Abstract
Since the onset of myocardial infarction and stroke has distinct circadian patterns, the disruption of circadian rhythms may contribute to cardiovascular disease. A recent clinical study, reporting that the severity of myocardial ischemia depends on the time-of-day when ischemia occurs, highlights the impact of circadian rhythms on cardiovascular disease. In support of these observations, we found a cardioprotective role of the circadian rhythm protein Period 2 (Per2) during myocardial ischemia in mice. In these studies, exposing mice to daylight induced cardiac Per2, which was associated with protection from myocardial ischemia. Recent epidemiological studies found sunlight to be the dominant regulator of the human circadian rhythm, suggesting sunlight cycles are critical for maintaining a healthy cardiovascular system. However, the impact of circadian rhythm proteins on human disease remains unclear. This current review aims to make a link to current and future clinical practice by targeting cardiac Per2.
Keywords: Period 2, Circadian rhythm, Heart ischemia, Cardiac metabolism, Light therapy, Cardiovascular disease, Adenosine, Adora2b, Ischemic preconditioning
1. Introduction
The first recorded observation of an endogenous circadian oscillation was made by the French scientist Jean-Jacques d‘Ortous de Mairan in 1729 (Edery, 2000). He noted that a 24-h pattern in the movement of Mimosa pudica plant leaves continued even when the plant was kept in constant darkness. Aschoff also applied these methods to studies in humans by building an underground “bunker” to isolate human subjects from any external environmental cues (Aschoff, 1965). After more than twenty years of tracking sleep-wake cycles, Aschoff concluded that humans indeed have endogenous circadian oscillators. This discovery has become the foundation for our understanding of many medical problems such as aging, sleep disorders, and jet lag. In the 1970s, researchers began experimenting with the circadian clock in Drosophila melanogaster, which led to the identification of gene loci such as Clock or Period (Per) as important players in these processes (Tei et al, 1997).
Many physiological and metabolic processes display day-night rhythms. Dysregulation of these pathways can result in the development of cholesterol abnormalities, insulin resistance, and obesity. These disorders often occur simultaneously and are subsumed under the term ‘metabolic syndrome’ (Staels, 2006). Interestingly, studies have shown that deletion of the circadian rhythm genes Clock or Bmal1, results not only in circadian disturbances, but also in metabolic abnormalities of lipid and glucose homeostasis (Staels, 2006). This phenotype resembles the abovementioned ‘metabolic syndrome’ that is often associated with cardiovascular diseases (Haffner and Taegtmeyer, 2003). Very recent studies found an important role for Period 2 (Per2) in the regulation of fatty acid metabolism in adipocytes (Grimaldi et al., 2010) or glucose metabolism of the heart (Eckle et al., 2012). In fact, our studies on cardiac Per2 (Eckle et al., 2012) showed that Per2 facilitated a metabolic switch from fatty acid to glucose metabolism during myocardial ischemia, which was protective against the ischemic injury. Moreover, while Per2−/− mice had larger infarct sizes when compared to controls, light exposure of wildtype mice was able to induce cardiac Per2, which was associated with elevated transcript levels of glycolytic enzymes and reduced infarct sizes. In the current review, we will recapitulate the consequences of disrupted circadian rhythm pathways on metabolic processes while focusing on the light dependent protein Per2 and its clinical implications for cardiovascular disease.
2. Pathogenesis
2.1. Regulatory control points of the circadian rhythm network
The ‘master’ internal clock in mammals is located in the hypothalamic suprachiasmatic nucleus (SCN). The primary environmental synchronizer of circadian rhythms in mammals is the daily light-dark cycle (Takahashi et al, 2008). Photosensitive retinal ganglion cells, expressing the photopigment melanopsin, integrate photic information for entrainment within the retina and project to the core region of the SCN via the retinohypothalamic tract (Fig. 1). These photoreceptors are most sensitive to blue light (Hattar et al., 2002) and if these receptors are completely depleted, the organism does not have light as a ‘Zeitgeber’, or time synchronizer (Guler et al., 2008).
Fig. 1.
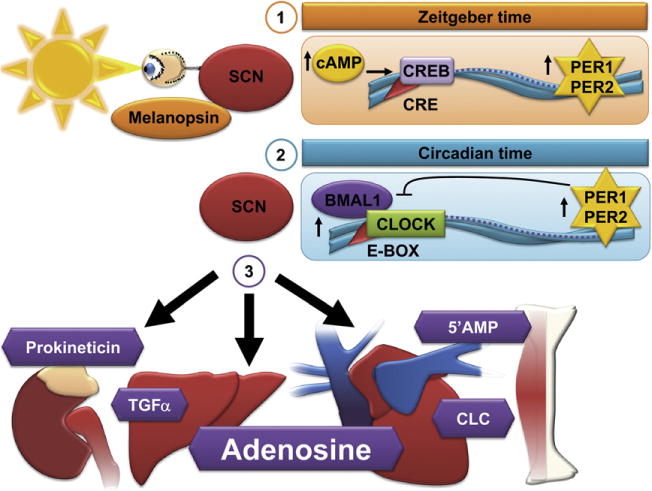
Regulatory mechanisms: Circadian vs. Zeitgeber time. The suprachiasmatic nucleus (SCN) in the brain is the central regulator of circadian rhythmicity. (1) External stimuli such as light determine Zeitgeber time. Light via melanopsin in the retinal ganglion cells increases cAMP and subsequent CREB (cyclic AMP responsive element-binding protein) binding to cAMP-responsive elements (CRE) within the Period 1 (Per1) and Period 2 (Per2) promotor, leading to the transcriptional induction of Per1 and Per2. (2) While circadian time is independent of any external stimuli, it consists of a transcriptional-translational feedback loop. During the day Clock/Bmal1 levels rise, bind to E-box elements within the promoter of Per1 and Per2 and increase Per1 and Per2 transcript levels. High Per1 and Per2 levels inhibit their own transcription via inhibition of Clock/Bmal1 activity. (3) Hormonal and humoral factors (e.g. transforming growth factor α (TGFα), cardiotrophin-like cytokine (CLC), prokineticin, 5′-AMP or adenosine) are supposed to control circadian rhythms in peripheral organs and are able to control metabolism (e.g. 5′-AMP or adenosine).
Both neuronal and humoral signals originate from the SCN and control diverse physiological functions. SCN-secreted circulating factors are likely to include signaling molecules such as transforming growth factor α (TGFα), cardiotrophin-like cytokine, prokineticin, 5′-AMP or adenosine (Zhang et al., 2006; Takahashi et al., 2008) (Fig. 1).
The endogenous circadian clock mechanism involves a cell autonomous transcription-translation feedback loop. During the day, the transcription factor Clock interacts with Bmal1 to activate transcription of the Per and Cryptochrome (Cry) genes, resulting in high levels of these transcripts. The resulting Per and Cry proteins translocate to the nucleus to inhibit their own transcription (Takahashi et al, 2008). The entire cycle takes approximately 24 h. This process is also active without external cues and takes approximately 25 h in humans (‘circadian’ time vs. ‘zeitgeber’ time, Fig. 1) (Aschoff, 1965).
Besides autonomous mechanisms, circadian clock resetting by photic induction of Per1 and Per2 genes is mediated by the binding of phosphorylated CREB (cyclic AMP responsive element-binding protein) to a cAMP-responsive element (CRE) in the respective promoters (Ginty et al., 1993). In our studies we identified a CRE, that was responsible for an adenosine receptor A2b (Adora2b) mediated induction of Per2 transcript (Fig. 2). Moreover, these studies showed that cardiac Per2 stabilization maybe achieved by daylight exposure in mice. This is in line with previous studies reporting that timing of light exposure determines the circadian profile of cardiac Per2 protein expression (Bendova and Sumova, 2006). The mechanisms linking central circadian rhythm regulation and peripheral Per2 stabilization may involve humoral pathways such as cyclic alterations in adenosine concentrations (Zhang et al., 2006) (Figs. 1 and 2).
Fig. 2.
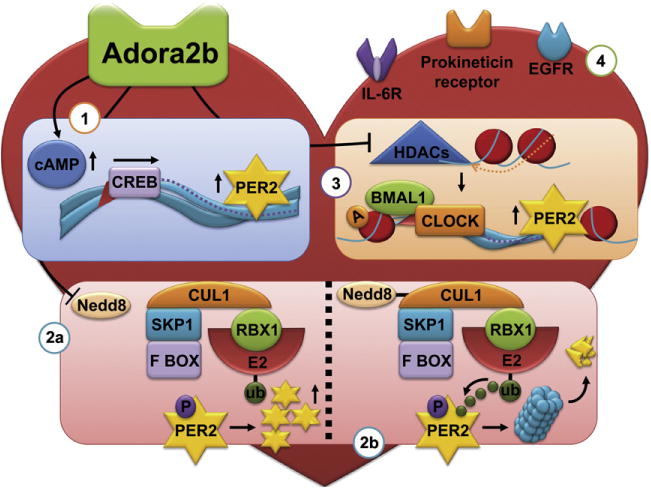
Proposed regulation of cardiac Per2. (1,2) Adenosine A2b receptor (Adora2b) signaling is able to induce cardiac Period 2 (Per2) transcript via the cAMP/CREB (cyclic AMP responsive element-binding protein) pathway (1) or Per2 protein via the inhibition of the SKP1–Cullin1–F-box (SCF) ubiquitin E3 ligase (2a). The SCF complex consists of the Skp1 bridging protein, Cullin 1 (Cul1, which forms the major structural scaffold), the F-box protein (contributes to the specificity of SCF by aggregating to target proteins) and Rbx1 (contains a small zinc-binding domain called the RING Finger, to which the E2-ubiquitin conjugate binds, allowing the transferral of the ubiquitin to a lysine residue on the target protein). As Per2 is phosphorylated during the day, it becomes a target for the SCF ubiquitin ligase with subsequent degradation via the proteasome (2b). Adora2b signaling deneddylates Cullin1 (Cul1) which inhibits the ubiquitin ligase activity, thereby increasing Per2 protein levels (2a). (3) Adora2b and Per2 levels are increased in patients with ischemic heart failure. As such, Adora2b signaling could also lead to the induction of Per2 transcript and protein via the inhibition of histone deacetylases (HDACs). Acetylation of histone H3 increases the binding of polymerase polII to the Per2 promotor, increasing its transcription (3). (4) Besides Adora2b signaling other humoral pathways could be involved in the regulation of cardiac Per2, e.g. TGFα via Il-6 receptors, cardiotrophin-like cytokine (CLC) viaEGFR receptors or prokineticin via its respective receptors.
Furthermore, post-translational degradation of circadian clock proteins are crucial steps for a circadian periodicity. As seen in Drosophila, mammalian Per proteins are phosphorylated as they accumulate during the day. Phosphorylated Per becomes a target for the SCF (SKP1-Cullin1-F-box protein) E3 ubiquitin ligase leading to degradation by the 26S proteasome (Gallego and Virshup, 2007). The SCF complex is active only when Cullin1 (Cul1) is covalently modified by the ubiquitin-like protein Nedd8. We found that deneddylation of Cul1 was greatly enhanced after Adora2b agonist treatment, leading to Per2 stabilization in human cells as well as in isolated murine cardiomyocytes (Fig. 2). Interestingly, enhanced Adora2b signaling is one of several pathways implicated in cardiac adaptation to ischemia. In fact, cardioprotective responses elicited by ischemic preconditioning (IP) are abolished following genetic ablation of Adora2b signaling (Eckle et al., 2007). IP consists of short and repeated episodes of ischemia and reperfusion prior to myocardial infarction resulting in attenuation of infarct size (Murry et al., 1986). Despite it long being the focus of extensive studies, including its replication in humans, the fundamental mechanism of IP remains unclear. So far, Adora2b is the only one of the four-adenosine receptors whose cardiac expression is induced by ischemia in both mice and humans and whose function is implicated in ischemic preconditioning (Eckle et al., 2012). As Adora2b signaling increases Per2 expression by altering both Per2 transcription and Per2 protein stability, Per2 is a strong candidate for mediating ischemic preconditioning (Fig. 2).
Histone acetylation is another important regulator of the mammalian clock (Etchegaray et al., 2003). The level of histone 3 (H3) acetylation varies throughout the day, as does the recruitment of RNA polymerase II (polII) to Per promoters. Per transcript levels are at their highest when H3 acetylation and polII binding are greatest, indicating that acetylation enhances transcription by increasing the recruitment of polII to the Per promoter. Deacetylase activity is therefore associated with inhibition of Per transcription. Deacetylase activity can be mediated by histone deacetylases (HDACs). Interestingly, HDACs play a crucial role in heart failure and as such, HDAC inhibitors are able to prevent heart failure in animal models (McKinsey, 2012). One possible explanation could be the upregulation of Per2 in the heart via HDAC inhibition (Fig. 2). This would support our findings of Per2 overexpression in heart samples obtained from patients with ischemic heart failure (Eckle et al., 2012). However, determining direct effects of Per2 on heart failure will require future studies.
2.2. Deficient circadian rhythm pathways increase the risk for cardiovascular disease
The circadian clock in metabolism is one of the most studied areas in the field, outside of the central clock (Richards and Gumz, 2012). For example, Bmal1−/− and Clock−/− mice are diabetic. Clock−/− mice display a metabolic syndrome and Cry1−/−mice develop hypertension. Per2−/− animals have an increased senescence and a dysfunction of the endothelium leading to autoamputation in a mouse model for hind limb ischemia (Wang et al., 2008). Recent studies implicated Per2 in the regulation of fatty acid metabolism with increased oxygen consumption in Per2−/− mice (Grimaldi et al, 2010). Our studies showed impaired glycolysis during myocardial ischemia and severe depletion of glycogen storages leading to dramatically increased infarct sizes in Per2−/− mice (Eckle et al., 2012). Another study on Krueppel-like-factor 15 indicated that circadian rhythms also play a critical role in developing cardiac arrhythmias (Jeyaraj et al., 2012), which could cause sudden cardiac death. In fact, these studies found that Bmal1−/− or Per2−/−/Cry1−/− mice exhibited deregulation of cardiac ion channels in conjunction with marked action potential prolongation (Fig. 3).
Fig. 3.
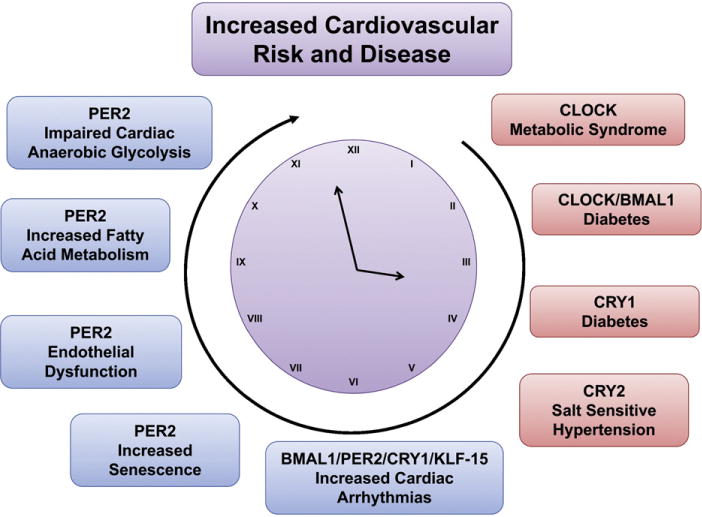
Disruption of circadian pathways increases the risk for cardiovascular disease. Disruption of different circadian proteins leads to a huge variety of metabolic, vascular or cardiac phenotypes, which all are known risk factors for the development of cardiovascular disease. Blue box: Per2 associated; red box: other clock proteins than Per2.
Even though deficiency of different circadian rhythm proteins leads to a variety of metabolic or vascular phenotypes, all phenotypes represent well-known risk factors for the development of cardiovascular disease. Thus, an intact circadian rhythm seems to be crucial to maintain a healthy cardiovascular system (Fig. 3).
2.3. Diurnal variations of cardiovascular disease
Throughout the 1970s reports accumulated on the circadian variation of nocturnal stroke in association with hypertension. By 1985, a multicenter analysis of the limitations of infarct size observed circadian periodicity in the time of onset of the clinical manifestations of acute myocardial infarction, with a peak incidence between 6:00 a.m. and noon. This increased morning incidence has been confirmed repeatedly in individual populations and in a meta-analysis of more than 60,000 patients. Other studies reported an increased incidence during early morning hours in unstable angina, sudden death, stroke, ventricular arrhythmias, cardiogenic shock, aortic aneurysm rupture, stent thrombosis, and transient myocardial ischemia (Braunwald, 2012). In fact, two recent studies found that patients have larger infarct sizes in early morning hours compared to other times of the day (Reiter et al., 2012; Suarez-Barrientos et al., 2011; Ibanez et al., 2012).
As cardiac metabolism is critical for the heart to adapt to ischemia, circadian variations in metabolism could explain the above findings. In order to adapt its metabolism, the cardiomyocyte has a number of important adaptive transcription factor pathways controlling cardiac energy metabolism, including the Hypoxia inducible factor-1 α (Hif1α) pathway (Lopaschuk and Jaswal, 2010). Hif1a is an important mediator of anaerobic glycolysis in mammalian cells. In our studies, Per2 stabilization was associated with the induction of Hif1α dependent anaerobic glycolysis, where hearts from Per2−/− mice were unable to produce lactate during ischemia. Interestingly, earlier studies found that Per2 serves as a co-regulator of transcription. Transcription factors involved in the regulation of metabolic pathways and are able to interact with Per2 in vitro include Rev-Erbα, or Pparγ (Ripperger et al, 2010), whereas our studies indicated Per2 as coactivator of Hif1α. Nevertheless, the tight regulation of fatty acid and glucose metabolism is crucial during myocardial ischemia or reperfusion. Therefore, the circadian, Per2 dependent regulation of Hif1a indicates that targeting these pathways would require a finely tuned and time-of-day dependent drug administration (Fig. 4).
Fig. 4.
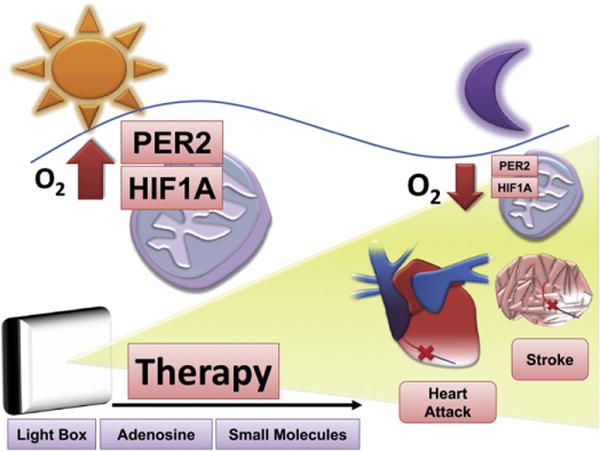
Novel and innovative therapeutic concepts for cardiovascular disease. Potential therapeutics to enhance circadian rhythm pathways during myocardial ischemia or stroke could be light boxes, adenosine (e.g. adenosine receptor A2b agonists) or small molecules that are able to specifically enhance Period 2 (Per2) levels or Per2 dependent pathways (Hif1α). Based on the circadian nature of cardiovascular diseases, infarct sizes, Per2 or Hif1α levels, future therapies have to consider the time-of-day when and what type of treatment is initiated.
3. Therapy
3.1. Current clinical practice and applications
Circadian rhythmicity of cardiac metabolism and susceptibility to ischemia suggests that the outcome of experiments at the bench or pharmacotherapy and interventions in a clinical setting are time-of-day dependent. In fact, chronotherapeutics has already proven to be promising for improving the therapeutic index of several drugs. An example relates to the circadian variations in cholesterol synthesis. For this reason, statins, widely prescribed cholesterol-lowering drugs that inhibit this enzyme, are most efficient when taken before bedtime (Staels, 2006). Thus, the observation that cardiac Per2 controls cardiac energy homeostasis may lead to the development of more finely tuned and hopefully more optimal treatments of myocardial ischemia and its related complications (Fig. 4).
So far, no specific Per2 activator exists. However, specific Adora2b agonists or specific Hif1α activators are available and could be an effective intervention in a clinical setting. Along with colleagues, we have shown the beneficial effect of Adora2b or Hif1a activation on myocardial infarct sizes (Eckle et al., 2007, 2008) that seems to be strongly associated with Per2 mediated cardioprotection (Eckle et al., 2012). Future studies need to investigate if the time-of-day these drugs are administered could increase their therapeutic index. However, since both targets would depend on the function of Per2 these therapies might not work in diseased hearts with a Per2 dysfunction. Therefore, pharmacological approaches targeting cardiac metabolism downstream of Per2 might be the better strategy in the treatment or prevention of myocardial ischemia. Indeed, several clinical studies using a metabolic approach (e.g. targeting fatty acid pathways) to patients with myocardial ischemia have revealed promising results in the recent years (Jaswal et al., 2011; Frank et al, 2012). As our studies just scratched the surface by examining how Per2 regulates cardiac metabolism, future in depth studies will be necessary to define cardiac metabolism downstream of Per2.
3.2. Light as therapy
Recent epidemiological studies found that the circadian rhythm in humans is dominantly regulated by sunlight (Roenneberg et al., 2007). This implicates sunlight cycles as being critical for the human organism. Diurnal variations of heart attacks suggest lack of daylight as a key player in cardiovascular disease. Only daylight with an intensity >2500 lx is able to synchronize the human circadian system (Lewy et al., 1980). Light boxes (>10,000 lx) with built-in UV filters, used to treat seasonal disorders, are able to ensure synchronization of the human circadian system. As light receptors in the retinal ganglion cells are most sensitive to blue light (Hattar et al., 2002), newer light boxes mainly emit blue light to shorten exposure times. Surprisingly, a very recent study using UV light showed that ultra-violet sensitive cones might also play an important role for circadian and sleep regulation in mice (van Oosterhout et al, 2012). However, exploring whether UV light is a safe approach in the context of resetting the circadian clock would require further evaluation. In our animal studies, full spectrum daylight (>10,000 lx; excluding UV light) exposure was able to induce cardiac Per2, which was associated with metabolic changes and protection from myocardial ischemia. In this setting, we exposed mice to daylight prior to the onset of ischemia. However, it needs to be elucidated whether light exposure after ischemia is also beneficial. Moreover, based on the circadian pattern of Per2 with the levels lowest during late night and early morning hours, light exposure could be most effective at these times (Fig. 4). Indeed, our studies of light exposure in animals were performed during times where cardiac Per2 levels were found to be the lowest. Whether light exposure during periods where Per2 levels are high could potentiate the cardioprotective effect of Per2, will require additional studies. Nevertheless, translating our findings into a human system would indicate daylight boxes as future strategies in the treatment or prevention of myocardial ischemia or other cardiovascular disease such as stroke (Fig. 4).
3.3. Summary
Whether daylight application in hospitals will improve the outcome after myocardial ischemia or stroke will require future studies. Beside light therapy, new drugs such as small molecules (Hirota et al, 2012) that are able to enhance circadian proteins (e.g. Per2) or circadian protein pathways (e.g. Hif1α) could represent novel treatment interventions in myocardial ischemia and beyond. Future studies will have to consider the time-of-day for a treatment strategy – regardless if it is light, a drug or an intervention (Fig. 4).
References
- Aschoff J. Circadian rhythms in man. Science. 1965;148:1427–32. doi: 10.1126/science.148.3676.1427. [DOI] [PubMed] [Google Scholar]
- Bendova Z, Sumova S. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiological Research. 2006;55:623–32. doi: 10.33549/physiolres.930849. [DOI] [PubMed] [Google Scholar]
- Braunwald E. On circadian variation of myocardial reperfusion injury. Circulation Research. 2012;110:6–7. doi: 10.1161/CIRCRESAHA.111.260265. [DOI] [PubMed] [Google Scholar]
- Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nature Medicine. 2012;18:774–82. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, et al. Cardioprotection by ecto-5′nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- Edery I. Circadian rhythms in a nutshell. Physiological Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–82. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial Ischemia reperfusion injury: from basic science to clinical bedside. Seminars in Cardiothoracic and Vascular Anesthesia. 2012 doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nature Reviews Molecular Cell Biology. 2007;8:139–48. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, et al. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–41. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, et al. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metabolism. 2010;12:509–20. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108:1541–5. doi: 10.1161/01.CIR.0000088845.17586.EC. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–7. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez B, Suarez-Barrientos A, Lopez-Romero P. Circadian variations of infarct size in STEM1. Circulation Research. 2012;110:e22. doi: 10.1161/CIRCRESAHA.111.262816. (author reply e23). [DOI] [PubMed] [Google Scholar]
- Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation—a novel therapeutic intervention in the ischemic and failing heart. Biochimica et Biophysica Acta. 2011;1813:1333–50. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–9. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. Journal of Cardiovascular Pharmacology. 2010;56:130–40. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annual Review of Pharmacology and Toxicology. 2012;52:303–19. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circulation Research. 2012;110:105–10. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB Journal. 2012;26:3602–13. doi: 10.1096/fj.12-203554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripperger JA, Schmutz I, Albrecht U. PERsuading nuclear receptors to dance the circadian rhythm. Cell Cycle. 2010;9:2515–21. doi: 10.4161/cc.9.13.12075. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Current Biology. 2007;17:R44–5. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nature Medicine. 2006;12:54–5. doi: 10.1038/nm0106-54. (discussion 55). [DOI] [PubMed] [Google Scholar]
- Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, et al. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011 doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Reviews Genetics. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, et al. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–6. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- van Oosterhout F, Fisher SP, van Diepen HC, Watson TS, Houben T, VanderLeest HT, et al. Ultraviolet light provides a major input to non-image-forming light detection in mice. Current Biology. 2012;22:1397–402. doi: 10.1016/j.cub.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, et al. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–73. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–3. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]


