Abstract
Bacterial infections caused by Shigella flexneri, Salmonella typhimurium and Burkholderia pseudomallei are currently difficult to prevent due to the lack of a licensed vaccine. Here we present formulation and immunogenicity studies for the three type III secretion system (TTSS) needle proteins MxiHΔ5, PrgIΔ5 and BsaLΔ5 (each truncated by five residues at its C terminus) as potential candidates for vaccine development. These antigens are found to be thermally stabilized by the presence of carbohydrates and polyols. Additionally, all adsorb readily to aluminum hydroxide apparently through a combination of hydrogen bonds and/or Van der Waals forces. The interaction of these proteins with the aluminum-based adjuvant changes with time to resulting in varying degrees of irreversible binding. Peptide maps of desorbed protein, however, suggest that chemical changes are not responsible for this irreversible association. The ability of MxiHΔ5 and PrgIΔ5 to elicit strong humoral immune responses was tested in a murine model. When administered intramuscularly as monomers, the needle components exhibited dose dependent immunogenic behavior. The polymerized version of MxiH was exceptionally immunogenic even at low doses. The responses of both monomeric and polymerized forms were boosted by adsorption to an aluminum salt adjuvant.
Keywords: Type III secretion, Shigella flexneri, Salmonella typhimurium, Burkholderia pseudomallei, fluorescence spectroscopy, circular dichroism spectroscopy, absorbance spectroscopy, Empirical Phase Diagram, Immunogenicity
INTRODUCTION
Shigella flexneri, Salmonella typhimurium and Burkholderia pseudomallei are three pathogens responsible for an extensive number of potentially preventable diseases. For example, Shigella is one of the leading causes of infant mortality in developing countries, and is responsible for the infection of more than 165 million people each year worldwide.1 Salmonella is best known for its high-profile outbreaks in the developed world and is associated worldwide with more than 200 million cases every year.2–4 Burkholderia is endemic to tropical regions, and is listed as a Category B Bioterrorism Agent by the CDC due to the severity of acute disease , the potential for aerosol delivery and worldwide availability.5 In all of these cases, there are no licensed vaccines for prevention of infection by these pathogens, which are also becoming increasingly resistance to antibiotic treatment.6–9 The need for vaccine development in this area is widely recognized and the work presented here targets this effort.
S. flexneri, S. typhimurium and B. pseudomallei each relies upon a type three secretion system (TTSS) as an essential virulence component. The type III secretion apparatus (TTSA) is appropriately referred to as a molecular ‘injectisome’ and is composed of more than 20 proteins which assemble to form basal and extracellular components.10 This macromolecular conduit allows the bacteria direct physical contact with a host cell and is responsible for the transport of effector proteins from the bacterial cytoplasm directly into target cells where they subvert normal cellular functions.11–13 The surface exposed ‘needle’ portion of the structure is composed of a defined number of monomeric subunits polymerized into a hollow helical array with an inner pore diameter of approximately 1.5 to 3.0 nm.14–17 This appendage is required for virulence and is intimately involved in the initial stages of an infection10,18. Therefore we have explored the use of these surface exposed proteins as potential vaccine antigens.
The vaccine antigens studied here are the monomeric subunits of the oligomerized needle appendage from each bacterial system. These small (~10 kDa), acidic (pI <5) proteins are characterized by distinct patches of both positive and negative surface charge, which probably contribute to their intrinsic polymerization properties.19 When some of these proteins are expressed recombinantly, their propensity to oligomerize results in a viscous solution of highly associated products; however, it is possible to prepare them as soluble monomers when five residues are deleted from the C terminus.20 The resulting recombinant proteins, MxiHΔ5, PrgIΔ5 and BsaLΔ5, from the gram-negative bacteria S. flexneri, S. typhimurium and B. pseudomallei, respectively, have been shown to retain native structure.20
Previously, we characterized the solution stability of these mutant monomeric needle antigens, and found them to be pH sensitive, thermally-labile proteins with reversible transitions and molten globule-like behavior in the physiological temperature range.21 Here we describe formulation studies of these vaccine candidates accompanied by examination of the immunogenicity of MxiH Δ5 and PrgI Δ5 in a murine model. BsaL Δ5 was not studied due to material limitations. We have also formulated a recombinant polymeric needle construct of MxiH which mimics the actual polymerized TTSS needle structure. In many cases, antigens with repeating epitopes display greater immunogenicity than those possessing only a single recognition site. Increased antigen size (as occurs upon polymerization) has also been shown to likewise increase immunogenicity.22 It should be emphasized that the intention of this work is not to propose a final clinical formulation, but rather to examine aspects of potential future formulations which may be useful for further development of these and related vaccines based on the needle proteins.
MATERIALS AND METHODS
Materials
C-terminal truncated proteins were expressed in E. coli with a C-terminal His6 tag as described previously.17,20 Affinity purification was performed using nickel chelation chemistry, and the samples were stored in 20mM isotonic pH 6 citrate phosphate buffer and stored at −80 °C until use. For immunogenicity studies, MxiH was also expressed in its full length form, resulting in largely polymerized material referred to as ‘Needle’ hereafter (data not shown). Alhydrogel® (2%) and Adjuphos® were acquired from E.M. Sergeant Pulp and Co., Inc. (Clifton, NJ).
Screening for Excipients
Potential excipients were screened for stabilizing effects using far-UV circular dichroism spectroscopy. Secondary structure was used as the stability indicating parameter in this case because of the lack of other useable temperature sensitive signals (i.e., Trp fluorescence, static light scattering, etc.) exhibited by these proteins.21 Thermal melts were conducted using a Jasco J-720 spectropolarimeter equipped with a six position sample holder and a Peltier temperature control device (Easton, MD). Initially, individual far-UV CD spectra were recorded for each of 6 samples at 10 °C from 260 to 190 nm in a 0.1 cm path length cell. The cell holder temperature was then increased from 10 to 85 °C in increments of 0.5 °C. At each temperature step the cell holder was incubated for 5 min to ensure thermal equilibration of the samples before a spectrum was obtained. For each thermal melt, a control protein sample containing 0.25 mg/mL protein in 20mM isotonic citrate phosphate buffer at pH 6.0 was run simultaneously with 5 samples containing test compounds at the indicated concentrations. Spectra of the compounds alone were also collected and subtracted from the protein spectra when necessary. Using the instrument software, the resulting data were converted to molar ellipticity as a function of temperature. Midpoints of thermal transitions (Tms) were determined using Microcal Origin® sigmoidal fit graphing tools.
Adsorption Isotherms
Protein was dialyzed into isotonic 10 mM histidine buffer at pH 6 overnight at 4 °C using 3,500 MWCO Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific, Rockford, IL). Upon completion, the protein concentration was determined by UV absorbance spectroscopy using an Agilent 8453 UV/Vis spectrophotometer equipped with a diode array detector (Agilent Technologies, Santa Clara, CA). Extinction coefficients of 9,970, 11,460, and 12,950 M−1 cm−1 at 280 nm were used for MxiH Δ5, PrgI Δ5 and BsaL Δ5, respectively.21 Protein was then adsorbed to Alhydrogel by combining a pre-determined volume of Alhydrogel stock, protein stock and histidine buffer to produce a range of protein concentrations (0–1.25 mg/mL) and a constant aluminum concentration (0.5 mg/mL), and allowing it to rotate end-over-end at 4 °C for 1 hour. Samples were then centrifuged at 14,000× g for 30 sec, and the resulting supernatant was removed and assayed for protein content by UV absorbance spectroscopy. The amount of protein adsorbed was determined by subtracting the amount found in the supernatant from the original amount added.
Adsorption Mechanism
‘Elution’ solutions (see Table 1) were prepared in 20mM isotonic pH 6 histidine buffer using ACS grade reagents and were not pH adjusted. The adsorption protocol described above was used to prepare several 1.5 mL samples containing 0.2 mg/mL protein per 0.5 mg/mL aluminum, a ratio at which > 95% of the protein was found to be adsorbed. The samples were then centrifuged for 2 min at 3,000× g to pellet the aluminum-protein complex, and the resulting supernatant was removed. A control sample was prepared by adding 1 mL of isotonic 10 mM histidine buffer at pH 6 to an aluminum-protein pellet. In the same way, 1 mL of an ‘elution’ solution was added to each tube containing a centrifuged aluminum-protein pellet (The pellets were redispersed in the elution solution by flicking the sides of the tubes and centrifuging for 5 sec). All vials were then rotated end-over-end at 4 °C for 48 hours. The samples were then once again centrifuged, and the supernatant of each evaluated for protein content.
Table 1.
Protein (mg/mL) present in sample supernatant as assayed by UV absorbance spectroscopy following ‘elution’ treatment. All samples originally contained 0.2 mg/mL protein in the presence of 0.5 mg/mL Aluminum. Where a dash is present no sample was analyzed. Error was estimated at approximately 0.05 mg/mL.
| MxiHΔ5 | Freshly Prepared | 2 wks, 4 °C | 2 wks, 40 °C |
|---|---|---|---|
| Prior to Elution | 0.001 | 0.001 | 0.000 |
| 10 mM Histidine | 0.003 | 0.002 | 0.014 |
| 0.5 M NaCl | 0.001 | - | - |
| 0.75 M NaCl | 0.002 | - | - |
| 1.0 M NaCl | 0.002 | - | - |
| 2.0 NaCl | 0.005 | - | - |
| 3.0 M NaCl | 0.001 | 0.000 | - |
| 1.0 M Gdn HCl | 0.003 | 0.003 | 0.001 |
| 1.0 M NaCit | 0.143 | 0.091 | 0.039 |
| 1.0 M NaPhos | 0.201 | 0.160 | 0.078 |
| 70 mM SDS | 0.088 | - | - |
| PrgIΔ5 | |||
| Prior to Elution | 0.001 | 0.002 | 0.002 |
| 10 mM Histidine | 0.003 | 0.003 | 0.000 |
| 0.5 M NaCl | 0.001 | - | - |
| 0.75 M NaCl | 0.002 | - | - |
| 1.0 M NaCl | 0.004 | - | - |
| 2.0 NaCl | 0.003 | - | - |
| 3.0 M NaCl | 0.000 | 0.006 | - |
| 1.0 M Gdn HCl | 0.005 | 0.006 | 0.004 |
| 1.0 M NaCit | 0.137 | 0.081 | 0.036 |
| 1.0 M NaPhos | 0.197 | 0.151 | 0.092 |
| 70 mM SDS | 0.056 | - | - |
| BsaLΔ5 | |||
| Prior to Elution | 0.001 | 0.002 | 0.002 |
| 10 mM Histidine | 0.004 | 0.003 | 0.003 |
| 0.5 M NaCl | 0.000 | - | - |
| 0.75 M NaCl | 0.001 | - | - |
| 1.0 M NaCl | 0.006 | - | - |
| 2.0 NaCl | 0.003 | - | - |
| 3.0 M NaCl | 0.000 | 0.000 | - |
| 1.0 M Gdn HCl | 0.002 | 0.005 | 0.002 |
| 1.0 M NaCit | 0.171 | 0.105 | 0.036 |
| 1.0 M NaPhos | 0.196 | 0.154 | 0.082 |
| 70 mM SDS | 0.100 | - | - |
Capillary liquid chromatography-mass spectrometry analysis and protein mapping
A 0.1 mg/mL trypsin solution was prepared by dissolving 5.2 mg of trypsin in 52 mL of 0.05 M ammonium bicarbonate buffer at pH 6.6. Protein was exposed to the trypsin solution at a 1:200 concentration ratio, and incubated at 37 °C for > 1 hour to permit adequate digestion. Samples were introduced into an LTQ-FT Ultra Hybrid Mass Spectrometer (ThermoFinnigan) via capillary liquid chromatography as described.23 A data-dependant acquisition method was used for setting up the experiments. The five most intensive precursor ions in a survey MS1 mass spectrum acquired in the Fourier transform-ion cyclotron resonance over a mass range of 300–2000 m/z were selected and sequentially fragmented for MS2 analysis in the linear ion trap by collision-induced dissociation. Experimental raw files were processed by TurboSequest batch search using BioWorks 3.2 software. All text files generated by BioWorks 3.2 for every MS2 fragmentation spectrum were combined within each experiment using an in-house written Perl script. The resulted files were submitted for peptide/protein identification to the Mascot (v2.2, Matrix Science) database-searching program. Parameters set during the searches were as follows: custom protein database, a peptide mass tolerance of 2.2 u to account for higher isotopes of large peptides and MS/MS tolerance of 0.6 Da, semi-trypsin specificity with up to two missed cleavages. Variable modification was set to consider oxidation on methionine residues. Sequest and Mascot results were imported into Scaffold 2.2.03 software (Proteome Software Inc.) for analyzing with the X!Tandem search algorithm and statistical validation of peptide/protein identities. Peptides validated by Scaffold at a confidence level of 50% and greater were taken to calculate protein coverage. Identification of peptides at a confidence level of less than 50% was considered positive if their corresponding m/z value from the survey MS1 scans were found to be within 10 ppm mass tolerance.
Animals and Immunization
Animal studies were performed in accordance with regulations set forth by the Institutional Animal Care and Use Committee and guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. For all experiments, BALB/c mice were acquired from the Charles River Breeding Laboratory (Wilmington, MA) and quarantined for 7 days prior to study start. Inoculations (100 µL) were administered intramuscularly on study days 1, 14 and 28 and mice were bled via the submandibular vein on days 0, 7, 21, 35 and 49. Weights were recorded at each time-point and monitored for indications of adverse effects due to dosing, although none were observed. Mice were euthanized following the final bleed on day 49.
In the first study, each group contained 10 mice (5 female and 5 male) with the exception of the histidine buffer control group which contained only 7 mice. No significant differences were observed in end-point titers between male and female mice. Formulations of MxiH Δ5 were dosed at 1 (M1), 10 (M10) and 50 (M50) µg/dose. The two control groups were dosed with either formulation buffer or 0.5 mg/mL aluminum in formulation buffer.
Study 2 contained groups of 7 mice (female), and a single control group containing 5 mice (0.5 mg/mL aluminum in 10mM isotonic His buffer). MxiH Δ5 and MxiH Needle were dosed separately at 10 µg, both in the formulation buffer as well as adsorbed to Alhydrogel (M-Soln, M-His, N-Soln, and N-His, respectively). The third study, conducted concurrently with Study 2, involved groups of 8 mice (female), and a control group of 5 mice (0.5 mg/mL aluminum in 10mM isotonic His buffer). Formulations of PrgI Δ5 were administered at 1, 10 and 50 µg/dose (P1, P10, and P50). Non-adsorbed formulations (i.e., formulations which did not contain adjuvant) were also administered at 50 µg for PrgI Δ5 (P50-Soln).
ELISA / IgG Detection in Serum Samples
A standard Enzyme Linked Immunosorbent Assay (ELISA) was used to evaluate the stability of the adsorbed PrgI Δ5 vaccine. Only anti-PrgI Δ5 antibodies (polyclonal) were available and therefore only the PrgI formulation stability was studied in this manner. In addition, the antigen specific IgG titers for individual sera samples of MxiH Δ5, MxiH needle, and PrgI Δ5 was determined using this assay. All solutions and incubated adsorbed samples were plated (100 µL) onto Nunc-Immuno™ MediSorp™ (Nalge Nunc International, Wiesbaden, Germany) 96-well plates at 10 µg/mL in carbonate buffer (pH 9.6) overnight at 4 °C. Plates were blocked with Superblock (ThermoScientific, Rockford, IL) per product instructions. A dilution of Anti-PrgIΔ5 IgG (1:10,000) and Sera dilutions (100 µL) were prepared in Superblock and allowed to incubate on the plate for 2 hours at RT. For stability studies, the plates were washed 4 times using a 0.85% sodium chloride solution while for immunogenicity analysis, the plates were washed three times using PBS containing 0.05% Tween 20, and finally with PBS buffer alone. HRP-conjugated goat anti-Mouse monoclonal antibody (Sigma, St. Louis, MO) was diluted 1:500 in blocking buffer, plated (100 µL) and incubated for 1 hour at RT. The plates were then washed as above, and 3,3’5,5’-TetraMethylBenzidine (TMB) substrate (100 µL) (Sigma, St. Louis, MO) was added. After 30 minutes at RT, 2 M HCl (100 µL) was added to quench the reaction and plates were read using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) at 450 nm.
Statistical Analysis
The geometric mean was used to represent group responses, and error was determined as the standard deviation. Statistical significance was determined with Student’s t-test using Microsoft Excel. Significance was determined as p ≤ 0.05.
RESULTS
Screening for Excipients
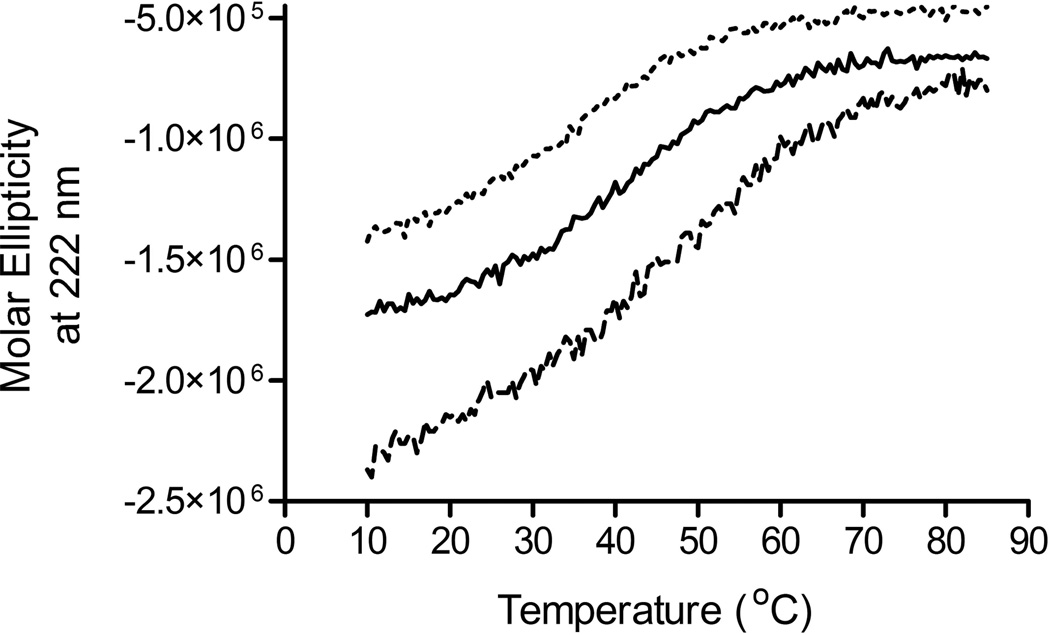
A panel of Generally Regarded as Safe (GRAS) compounds was screened for potential stabilizing effects on the secondary structure of all three recombinant proteins using temperature dependent circular dichroism spectroscopy. As shown in Figure 1, MxiH Δ5 and PrgI Δ5 displayed broad yet distinctly sigmoidal transitions with midpoints of thermal melting (Tm) of 40.6 +/− 0.2 and 34.8 +/− 0.5 °C, respectively. For the purposes of excipient selection, we used changes in Tm as a quantitative measure of potential excipient effects. The melting curve for BsaL Δ5 did not display sigmoidal behavior and as a result no thermal midpoint could be assigned. This difference observed in the melting curves may be attributed to the absence of a critical salt bridge in BsaL Δ5 which is present in both MxiH Δ5 and PrgI Δ5.19
Figure 1.
Circular dichroism thermal melting curves for each MxiH Δ5 ( ), PrgI Δ5 (
), PrgI Δ5 ( ) and BsaL Δ5 (
) and BsaL Δ5 ( ) at 0.2 mg/mL in isotonic 10 mM Histidine buffer at pH 6.0.
) at 0.2 mg/mL in isotonic 10 mM Histidine buffer at pH 6.0.
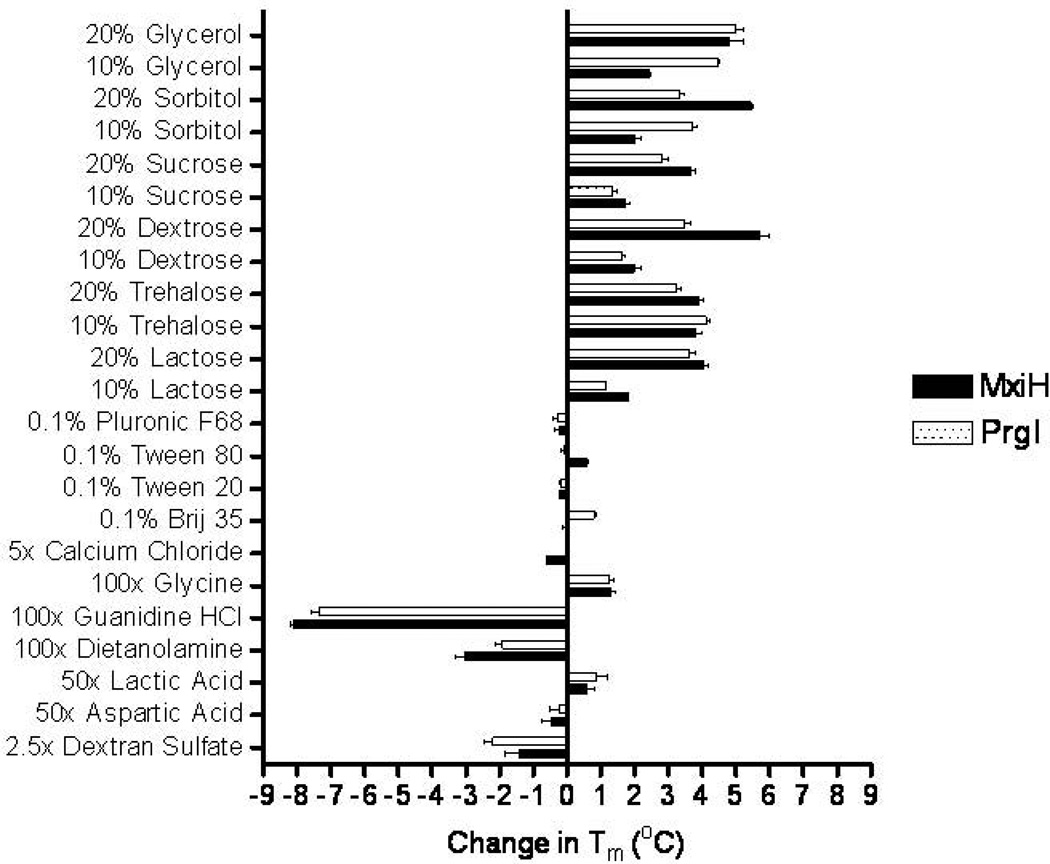
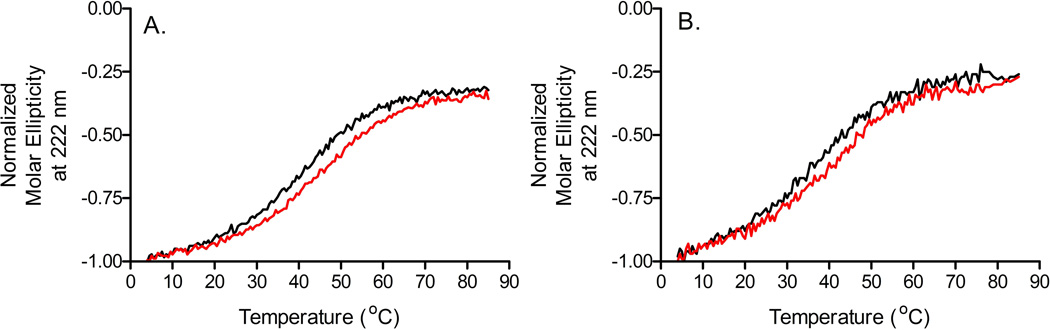
For both MxiH Δ5 and PrgI Δ5, stabilization, as represented by an increase in Tm, was observed in the presence of polyols and carbohydrates including glycerol, sorbitol, dextrose, trehalose, and sucrose. As shown in Figure 2, greater stabilization was typically a function of increasing excipient concentration. Although it was not possible to confirm with Tm values, visual comparison of the BsaL Δ5 melting curves demonstrated similar stabilization trends (data not shown). Combinations of excipients were also screened using circular dichroism spectroscopy where the proteins demonstrated increased thermal stability in the presence of the chosen excipient combinations (data not shown). Shown in Figure 3 are the thermal melting curves for both MxiH Δ5 and PrgI Δ5 in the presence and absence of 10% sucrose and 5% dextrose, an excipient combination which was identified as optimal for all three proteins. In both cases, approximately 4 °C of additional thermal stabilization was gained in the presence of 10% sucrose and 5% dextrose (MxiH Δ5, 40.4 to 44.4 °C and PrgI Δ5, 35.7 to 39.3 °C)
Figure 2.
Changes in Tm (°C) as measured by circular dichroism spectroscopy for MxiH Δ5 ( ) and PrgI Δ5 (
) and PrgI Δ5 ( ).
).
Figure 3.
Circular dichroism thermal melting curves for (A) MxiH Δ5 and (B) PrgI Δ5 at 222 nm in the presence ( ) and absence (-) of 10% Sucrose and 5% Dextrose.
) and absence (-) of 10% Sucrose and 5% Dextrose.
Adsorption Isotherms
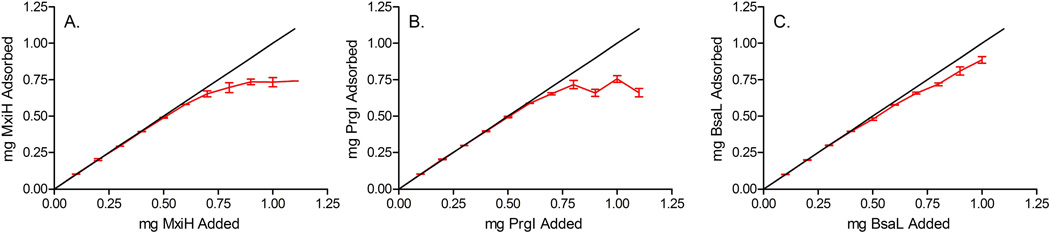
The three proteins studied in this work are acidic, with iso-electric points in the region of 4.5–5. The two most commonly employed aluminum salt adjuvants are aluminum hydroxide which is positively charged at neutral pH and has a point of zero charge (PZC) of 11, and aluminum phosphate which is negatively charged at neutral pH and has a PZC of approximately 5–7.24 As one would expect, minimal adsorption of the needle proteins occurs in the presence of aluminum phosphate (data not shown), while substantial interaction is observed with aluminum hydroxide. This phenomenon is presumably due to the electrostatic repulsive forces present between the aluminum phosphate and the protein at neutral pH, and the electrostatic attractive forces present between the aluminum hydroxide and the proteins. As shown in Figure 4, the three proteins display similar adsorption isotherms with high levels of protein loading. When the data were plotted according to the Langmuir equation,25 straight lines with R2 values in the range of 0.984 to 0.999 were obtained. The adsorptive capacities were calculated to be 1.56, 1.72 and 1.62 mg/mg aluminum for PrgI Δ5, BsaL Δ5 and MxiH Δ5, respectively. The adsorption coefficients for 0.85 mg/mL aluminum were also calculated to be 119, 116 and 96 mL/mg for PrgI Δ5, BsaL Δ5 and MxiH Δ5, respectively.
Figure 4.
Adsorption isotherms for MxiH Δ5 (A), PrgI Δ5 (B) and BsaL Δ5 (C) with 0.5 mg/mL aluminum in isotonic 10 mM histidine buffer at pH 6. Solid lines represent 100% adsorption.
Adsorption Mechanism
To investigate the forces responsible for adsorption of the needle proteins to aluminum hydroxide, a series of ‘elution’ solutions (Table 1) were prepared and the adsorbed samples were treated with each. The elution solutions were expected to primarily inhibit a specific type of protein-adjuvant interaction. Adsorbed samples were prepared as described above and supernatants were assayed for protein content prior to treatment. In all cases, < 2% residual protein was observed indicating that approximately 98% of the protein was adsorbed prior to ‘elution’ treatment. As shown in Table 1, when freshly adsorbed samples (< 24 h incubation) were treated, nearly all of the protein was desorbed from the surface in the presence of 1.0 M guanidine hydrochloride, 1.0 M sodium citrate, 1.0 M monobasic sodium phosphate or 1.0 M dibasic sodium phosphate. Although the proteins have highly charged surfaces,19 they do not appear to interact with the adjuvant directly through electrostatic interactions since they are not eluted in the presence of up to 3.0 M NaCl. If the adjuvant-adsorbed formulations were aged prior to analysis, the results differed significantly. After 2 weeks of incubation at 4 °C, only the sodium phosphate and sodium citrate solutions were able to desorb the protein. In both cases, the quantity eluted represented only approximately 75% of the total protein. At an increased incubation temperature of 40 °C, the results were similar, but the quantity eluted was further reduced to less than half of the original amount. These observations suggest that interactions occurring initially upon adsorption change with time as the protein establishes new contacts with the surface. Given the lack of elution in the presence of high salt concentrations and the strongly hydrophilic nature of the proteins and the surface, we hypothesize that interaction with the surface at longer times probably involves Van der Waals forces or hydrogen bonding.
Stability of Vaccine Antigens Adsorbed to Adjuvants
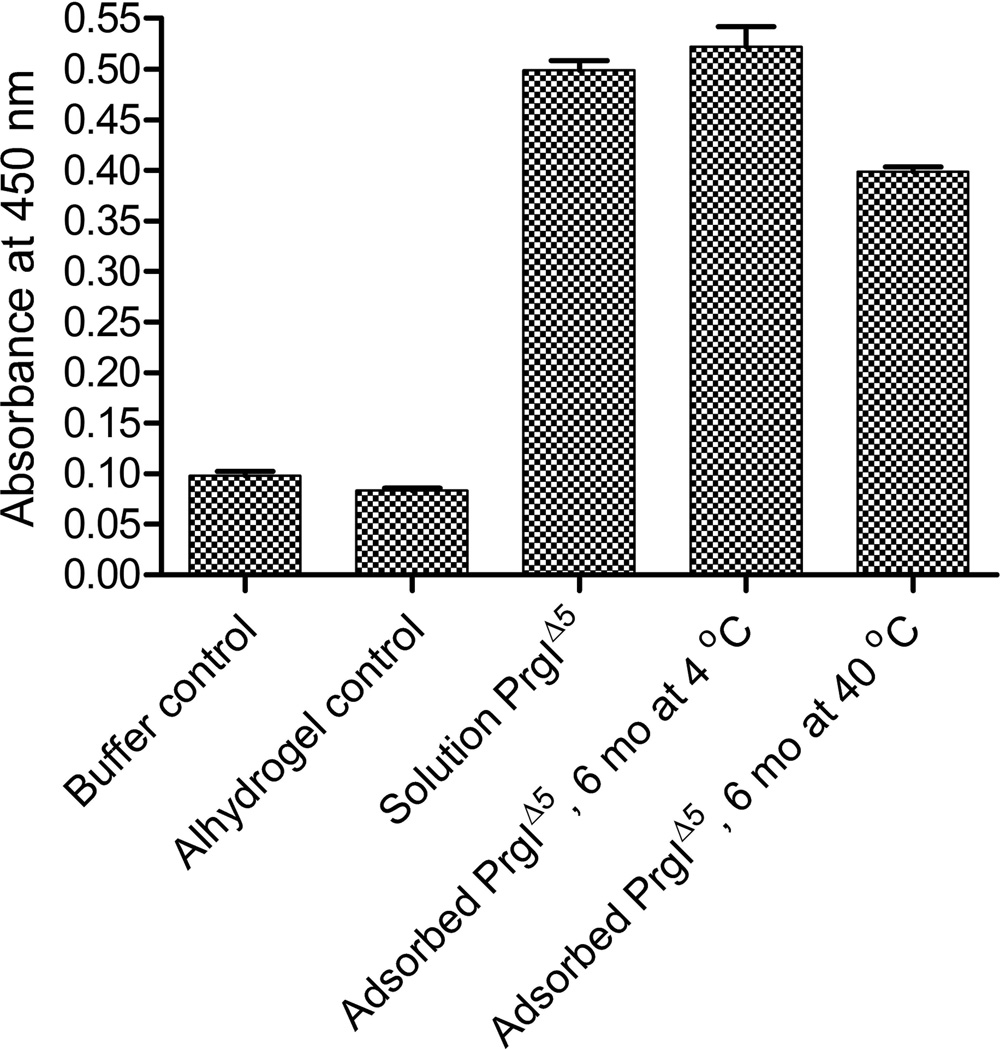
To examine the stability of the adsorbed product, we employed a standard ELISA method. We were particularly interested in evaluating the effect of decreased protein desorption over time in terms of changes in the protein as detected by interaction with a specific antibody. We therefore compared the antibody binding of solution protein to that of adsorbed protein that had either been stored at 4 or 40 °C for over 6 months. As shown in Figure 5, no significant difference (p<0.0001) in interaction with antibody was observed for PrgI Δ5 when present in solution or adsorbed on the adjuvant and stored for 6 months at 4 °C. A second sample which had been stored at 40 °C, however, did display a significantly lower response than both the solution material and the adsorbed material stored at 4 °C. This result suggests loss of at least one epitope upon long-term storage at elevated temperatures.
Figure 5.
Absorbance values observed at 450 nm when each sample was plated and analyzed by a standard ELISA protocol.
Capillary liquid chromatography-mass spectrometry analysis and protein mapping
To further examine the difference observed by ELISA between the adsorbed PrgI Δ5 formulations stored at 4 vs. 40 °C, we eluted protein from the adjuvant surface using 1.0 M sodium phosphate and obtained a peptide map for both samples. We also acquired a map of the soluble parent protein which had been frozen since production. Although the coverage was not optimal due to the presence of several small peptides, all residues commonly associated with chemical degradation reactions were present and no changes were observed in either of the adsorbed samples. This suggests that the decrease in antibody binding observed in the ELISA assay was due to a change in protein conformation as opposed to chemical modification of the amino acid sequence.
Generation of Humoral Responses
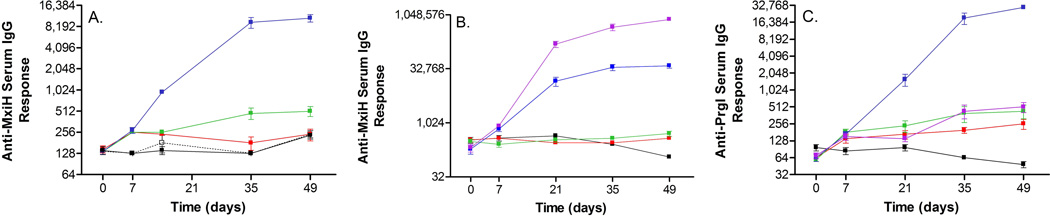
Mice were intramuscularly immunized three times at two week intervals (study days 1, 14 and 28), and blood samples were collected via the submandibular vein on days 0, 7, 21, 35, and 49. For all cases in which significant increases in IgG were observed, the mice had endpoint titers within two dilutions of the geometric mean. Figures displaying individual titers for each experiment can be found in the Supplemental Data. On day 0 of the first experiment, all mice had anti-MxiH IgG titers near 128 (Figure 6A). Mice in the control group maintained a similar value throughout the study. Animals dosed with 1 µg of MxiH Δ5 (M1) showed only a slight increase in antibody response throughout the study (p<0.02, day 49 compared to day 0), while those administered a 10 µg dose (M10) showed some response after the second boost compared to the control groups (p<0.0015). Mice who received the highest dose of 50 µg (M50) displayed an increase in serum IgG following the initial dose (p=0.0002) which continued to increase following the two booster injections. In all cases, the responses observed following the second boost were sustained for three weeks after the last injection.
Figure 6.
Anti MxiH (A and B) and PrgI (C) IgG serum responses following intramuscular injections on study days 1, 14, 28 of Buffer Control ( ), Alhydrogel Control (
), Alhydrogel Control ( ), M1/M-His/P1 (
), M1/M-His/P1 ( ), M10/M-Al/P10 (
), M10/M-Al/P10 ( ), M50/N-His/P50 (
), M50/N-His/P50 ( ). N-Al/P-Soln (
). N-Al/P-Soln ( ).
).
The second and third studies followed the same immunization and blood collection schedule as the initial experiment. In the second study, the formulation doses were 10 µg. Similar to the initial experiment, all mice had anti-MxiH IgG endpoint titers of approximately 256 on day 0, and the control group maintained similar values throughout the study (Figure 6B). Those animals who received a 10 µg dose of MxiH Δ5 in formulation buffer (M-Soln) did not show a significant response throughout the entire study. When the same dose was administered adsorbed to Alhydrogel (M-Al), again no response was observed. A significant anti-MxiH IgG response was observed following the initial injection and further increased following the first booster when mice were inoculated with the polymerized form of MxiH in solution (N-Soln.). The responses observed following the second booster increased only slightly (p<0.05). When the same dose was administered adsorbed to the adjuvant (N-Al), a similar trend was observed of increased magnitude.
The final study involved the needle protein PrgI Δ5 from S. typhimurium. At the start of the study, all mice displayed PrgI specific IgG endpoint titers of approximately 64 (Figure 6C). The control group displayed a similar value throughout the study. Mice given the 1 µg dose of PrgI Δ5 (P1) demonstrated an increase in titer over the course of the experiment (p<0.005, day 49 compared to day 0). The mice inoculated with 10 µg (P10) displayed an increase following the first dose, and levels rose somewhat during the remainder of the experiment. The mice in the highest Alhydrogel adsorbed dose group (P50), had increased titers following each injection, while in the absence of Alhydrogel (P50-Soln), the response was comparable to that of P1 and P10.
DISCUSSION
As described above, previous work has thoroughly defined the solution stability of each of the three proteins over a range of pH 3–8 and under thermal stress. We found that these mutant proteins, which are composed of helix-turn-helix structural elements, are sensitive to pH, are thermally labile and have reversible thermal transitions. They also display molten globule-like behavior in the physiological temperature range which may be critical to their transport through the growing needle structure given its limited diameter of 1.5–3.0 nm for the pathogens studied here. In this study, we have further characterized these proposed vaccine candidates for the purpose of formulation development. Excipient screening indicated that their thermal stability is increased by the presence of polyols and carbohydrates probably by the well described mechanism of preferential hydration.26 Binding isotherms for each antigen to aluminum hydroxide were also obtained. In all three cases, data were well described by the Langmuir equation with adsorptive capacities in the range of 1.56–1.72 mg/mg aluminum. Although the proteins may initially interact with aluminum hydroxide through electrostatic interactions, hydrogen bonds and Van der Waals (apolar) forces may be involved at longer incubation times. Additionally, a time-dependent change was observed in the desorption profile of adsorbed proteins. These changes in adsorption behavior were characterized over time using both ELISA and peptide mapping methods for MxiH. ELISA results indicated a difference in the antibody binding ability of the adsorbed protein stored for ~ 6 months at 40 °C while no change was observed at 4 °C. Peptide maps of the adsorbed protein indicate that relative to the protein primary structure prior to adsorption, no chemical changes were observed.
Upon examining the antigenic potential of the TTSS needle proteins, the MxiH monomer was found to be weakly immunogenic and dose dependent, requiring large doses of antigen, multiple boosts and the presence of an adjuvant to elicit a strong immune response. The polymerized form of the needle protein, expressed without the C-terminal truncation, is much more immunogenic than the monomeric version. There exists a well established correlation between antigen size, multivalency and immune response.22 Thus, it is possible that the increased immunogenicity observed here results from the increased size and epitope valency of the polymerized needle. Although these oligomers are perhaps ideal vaccine candidates, they need to be of defined, reproducible and stable size. Production of such uniform and homogeneous polymerized needles is currently under investigation. The current studies assume that excipients which stabilize the monomers will most probably be effective in stabilizing the oligomers as well. It is also possible that the five-residue C-terminal region which is absent in the monomeric form constitutes a potent epitope, though this is unlikely. As with the monomeric version, the response of the polymerized form of MxiH is boosted by adsorption to an aluminum salt adjuvant. Additionally, no adverse effects were observed throughout the immunization regimen as indicated by normal weight gain patterns (data not shown). In all studies where increases in specific antibody titers were observed, there were no non-responders suggesting that the formulations may be efficacious if IgG levels are predictive of protection. Antigens from the Salmonella system also appear to elicit substantial immune responses. PrgI displayed dose dependent behavior, and titers increased markedly when booster doses were administered.
While the studies presented here support further vaccine development utilizing TTSS needle proteins, further work is clearly necessary. Additional studies will focus on tailoring the formulation to elicit an appropriate response based on the pathogenesis of each species to ensure protection. The current immunogenicity studies support the development of TTSS proteins as antigens in multivalent subunit vaccines.
Concurrent with this work, a second protein integral to the TTSS has also been developed as a potential vaccine candidate. This protein, often referred to as the TTSS ‘tip’ protein, has been shown in Shigella to reside at the distal tip during the initial stages of infection.27 Other groups have shown this protein from the Shigella system, IpaD, to be highly immunogenic.28 Additionally, antibodies specific to this protein have been shown to neutralize host-cell infection by Shigella suggesting protective behavior.27 It is interesting to note that the excipients which provided the greatest degree of thermal stabilization for the needle proteins were similar to those which stabilize the tip proteins (unpublished data), and that both groups of proteins strongly adsorb to aluminum hydroxide. In the following paper, we describe the formulation and antigenicity of representative tip proteins and their combination with TTSS needle proteins as a next step to the creation of a general form of gram negative bacterial vaccine.
Supplementary Material
Supplemental Data.
Individual titers for Anti MxiH (Studies 1 & 2) and PrgI (Study 3) IgG serum responses following intramuscular injections on study days 1, 14, 28 of Buffer Control ( ), M1/M-His/P1 (
), M1/M-His/P1 ( ), M10/M-Al/P10 (
), M10/M-Al/P10 ( ), M50/N-His/P50 (
), M50/N-His/P50 ( ). N-Al/P-Soln (
). N-Al/P-Soln ( ), respectively. Blood samples were collected via the submandibular vein on days 0, 7, 21, 35, and 49.
), respectively. Blood samples were collected via the submandibular vein on days 0, 7, 21, 35, and 49.
ACKNOWLEDGEMENTS
The authors acknowledge funding from NIH Biotechnology Training Grant GM08359 to B.S.B. as well as support from Nadezhda Galeva in the KU Analytical Proteomics Lab. This work was supported by the Gates Foundation as part of the Grand Challenge Exploration Initiative.
Footnotes
Work was performed at The University of Kansas
REFERENCES
- 1.Shigella. 2009 [cited 2009 February 19]; Available from: http://www.who.int/vaccine_research/diseases/shigella/en/
- 2.World Health Organiation Drug-Resistant Salmonella. 2005 [cited 2009 February 19]; Available from: http://www.who.int/mediacentre/factsheets/fs139/en/print.html.
- 3.Salmonella Saintpaul Outbreak. 2008 [cited 2009 February 19]; Available from: http://www.fda.gov/oc/opacom/hottopics/tomatoes.html.
- 4.Peanut Product Recalls: Salmonella Typhimurium. 2008 [cited 2009 February 19]; Available from: http://www.fda.gov/oc/opacom/hottopics/Salmonellatyph.html.
- 5.National Center for Zoonotic V-B, and Enteric Diseases (ZVED). Melioidosis. 2008 March 27 2008 [cited 2009 February 20]; Available from: http://www.cdc.gov/nczved/dfbmd/disease_listing/melioidosis_gi.html.
- 6.Ashkenazi S, Levy I, Kazaronovski V, Samra Z. Growing antimicrobial resistance of Shigella isolates. J Antimicrob Chemother. 2003;51(2):427–429. doi: 10.1093/jac/dkg080. [DOI] [PubMed] [Google Scholar]
- 7.Avgeri SG, Matthaiou DK, Dimopoulos G, Grammatikos AP, Falagas ME. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int J Antimicrob Agents. 2009;33(5):394–404. doi: 10.1016/j.ijantimicag.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Ergönül Ö, Imre A, Çelikbas A, Dokuzoguz B. Drug resistance of Shigella species: changes over 20 years in Turkey. International Journal of Antimicrobial Agents. 2004;23(5):527–528. doi: 10.1016/j.ijantimicag.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Melloul AA, Hassani L. Antibiotic resistance of Salmonella strains isolated from children living in the wastewater-spreading field of Marrakesh city (Morocco) World. J Microbiol Biotechnol. 1999;15(1):81–85. [Google Scholar]
- 10.Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444(7119):567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 11.Blocker A, Jouihri N, Larquet E, Gounon P, Ebel F, Parsot C, et al. Structure and composition of the Shigella flexneri 'needle complex', a part of its type III secreton. Molec Microbiol. 2001;39(3):652–663. doi: 10.1046/j.1365-2958.2001.02200.x. [DOI] [PubMed] [Google Scholar]
- 12.Tamano K, Aizawa S, Katayama E, Nonaka T, Imajoh-Ohmi S, Kuwae A, Nagai, et al. Supramolecular structure of the Shigella type III secretion machinery: The needle part is changeable in length and essential for delivery of effectors. EMBO J. 2000;19(15):3876–3887. doi: 10.1093/emboj/19.15.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epler CR, Dickenson NE, Olive AJ, Picking WL, Picking WD. Liposomes recruit IpaC to the Shigella flexneri type III secretion apparatus needle as a final step in secretion induction. Infection and Immunity. 2009;77(7):2754–2761. doi: 10.1128/IAI.00190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordes FS, Komoriya K, Larquet E, Yang S, Egelman EH, Blocker A, et al. Helical structure of the needle of the type III secretion system of Shigella flexneri. J Biol Chem. 2002;278(19):17103–17107. doi: 10.1074/jbc.M300091200. [DOI] [PubMed] [Google Scholar]
- 15.Deane JE, Roversi P, Cordes FS, Johnson S, Kenjale R, Daniell S, et al. Molecular model of a type III secretion system needle: Implications for host-cell sensing. Proc Natl Acad Sci USA. 2006;103(33):12529–12533. doi: 10.1073/pnas.0602689103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280(5363):602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Wang Y, Picking WL, Picking WD, De Guzman RN. Solution structure of monomeric BsaL, the type III secretion needle protein of Burkholderia pseudomallei. J Mol Biol. 2006;359(2):322–330. doi: 10.1016/j.jmb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 18.He SY, Nomura K, Whittam TS. Type III protein secretion mechanism in mammalian and plant pathogens. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2004;1694(1–3):181–206. doi: 10.1016/j.bbamcr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Ouellette AN, Egan CW, Rathinavelan T, Im W, De Guzman RN. Differences in the electrostatic surfaces of the type III secretion needle proteins PrgI, BsaL, and MxiH. J Mol Biol. 2007;371(5):1304–1314. doi: 10.1016/j.jmb.2007.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darboe N, Kenjale R, Picking WL, Picking WD, Middaugh CR. Physical characterization of MxiH and PrgI, the needle component of the type III secretion apparatus from Shigella and Salmonella. Protein Sci. 2005;15(3):543–552. doi: 10.1110/ps.051733506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett BS, Picking WD, Picking WL, Middaugh CR. The response of type three secretion system needle proteins MxiHΔ5, BsaLΔ5, and PrgIΔ5 to temperature and pH. Proteins. 2008;73(3):632–643. doi: 10.1002/prot.22085. [DOI] [PubMed] [Google Scholar]
- 22.Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HM. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989;143(4):1239–1244. [PubMed] [Google Scholar]
- 23.Ikehata K, Duzhak TG, Galeva NA, Ji T, Koen YM, Hanzlik RP. Protein targets of reactive metabolites of Thiobenzamide in rat liver in Vivo. Chemical Research in Toxicology. 2008;21(7):1432–1442. doi: 10.1021/tx800093k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hem SL, White JL. Structure and properties of aluminum containing adjuvants. In: Powell MF, Newman MJ, editors. Vaccine Design: The Subunit and Adjuvant Approach. New York: Plenum Press; 1995. pp. 249–276. [DOI] [PubMed] [Google Scholar]
- 25.Martin A. Physical Pharmacy. Philadelphia: Lippincott Williams and Wilkins; 1992. Interfacial Phenomena; pp. 380–381. [Google Scholar]
- 26.Lee JC, Timasheff SN. The stabilization of proteins by sucrose. J Biol Chem. 1981;256(14):7193–7201. [PubMed] [Google Scholar]
- 27.Espina M, Olive AJ, Kenjale R, Moore DS, Ausar SF, Kaminski RW, et al. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect Immun. 2006;74(8):4391–4400. doi: 10.1128/IAI.00440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turbyfill KR, Mertz JA, Mallett CP, Oaks EV. Identification of epitope and surface-exposed domains of Shigella flexneri i3nvasion plasmid antigen D (IpaD) Infect Immun. 1998;66(5):1999–2006. doi: 10.1128/iai.66.5.1999-2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data.
Individual titers for Anti MxiH (Studies 1 & 2) and PrgI (Study 3) IgG serum responses following intramuscular injections on study days 1, 14, 28 of Buffer Control ( ), M1/M-His/P1 (
), M1/M-His/P1 ( ), M10/M-Al/P10 (
), M10/M-Al/P10 ( ), M50/N-His/P50 (
), M50/N-His/P50 ( ). N-Al/P-Soln (
). N-Al/P-Soln ( ), respectively. Blood samples were collected via the submandibular vein on days 0, 7, 21, 35, and 49.
), respectively. Blood samples were collected via the submandibular vein on days 0, 7, 21, 35, and 49.