which preferentially localizes in mitochondria. In intact cells an increased calcium signal was induced by the addition of 100 µM ATP to HepG2 or HeLa cells. The application of this physiological calcium stimulus increases the cytosolic calcium concentration leading to mitochondrial calcium-uptake. Calcium response to ATP is highly variable and depends on the age of the culture, the expression of purine receptors, and the availability of calcium in ER. For this reason, the calcium uptake difference between control and mPhaZ7 expressing cells was compared to the cells of the same preparation in the same imaging field. In both cell types examined (HepG2 and HeLa), the expression of mPhaZ7 significantly reduced mitochondrial calcium uptake (Fig. 4A and B). In cells expressing mPhaZ7 point mutant (Ser136Ala) lacking depolymeraze activity, the calcium uptake was similar to the non-transfected cells (Fig. 4C). mPhaZ7-expressing mitochondria showed a 7.54-fold lower mitochondrial calcium uptake compared to control cells (n = 54 mPhaZ7 transfected cells; n = 45 control HepG2; p < 0.001) in HepG2 cells, and a 5-fold lower calcium uptake in HeLa cells (n = 4 mPhaZ7; n = 4 control; p < 0.05). Importantly, in the case of HeLa cells expressing mPhaZ7, the inhibition of mitochondrial calcium uptake was not due to a lower mitochondrial membrane potential, as HeLa cells displayed no difference in the value of ΔΨm between control and mPhaZ7 cells (Fig. 2B). The addition of 20 µM ferutinin, an electrogenic calcium ionophore, whose potential-dependently increases [Ca2+]m, mimicking the calcium uniporter [16,17,24], increased mitochondrial X-rhod-1 fluorescence in both HepG2 and HeLa expressing mPhaZ7 (Fig. 4A and B). This suggests that mPhaZ7 expression does not compromise mitochondrial function or the ability to accumulate the fluorescent probe, but rather decreases the mitochondrial calcium permeability.
Fig. 4.
Effect of mPhaZ7 expression on mitochondrial calcium uptake. Expression of mPhaZ7 inhibits mitochondrial calcium uptake in the intact HepG2 (A) and HeLa (B) cells induced by the addition of 100 µM ATP. The expression of inactive point mutant of mPhaZ7 (Ser136Ala) labeled as mPhaZ7-PM, did not inhibit calcium uptake (C). Application of calcium to mitochondria of digitonin-permeabilized cells induces uptake of this cation in control but not in mPhaZ7-expressing mitochondria in HepG2 (D) and HeLa (E) cells. Ferutinin was used at a concentration 20 µM. In panel (E) calcium concentrations are in nM.
To further confirm that the effects observed in the experiments with intact cells are directly related to changes in the activity of the mitochondrial calcium uniporter, we measured calcium uptake in the mitochondria of permeabilized cells [13]. These experiments allow the direct measurement of the kinetics of calcium uptake as a function of well-controlled concentrations of calcium in the recording solution. Similar to experiments with intact cells, in permeabilized HepG2 cells the application of 7 µM calcium induced a 6.3-fold increase in [Ca2+]m in mitochondria of control cells, compared to mitochondria expressing mPhaZ7 (n = 4 experiments; Fig. 4D (p < 0.001)). Importantly, the application of 20 µM ferutinin in the presence of 7 µM calcium induced a significant [Ca2+]m increase in both control and mPhaZ7 mitochondria (Fig.4D; n = 3 experiments), confirming their functional integrity. A similar inhibition of mitochondrial calcium uptake by the expression of the mPhaZ7 was observed in HeLa cells (n = 3 experiments; Fig. 4E). Interestingly, unlike the case of HepG2 cells, in HeLa cells addition of ferutinin induced a rapid decrease of X-Rhod-1 fluorescence (compare Fig. 4D and E). Taking into account that ferutinin can transport calcium along its electrochemical gradient, these differences likely arise from the different values of membrane potential, mitochondrial calcium load, and concentrations of the free calcium in the recording media in these two sets of experiments.
3.4. Effect of PHB binding dye – Nile Red on mitochondrial calcium uptake
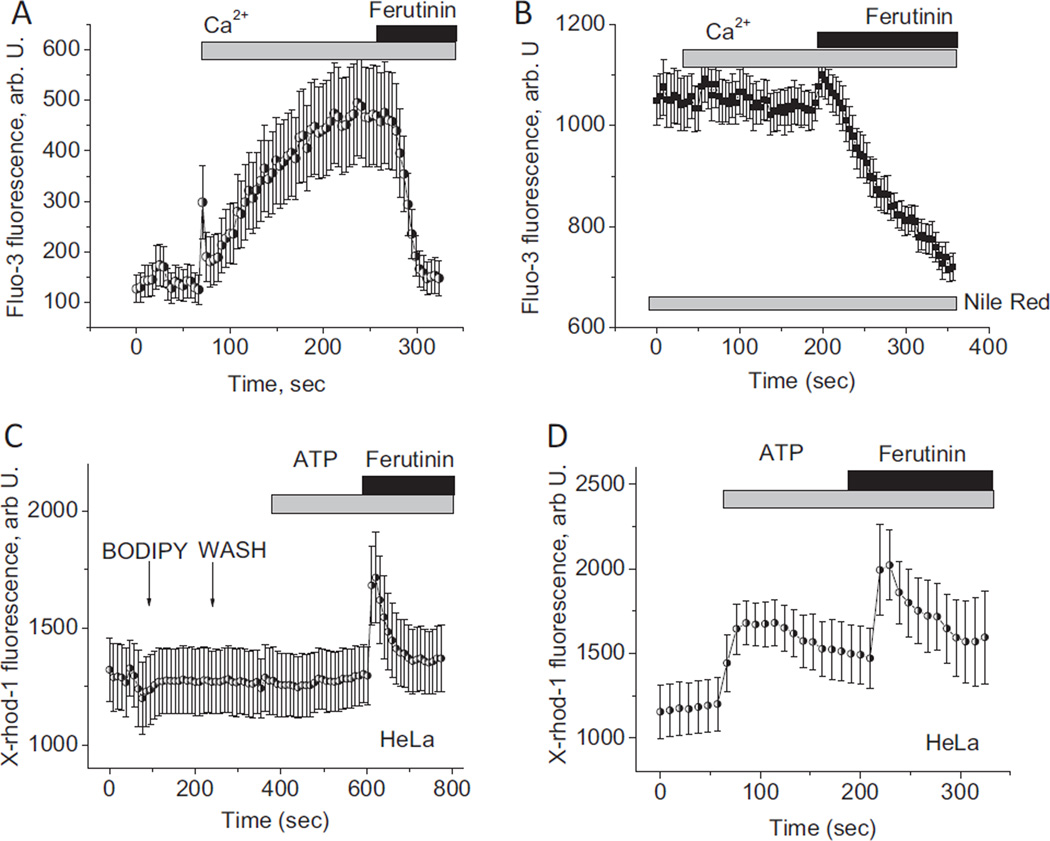
Nile Red can readily bind to PHB and is used in research as a fluorescent indicator for this polymer [25,26]. We used this property of Nile Red to bind PHB in mitochondria of permeabilized HeLa cells to investigate how a chemical interference with PHB may affect mitochondrial calcium uptake. In the control mitochondria of permeabilized cells loaded with Fluo-3, the addition of calcium caused a rise in fluorescence from 140 ±30 to 500 ±100 A.U., n = 4 experiments (Fig. 5A) indicating active calcium uptake. The application of 10 µM ferutinin induced loss of the fluorescent signal indicating opening of the PTP (Fig. 5A). Preincubation of permeabilized cells with 10 µM Nile Red completely blocked the mitochondrial calcium uptake induced by 15 µM calcium (n = 3 experiments; Fig. 5B). The application of 10 µM ferutinin to these mitochondria induced transient calcium rise (Fig. 5B) followed by the abrupt loss of fluorescence suggesting that the mitochondria are not destroyed by incubation with Nile Red and that they are still able to electrogenically uptake calcium, which is followed by its release due to the calcium induced PTP. Thus, the presence of the PHB binding molecule, Nile Red in mitochondrial membranes, inhibits mitochondrial calcium uptake. A similar effect of inhibition of the mitochondrial calcium uptake was seen in intact cells. In this case we used another lipophilic dye, BODIPY 493/503, which also interacts with PHB [27,28] but is characterized by green fluorescence which makes it possible to use it in combination with calcium sensitive red X-Rhod-1 probe. As can be seen in Fig. 5C, in the presence of BODIPY 493/503 the addition of the ATP did not induce the rise in mitochondrial calcium, whereas significant increase was detected in control experiments shown in Fig. 5D.
Fig. 5.
The effect of Nile Red and BODIPY 493/503 addition on mitochondrial calcium uptake in HeLa cells. Effect of 15 µM calcium and 20 µM ferutinin on mitochondrial calcium concentration in control (A) and Nile Red-treated mitochondria of digitonin-permeabilized HeLa cells (B). Effect of ATP and ferutinin addition on the mitochondrial calcium uptake in intact HeLa cells treated with BODIPY 493/503 (C) and in control cells (D).
3.5. Cell survival
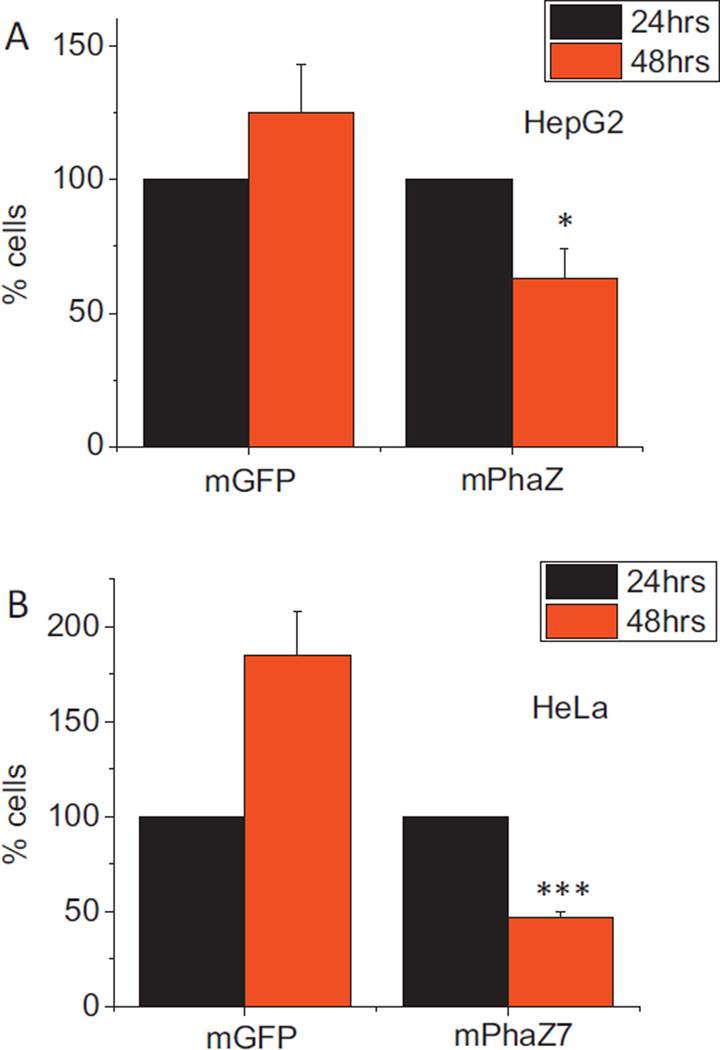
The effect on cell survival of expressing mPhaZ7 was also investigated. HepG2 and HeLa cells were transfected with the mPhaZ7 or mGFP control, and the number of green cells at 24 and 48 h post-transfection were counted. The number of HepG2 and HeLa cells expressing mGFP increased from 24 to 48 h, suggesting active cell division (Fig. 6). In contrast the number of cells expressing the mPhaZ7 construct decreased from 24 to 48 h. For HepG2 cells, the number of cells expressing mGFP increased by 125% ± 18% by 48 h, whereas the number of cells expressing mPhaZ7 fell by 47% ±11% (Fig. 6A; n = 3 experiments, 10 fields per experiment). In HeLa, the number of cells expressing mGFP increased by 85% ± 23% (Fig. 6B; n = 3 experiments, 10 fields per experiment). The number of cells expressing mPhaZ7 fell by 53% ± 3% in the same time period. Therefore, expression of mPhaZ7 significantly decreases survival in HeLa and HepG2 transfected cells suggesting that PHB play an important role in cell physiology. While the exact mechanisms of PHB function remain elusive, one of the possible roles of this polymer might be its ability to mediate calcium transport in mitochondria. Taking into account that mitochondrial calcium transport is believed to be one of the most fundamental processes in cell physiology it is conceivable to suggest that its alternation by changes in PHB can have profound effect on normal cell function.
Fig. 6.
Expression of dPHB in HepG2 and HeLa cells decreases survival. Cells were transfected with either mGFP or a mPhaZ7 construct and green cells were counted at 24 and 48 h post transfection. Expression of mPhaZ7 in HepG2 (A) and HeLa (B) decreased survival compared to control transfected cells. The number of cells is expressed as a percentage of the 24h count. *p < 0.05; ***p < 0.001.
4. Discussion
We have found that the expression of a mitochondrially targeted mPhaZ7, or chemical binding of PHB with Nile Red, inhibits mitochondrial calcium influx. Currently available methods for PHB assay did not allow us to directly compare levels of PHB in non-transfected and transfected cells. However, taking into account that the observed effects were not seen in mitochondria expressing a point mutant of the enzyme lacking PHB depolymeraze activity we suggest that the effects observed are likely related to the hydrolysis of endogenous PHB. At present very little is known about the role of PHB in the mammalian mitochondria. To the best of our knowledge, the present work provides the first experimental evidence of the involvement of this polymer in mitochondrial function. Thus, at this stage, all potential explanations for the effect of PHB hydrolysis on mitochondrial calcium uptake should be considered. First, changes in PHB homeostasis may interfere with mitochondrial metabolism. Expression of the enzyme led to decrease in ΔΨm and, as a result, lower calcium influx. However, this explanation may be possible for HepG2 cells only, but not for HeLa or astroglioma U87 cells, where mitochondrial membrane potential was comparable between controls and cells expressing the PHB depolymeraze (Fig. 2A – C). Second, changes in the level of PHB in the mitochondrial membrane may lead to changes in membrane fluidity and affect the activity of the calcium uptake machinery, in a similar way to the changes observed in calcium permeability of the plasma membrane due to different content of cholesterol [29,30]. The change in the membrane fluidity in the presence of PHB has been documented before in experiments with Escherichia coli as well as with artificial liposomes. Specifically it was suggested that release of PHB from its form complexed to polyphosphate results in significant decrease in the membrane fluidity [1,31]. However, we should note that the same scenario is less likely present in mitochondria due to much lower levels of PHB comparing to bacteria and experiments with artificial polymer [3].Third, PHB has been shown to be an essential part of several proteins including mammalian ion channels [7]. Recent studies indicate that the calcium uniporter is most likely a supramolecular complex with at least two essential protein components, MCU presumably forming the pore part of the calcium uniporter complex [32,33] and MICU1 playing a role in calcium sensing [34]. PHB may be another important structural part of the calcium uniporter in a similar way to the bacterial or TRP channels [5–7]. In this case, the enzymatic or chemical hydrolysis of the PHB pool that is associated with the protein channel should inhibit transport through this channel. Of special interest here is an earlier finding that uniporter activity linked to 40 kDa mitochondrial protein required the presence of a low-molecular weight membrane component of unknown nature [35,36]. Finally, it should be noted that PHB in its free form is a known ionophore, which is capable of transporting cations through hydrophobic organic phases as well as mediating calcium transport through lipid bilayers [2]. This property of PHB can be explained by its amphyphilic nature. PHB is a polyester within alternating hydrophobic and hydrophillic groups. It has been proposed that PHB can span the bilayer with calcium ion being able to transfer along carbonyl groups inside the passage way formed by the polymer [2,15]. In this case, PHB mediates calcium uptake by a carrier mechanism with kinetics similar to the known calcium carrier - calcimycin [15] and much slower comparing to kinetics of typicalion channel. It should be noted here that although MCU (key protein of the mitochondria calcium uptake system) can function as an ion channel in the model membranes, the experimental support for this is based on the channel activity of the recombinant MCU protein expressed and purified from bacteria and recorded in model bilayers in the presence of free calcium in concentrations 5–6 orders of magnitude above that of the physiological [33]. However, the rate of calcium uptake by intact mitochondria is significantly lower than would be expected from the function of the ion channel. Indeed, the maximal rate of the uniporter in isolated mitochondria is at the level of 1200 nmol/mg/min [37] to 1400 nmol/mg/min [38] with typical values of 100–300 nmol/mg/min. Thus, taking into account the number of 109 mitochondria in 1 mg of protein of a preparation [39], the maximal rate of integral current per whole mitochondrion through the uniporter mechanism is expected to be at the levels of 500 fA (or about 10–20 fA per single channel, assuming that this conductance is due to the MCU activity in a channel mode). This is several orders of magnitude smaller than what would be expected if this uptake was occurring through calcium selective channel, and in fact below the resolution of electrophysiological approach. It is possible that in the intact mitochondria channel functions in much lower conductance mode comparing to that observed in model system. However, this also raises the possibility that, at least under conditions of isolated mitochondria, calcium uptake might occur by a mechanism with kinetics consistent to that of an ion carrier. In the context of current work, we propose the possibility that slow conductance carrier mediated uptake may be provided by PHB. With respect to this it is important to note that the rate of calcium uptake by mitochondria is similar to the rate of calcium uptake induced by another carrier – ferutinin, an electrogenic natural calcium ionophore isolated from the plant of Ferula genus [16,17,24]. Indeed, one would expect that if this channel was involved in the uniporter function, this uptake rate would be considerably faster compared to ferutinin. We should also point out that in this scenario PHB would provide the conduit for calcium transport across the bilayer which can assume conformations favorable to this transport, but it is not clear whether it is particularly discriminative between different cations. Although selectivity of PHB mediated transport across lipid bilayers to calcium has never been compared to selectivity of other ions, in case of PHB induced ion transport across the hydrophobic chloroform phase (U-tube) experiment it has been shown that selectivity follows Hofmeister series, which favors transport of ions with smallest surface-charge density [2]. In case of biological environment it is very likely that the presence of multiple protein components is still required to account for uniporter properties including calcium selectivity and sensitivity to the ruthenium red. It is also conceivable that mitochondrial calcium uniporter uptake can function in a set of different kinetic modes, some involving different components of the uniporter complex. The possibility of a multitude of kinetic modes as well as a number of other molecular compounds which might be critically involved in the uniporter function is described in a recent review [40] and is also evidenced by recent patch-clamp study [41].
mPhaZ7 overexpression dramatically decreased mitochondrial membrane potential only in HepG2 cells (Fig. 2) but not in HeLa or U87 cells, suggesting a tissue specificity of the roles played by the polymer. This effect on mitochondrial membrane potential and presumably bioenergetics can be explained by the fact that in certain cell types PHB might be an active participant in energy metabolism in a way similar to bacteria. It should be noted that acetoacetate and hydroxyl butyrate, also known as ketone bodies, are important energy metabolites in mitochondria and are known intermediate products of the PHB metabolism cycle in simple organisms [21]. It is possible that levels of PHB might be directly linked to the levels of either acetoacetate or ketone bodies. This can provide a possible explanation to the changes in mitochondrial membrane potential seen in mPhaZ7 expressing mitochondria. These changes are the reflection of the changed energetic status of these mitochondria due to the change in the balance of energy metabolites. In this context it is worth mentioning that HepG2 cells are derived from the liver cell line known to have active ketone bodies metabolism. However, mPhaZ7 activity inhibits mitochondrial calcium uptake in all cells studied, indicating its direct effect on membrane transport. It is likely that the disruption of normal mitochondrial calcium homeostasis in these cells is the key reason for the cell death seen in our experiments (Fig. 6).
In conclusion, our data indicate that PHB is involved in mitochondrial calcium uptake. The ability of PHB to mediate this transport is likely linked to either the ability of PHB to function as a calcium ionophore or as regulator of the protein ion channels.
Acknowledgements
We thank Dr. Jendrossek (University of Stuttgart) for providing bacterial PhaZ7 DNA. Authors declare no conflict of interests. This work was supported by the Heart and Stroke Foundation of Nova Scotia and Natural Sciences and Engineering Research Council of Canada (to E.P.). We also thank Anna Galeta for help proofing and correcting this manuscript.
References
- 1.Reusch RN. Poly-beta-hydroxybutyrate/calcium polyphosphate complexes in eukaryotic membranes. Proceedings of the Society for Experimental Biology and Medicine. 1989;191:377–381. doi: 10.3181/00379727-191-42936. [DOI] [PubMed] [Google Scholar]
- 2.Seebach D, Fritz MG. Detection, synthesis, structure and function of oligo(3-hydroxyalkanoates): contributions by synthetic organic chemists. International Journal of Biological Macromolecules. 1999;25:217–236. doi: 10.1016/s0141-8130(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 3.Seebach D, Brunner A, Burger HM, Schneider J, Reusch RN. Isolation and 1H NMR spectroscopic identification of poly(3-hydroxybutanoate) from prokaryotic and eukaryotic organisms. Determination of the absolute configuration (R) of the monomeric unit 3-hydroxybutanoic acid from Escherichia coli and spinach. European Journal of Biochemistry. 1994;224:317–328. doi: 10.1111/j.1432-1033.1994.00317.x. [DOI] [PubMed] [Google Scholar]
- 4.Reusch RN, Sadoff HL. Putative structure and functions of a poly-beta-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4176–4180. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakharian E, Reusch RN. Functional evidence for a supramolecular structure for the Streptomyces lividans potassium channel KcsA. Biochemical and Biophysical Research Communications. 2004;322:1059–1065. doi: 10.1016/j.bbrc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Zakharian E, Reusch RN. Haemophilus influenzae outer membrane protein P5 is associated with inorganic polyphosphate and polyhydroxybutyrate. Biophysical Journal. 2007;92:588–593. doi: 10.1529/biophysj.106.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakharian E, Thyagarajan B, French RJ, Pavlov E, Rohacs T. Inorganic polyphosphate modulates TRPM8 channels. PLoS ONE. 2009;4:e5404. doi: 10.1371/journal.pone.0005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlov E, Zakharian E, Bladen C, et al. A large voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophysical Journal. 2005;88 doi: 10.1529/biophysj.104.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinnally KW, Lohret TA, Campo ML, Mannella CA. Perspectives on the mitochondrial multiple conductance channel. Journal of Bioenergetics and Biomembranes. 1996;28:115–123. doi: 10.1007/BF02110641. [DOI] [PubMed] [Google Scholar]
- 10.Szabo I, Zoratti M. The mitochondrial megachannel is the permeability transition pore. Journal of Bioenergetics and Biomembranes. 1992;24:111–117. doi: 10.1007/BF00769537. [DOI] [PubMed] [Google Scholar]
- 11.Seidlmayer LK, Blatter LA, Pavlov E, Dedkova EN. Inorganic polyphosphate - an unusual suspect of the mitochondrial permeability transition mystery. Channels (Austin) 2012;6 doi: 10.4161/chan.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidlmayer LK, Gomez-Garcia MR, Blatter LA, Pavlov E, Dedkova EN. Inorganic polyphosphate is a potent activator of the mitochondrial permeability transition pore in cardiac myocytes. Journal of General Physiology. 2012;139:321–331. doi: 10.1085/jgp.201210788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramov AY, Fraley C, Diao CT, et al. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18091–18096. doi: 10.1073/pnas.0708959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das S, Kurcok P, Jedlinski Z, Reusch RN. Ion channels formed by biomimetic oligo-(R)-3-hydroxybutyrates and inorganic polyphosphates in planar lipid bilayers. Macromolecules. 1999;32:8781–8785. [Google Scholar]
- 15.Fritz MG, Walde P, Seebach D. Oligoesters of (R)-3-hydroxybutanoic acid: transmembrane transport of Ca2+ across vesicle bilayers. Macromolecules. 1999;32:574–580. [Google Scholar]
- 16.Zamaraeva MV, Hagelgans AI, Abramov AY, et al. Ionophoretic properties of ferutinin. Cell Calcium. 1997;22:235–241. doi: 10.1016/s0143-4160(97)90062-2. [DOI] [PubMed] [Google Scholar]
- 17.Abramov AY, Zamaraeva MV, Hagelgans AI, Azimov RR, Krasilnikov OV. Influence of plant terpenoids on the permeability of mitochondria and lipid bilayers. Biochimica et Biophysica Acta. 2001;1512:98–110. doi: 10.1016/s0005-2736(01)00307-8. [DOI] [PubMed] [Google Scholar]
- 18.Braaz R, Handrick R, Jendrossek D. Identification and characterisation of the catalytic triad of the alkaliphilic thermotolerant PHA depolymerase PhaZ7 of Paucimonas lemoignei . FEMS Microbiology Letters. 2003;224:107–112. doi: 10.1016/S0378-1097(03)00425-7. [DOI] [PubMed] [Google Scholar]
- 19.Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- 20.Papageorgiou AC, Hermawan S, Singh CB, Jendrossek D. Structural basis of poly(3-hydroxybutyrate) hydrolysis by PhaZ7 depolymerase from Paucimonas lemoignei . Journal of Molecular Biology. 2008;382:1184–1194. doi: 10.1016/j.jmb.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 21.Trainer MA, Charles TC. The role of PHB metabolism in the symbiosis of rhizobia with legumes. Applied Microbiology and Biotechnology. 2006;71:377–386. doi: 10.1007/s00253-006-0354-1. [DOI] [PubMed] [Google Scholar]
- 22.McCormack JG. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochemical Journal. 1985;231:581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlov E, Aschar-Sobbi R, Campanella M, Turner RJ, Gomez-Garcia MR, Abramov AY. Inorganic polyphosphate and energy metabolism in mammalian cells. Journal of Biological Chemistry. 2010;285(13):9420–9428. doi: 10.1074/jbc.M109.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abramov AY, Duchen MR, Actions of ionomycin. 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium. 2003;33:101–112. doi: 10.1016/s0143-4160(02)00203-8. [DOI] [PubMed] [Google Scholar]
- 25.Jendrossek D. Fluorescence microscopical investigation of poly(3-hydroxybutyrate) granule formation in bacteria. Biomacromolecules. 2005;6:598–603. doi: 10.1021/bm049441r. [DOI] [PubMed] [Google Scholar]
- 26.James BW, Mauchline WS, Dennis PJ, Keevil CW, Wait R. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Applied and Environment Microbiology. 1999;65:822–827. doi: 10.1128/aem.65.2.822-827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kacmar J, Carlson R, Balogh SJ, Srienc F. Staining and quantification of poly-3-hydroxybutyrate in Saccharomyces cerevisiae and Cupriavidus necator cell populations using automated flow cytometry. Cytometry A. 2006;69:27–35. doi: 10.1002/cyto.a.20197. [DOI] [PubMed] [Google Scholar]
- 28.Elustondo P, Zakharian E, Pavlov E. Identification of the polyhydroxybutyrate granules in mammalian cultured cells. Chemical and Biodiversity. 2012;9:2597–2604. doi: 10.1002/cbdv.201200294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abramov AY, Ionov M, Pavlov E, Duchen MR. Membrane cholesterol content plays a key role in the neurotoxicity of beta-amyloid: implications for Alzheimer’s disease. Aging Cell. 2011;10:595–603. doi: 10.1111/j.1474-9726.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 30.Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. Cholesterol and ion channels. Sub-Cellular Biochemistry. 2010;51:509–549. doi: 10.1007/978-90-481-8622-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das S, Lengweiler UD, Seebach D, Reusch RN. Proof for a nonproteinaceous calcium-selective channel in Escherichia coli by total synthesis from (R)-3-hydroxybutanoic acid and inorganic polyphosphate. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9075–9079. doi: 10.1073/pnas.94.17.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360):341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De SD, Raffaello A, Teardo E, Szabo I, Rizzuto R. A 40-kDa protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perocchi F, Gohil VM, Girgis HS, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saris NE, Sirota TV, Virtanen I, et al. Inhibition of the mitochondrial calcium uniporter by antibodies against a 40-kDa glycoprotein T. Journal of Bioenergetics and Biomembranes. 1993;25:307–312. doi: 10.1007/BF00762591. [DOI] [PubMed] [Google Scholar]
- 36.Mironova GD, Baumann M, Kolomytkin O, et al. Purification of the channel component of the mitochondrial calcium uniporter and its reconstitution into planar lipid bilayers. Journal of Bioenergetics and Biomembranes. 1994;26:231–238. doi: 10.1007/BF00763072. [DOI] [PubMed] [Google Scholar]
- 37.Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochimica et Biophysica Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiological Reviews. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 39.Schwerzmann K, Cruz-Orive LM, Eggman R, Sanger A, Weibel ER. Molecular architecture of the inner membrane of mitochondria from rat liver: a combined biochemical and stereological study. Journal of Cell Biology. 1986;102:97–103. doi: 10.1083/jcb.102.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchi J, Pan S, Sheu SS. Perspectives on: SGP symposium on mitochondrial physiology and medicine: molecular identities of mitochondrial Ca2+ influx mechanism: updated passwords for accessing mitochondrial Ca2+-linked health and disease. Journal of General Physiology. 2012;139:435–443. doi: 10.1085/jgp.201210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bondarenko AI, Jean-Quartier C, Malli R, Graier WF. Characterization of distinct single-channel properties of Ca(2+) inward currents in mitochondria. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]